Abstract
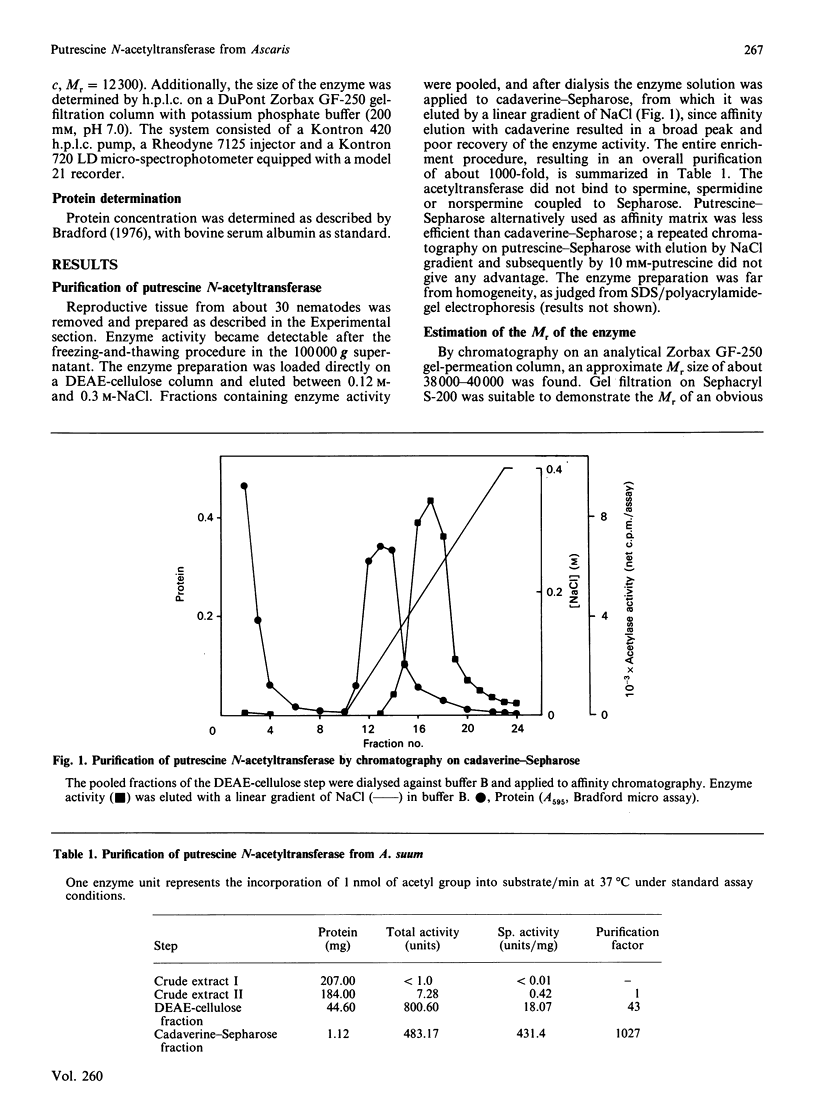
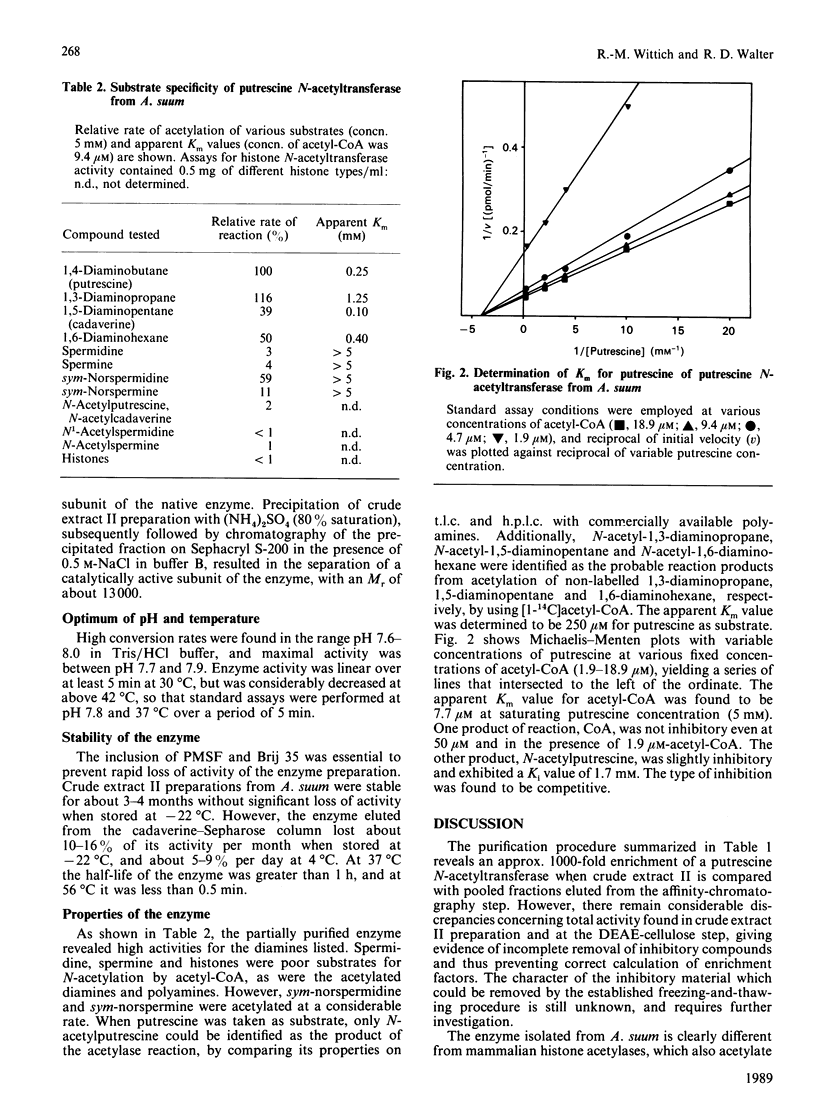
A cytosolic enzyme catalysing the acetylation of the diamines putrescine, cadaverine, 1,3-diaminopropane and 1,6-diaminohexane has been partially purified from reproductive tissue of the intestinal parasitic nematode Ascaris suum. The enzyme formed N-acetylated derivatives of the above diamines when incubated in the presence of acetyl-CoA. The Michaelis constants (Km) for the above diamines were 0.25 nM, 0.1 mM, 1.25 mM and 0.4 mM respectively, and the apparent Km for acetyl-CoA was 7.7 microM. sym-Norspermidine was also acetylated by this enzyme preparation, and, at a much lower rate, the enzyme acted on sym-norspermine. The common polyamines, spermidine and spermine, and histones were not substrates. Purification steps involved a freezing-and-thawing procedure to release enzyme activity from unknown inhibitors, DEAE-cellulose chromatography and affinity chromatography on cadaverine-Sepharose, from which the enzyme was eluted by increasing ionic strength. The enzyme exhibited an apparent Mr of about 38,000-40,000, and it consisted of at least two subunits, of which the catalytic one had an Mr of about 13,000. The partially purified enzyme showed no deacetylase activity, and its activity was competitively inhibited by the product N-acetylputrescine, but not by CoA. The name putrescine N-acetyltransferase is suggested for this enzyme, which may have an important function in the degradation of diamines of lower eukaryotes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belikoff E., Wong L. J., Alberts B. M. Extensive purification of histone acetylase A, the major histone N-acetyl transferase activity detected in mammalian cell nuclei. J Biol Chem. 1980 Dec 10;255(23):11448–11453. [PubMed] [Google Scholar]
- Blankenship J., Walle T. Acetylation of spermidine and spermine by rat liver and kidney chromatin. Arch Biochem Biophys. 1977 Feb;179(1):235–242. doi: 10.1016/0003-9861(77)90108-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Della Ragione F., Erwin B. G., Pegg A. E. Studies of the acetyl-CoA-binding site of rat liver spermidine/spermine N1-acetyltransferase. Biochem J. 1983 Sep 1;213(3):707–712. doi: 10.1042/bj2130707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Ragione F., Pegg A. E. Studies of the specificity and kinetics of rat liver spermidine/spermine N1-acetyltransferase. Biochem J. 1983 Sep 1;213(3):701–706. doi: 10.1042/bj2130701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. R. Calf liver nuclear N-acetyltransferases. Purification and properties of two enzymes with both spermidine acetyltransferase and histone acetyltransferase activities. J Biol Chem. 1978 Jan 10;253(1):233–237. [PubMed] [Google Scholar]
- Libby P. R. Rat liver nuclear N-acetyltransferases: separation of two enzymes with both histone and spermidine acetyltransferase activity. Arch Biochem Biophys. 1980 Aug;203(1):384–389. doi: 10.1016/0003-9861(80)90190-3. [DOI] [PubMed] [Google Scholar]
- Matsui I., Wiegand L., Pegg A. E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981 Mar 10;256(5):2454–2459. [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragione F. D., Pegg A. E. Purification and characterization of spermidine/spermine N1-acetyltransferase from rat liver. Biochemistry. 1982 Nov 23;21(24):6152–6158. doi: 10.1021/bi00267a020. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Rennert O. M. Interconversion, catabolism and elimination of the polyamines. Med Biol. 1981 Dec;59(5-6):334–346. [PubMed] [Google Scholar]
- Seiler N., Knödgen B. Determination of the naturally occurring monoacetyl derivatives of di- and polyamines. J Chromatogr. 1979 Oct 11;164(2):155–168. doi: 10.1016/s0378-4347(00)81184-6. [DOI] [PubMed] [Google Scholar]
- Seiler N., al-Therib M. J. Acetyl-CoA: 1,4-diaminobutane N-acetyltransferase. Occurrence in vertebrate organs and subcellular localization. Biochim Biophys Acta. 1974 Jul 4;354(2):206–212. doi: 10.1016/0304-4165(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Wiktorowicz J. E., Bonner J. Studies on histone acetyltransferase. Partial purification and basic properties. J Biol Chem. 1982 Nov 10;257(21):12893–12900. [PubMed] [Google Scholar]
- Wittich R. M., Kilian H. D., Walter R. D. Polyamine metabolism in filarial worms. Mol Biochem Parasitol. 1987 Jun;24(2):155–162. doi: 10.1016/0166-6851(87)90102-2. [DOI] [PubMed] [Google Scholar]


