Abstract
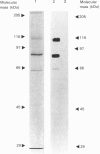
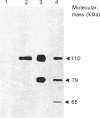
Progesterone receptor was purified in a single step from human uteri using immunoaffinity chromatography with monoclonal antibodies raised against the rabbit receptor. A total of 39 monoclonal antibodies were prepared against the human receptor and characterized. Immunoblot experiments using crude uterine cytosol or purified receptor showed that the antibodies belonged to three groups: they recognized either a single receptor species (apparent molecular mass 110 kDa), two species (110 and 79 kDa) or three species (110, 79 and 65 kDa). The species specificity of the antibodies was very variable; some recognized only the human receptor, others interacted with several mammalian receptors (rabbit, guinea pig and rat), while a single one also cross-reacted with the chick receptor. The epitopes recognized by 15 of the antibodies showing the strongest affinity for the human receptor were mapped using a method recently described [Lorenzo, Jolivet & Milgrom (1988) Eur. J. Biochem. 176, 53-60] which involves immunoprecipitation of C-terminally truncated proteins obtained by transcription and translation of cloned cDNA in vitro. The antibodies recognized five different regions of the receptor, all localized on the N-terminal half of the protein. None of the antibodies interacted with an epitope present in the DNA-binding or steroid-binding regions of the receptor. Comparison of the pattern of receptor species recognized by the antibodies and the localization of their epitopes showed that the 79 and 65 kDa receptor species were derived from the 110 kDa form by deletion of its N-terminal part. The N-terminus of the 79 kDa species was found to lie between amino acids 121 and 208, and that of the 65 kDa species between amino acids 208 and 296.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brailly S., Lorenzo F., Jolivet A., Logeat F., Pallud C., Milgrom E. Radioimmunoassay of progesterone receptor in human tissues: application to breast cancer. J Endocrinol. 1988 Mar;116(3):427–434. doi: 10.1677/joe.0.1160427. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J., Okret S., Wrange O., Gustafsson J. A. Immunochemical analysis of the glucocorticoid receptor: identification of a third domain separate from the steroid-binding and DNA-binding domains. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4260–4264. doi: 10.1073/pnas.79.14.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. L., Zaino R. J., Feil P. D., Miller J. V., Steck M. E., Ohlsson-Wilhelm B. M., Satyaswaroop P. G. Monoclonal antibodies to human progesterone receptor: characterization by biochemical and immunohistochemical techniques. Endocrinology. 1987 Sep;121(3):1123–1132. doi: 10.1210/endo-121-3-1123. [DOI] [PubMed] [Google Scholar]
- Estes P. A., Suba E. J., Lawler-Heavner J., Elashry-Stowers D., Wei L. L., Toft D. O., Sullivan W. P., Horwitz K. B., Edwards D. P. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987 Sep 22;26(19):6250–6262. doi: 10.1021/bi00393a045. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legrain P., Juy D., Buttin G. Rosette-forming cell assay for detection of antibody-synthesizing hybridomas. Methods Enzymol. 1983;92:175–182. doi: 10.1016/0076-6879(83)92017-7. [DOI] [PubMed] [Google Scholar]
- Logeat F., Hai M. T., Milgrom E. Antibodies to rabbit progesterone receptor: crossreaction with human receptor. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1426–1430. doi: 10.1073/pnas.78.3.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeat F., Pamphile R., Loosfelt H., Jolivet A., Fournier A., Milgrom E. One-step immunoaffinity purification of active progesterone receptor. Further evidence in favor of the existence of a single steroid binding subunit. Biochemistry. 1985 Feb 12;24(4):1029–1035. doi: 10.1021/bi00325a034. [DOI] [PubMed] [Google Scholar]
- Logeat F., Vu Hai M. T., Fournier A., Legrain P., Buttin G., Milgrom E. Monoclonal antibodies to rabbit progesterone receptor: crossreaction with other mammalian progesterone receptors. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6456–6459. doi: 10.1073/pnas.80.21.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosfelt H., Logeat F., Vu Hai M. T., Milgrom E. The rabbit progesterone receptor. Evidence for a single steroid-binding subunit and characterization of receptor mRNA. J Biol Chem. 1984 Nov 25;259(22):14196–14202. [PubMed] [Google Scholar]
- Lorenzo F., Jolivet A., Loosfelt H., Thu vu Hai M., Brailly S., Perrot-Applanat M., Milgrom E. A rapid method of epitope mapping. Application to the study of immunogenic domains and to the characterization of various forms of rabbit progesterone receptor. Eur J Biochem. 1988 Sep 1;176(1):53–60. doi: 10.1111/j.1432-1033.1988.tb14250.x. [DOI] [PubMed] [Google Scholar]
- Misrahi M., Atger M., d'Auriol L., Loosfelt H., Meriel C., Fridlansky F., Guiochon-Mantel A., Galibert F., Milgrom E. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun. 1987 Mar 13;143(2):740–748. doi: 10.1016/0006-291x(87)91416-1. [DOI] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., Groyer-Picard M. T., Lorenzo F., Jolivet A., Vu Hai M. T., Pallud C., Spyratos F., Milgrom E. Immunocytochemical study with monoclonal antibodies to progesterone receptor in human breast tumors. Cancer Res. 1987 May 15;47(10):2652–2661. [PubMed] [Google Scholar]
- Press M. F., Greene G. L. Localization of progesterone receptor with monoclonal antibodies to the human progestin receptor. Endocrinology. 1988 Mar;122(3):1165–1175. doi: 10.1210/endo-122-3-1165. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Beito T. G., Proper J., Krco C. J., Toft D. O. Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology. 1986 Oct;119(4):1549–1557. doi: 10.1210/endo-119-4-1549. [DOI] [PubMed] [Google Scholar]
- Vu Hai M. T., Milgrom E. Characterization and assay of the progesterone receptor in rat uterine cytosol. J Endocrinol. 1978 Jan;76(1):21–31. doi: 10.1677/joe.0.0760021. [DOI] [PubMed] [Google Scholar]