Abstract
Medical technology plays a significant role in the reduction of disability and mortality due to the global burden of disease. The lack of diagnostic technology has been identified as the largest gap in the global health care pathway and the cost of this technology is a driving factor for its lack of proliferation. Technology developed in high-income countries is often focused on producing high quality, patient specific data at a cost high-income markets can pay. Little evidence exists to demonstrate incremental improvement to patient outcome for incremental increases in cost. Magnetic Resonance Imaging, and the development of portable and affordable versions of this technology provide a framework for understanding the role of engineering for global health. We focus on 2 key challenges which can be met using engineering principles: 1) development of appropriate and sustainable technology, and 2) reducing the need for expert interpretation of clinical results using machine learning methods. Engineering technologies for global health, where resources are often severely limited, requires a paradigm shift where the value of information to improve patient outcome drives technological development. While machine learning plays an important role in this process, great care must be taken to ensure appropriate translation to clinical practice.
Keywords: low field MRI, engineering in global health, open source medical technology, sustainable health technology, global health diagnostics
Graphical Abstract
Introduction
Engineering has played an active role in medical practice since such practice was first recorded. The first known prosthetics were thought to be developed in the 15th century B.C. in ancient Egypt where an ancient mummy shows evidence of the replacement of an amputated toe with a prosthetic one [32].
Industrialization has provided engineering solutions to medical problems at unprecedented depth and scale. The pharmaceutical industry alone is now worth an estimated $1.4 trillion USD worldwide, while the global medical device industry was valued at over $500 billion USD in 2023 [23]. This enormous worth has in some ways made possible incredible advances such as CRISPR, a genetic editing technology which promises to usher in a new era of personalized medicine in which technologies and therapies can be developed at the patient level [33]. Robotic assisted surgery has become common practice with at least 10 companies having already obtained FDA approval [30]. Advanced use of machine learning and artificial intelligence has captured the imagination of researchers around the globe. Combined with the speed and affordability of modern computing, data can be assimilated, analyzed, and put into use in ways that were never before possible [9]. While these advances in technology are sure to reduce disability and death for many, this has not been the case for most.
In high income countries (HIC), technological advancement in medicine is often a product of market driving forces as much as it is medical need. Indeed, most health technology is produced in HIC for high income markets [11]. Much of the success of this sector has been driven by federal research funding. In the United States, National Institutes of Health funding was involved in every drug approved by the FDA from 2010–2016, totalling over $200 billion USD in investment [4]. Without this enormous injection of high-risk funding, it is unlikely that private investors would have funded the additional research and development (R&D) and clinical trials required to bring these products to market.
In low- and middle-income countries (LMIC), few resources exist for high-risk R&D focused on local problems, regardless of the market size. There are some examples of successful medical technologies developed by and for LMIC, such as Chhabra ventriculoperitoneal shunts for hydrocephalus [3], but in general efforts have been inadequate and unfocused. There are excellent targeted funding mechanisms championed by HIC governments and organizations which expressly require the inclusion of LMIC researchers and entrepreneurs in the development phase of projects; however, these mechanisms often represent the health priorities of the HIC organization providing the funding, and can be difficult to align with local governmental healthcare systems and policies. While important, these cannot substitute for lack of local support and infrastructure to sustain development and incorporate new technology into healthcare.
Given the high cost of medical technology, many LMIC rely on donations from HIC governments or charitable organizations. Donations are an ineffective way to transfer technology from HIC to LMIC. A 2011 study estimated that between 40%−80% of medical technologies donated to LMIC were in a state of dysfunction [11,29]. A recent 2019 study estimates that 50% of all donated x-ray technology in LMIC is nonfunctional [19]. It appears that despite the technological advancement of HIC medical devices, disappointingly little may have improved within the past decade in terms of global accessibility to critical devices.
The Lancet commission on Technologies for Global Health (2012) suggests that the development of frugal technologies should be prioritized to improve global access. They highlight three main barriers to the use of technology in global health: 1) the technology does not exist, 2) it exists but is not accessible, or 3) it exists and is accessible, but is not adopted by the target health system [11].
Following the 2015 report on Global Burden of Disease, Injuries, and Risk Factors, Barber et al. (2017) showed that there has been global improvement in access to and quality of healthcare service related to so-called “amenable” deaths from 1990 to 2015. An amenable death is a death that could be avoided if current medical knowledge or technology were available and optimally applied at the time of care. Although improvement is encouraging, they also show that the pace of improvement has been significantly slower in LMIC, suggesting that the gap in treatment quality and access even for diseases we know how to treat is not sufficiently narrowing [1]. It is reasonable to conclude that the well documented lack of access to technology faced by most of the population of the world plays a significant role in this avoidable loss of life.
While most investment in technology for health is focused on pushing scientific boundaries, engineering in global health, including HIC regions, faces an entirely different challenge. How do we provide universal access to the treatments and technologies that we already understand well?
In order to answer this basic question, engineers must understand priorities for greatest impact. Following the 2019 COVID pandemic the importance of affordable and accessible diagnostics has risen to the forefront of global health. The 2021 Lancet commission on diagnostics identified diagnostic testing and diagnostic imaging as the largest gap in the care pathway for five of six considered conditions in a global study. In this study, 35–62% of patients in the care pathway who are screened for a disease do not pass to the diagnosis stage due to lack of access to diagnostics. The study estimates that if this gap were reduced to 10%, disability adjusted life years (DALYs) lost to these conditions could be reduced by more than 38 million life years [8]. Presently, the commission estimates that 47% of the world has little or no access to diagnostics.
Availability of diagnostic testing is a crucial part of the care pathway. Treatments are often known, affordable, and achievable. In many cases, treatment can be as simple as prescribing the correct antimicrobial medication following identification of the infecting organism. Diagnostic imaging, such as magnetic resonance imaging (MRI), computed tomography (CT), and endoscopy, are technologies that are often crucial to care selection and often lacking.
In the present article, we focus on the highly important role of diagnostic imaging in global health from an engineering perspective. We focus on 2 key challenges which can be met using engineering principles: 1) development of appropriate and sustainable technology, and 2) reducing the need for expert interpretation of clinical results using machine learning methods. We develop these ideas by pointing out recent advances in the field of MRI and offering suggestions for future work.
Low-field magnetic resonance imaging
MRI is one of the safest and most versatile diagnostic tools in modern medicine, but it is also one of the most costly. A commercial MRI scanner can cost around $1 million USD per tesla of field strength [27], not including the high cost of maintaining or building the specialized facility required to house it. Lower cost systems have recently been developed which prioritize affordability and portability at the cost of image quality. However, there is rigorous and extensive evidence that the image quality from such systems is sufficient to guide clinical decision making for certain diseases [14]. In terms of cost to benefit ratio for patients, it is likely that for diseases such as stroke, head trauma and infant hydrocephalus, this technology will emerge as an effective option for diagnostic care in low resource settings, and even in high resource settings where the added benefit of point-of-care and radiation free imaging may outweigh image quality decrement.
This is a critical concept in engineering for global health. Diagnostic technologies have historically been developed in high resource settings for high resource medical systems. As such, researchers and engineers have sought to maximize the data delivered by these devices. Yet there is a cost of each incremental increase in information, and that marginal cost of additional information [12] is typically ignored in evaluating the additional benefit for patient outcome. Few studies have been performed to link advances in diagnostic data with superior clinical outcomes. There is an important distinction between data and information that we make here. Data may be sparse or vast but contain sufficient diagnostic information for a clinician to plan a proper course of treatment.
Commercially available low-field MRI
The recently released 64 mili-tesla permanent magnet based portable MRI system from Hyperfine has broken many use barriers in very little time. It is currently the only FDA approved low-field system of its kind. Mazurek [21] demonstrated one of the first use cases in adult head imaging. The Hyperfine system was used to investigate stroke comparatively with CT and 1.5 tesla MRI systems. It was shown that the 64 mili-tesla system had comparable diagnostic accuracy as compared to CT and 1.5 tesla systems, however small bleeds in the brain remained undetected in some cases by the low-field system. The group extended this work by showing a detailed comparison between the low-field Hyperfine scanner and the gold standard in stroke care, CT, in a patient group of 177 who presented at the Yale New Haven Hospital with Stroke symptoms. Sensitivity and specificity to intracerebral hemorrhage (ICH) was calculated to be 96% [22]. This number was an improvement over the initial work which showed sensitivity and specificity of only 80% to small ICH. This drastic improvement is likely a product of an updated deep learning based post processing technique which was employed in the later study.
Mobile MRI has also been explored with the 64 mT hyperfine system [6]. The entire system was installed in a large cargo van and MRI imaging was performed outside the hospital setting. Image quality in the van was comparable to that in the hospital demonstrating the capacity of this technology to be used in a variety of settings. This same workflow has been performed with portable CT and is an important strategy for global health where mobility of patients is often hampered by road conditions and the cost of travel. Such traveling diagnostic centers could be extended to other types of technologies as long as proper consideration is placed in the appropriate design of the technology for portability.
Though the 64 mT Hyperfine system has demonstrated the clinical utility of lower quality brain imaging, it still potentially suffers from the same failures in global health that other medical technologies face. The costs, about $500,000 USD to lease for 5 years, may still be out of reach for many health ministries in LMIC. This also reduces the cost/benefit to patients, especially considering the recent release of higher field systems (0.5 tesla) that require no cryogenic replacement and can be purchased for a similar price. The hyperfine system is portable on a hospital floor, but is not so portable that it could navigate an extremely uneven or sloping floor, and the weight of its fixed magnet may render it incompatible with elevators in certain settings.
Open-Source Imaging Initiative
The open-source imaging initiative (OSII), launched in 2016, was formed to address this problem of accessibility and cost related to MRI research and use. The goal of the initiative is to create an expert knowledge base that has a unified goal of making available open-source hardware and software related to MRI [34]. In 2023, the first head only open-source MRI scanner was presented at the 2022 International Society of Magnetic Resonance in Medicine workshop on low-field MRI, following successful prototype builds at the Leiden University Medical Center in Leiden, The Netherlands [26], and the Mbarara University of Science and Technology in Mbarara, Uganda [24].
The original description of the device by O’Reilly et al [27] and subsequent publications detailing improvements in homogeneity of the main magnet [31], gradient design [7], and in vivo imaging [26,28] show its potential to achieve clinically relevant brain imaging at a very low cost.
Importantly, in 2022, the system was successfully reproduced in less than two weeks at the Mbarara University of Science and Technology in Mbarara, Uganda [24]. This was achieved by pre-fabricating the majority of the modular components in the Netherlands, and shipping all that was required to build a system as a “kit” to the lab in Uganda. Then, in collaboration with local students and researchers, the components were assembled into a working low-field MRI system. Efforts are ongoing in Mbarara to further develop this project into a device that can be used in clinical applications. Following this project, there are recent efforts in Asuncion, Paraguay to replicate the open-source imaging system using primarily local tools and components.
This is an important milestone for diagnostic imaging in global health. Though there are still unanswered questions surrounding the role of open-source in medical technology, this project demonstrates what is achievable through the free sharing of knowledge and information. Echoing the recent call from the seventy-sixth world health assembly to strengthen diagnostics capacity, where diagnostic imaging was particularly noted to be lacking in the “developing world” [35], this is a technology which, by design, is affordable, portable, and transferable to the region of use - all necessary requirements for a sustainable solution.
Open source architecture and technology does not obviate the need for commercial development for dissemination, service, maintenance and training. It also does not obviate the need for meeting all local medical safety regulatory requirements. But shipping technologies that meet the regulatory requirements in HIC to LMIC is both not a requirement, but also another factor in escalating costs. It also is a practice that prevents the LMIC development of the industrial infrastructure to produce its own effective technologies to meet its own local needs.
Other low-field Systems
While we have chosen to highlight the Hyperfine and OSII low-field MRI systems, there are several other important projects which have contributed extensively to the field and show substantial promise for future deployment. One such is an 80 mT brain only scanner developed at the Martinos Center of Massachusetts General Hospital [5]. This system has a unique built-in readout gradient in one direction, reducing the need for high powered gradient amplifiers to perform imagining. Field inhomogeneities and gradient non-linearity make distortion free imaging a challenge; however, it is likely that future work with machine learning can resolve these issues.
Another emerging low-field system is described in [17]. This brain imaging system has a 0.055 tesla ‘double pole’ permanent homogeneous magnet and is similar in concept to the Hyperfine system. A deep learning based electromagnetic interference (EMI) canceling scheme is used to remove noise from the raw signal data, forgoing the use of external shielding. The use of two disc shaped magnets also keeps the design open for patient comfort, unlike the cylindrical shell designs of the OSII system and the Martinos Center system. The system is capable of T1, T2, FLAIR and Diffusion weighted imaging and has impressive data showing the utility for diseases such as Stroke and tumor detection.
Image processing and enhancement with Machine Learning
The increased affordability of high quality electronics is largely responsible for the recent success of affordable medical technology. Beyond the affordability of the hardware itself, cost-effective electronics have made computational power more affordable, opening the door for the widespread use of machine learning in nearly every sector of society. Low-field MRI is no exception.
Data collection in MRI is a constant trade-off between time required for image acquisition, image quality, and signal-to-noise ratio (SNR). Faster imaging sequences are far more valuable in the clinic, for uncooperative young children, and for organizational workflows, but often suffer from reduced SNR, contrast, or resolution. In low-field MRI, this problem is compounded by the intrinsically lower SNR that comes with lower field strength. Advanced reconstruction, segmentation, and enhancement techniques have been developed to improve image quality and interpretation, in no small part driven by these factors.
Conventional MRI sequences make elegant use of the Fourier transform to convert the phase and frequency of the signal in spatial frequency space to image space. Unlike other bioimaging methods such as CT or XRay where image contrast is quantitative, image interpretation in MRI is largely reliant upon knowledge of the specific tissue properties (called T1 and T2 signal relaxation times) at the particular field strength of the system and for the imaging sequence being used. Magnetic resonance fingerprinting (MRF) has been shown to be a reliable approach to quantitative MRI [18]. MRF is an extension of compressed sensing, where multiple independent signal evolutions are used to identify the unique chemical properties, or fingerprint, of distinct tissues. It has been shown to be as fast as traditional clinical imaging sequences and potentially more robust to noise and bias. Beyond the potential use in clinical practice, MRF and other quantitative MRI methods offer the important potential for collecting large libraries of MRIdata that can be used to train machine learning networks.
Another important advancement in the use of machine learning in image reconstruction is called automated transform by manifold approximation (AUTOMAP). AUTOMAP uses a supervised deep learning methodology to transform raw signal in frequency space to image space instead of the standard method that relies on the Fourier transform [36]. This is a powerful tool, since it allows for the possibility of removing noise and image artifacts directly and during the reconstruction process. It also enables the utilization of less perfectly smooth magnetic field gradients (linear) required to use the Fourier transform, requiring only that the matching of the object components in the MRI machine can be mapped one-to-one with a unique location of the image space. The quantitative nature also opens the door to repositories of learning libraries that are not specific to a particular MRI system or imaging sequence, allowing for the transfer of learning from one image dataset to another, a task that is difficult with conventional MRI techniques. AUTOMAP has been successfully applied to low field MRI as well, demonstrating the ability of deep learning based image reconstruction techniques to improve upon the achievable SNR in images that have inherently low signal and high noise [15]
Deep learning methods have also been applied to a 55 mili-tesla low field MRI system to remove noise and enhance images during reconstruction [17,20]. This shielding free system has produced remarkably high SNR images which appear to show high anatomical accuracy with matched high field (3 tesla) scans, however some error is always present.
The error between machine learning enhanced images and conventional “ground truth” images is a point of concern for clinical practice. In the treatment planning of infant hydrocephalus, which occurs congenitally or following brain hemorrhages in premature infants (HIC dominant) or post-infection (LMIC dominant), knowledge of brain and cerebrospinal fluid (CSF) volumes are key to understanding the condition of the patient before and after surgical intervention [16]. Recent work has shown that deep learning methods can segment brain from CSF in CT images of hydrocephalus [2,10] with remarkable volumetric accuracy. In the same work, information from a learning library was used to perform super resolution and improve the quality of low SNR and low resolution images [10]. Traditional image quality metrics (i.e. the Dice score) show significant agreement between enhanced images and ground truth in most cases, however in the same study a panel of experts considered small errors between the enhanced images and ground truth to be potentially treatment altering, and it was concluded that machine learned enhancement could at times be risky in the application of hydrocephalus treatment planning.
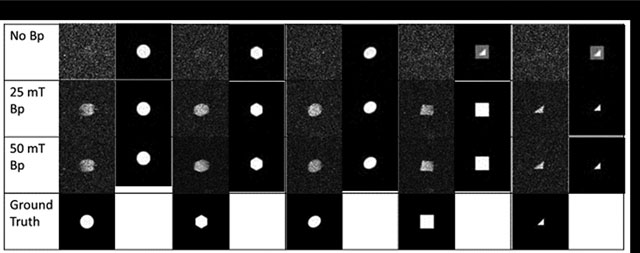
We illustrate how some of these risks arise in machine learning for medical images. For example, in facial recognition, the image to be recognized is already in the machine learning database. A noisy low-resolution picture just needs to be matched with the library’s ground truth images. But in medical image analysis, each patient can present new one-off structures which are not present in the existing database. This enables algorithms to hallucinate structures that are not actually present as the machine learning attempts to bring information from its library of data to enhance the current image. Consider this simple imaging experiment performed in our laboratory on a 4 mili-tesla low field MRI system described in [25]. We constructed simple geometric phantoms as seen in Figure 1. We trained a deep learning network on a library of ideal perfect geometrical shapes without noise representing the phantom shapes (squares, triangles, circles) as described in [10]. By varying the field strength of an additional (prepolarizing field) between 0 tesla (off), 25 mili-tesla, and 50 mili-tesla we produced images with increasing improvement in signal-to-noise. Notice that when the prepolarizing field was turned off, there is maximal noise in the raw images. At higher prepolarizing field, the deep learning network is able to reproduce the correct image. But when the signal-to-noise is low (the prepolarizing field is off), the deep learning network overlays the shapes in its library and produces an image that could exist, but in fact, does not. It hallucinates when bringing the library information to handle noisy and uncertain parts of the images.
Figure 1:

The results of an imaging experiment on a prepolarized low field MRI with a 4.23 mT readout field. Five geometrical phantoms with cross sections of a circle, hexagon, oval, square, and triangle, were constructed and filled with water. Images were collected with the prepolarized field (Bp) off, at 25 mT, and 50 mT. Ground truth was synthetically generated using a computer. A deep learning library was trained with artificially degraded images at the SNR levels calculated from the experimental images. Enhanced images are displayed in each column next to the experimental images for comparison. Note the production of hallucinations in the images at upper right.
In [10], small errors in image enhancement of infant hydrocephalus images were deemed to be potentially treatment altering by the panel of experts. A common machine learning hallucination was smoothing out the boundary of the fluid containing ventricles within the brain, suggesting a subtle structural change used by clinicians to indicate that the pressure within the brain is increasing and may require surgery. Nevertheless, the unenhanced noisier and low resolution images could be acceptable for treatment planning in many of these same cases.
In recent findings of Man et al [20], other examples of structural deep learning hallucinations were readily seen in the deep learning enhanced imagery [13].
These examples point to the need for further improvement in machine learning algorithms to preserve structural fidelity. At present there may be a need for a cultural shift in the acceptability of noisy images by clinicians from low-field MRI, until the rapidly advancing state of the art of machine learned image enhancement enables more reliable anatomic fidelity. Presently, caution is required when applying machine learning techniques to enhance clinical data, and there is need to show that enhancement adds clinical value beyond the risk of its use.
Conclusions
The important role of the engineering of diagnostic technology in the health care pathway has gained increased attention. Simultaneously, the emergence of high powered computing and machine learning in medicine means that the way we use diagnostic tools may be shifting. A diagnostic tool, such as an MRI scan of the brain, genetic screening, or even a simple blood test, is designed to deliver information in the form of data to clinicians to improve treatment specificity and patient outcome. Conventional technology has relied on the collection of high quality, patient specific data which must be interpreted by an expert to generate a diagnosis. Yet the amount and quality of data required to improve patient outcome from a particular disease is often unclear. This uncertainty means that we have lacked an important component needed for global health engineering design – the ratio of outcome improvement to cost, and the incremental improvement from incremental increases in cost. These concepts have long been embraced in the pharmaceutical industry, and we posit that developing such standards would be transformative for sustainable health engineering of medical devices.
The use of machine learning in healthcare enables a shift in the way we will look at diagnostic technology in the future. Firstly, how much information is actually required to accurately diagnose a patient? This may allow for a significant reduction in the costs associated with producing a technology. Secondly, what are the potential roles for machine learning to improve the diagnostic accuracy of lower quality diagnostic tests? We have explored the lower limits of diagnostic useability for medical decision making from lower quality image data, but defining the relationship of outcome improvement to cost, and the incremental cost of improving data quality, are fundamental properties that will be critical to improve the impact of medical technology on global health.
Clinics Care Points.
In global health, lower quality information may often be better than no information.
Lower quality MRI images have been shown to be useful for many clinical diagnostic needs.
Machine learning is fallible, and clinical integration should be cautiously pursued.
Key Points.
A new paradigm for engineering of medical technology for low-resource settings is needed.
Low-field MRI can provide useful diagnostic information in many settings where high-field MRI is neither feasible nor affordable.
Machine learning algorithms are used to enhance the appearance of low-field MRI images, but caution is required because present versions can introduce structural artifacts that can affect clinical decision-making.
Acknowledgements
We are extremely grateful to Dr. Vishal Monga and Dr. Venkat Cherukuri for developing and implementing the deep learning algorithms used in the illustrative experiment with geometric phantom imaging in this work, as well as the ongoing discussion surrounding the appropriate use of machine learning in medical diagnostics.
Disclosures
Supported by NIH R01HD085853 (SJS, JRH), NIH Director’s Transformative Award R01AI145057 (SJS), R01HD096693 (SJS), U01NS107486 (SJS), an Equipment Transfer Agreement between Hyperfine and Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Barber RM, Fullman N, Sorensen RJD, Bollyky T, Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel …, Lancet (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cherukuri V, Guo T, Schiff SJ, Monga V, Deep MR Brain Image Super-Resolution Using Spatio-Structural Priors, IEEE Trans. Image Process (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chhabra DK, The saga of the “Chhabra” shunt, Neurol. India 67 (2019) 635–638. [DOI] [PubMed] [Google Scholar]
- [4].Cleary EG, Beierlein JM, Khanuja NS, McNamee LM, Ledley FD, Contribution of NIH funding to new drug approvals 2010–2016, Proceedings of the National Academy of Sciences 115 (2018) 2329–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cooley CZ, McDaniel PC, Stockmann JP, Srinivas SA, Cauley SF, Śliwiak M, Sappo CR, Vaughn CF, Guerin B, Rosen MS, Lev MH, Wald LL, A portable scanner for magnetic resonance imaging of the brain, Nat Biomed Eng 5 (2021) 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Deoni SCL, Medeiros P, Deoni AT, Burton P, Beauchemin J, D’Sa V, Boskamp E, By S, McNulty C, Mileski W, Welch BE, Huentelman M, Development of a mobile low-field MRI scanner, Sci. Rep 12 (2022) 5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Vos B, Fuchs P, O’Reilly T, Webb A, Gradient coil design and realization for a Halbach-based MRI system, IEEE Transactions on (2020). [Google Scholar]
- [8].Fleming KA, Horton S, Wilson ML, Atun R, DeStigter K, Flanigan J, Sayed S, Adam P, Aguilar B, Andronikou S, Boehme C, Cherniak W, Cheung AN, Dahn B, Donoso-Bach L, Douglas T, Garcia P, Hussain S, Iyer HS, Kohli M, Labrique AB, Looi L-M, Meara JG, Nkengasong J, Pai M, Pool K-L, Ramaiya K, Schroeder L, Shah D, Sullivan R, Tan B-S, Walia K, The Lancet Commission on diagnostics: transforming access to diagnostics, Lancet 398 (2021) 1997–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hamet P, Tremblay J, Artificial intelligence in medicine, Metabolism 69S (2017) S36–S40. [DOI] [PubMed] [Google Scholar]
- [10].Harper JR, Cherukuri V, O’Reilly T, Yu M, Mbabazi-Kabachelor E, Mulando R, Sheth KN, Webb AG, Warf BC, Kulkarni AV, Monga V, Schiff SJ, Assessing the utility of low resolution brain imaging: treatment of infant hydrocephalus, Neuroimage Clin 32 (2021) 102896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Howitt P, Darzi A, Yang G-Z, Ashrafian H, Atun R, Barlow J, Blakemore A, Bull AMJ, Car J, Conteh L, Cooke GS, Ford N, Gregson SAJ, Kerr K, King D, Kulendran M, Malkin RA, Majeed A, Matlin S, Merrifield R, Penfold HA, Reid SD, Smith PC, Stevens MM, Templeton MR, Vincent C, Wilson E, Technologies for global health, Lancet 380 (2012) 507–535. [DOI] [PubMed] [Google Scholar]
- [12].Jackson CH, Baio G, Heath A, Strong M, Welton NJ, Wilson ECF, Value of Information Analysis in Models to Inform Health Policy, Annu Rev Stat Appl 9 (2022) 95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Johnson PM, Lui YW, The deep route to low-field MRI with high potential, Nature 623 (2023) 700–701. [DOI] [PubMed] [Google Scholar]
- [14].Kimberly WT, Sorby-Adams AJ, Webb AG, Wu EX, Beekman R, Bowry R, Schiff SJ, de Havenon A, Shen FX, Sze G, Schaefer P, Iglesias JE, Rosen MS, Sheth KN, Brain imaging with portable low-field MRI, Nat Rev Bioeng 1 (2023) 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koonjoo N, Zhu B, Bagnall GC, Bhutto D, Rosen MS, Boosting the signal-to-noise of low-field MRI with deep learning image reconstruction, Sci. Rep 11 (2021) 8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kulkarni AV, Schiff SJ, Mbabazi-Kabachelor E, Mugamba J, Ssenyonga P, Donnelly R, Levenbach J, Monga V, Peterson M, MacDonald M, Cherukuri V, Warf BC, Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda, N. Engl. J. Med 377 (2017) 2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu Y, Leong ATL, Zhao Y, Xiao L, Mak HKF, Tsang ACO, Lau GKK, Leung GKK, Wu EX, A low-cost and shielding-free ultra-low-field brain MRI scanner, Nat. Commun 12 (2021) 7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA, Magnetic resonance fingerprinting, Nature 495 (2013) 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Malkin R, Teninty B, Medical Imaging in Global Public Health: Donation, Procurement, Installation, and Maintenance, in: Mollura DJ, Culp MP, Lungren MP (Eds.), Radiology in Global Health: Strategies, Implementation, and Applications, Springer International Publishing, Cham, 2019: pp. 77–83. [Google Scholar]
- [20].Man C, Lau V, Su S, Zhao Y, Xiao L, Ding Y, Leung GKK, Leong ATL, Wu EX, Deep learning enabled fast 3D brain MRI at 0.055 tesla, Sci Adv 9 (2023) eadi9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mazurek MH, Cahn BA, Yuen MM, Prabhat AM, Chavva IR, Shah JT, Crawford AL, Welch EB, Rothberg J, Sacolick L, Poole M, Wira C, Matouk CC, Ward A, Timario N, Leasure A, Beekman R, Peng TJ, Witsch J, Antonios JP, Falcone GJ, Gobeske KT, Petersen N, Schindler J, Sansing L, Gilmore EJ, Hwang DY, Kim JA, Malhotra A, Sze G, Rosen MS, Kimberly WT, Sheth KN, Portable, bedside, low-field magnetic resonance imaging for evaluation of intracerebral hemorrhage, Nat. Commun 12 (2021) 5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mazurek MH, Parasuram NR, Peng TJ, Beekman R, Yadlapalli V, Sorby-Adams AJ, Lalwani D, Zabinska J, Gilmore EJ, Petersen NH, Falcone GJ, Sujijantarat N, Matouk C, Payabvash S, Sze G, Schiff SJ, Iglesias JE, Rosen MS, de Havenon A, Kimberly WT, Sheth KN, Detection of Intracerebral Hemorrhage Using Low-Field, Portable Magnetic Resonance Imaging in Patients With Stroke, Stroke 54 (2023) 2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mikulic M, Topic: Global Pharmaceutical Industry, Statista; (2024). [Google Scholar]
- [24].Muhumuza I, Teeuwisse W, Harper J, On-site construction of a point-of-care low-field MRI system in Africa, NMR Biomed (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Obungoloch J, Harper JR, Consevage S, Savukov IM, Neuberger T, Tadigadapa S, Schiff SJ, Design of a sustainable prepolarizing magnetic resonance imaging system for infant hydrocephalus, MAGMA 31 (2018) 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].O’Reilly T, Teeuwisse WM, de Gans D, Koolstra K, Webb AG, In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array, Magn. Reson. Med 85 (2021) 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].O’Reilly T, Teeuwisse WM, Webb AG, Three-dimensional MRI in a homogenous 27 cm diameter bore Halbach array magnet, J. Magn. Reson 307 (2019) 106578. [DOI] [PubMed] [Google Scholar]
- [28].O’Reilly T, Webb AG, In vivo T1 and T2 relaxation time maps of brain tissue, skeletal muscle, and lipid measured in healthy volunteers at 50 mT, Magn. Reson. Med 87 (2022) 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Perry L, Malkin R, Effectiveness of medical equipment donations to improve health systems: how much medical equipment is broken in the developing world?, Med. Biol. Eng. Comput 49 (2011) 719–722. [DOI] [PubMed] [Google Scholar]
- [30].Peters BS, Armijo PR, Krause C, Choudhury SA, Oleynikov D, Review of emerging surgical robotic technology, Surg. Endosc 32 (2018) 1636–1655. [DOI] [PubMed] [Google Scholar]
- [31].Tewari S, O’Reilly T, Webb A, Improving the field homogeneity of fixed- and variable-diameter discrete Halbach magnet arrays for MRI via optimization of the angular magnetization distribution, J. Magn. Reson 324 (2021) 106923. [DOI] [PubMed] [Google Scholar]
- [32].Thurston AJ, Paré and prosthetics: the early history of artificial limbs, ANZ J. Surg 77 (2007) 1114–1119. [DOI] [PubMed] [Google Scholar]
- [33].Wang JY, Doudna JA, CRISPR technology: A decade of genome editing is only the beginning, Science 379 (2023) eadd8643. [DOI] [PubMed] [Google Scholar]
- [34].Winter L, Periquito J, Kolbitsch C, Pellicer-Guridi R, Nunes RG, Häuer M, Broche L, O’Reilly T, Open-source magnetic resonance imaging: Improving access, science, and education through global collaboration, NMR Biomed (2023) e5052. [DOI] [PubMed] [Google Scholar]
- [35].World Health Organization, First WHO model list of essential in vitro diagnostics: Volume 1017, World Health Organization, Genève, Switzerland, 2019. [Google Scholar]
- [36].Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS, Image reconstruction by domain-transform manifold learning, Nature 555 (2018) 487–492. [DOI] [PubMed] [Google Scholar]


