Abstract
Objectives
This study aims to identify COVID-19 breakthrough infections among people with HIV (PWH) across different phases of the pandemic and explore whether differential immune dysfunctions are associated with breakthrough infections.
Design and Methods
This retrospective population-based cohort study used data from an integrated electronic health record (EHR) database in South Carolina (SC). Breakthrough infection was defined as the first COVID-19 diagnosis documented in the state agency after the date an individual was fully vaccinated (i.e., two doses of Pfizer/BNT162b2 or Moderna/mRNA-1273, or one dose of Janssen/Ad26.COV2.S) through June 14, 2022. We analyzed the risk and associated factors of the outcome using Cox proportional hazards models.
Results
Among 7,596 fully vaccinated PWH, the overall rate of breakthrough infections was 118.95 cases per 1,000 person years. When compared with the Alpha dominant period, the breakthrough infection rate was higher during both Delta (HR: 1.50; 95%CI: 1.25, 1.81) and Omicron (HR: 2.86; 95%CI: 1.73, 4.73) dominant periods. Individuals who received a booster dose had a lower likelihood of breakthrough infections (HR: 0.19; 95%CI: 0.15, 0.24). There was no association of breakthrough infections with degree of HIV viral suppression, but a higher CD4 count was significantly associated with fewer breakthroughs among PWH (>500 vs <200 cells/mm3: HR: 0.68; 95%CI: 0.49, 0.94).
Conclusions
In our PWH population, the incidence of breakthrough infection was high (during both Delta and Omicron dominant periods) and mainly associated with the absence of a booster dose in patients over 50 years of age, with comorbidities and low CD4 count.
Keywords: COVID-19, HIV/AIDS, Breakthrough Infections, CD4 count, Viral suppression
Introduction
The remarkable achievement and efficacy of COVID-19 vaccines was observed in both large, randomized-controlled clinical trials and real-world settings,1–6 where several vaccines were found to be safe and efficacious in preventing symptomatic COVID-19. Despite the high level of vaccine efficacy, a considerable percentage of individuals with up to date vaccines (i.e., received all recommended doses of an FDA-authorized COVID-19 vaccine) will develop symptomatic or asymptomatic infections with SARS-CoV-2,7 which are referred to as COVID-19 vaccine breakthrough infections (hereafter as “breakthrough infections”). Understanding COVID-19 vaccine efficacy and durability of protection in real-world settings is critical to helping disease monitoring, informing immunization policy, and preventing the resurgence of COVID-19 cases. Breakthrough infections in the US trended much higher when the Delta and Omicron variants of SARS-CoV-2 became the dominant strains.8 Due to the varied vaccine responses and antibody kinetics, the occurrence of breakthrough infections might differ among different patients’ characteristics. It was reported that vaccine-induced antibody responses were persistently lower in susceptible populations, such as older adults, immunosuppressed populations, and individuals with other specific comorbidities.9–11 These underrepresented populations with compromised immune systems, therefore, might be more prone to have breakthrough infections.
Immunocompromised individuals, such as people with HIV (PWH), appear to be at an elevated risk of COVID-related hospitalization and death,12 although such elevated risk might disappear in the late stage of the pandemic as suggested in a recent study.13 Understanding the effectiveness of COVID-19 vaccination in immunocompromised populations is critical for disease monitoring and public health policy. However, immunocompromised individuals, such as organ transplant recipients or cancer patients, may have poor immune responses, even after full COVID-19 vaccination.14,15 The evidence for vaccine effectiveness among PWH is scarce because of the small number of studies and under-representativeness in the clinical trials of COVID-19 vaccine efficacy.16 Prior studies have shown mixed findings regarding the effectiveness of COVID-19 vaccine among PWH.17 Liu and colleagues suggested that immunocompromised individuals were among the highest risk groups experiencing breakthrough infections, yet such an association was not observed for PWH.18 Coburn et al and Sun et al both demonstrated that PWH had a higher risk of breakthrough infections with a much larger sample size.19,20 However, evaluations of real-world COVID-19 vaccine effectiveness in these studies were either conducted with a very small sample size18 or had a short period of postvaccination follow-up which could not capture the breakthrough infections during the circulation of Omicron dominant period,19,20 which makes it hard to compare breakthrough infection rates between different phases of the pandemic. To understand COVID-19 vaccine effectiveness among PWH amidst the evolving situation of the pandemic, we sought to explore dynamic breakthrough infections amid the emerging different variants of concern (e.g., Delta, Omicron) and whether different immune and HIV viral suppression status played a role in breakthrough infections among fully vaccinated PWH.
Methods
Data sources and study population
This study is a population-based cohort study, with data being retrieved from integrated statewide electronic health record (EHR) data in South Carolina (SC). Three data sources were used to define the study population. First, we retrieved the HIV diagnosis information from the electronic HIV/AIDS reporting system (eHARS) in the SC Department of Health and Environmental Control (SC DHEC) between 2005 and December 2020 to form an HIV cohort. Then the HIV cohort was merged with the statewide COVID-19 vaccine record (e.g., Statewide Immunization Online Network (SIMON)) to define vaccinated PWH. The most updated vaccine record released to our research team was from Jan 2, 2021 to June 14, 2022. Finally, the merged HIV and COVID-19 vaccine cohorts were further linked to the SC COVID-19 tester cohort, where a breakthrough infection were identified if the vaccinated PWH had a COVID-19 infection record in the COVID-19 tester cohort. The COVID-19 tester database consists of all SC residents who were ever tested for COVID-19 infection (by nasal swab or antibody test) and with results (both positive and negative) reported to SC DHEC between March 7, 2020 and April 14, 2022 (Figure 1). The SC Revenue and Fiscal Affairs Office (SC RFA) was the honest broker of all the data linkage. In our study, we used the vaccine record between Jan 2, 2021 to March 30, 2022, allowing us to have enough follow-up time to detect and record for breakthrough case detection. Specific inclusion and exclusion criteria and the cohort building diagram are displayed in Figure 1.
Figure 1.
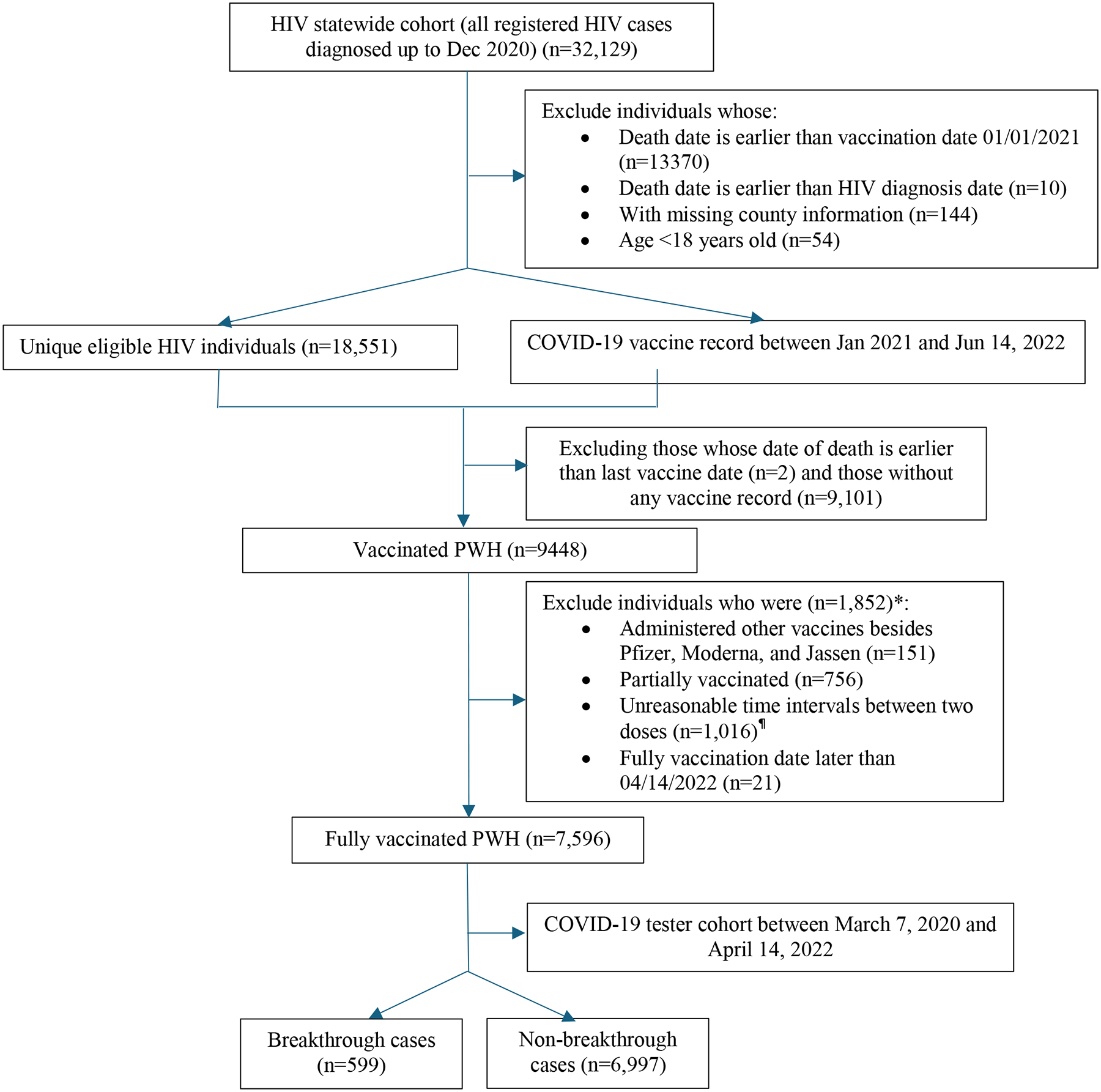
Study cohort flowchart
Note: * individuals might meet two exclusion criteria at the same time, thus, the total number of excluded individuals in each category is more than the unique number of excluded patients
¶ If a patient administers Pfizer vaccine, time intervals beyond 20–90 days were excluded; if a patient administers Moderna vaccine, time intervals beyond 28–90 days were excluded.
Eligible individuals were adult PWH (≥18 year old) and fully vaccinated against COVID-19 with one of three vaccines authorized in the US (i.e., BNT162b2 [Pfizer], mRNA-1273 [Moderna], Ad26.COV2.S [Janssen]). Vaccination status (merely refer to vaccine doses administrated) was classified based on the number of vaccine doses received before a reference date, which was either the date of symptom onset for breakthrough infections or the end date of this project for individuals without breakthrough infections. Individuals were considered fully vaccinated 14 days either after receipt of the second vaccine dose of Pfizer-BioNTech or Moderna or after a single-dose of the Janssen vaccine.21 Individuals with mixed doses between Pfizer and Moderna were also considered as fully vaccinated. Booster vaccination was defined as individuals who received additional doses after the primary series of vaccine (regardless of the time interval between the primary doses and the booster dose) and before the outcome occurrence.
Outcome: Breakthrough infection
Breakthrough case was defined as the first SARS-CoV-2 infection or COVID-19 diagnosis after the date an individual was fully vaccinated. Details were described in a previous protocol paper.22 Briefly, incident COVID-19 cases were identified using the information from the SC statewide Case Report Form (CRF) (“Human Infection with 2019 Novel Coronavirus Case Report Form”) for SARS-CoV-2 infection issued by SC DHEC, which contains information about lab-confirmed and probable cases of COVID-19, hospitalization, intensive care unit (ICU) admission, and death information. The criteria for case ascertainment were described in the standardized surveillance case definition of COVID-19.20 Briefly, we have included the lab confirmed cases (i.e., detection of SARS-CoV-2 RNA in a clinical specimen using a molecular amplification detection test) and probable COVID-19 cases (i.e., meeting clinical criteria AND epidemiologic evidence with no confirmatory lab testing; or meets presumptive lab evidence AND either clinical criteria OR epidemiological evidence; or meets vital records criteria with no confirmatory lab testing). Based on different presenting symptoms of breakthrough infections, disease severity was categorized into five groups using information from CRF: asymptomatic (no symptoms), mild symptoms (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell), moderate/severe symptoms (e.g., difficulty breathing or developed pneumonia or ARDS), hospitalization, and death. The detailed categorization of disease severity was described elsewhere.23
Covariates
In addition to demographic factors, covariates included the type of primary series vaccine (Pfizer/BNT162b2, Moderna/mRNA-1273, or Janssen/Ad26.COV2.S), additional/booster vaccine doses after full vaccination, and evidence of SARS-CoV-2 infection prior to the date of being fully vaccinated (history of COVID-19). We also adjusted the calendar periods differentiated by different dominant variants of concerns (e.g., Delta, Omicron). Based on the milestone of the national implementation of COVID-19 mitigation measures24 and different predominant variants in SC25, we categorized the study periods of the occurrence of breakthrough infections into three time windows, i.e., Alpha dominant period (Jan 2, 2021-June 30, 2021), Delta dominant period (July 1, 2021-November 30, 2021), and Omicron (December 1, 2021-April 14, 2022).26 Comorbidities were defined as the presence of corresponding ICD codes27 two years before the COVID-19 vaccination rollout (i.e., January 2019 and December 2020).
To examine the association between different immunity levels on the outcomes of interest, we used the most recent CD4 count and HIV viral load record closest to the fully vaccinated date as predictors. CD4 counts were categorized into <200 cells/mm3, 200–500 cells/mm3, and >500 cells/mm3. HIV viral loads were classified as <200 copies/ml (virally suppressed) and ≥200 copies/ml (virally unsuppressed). History of AIDS was retrieved based on the clinical diagnosis prior to the vaccine rollout policy.
Statistical analysis
Study entry for eligible patients was the date of being fully vaccinated. The primary outcome is the time from fully vaccination to date of breakthrough infection, death, 9 months after the fully vaccinated date, or the end of the study (April 14, 2022), whichever occurred first. To assess the socio-demographic distribution properties and clinical characteristics of PWH with and without breakthrough infections, Chi-square or Fisher’s exact tests were used for categorical variables. Incidence rates and 95% Confidence Intervals (CIs) of COVID-19 breakthrough infections were calculated per 1,000 person-years for each month overall, by different time periods and by vaccine brands. We compared the risk of breakthrough infections among PWH using Cox proportional hazards (PH) models to assess unadjusted hazard ratios (HRs) and adjusted hazard ratios (aHRs) with 95%CIs. Adjustment factors included sex, race and ethnicity, age, vaccine brands, booster dose, prior COVID-19 diagnosis, and calendar period differentiated by different dominant variants of concerns (e.g., Delta, Omicron). For HIV markers, CD4 counts and viral suppression status were assessed as risk factors for breakthrough infections. The PH assumption was verified by plotting the logarithm of the cumulative hazard function versus follow-up time. We used Kaplan-Meier cumulative incidence curves to demonstrate time to breakthrough infection by immune dysfunction status (i.e., CD4 count) and vaccine brands. Sensitivity analyses were conducted by only including lab-confirmed COVID-19 cases. Analyses were conducted with R version 4.1.2 (R Project for Statistical Computing). A 2-sided p<0.05 guided statistical significance interpretation.
Results
Study population characteristics
A total of 7,596 eligible fully vaccinated PWH were included in the current study. Among them, 7.9% (599/7,596) had breakthrough infections. Over half of them (57.2%) were aged 50 years old and older, 69.6% were male, 73.3% were Black, 45.5% self-reported men who have sex with men (MSM) status, and 82.1% resided in urban areas. Regarding COVID-19 related information, 12.7% of subjects had prior COVID-19 diagnosis before being fully vaccinated. The majority received either Pfizer/BNT162 (43.4%) or Moderna/mRNA-1273 (47.7%) vaccines; 8.7% received Janssen/Ad26.COV2.S; and 47.7% received additional/booster doses. At full vaccination, 64.0% had HIV viral suppression and 62.7% had CD4 counts >500 cells/mm3 (Table 1).
Table 1.
Baseline characteristics among overall PLWH with full vaccination
| Characteristic | Overall | Breakthrough infections | p-value | |
|---|---|---|---|---|
| N=7,596 | No | Yes | ||
| 6,997 (92.11%) | 599 (7.89%) | |||
| Age at fully vaccination (years) | <.0001 | |||
| 18–29 | 565 (7.44) | 491 (86.90) | 74 (13.10) | |
| 30–39 | 1,278 (16.82) | 1,135 (88.81) | 143 (11.19) | |
| 40–49 | 1,411 (18.58) | 1,269 (89.94) | 142 (10.06) | |
| 50–59 | 2,385 (31.40) | 2,230 (93.50) | 155 (6.50) | |
| 60+ | 1,957 (25.76) | 1,872 (95.66) | 85 (4.34) | |
| Sex | 0.008 | |||
| Female | 2,310 (30.41) | 2,099 (90.87) | 211 (9.13) | |
| Male | 5,286 (69.59) | 4,898 (92.66) | 388 (7.34) | |
| Race | 0.003 | |||
| White | 1,632 (21.48) | 1,535 (94.06) | 97 (5.94) | |
| Black | 5,571 (73.34) | 5,095 (91.46) | 476 (8.54) | |
| Hispanic | 231 (3.04) | —— | —— | |
| Other/unknown | 162 (2.13) | —— | —— | |
| HIV transmission mode | 0.32 | |||
| Heterosexual | 1,954 (25.72) | 1,794 (91.81) | 160 (8.19) | |
| MSM | 3,457 (45.51) | 3,177 (91.90) | 280 (8.10) | |
| IDU/MSM | 514 (6.77) | 484 (94.16) | 30 (5.84) | |
| Other/unknown | 1,671 (22.00) | 1,542 (92.28) | 129 (7.72) | |
| Residence | 0.01 | |||
| Rural | 1,357 (17.86) | 1,227 (90.42) | 130 (9.58) | |
| Urban | 6,239 (82.14) | 5,770 (92.48) | 469 (7.52) | |
| Prior COVID-19 infection before vaccination | 0.44 | |||
| No | 6,632 (87.31) | 6,103 (92.02) | 529 (7.98) | |
| Yes | 964 (12.69) | 894 (92.74) | 70 (7.26) | |
| Vaccine brands | 0.03 | |||
| Pfizer/BNT162b2 | 3,296 (43.39) | 3,002 (91.08) | 294 (8.92) | |
| Janssen/Ad26.COV2.S | 659 (8.68) | 607 (92.11) | 52 (7.89) | |
| Moderna/mRNA-1273 | 3,624 (47.71) | —— | —— | |
| Heterologous vaccination1 | 17 (0.22) | —— | —— | |
| Circulating variants of concern | <.0001 | |||
| Alpha | 5,237 (68.94) | 4,860 (92.80) | 377 (7.20) | |
| Delta | 2,008 (26.43) | 1,804 (89.84) | 204 (10.16) | |
| Omicron | 351 (4.62) | 333 (94.87) | 18 (5.13) | |
| Booster dose after fully vaccination | <.0001 | |||
| No | 3,976 (52.34) | 3,474 (87.37) | 502 (12.63) | |
| Yes | 3,620 (47.66) | 3,523 (97.32) | 97 (2.68) | |
| Cardiovascular disease | 0.2089 | |||
| No | 6,946 (91.44) | 6,390 (92.00) | 556 (8.00) | |
| Yes | 650 (8.56) | 607 (93.38) | 43 (6.62) | |
| Chronic pulmonary disease | 0.0982 | |||
| No | 6,655 (87.61) | 6,143 (92.31) | 512 (7.69) | |
| Yes | 941 (12.39) | 854 (90.75) | 87 (9.25) | |
| Diabetes mellitus | 0.0841 | |||
| No | 6,697 (88.16) | 6,182 (92.31) | 515 (7.69) | |
| Yes | 899 (11.84) | 815 (90.66) | 84 (9.34) | |
| Renal disease | 0.416 | |||
| No | 7,110 (93.60) | 6,554 (92.18) | 556 (7.82) | |
| Yes | 486 (6.40) | 443 (91.15) | 43 (8.85) | |
| Liver disease | 0.2591 | |||
| No | 7,303 (96.14) | 6,722 (92.04) | 581 (7.96) | |
| Yes | 293 (3.86) | 275 (93.86) | 18 (6.14) | |
| Peptic ulcer disease | 0.2525 | |||
| No | 7,542 (99.29) | —— | —— | |
| Yes | 54 (0.71) | —— | —— | |
| Dementia | 0.3119 | |||
| No | 7,530 (99.13) | —— | —— | |
| Yes | 66 (0.87) | —— | —— | |
| Recent CD4 counts (cells/mm3) 2 | 0.02 | |||
| <200 | 428 (5.63) | 379 (88.55) | 49 (11.45) | |
| 200–350 | 833 (10.97) | 778 (93.40) | 55 (6.60) | |
| 351–500 | 1,165 (15.34) | 1,074 (92.19) | 91 (7.81) | |
| >500 | 4,759 (62.65) | 4,379 (92.02) | 380 (7.98) | |
| Unknown | 411 (5.41) | 387 (94.16) | 24 (5.84) | |
| Recent HIV viral load (copies/ml) 2 | 0.14 | |||
| <200 | 4,864 (64.03) | 4,490 (92.31) | 374 (7.69) | |
| ≥200 | 2,130 (28.04) | 1,944 (91.27) | 186 (8.73) | |
| Unknown | 602 (7.93) | 563 (93.52) | 39 (6.48) | |
| AIDS at full vaccination date | 0.03 | |||
| No | 3,351 (44.12) | 3,061 (91.35) | 290 (8.65) | |
| Yes | 4,245 (55.88) | 3,936 (92.72) | 309 (7.28) | |
Notes: ---Small number less than 10 were masked due to DHEC’s policy. MSM: men who have sex with men; IDU: injection drug user.
Heterologous vaccination refers to individuals who received mixed doses between Pfizer/BNT162b2, Janssen/Ad26.COV2.S, and Moderna/mRNA-1273.
The most recent CD4 count or viral load closest to the fully vaccination date (between 2019 and 2021)
Incidence rates and severity of breakthrough infections
The incidence of breakthrough infection at the end of the study was 118.95 per 1000 person years. The median (IQR) time from full vaccination (14 days after full vaccination) to breakthrough infection was 180 (minimum: 1 days; maximum: 270 days) days. When stratified by vaccine brands, the time interval from fully vaccination to breakthrough infection (in days) was 179.5 (1–270) days for Pfizer/BNT162, 175 (10–270) days for Janssen/Ad26.COV2.S, and 185 (4–270) days for Moderna/mRNA-1273, respectively.
Among a total of 599 breakthrough infections, 83.47% were asymptomatic, 11.85% presented with mild symptoms, 4.67% with presented moderate/severe symptoms, 2.00% were hospitalized, while 0.5% died from the infection. The distribution of breakthrough infections was different across different waves of COVID-19 infection as well as by different vaccine types. The breakthrough rate was the highest at the beginning of the Omicron dominant period (Jan, 2022) (599.8[95% CI: 536.5–663.1] per 1000 person-years), followed by the Delta dominant period (Sep 2021) (141.2[95% CI: 109.0–173.3]) and the Alpha dominant period (March 2021) (19.6[95% CI: 0.0–58.1]). When stratified by vaccine brands, the rate of breakthrough was consistently higher among Janssen/Ad26.COV2.S vaccine recipients during the Delta dominant period (range from 39.9–251.3 per 1000 person years), followed by Pfizer/BNT162 (15.4–184.9 per 1000 person years) and Moderna/mRNA-1273 (13.3–79.0 per 1000 person years). During the Alpha dominant period, there was no clear pattern of the distribution of breakthrough rates with individuals vaccinated with Moderna/mRNA-1273 had a higher breakthrough rate first, followed by Janssen/Ad26.COV2.S vaccine. During the Omicron dominant period, individuals vaccinated with Pfizer/BNT162b2 consistently had a higher rate of breakthrough infections (Pfizer vs Janssen, Moderna: 692.5 vs 404.4, 554.3). (Figure 2)
Figure 2.

Trends in breakthrough infections by variants of concern and vaccine type
Factors associated with the risk of breakthrough infections
Older age (50–59 vs 18–29 years: HR: 0.55; 95%CI: 0.40, 0.74; 60+ vs 18–29 years: HR: 0.37; 95%CI: 0.26, 0.52) and male sex (HR: 0.68; 95%CI: 0.53, 0.88) were significantly associated with a lower likelihood of breakthrough infections. Regarding comorbidities, diabetes mellitus (HR: 1.47; 95%CI: 1.14, 1.90) was significantly associated with a higher likelihood of breakthrough infection.
Breakthrough infection rates were substantially higher during both Delta (HR: 1.50; 95%CI: 1.25, 1.81) and Omicron (HR: 2.86; 95%CI: 1.73, 4.73) dominant periods compared with the Alpha dominant period. Comparing individuals vaccinated with Pfizer/BNT162, those vaccinated with Moderna/mRNA-1273 were less likely to experience a breakthrough infection (HR: 0.78; 95%CI: 0.66, 0.93). Individuals who received a booster dose had a lower likelihood of experiencing breakthrough infections (HR: 0.19; 95%CI: 0.15, 0.24). In terms of HIV markers, PWH with higher most recent CD4 counts at the time of full vaccination had a lower odds of breakthrough infections (>500 vs <200 cells/mm3: HR: 0.68; 95%CI: 0.49, 0.94), while viral suppression status was not associated with this outcome (HR: 1.06; 95%CI: 0.69, 1.63) (Table 2). As observed in the Kaplan-Meier plot, PWH with lower CD4 counts (log-rank test=12.38, p=0.0062) and vaccinated with Pfizer/BNT162 (log-rank test=11.31, p=0.0035) experienced a substantially faster time to breakthrough infection (Figure 3). The sensitivity analyses using the lab-confirmed COVID-19 cases only to define breakthrough infections yielded similar results as the main analysis (Supplementary Table 1, Table 2, and Figure 1).
Table 2.
Cox PH regression analyses for factors associated with breakthrough infections among overall population
| Effect | aHR (95% CI) | p-value |
|---|---|---|
| Age at fully vaccination (Years) | ||
| 18–29 | Ref. | |
| 30–39 | 0.82 (0.62, 1.09) | 0.17 |
| 40–49 | 0.78 (0.58, 1.05) | 0.11 |
| 50–59 | 0.55 (0.40, 0.74) | <.0001 |
| 60+ | 0.37 (0.26, 0.52) | <.0001 |
| Sex | ||
| Female | Ref. | |
| Male | 0.68 (0.53, 0.88) | 0.003 |
| Race | ||
| White | Ref. | |
| Black | 1.18 (0.94, 1.48) | 0.16 |
| Hispanic | 1.01 (0.61, 1.68) | 0.98 |
| Other/unknown | 0.56 (0.27, 1.16) | 0.12 |
| HIV transmission risk | ||
| Heterosexual | Ref. | |
| IDU/MSM | 0.99 (0.66, 1.50) | 0.97 |
| MSM | 1.12 (0.83, 1.50) | 0.46 |
| Other/unknown | 0.95 (0.75, 1.21) | 0.67 |
| Residence | ||
| Rural | Ref. | |
| Urban | 0.83 (0.68, 1.01) | 0.07 |
| History of covid infection prior vaccination | ||
| No | Ref. | |
| Yes | 0.82 (0.64, 1.06) | 0.13 |
| Vaccine brands | ||
| PFR | Ref. | |
| JSN | 0.86 (0.64, 1.17) | 0.3427 |
| MOD | 0.78 (0.66, 0.93) | 0.0053 |
| Mix | 1.62 (0.23, 11.65) | 0.6329 |
| Period | ||
| Alpha | Ref. | |
| Delta | 1.50 (1.25, 1.81) | <.0001 |
| Omicron | 2.86 (1.73, 4.73) | <.0001 |
| Booster received | ||
| No | Ref. | |
| Yes | 0.19 (0.15, 0.24) | <.0001 |
| Cardiovascular disease | ||
| No | Ref. | |
| Yes | 0.78 (0.55, 1.11) | 0.17 |
| Chronic pulmonary disease | ||
| No | Ref. | |
| Yes | 1.24 (0.98, 1.59) | 0.08 |
| Diabetes mellitus | ||
| No | Ref. | |
| Yes | 1.47 (1.14, 1.90) | 0.00 |
| Renal disease | ||
| No | Ref. | |
| Yes | 1.32 (0.93, 1.87) | 0.12 |
| Liver disease | ||
| No | Ref. | |
| Yes | 0.87 (0.54, 1.41) | 0.58 |
| Peptic ulcer disease | ||
| No | Ref. | |
| Yes | 0.51 (0.13, 2.06) | 0.34 |
| Dementia | ||
| No | Ref. | |
| Yes | 0.66 (0.21, 2.10) | 0.49 |
| Recent CD4 counts (cells/mm3) * | ||
| <200 | Ref. | |
| 200–350 | 0.61 (0.42, 0.91) | 0.01 |
| 351–500 | 0.69 (0.48, 0.99) | 0.05 |
| >500 | 0.68 (0.49, 0.94) | 0.02 |
| Unknown | 0.43 (0.23, 0.80) | 0.01 |
| Recent HIV viral load (copies/ml) * | ||
| <200 | Ref. | |
| ≥200 | 1.05 (0.87, 1.26) | 0.63 |
| Unknown | 1.06 (0.69, 1.63) | 0.79 |
| AIDS at full vaccination date | ||
| No | ||
| Yes | 0.91 (0.76, 1.10) | 0.33 |
Note.
The most recent CD4 count or viral load closest to the fully vaccination date (between 2019 and 2021)
Figure 3.

Time to COVID-19 breakthrough infection by CD4 count and vaccine type
Discussion
As the submission of this article, our study is one of the first studies examining the rate of breakthrough infections among PWH over a long follow-up period across waves of infection with SARS-CoV-2 variants of concern. Among 7,596 fully vaccinated PWH in SC, breakthrough infections in PWH 9 months after full vaccination were not common and lower than previous study,28 suggesting persistent vaccine effectiveness against SARS-CoV-2 variants. However, the rate of breakthrough infection in this study is much higher than that observed in previous studies (7.9% vs 3.8%).19 Given the later circulating time of Omicron strain, the number of eligible participants in this period was the lowest and the proportion of breakthrough infections was low as well. However, the incidence of breakthrough infections was the highest when taking the duration of follow up time into account. The high rate of breakthrough infections in this study can be primarily attributed to the significant increase in the rate of breakthrough infections during the Omicron period. Previous studies did not extend into the Omicron period.19,20 The Omicron lineage features strongly enhanced transmissibility compared to Delta and other pre-Omicron variants,29 which leads to a significant risk of vaccine immune evasion potential and subsequently to an increased incidence of breakthrough infections as suggested in previous studies.30,31 The vaccine effectiveness against symptomatic disease caused by the Omicron variant is substantially lower than with the Delta variant (the results were not shown in the Tables)32, and vaccine effectiveness waned rapidly. Considering the waning effect, we have selected 270 days as the maximum observation period after full vaccination date for each participant. The median time from full vaccination to breakthrough occurrence was 180 days, which indirectly reflected the waning effect after around 6 months. However, this waning effect was intertwined with the evolving virus strains circulating at different time periods. Booster doses resulted in a substantial increase in protection against mild infection; however, waning of protection against symptomatic disease was also seen after booster doses.
Consistent with prior studies,33,34 our results suggest that PWH with moderate or severe immune suppression (i.e., lower CD4 counts) had higher odds of breakthrough infection. However, we did not observe the association between HIV viral suppression status and breakthrough infections, which is also supported in another study.19 There has been a consensus that PWH with HIV viral suppression may have chronic immune impairment such as a partially recovered CD4 count and persistent immune activation, which may lead to suboptimal antibody production and ultimately to diminished vaccine efficacy,35 which support the CDC’s recommendation of 3 doses of COVID-19 vaccine as the primary doses for people who are moderately or severely immunocompromised.36 A growing body of evidence suggests that adult PWH who receive stable ART and have suppressed HIV viral loads generally mount robust immune responses to COVID-19 vaccines.37–41 Our study extends these prior observations and demonstrated that HIV viral stable PWH might still mount robust humoral immune responses even during the more contagious Delta and Omicron circulating periods. Nevertheless, it is very important for PWH with moderate or severe immune suppression to follow recommended booster vaccination recommendations to maintain protection.
Our study demonstrates that breakthrough infection rates varied during different circulating variants period and differed by vaccine brands. During the Alpha dominant period, there was no clear pattern of the distribution of breakthrough rates when stratified by vaccine brands. This changing pattern of breakthrough risks might be explained by the varied rollout time of each vaccine brand, where the first date of Janssen/Ad26.COV2.S recipients fully vaccinated would be in the amidst of the Alpha dominant period. During the Delta dominant period, breakthrough rates were persistently higher among PWH vaccinated with the Janssen/Ad26.COV2.S vaccine. In contrast, different from the other two circulating periods, individuals vaccinated with Pfizer/BNT162b2 during Omicron dominant period had consistently higher rates of breakthrough infections compared to the other two vaccines. The Omicron variant significantly compromised vaccine effectiveness due to viral escape from Pfizer/BNT162b2 neutralization.42 It has been reported that COVID-19 vaccine effectiveness was lower against symptomatic Omicron infection than Delta infection.43 Omicron was shown to escape antibody neutralization by the Pfizer/BNT162b2 messenger RNA vaccine.30 Moderna/mRNA-1273 was more effective than Pfizer/BNT162b2 or Janssen/Ad26.COV2.S following a primary series during the Delta-dominant period, but was also more effective as a booster than Pfizer/BNT162b2 during the Omicron-dominant period.44
Consistent with Coburn et.al.’s study,19 we found an inverse association between older age and breakthrough risk, which may be partially explained by behavioral modifications taken by older patients, including the adoption of masking and social distancing. Females were found to be associated with higher risk of breakthrough infections, corroborating to a study performed by Sun et al.20 and other national reports in the general population.45 It is well known that females mount a more robust humoral and cellular immune responses than males but their susceptibility to COVID-19 breakthrough can vary depending their underlying comorbidities. A clearly pronounced effect of sex for breakthrough infections at the population level needs further investigation. Our study did not find an association between a history of COVID-19 and breakthrough risk. Mixed findings were observed in prior studies, with one study observing an increased breakthrough risk for individuals with a prior COVID-19 diagnosis,19 while another one found the opposite result.20 Differential follow-up times might account for such discrepancies, such as the waning immunity from the natural infection during the Omicron circulating period.
Several limitations of this study are worth noting. First, although our study findings are generalizable to the state of SC, they are likely not generalizable to all PWH in the US. Those without regular access to health care (who may be at greater risk for COVID-19 infection) were less likely to be included. Individuals engaged in regular health care may have greater health-seeking behaviors, including a greater frequency of COVID-19 testing, leading to higher detection of breakthrough infections than in the general population. Second, the absence of data on patient antibodies against SARS-CoV-2 limited our ability to accurately estimate the vaccine protection effect and make the interpretations challenging. In the same time, COVID-19 diagnosis status was captured in the electronic health record (EHR) data, which may not fully account for COVID-19 testing that occurs outside of healthcare settings, including self-testing. Thus, breakthrough infections might be underreported. Third, we were unable to determine vaccine protection against severe forms of breakthrough infection owing to the small number of breakthrough cases resulting in hospitalization or death in our data set. Fourth, caution is needed when interpreting vaccine effectiveness among PWH across different vaccines. The breakthrough cases among individuals vaccinated with Janssen is relatively small, which might compromise the robustness of the finding of lower effectiveness of the Janssen/Ad26.COV2.S vaccine against COVID-19 infections, particularly in the early phases of the pandemic (e.g., Alpha circulating period). Fifth, the completeness and accuracy of the data sources that we used were not validated, which might introduce bias in the findings – though likely underestimating true infection rates. Further investigations are needed to confirm the findings in this study. Lastly, some key variables that might influence the outcome were not available in our dataset. For example, a certain population might have received monoclonal antibodies (evusheld) for COVID prophylaxis, which could have a protection effect against breakthrough infections. Furthermore, some key sociodemographic variables, such as employment status and socioeconomic status, might affect the SARS-CoV-2 exposure, yet were not available in our dataset. Future studies should take these variables into consideration.
This cohort study has extended prior observations by quantifying the breakthrough infection rates among PWH during the Omicron dominant period with real-world evidence. Breakthrough rates among PWH during Omicron period was much higher than the other two circulating periods due to the high pathogenicity and enhanced transmissibility of VOC as well as waning immunity. Immune suppressed individuals (i.e., lower CD4 counts) had a greater likelihood of breakthrough infections, while there was no association between HIV viral suppression status and breakthrough infections, demonstrating that viral stable PWH might still mount robust humoral immune responses even during the more contagious Delta and Omicron circulating periods. Booster doses definitely confer further protection against infection. Although additional dose/booster doses have been recommended for certain immunocompromised groups, our findings suggest that booster doses may be even more important for PWH with poor immune reconstitution (i.e. lower CD4 counts) to prevent breakthrough infections, particularly during surges of highly transmissible variants such as during the Omicron dominant period. Longitudinal monitoring of immune responses in PWH is critical to inform vaccine policy in this group of patients.
Supplementary Material
Acknowledgements
The authors thank the SC Department of Health and Environmental Control (DHEC), the office of Revenue and Fiscal Affairs (RFA), and other SC agencies for contributing the data in South Carolina.
Funding
This work was made possible with funding to the University of South Carolina through R21AI170159-01A1 from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH), with support from the NIH Office of AIDS Research. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
Conflict of interest:
Dr. Poland is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland provides consultative advice to AiZtech; Emergent Biosolutions; GlaxoSmithKline; Invivyd; Janssen Global Services, LLC; Merck & Co. Inc.; Moderna; Novavax; and Syneos Health.
These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies.
Dr. Poland holds patents related to vaccinia, influenza, and measles peptide vaccines. Dr. Poland has received grant funding from ICW Ventures for preclinical studies on a peptide-based COVID-19 vaccine. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Dr. Poland is an adviser to the White House and World Health Organization on COVID-19 vaccines and monkeypox, respectively.
Data Availability Statement
The authors are prohibited from making individual-level data available publicly due to provisions in our data use agreements with state agencies/data providers, institutional policy, and ethical requirements. To facilitate research, we make access to such data available via approved data access requests through the data owners. The data is unavailable externally or for re-release due to prohibitions in data use agreements with our state agencies or other data providers. Institutional policies stipulate that all external requests for data access require collaboration with a USC researcher. For more information or to make a request, please contact (Bankole Olatosi, PhD):Olatosi@mailbox.sc.edu. The underlying analytical codes are available from the authors on request.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. Dec 31 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. Feb 4 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. Feb 2021;26(6)doi: 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. May 15 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt AA, Omer SB, Yan P, Shaikh OS, Mayr FB. SARS-CoV-2 Vaccine Effectiveness in a High-Risk National Population in a Real-World Setting. Ann Intern Med. Oct 2021;174(10):1404–1408. doi: 10.7326/M21-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. MedRxiv. 2021; [Google Scholar]
- 7.Cdc Covid- Vaccine Breakthrough Case Investigations Team. COVID-19 Vaccine Breakthrough Infections Reported to CDC - United States, January 1-April 30, 2021. MMWR Morb Mortal Wkly Rep. May 28 2021;70(21):792–793. doi: 10.15585/mmwr.mm7021e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covid C, Team VBCI, Birhane M, et al. COVID-19 vaccine breakthrough infections reported to CDC—United States, January 1–April 30, 2021. Morbidity and Mortality Weekly Report. 2021;70(21):792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. Sep 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkelman TNA, Rai NK, Bodurtha PJ, et al. Trends in COVID-19 Vaccine Administration and Effectiveness Through October 2021. JAMA Netw Open. Mar 1 2022;5(3):e225018. doi: 10.1001/jamanetworkopen.2022.5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Mortality in Vaccinated Patients With Cancer in the US Between December 2020 and November 2021. Jama Oncology. Jul 2022;8(7):1027–1034. doi: 10.1001/jamaoncol.2022.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosioni J, Blanco JL, Reyes-Uruena JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. May 2021;8(5):e294–e305. doi: 10.1016/S2352-3018(21)00070-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sales TLS, Souza-Silva MVR, Delfino-Pereira P, et al. COVID-19 outcomes in people living with HIV: Peering through the waves. Clinics. 2023;78:100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. Jun 1 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barriere J, Chamorey E, Adjtoutah Z, et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. Aug 2021;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. May 20 2021;384(20):1899–1909. doi: 10.1056/NEJMoa2103055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costiniuk CT, Singer J, Lee T, et al. COVID-19 vaccine immunogenicity in people with HIV. AIDS (London, England). 2023;37(1):F1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Lee J, Ta C, et al. A Retrospective Analysis of COVID-19 mRNA Vaccine Breakthrough Infections - Risk Factors and Vaccine Effectiveness. medRxiv. Oct 7 2021;doi: 10.1101/2021.10.05.21264583 [DOI] [Google Scholar]
- 19.Coburn SB, Humes E, Lang R, et al. Analysis of Postvaccination Breakthrough COVID-19 Infections Among Adults With HIV in the United States. Jama Network Open. Jun 2022;5(6)e2215934. doi: 10.1001/jamanetworkopen.2022.15934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Zheng QL, Madhira V, et al. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. Jama Internal Medicine. Feb 2022;182(2):153–162. doi: 10.1001/jamainternmed.2021.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID19 vaccines currently authorized in the United States.. Accessed June 1, 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccinesus.html#footnote-01.
- 22.Xueying Y, Zhang J, Olatosi B, Weissman S, Li X. COVID-19 vaccine effectiveness among people living with and without HIV in South Carolina, USA: protocol of a population-based cohort study. BMJ Open. Aug 23 2022;12(8):e067095. doi: 10.1136/bmjopen-2022-067095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XA-O, Zhang JA-O, Chen S, et al. Demographic Disparities in Clinical Outcomes of COVID-19: Data From a Statewide Cohort in South Carolina. (2328–8957 (Print)) [DOI] [PMC free article] [PubMed]
- 24.U.S. Department of Health & Human Services. COVID-19 vaccines. Accessed Jun 6, 2022. https://www.hhs.gov/coronavirus/covid-19-vaccines/index.html
- 25.South Carolina Department of Health and Environmental Control. COVID-19 Variant Dashboard. Accessed Jun 6, 2022. https://scdhec.gov/covid19/covid-19-variants
- 26.Yang X, Shi F, Zhang J, et al. Disease severity of COVID-19 in different phases of the pandemic: Do healthcare workers have better outcomes? (2590–1362 (Electronic)) [DOI] [PMC free article] [PubMed]
- 27.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. Mar 15 2011;173(6):676–82. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 28.Lang R, Humes E, Coburn SB, et al. Analysis of severe illness after postvaccination COVID-19 breakthrough among adults with and without HIV in the US. JAMA Network Open. 2022;5(10):e2236397–e2236397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. Aug 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulliam JR, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376(6593):eabn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhlmann C, Mayer CK, Claassen M, et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. The Lancet. 2022;399(10325):625–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B. 1.1. 529) variant. New England Journal of Medicine. 2022;386(16):1532–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riou C, du Bruyn E, Stek C, et al. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest. Jun 15 2021;131(12)doi: 10.1172/JCI149125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nault L, Marchitto L, Goyette G, et al. Covid-19 vaccine immunogenicity in people living with HIV-1. bioRxiv. 2021-01-01 00:00:00 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy I, Rahav G. The effect of HIV on COVID-19 vaccine responses. Curr Opin HIV AIDS. May 1 2023;18(3):135–141. doi: 10.1097/COH.0000000000000790 [DOI] [PubMed] [Google Scholar]
- 36.Association HM. COVID-19 Vaccination Recommendations for People With HIV. Accessed November 6, 2023. https://www.idsociety.org/globalassets/idsa/public-health/covid-19/covid-19-vaccines-pwh-table.pdf
- 37.Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with HIV. Clin Infect Dis. Jul 22 2021;doi: 10.1093/cid/ciab648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruddy JA, Boyarsky BJ, Bailey JR, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS. Nov 15 2021;35(14):2399–2401. doi: 10.1097/QAD.0000000000003017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect. Dec 2021;27(12):1851–1855. doi: 10.1016/j.cmi.2021.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergman P, Blennow O, Hansson L, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. Nov 29 2021;74:103705. doi: 10.1016/j.ebiom.2021.103705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brumme ZL, Mwimanzi F, Lapointe HR, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. medRxiv. Oct 15 2021;doi: 10.1101/2021.10.03.21264320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. Feb 2022;602(7898):654–656. doi: 10.1038/s41586-021-04387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchan SA, Chung H, Brown KA, et al. Estimated effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. JAMA network open. 2022;5(9):e2232760–e2232760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen VH, Boileau C, Bogdanov A, et al. Relative effectiveness of BNT162b2, mRNA-1273, and Ad26. COV2. S vaccines and homologous boosting in preventing COVID-19 in adults in the US. medRxiv. 2023:2023.02. 10.23285603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stouten VA-O, Hubin P, Haarhuis F, et al. Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium. LID - 802 [pii] LID - 10.3390/v14040802 [doi]. (1999–4915 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors are prohibited from making individual-level data available publicly due to provisions in our data use agreements with state agencies/data providers, institutional policy, and ethical requirements. To facilitate research, we make access to such data available via approved data access requests through the data owners. The data is unavailable externally or for re-release due to prohibitions in data use agreements with our state agencies or other data providers. Institutional policies stipulate that all external requests for data access require collaboration with a USC researcher. For more information or to make a request, please contact (Bankole Olatosi, PhD):Olatosi@mailbox.sc.edu. The underlying analytical codes are available from the authors on request.


