Abstract
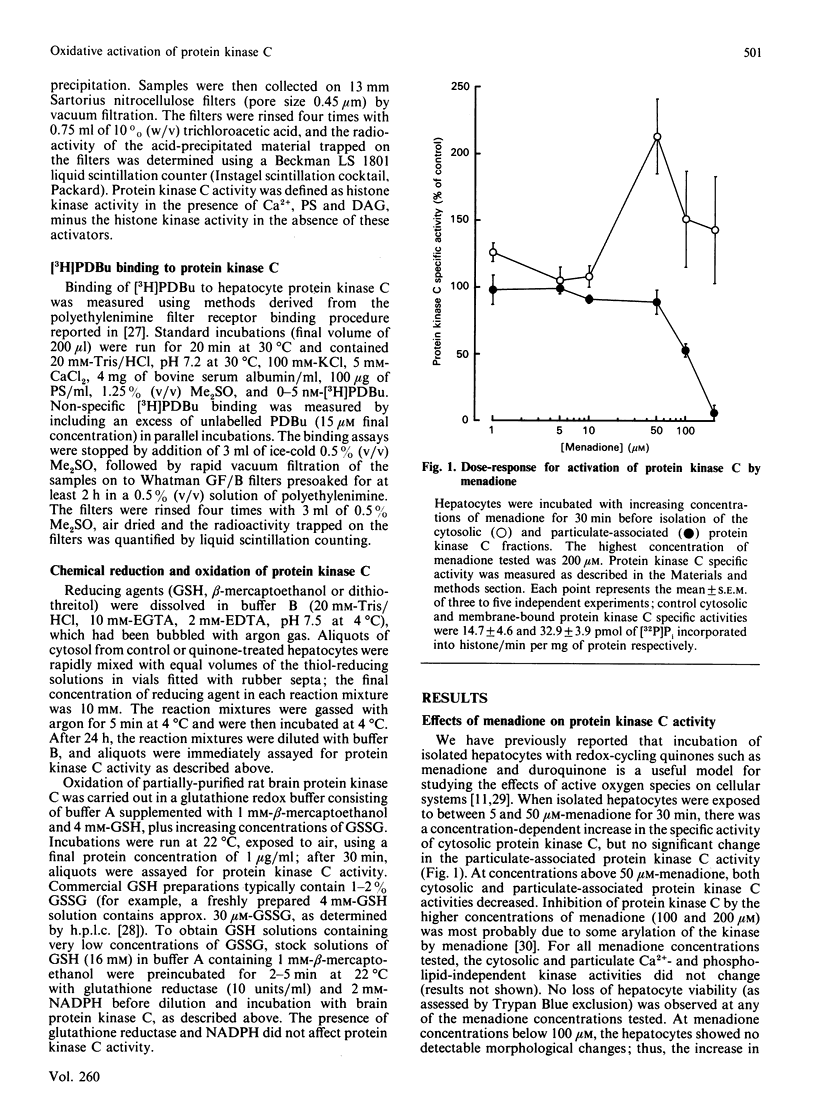
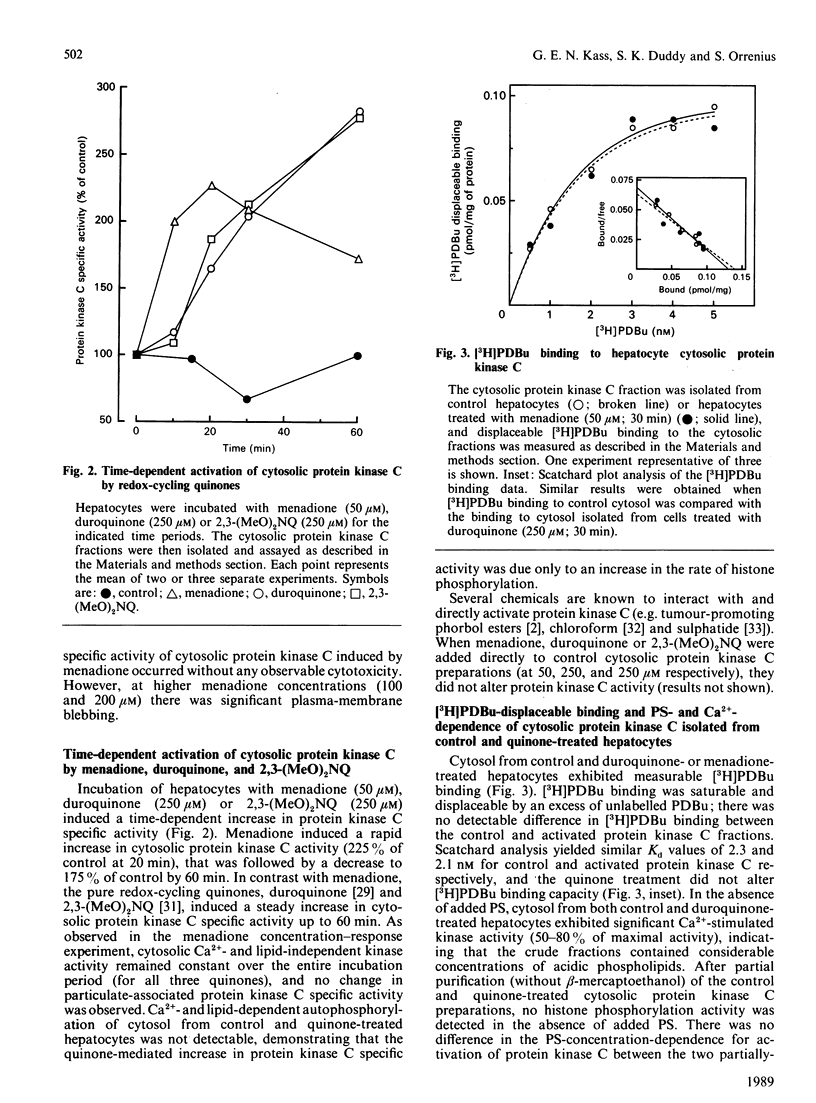
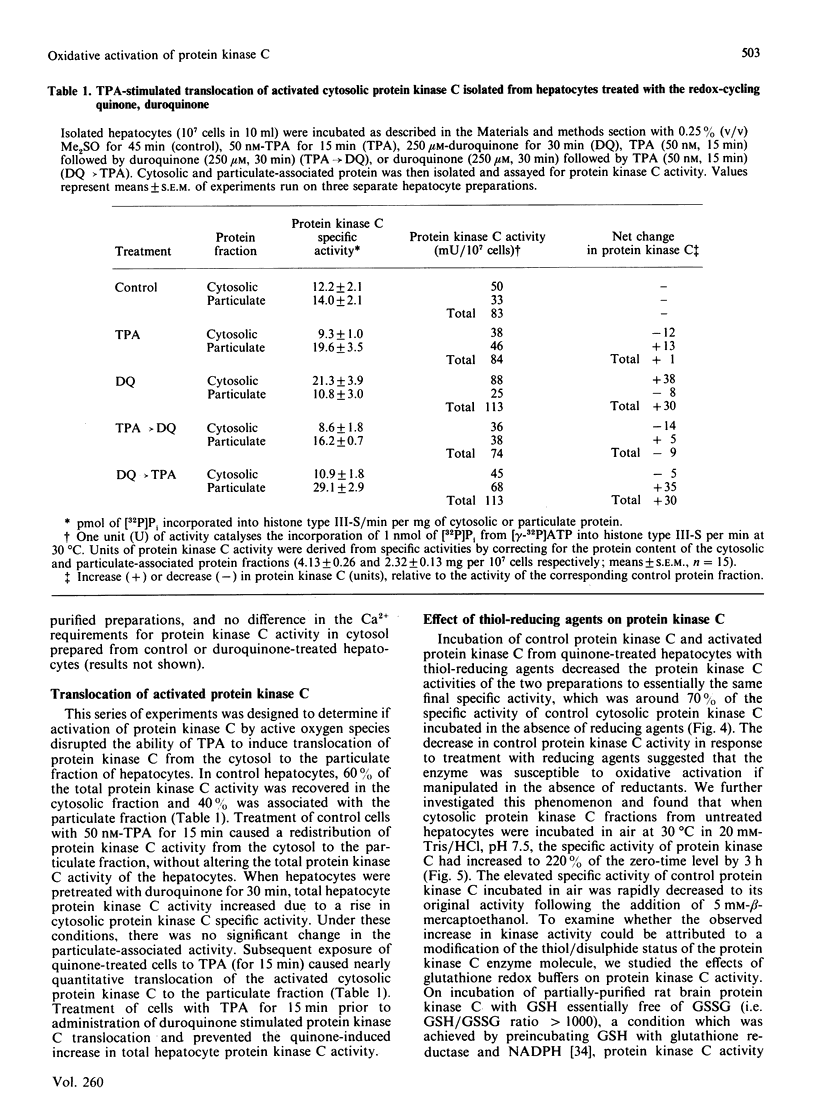
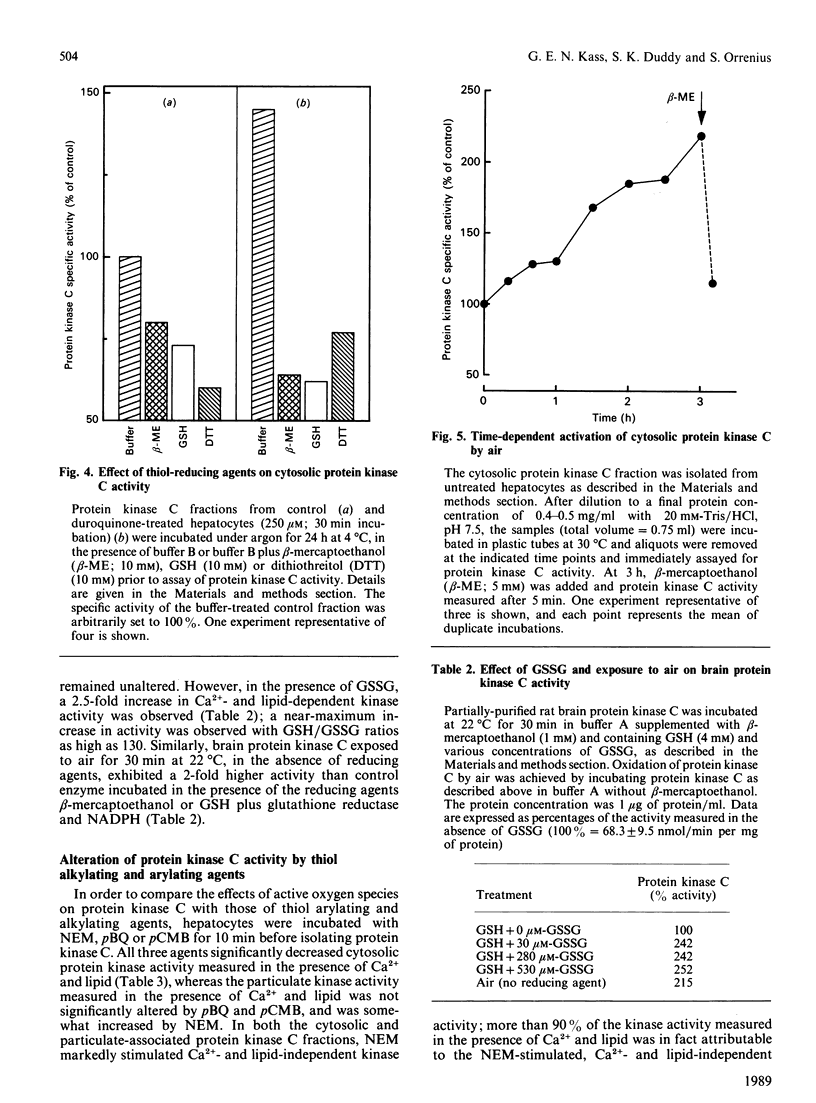
The effects of quinone-generated active oxygen species on rat hepatocyte protein kinase C were investigated. The specific activity of cytosolic protein kinase C was increased 2-3-fold in hepatocytes incubated with the redox-cycling quinones, menadione, duroquinone or 2,3-dimethoxy-1,4-naphthoquinone, without alterations in particulate protein kinase C specific activity or Ca2+- and lipid-independent kinase activities. Redox-cycling quinones did not stimulate translocation of protein kinase C; however, activated protein kinase C was redistributed from cytosol to the particulate fraction when quinone-treated hepatocytes were exposed to 12-O-tetradecanoylphorbol 13-acetate (TPA). Quinone treatment did not alter cytosolic phorbol 12,13-dibutyrate (PDBu) binding capacity, and the cytosol of both control and quinone-treated hepatocytes exhibited a Kd for PDBu binding of 2 nM. Quinone-mediated activation of cytosolic protein kinase C was reversed by incubation with 10 mM-beta-mercaptoethanol, dithiothreitol or GSH, at 4 degrees C for 24 h. Furthermore, protein kinase C specific activity in control cytosol incubated in air increased by over 100% within 3 h; this increase was reversed by thiol-reducing agents. Similarly, incubation of partially-purified rat brain protein kinase C in air, or with low concentrations of GSSG in the presence of GSH, resulted in a 2-2.5-fold increase in Ca2+- and lipid-dependent kinase activity. In contrast with the effects of the redox-cycling quinones, when hepatocytes were treated with the thiol agents N-ethylmaleimide (NEM), p-benzoquinone (pBQ) or p-chloromercuribenzoic acid (pCMB), the cytosolic Ca2+- and lipid-dependent kinase activity was significantly inhibited, but the particulate-associated protein kinase C activity was unaffected. The Ca2+- and lipid-independent kinase activity of both the cytosolic and particulate fractions was significantly stimulated by NEM, but was unaffected by pBQ and pCMB. These results show that hepatocyte cytosolic protein kinase C is activated to a high-Vmax form by quinone-generated active oxygen species, and this effect is due to a reduction-sensitive modification of the thiol/disulphide status of protein kinase C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellomo G., Mirabelli F., DiMonte D., Richelmi P., Thor H., Orrenius C., Orrenius S. Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. A study with isolated hepatocytes and menadione (2-methyl-1,4-naphthoquinone). Biochem Pharmacol. 1987 Apr 15;36(8):1313–1320. doi: 10.1016/0006-2952(87)90087-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Cappel R. E., Gilbert H. F. Thiol/disulfide exchange between 3-hydroxy-3-methylglutaryl-CoA reductase and glutathione. A thermodynamically facile dithiol oxidation. J Biol Chem. 1988 Sep 5;263(25):12204–12212. [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chan T. M., Chen E., Tatoyan A., Shargill N. S., Pleta M., Hochstein P. Stimulation of tyrosine-specific protein phosphorylation in the rat liver plasma membrane by oxygen radicals. Biochem Biophys Res Commun. 1986 Sep 14;139(2):439–445. doi: 10.1016/s0006-291x(86)80010-9. [DOI] [PubMed] [Google Scholar]
- Cooper D. R., Konda T. S., Standaert M. L., Davis J. S., Pollet R. J., Farese R. V. Insulin increases membrane and cytosolic protein kinase C activity in BC3H-1 myocytes. J Biol Chem. 1987 Mar 15;262(8):3633–3639. [PubMed] [Google Scholar]
- Cotgreave I. A., Moldéus P., Orrenius S. Host biochemical defense mechanisms against prooxidants. Annu Rev Pharmacol Toxicol. 1988;28:189–212. doi: 10.1146/annurev.pa.28.040188.001201. [DOI] [PubMed] [Google Scholar]
- Darbon J. M., Issandou M., Delassus F., Bayard F. Phorbol esters induce both intracellular translocation and down-regulation of protein kinase C in MCF-7 cells. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1159–1166. doi: 10.1016/0006-291x(86)90347-5. [DOI] [PubMed] [Google Scholar]
- Del Principe D., Menichelli A., De Matteis W., Di Corpo M. L., Di Giulio S., Finazzi-Agro A. Hydrogen peroxide has a role in the aggregation of human platelets. FEBS Lett. 1985 Jun 3;185(1):142–146. doi: 10.1016/0014-5793(85)80758-4. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys. 1984 Dec;235(2):334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Inhibition of protein synthesis initiation by oxidized glutathione: activation of a protein kinase that phosphorylates the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4110–4114. doi: 10.1073/pnas.75.9.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsbeck K., Nilsson K., Hansson A., Skoglund G., Ingelman-Sundberg M. Phorbol ester-induced alteration of differentiation and proliferation in human hematopoietic tumor cell lines: relationship to the presence and subcellular distribution of protein kinase C. Cancer Res. 1985 Dec;45(12 Pt 1):6194–6199. [PubMed] [Google Scholar]
- Fujiki H., Yamashita K., Suganuma M., Horiuchi T., Taniguchi N., Makita A. Involvement of sulfatide in activation of protein kinase C by tumor promoters. Biochem Biophys Res Commun. 1986 Jul 16;138(1):153–158. doi: 10.1016/0006-291x(86)90259-7. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant T. W., Rao D. N., Mason R. P., Cohen G. M. Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem Biol Interact. 1988;65(2):157–173. doi: 10.1016/0009-2797(88)90052-x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Susceptibility of protein kinase C to oxidative inactivation: loss of both phosphotransferase activity and phorbol diester binding. FEBS Lett. 1987 Dec 10;225(1-2):233–237. doi: 10.1016/0014-5793(87)81164-x. [DOI] [PubMed] [Google Scholar]
- Halsey D. L., Girard P. R., Kuo J. F., Blackshear P. J. Protein kinase C in fibroblasts. Characteristics of its intracellular location during growth and after exposure to phorbol esters and other mitogens. J Biol Chem. 1987 Feb 15;262(5):2234–2243. [PubMed] [Google Scholar]
- Hayes G. R., Lockwood D. H. Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8115–8119. doi: 10.1073/pnas.84.22.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Tsuda T., Kikuchi A., Tanimoto T., Yamashita T., Takai Y. Possible involvement of protein kinase C and calcium ion in growth factor-induced expression of c-myc oncogene in Swiss 3T3 fibroblasts. J Biol Chem. 1986 Jan 25;261(3):1187–1192. [PubMed] [Google Scholar]
- Kappus H. Overview of enzyme systems involved in bio-reduction of drugs and in redox cycling. Biochem Pharmacol. 1986 Jan 1;35(1):1–6. doi: 10.1016/0006-2952(86)90544-7. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Minakuchi R., Takai Y., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase (protein kinase C) from rat brain. Methods Enzymol. 1983;99:288–298. doi: 10.1016/0076-6879(83)99064-x. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Koshio O., Akanuma Y., Kasuga M. Hydrogen peroxide stimulates tyrosine phosphorylation of the insulin receptor and its tyrosine kinase activity in intact cells. Biochem J. 1988 Feb 15;250(1):95–101. doi: 10.1042/bj2500095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCaffrey P., Ran W., Campisi J., Rosner M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J Biol Chem. 1987 Feb 5;262(4):1442–1445. [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Muehlematter D., Larsson R., Cerutti P. Active oxygen induced DNA strand breakage and poly ADP-ribosylation in promotable and non-promotable JB6 mouse epidermal cells. Carcinogenesis. 1988 Feb;9(2):239–245. doi: 10.1093/carcin/9.2.239. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Pontremoli S., Horecker B. L. Activation of rabbit liver fructose diphosphatase by coenzyme A and acyl carrier protein. Proc Natl Acad Sci U S A. 1969 Nov;64(3):947–951. doi: 10.1073/pnas.64.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., McConkey D., Svensson S. A., Bellomo G., Orrenius S. Correlation between cytosolic Ca2+ concentration and cytotoxicity in hepatocytes exposed to oxidative stress. Toxicology. 1988 Nov 14;52(1-2):55–63. doi: 10.1016/0300-483x(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Perchellet J. P., Kishore G. S., Perchellet E. M. Enhancement by adriamycin of the effects of 12-O-tetradecanoylphorbol-13-acetate on mouse epidermal glutathione peroxidase activity, ornithine decarboxylase induction and skin tumor promotion. Cancer Lett. 1985 Nov;29(2):127–137. doi: 10.1016/0304-3835(85)90151-x. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Roghani M., Da Silva C., Castagna M. Tumor promoter chloroform is a potent protein kinase C activator. Biochem Biophys Res Commun. 1987 Feb 13;142(3):738–744. doi: 10.1016/0006-291x(87)91476-8. [DOI] [PubMed] [Google Scholar]
- Rossi L., Moore G. A., Orrenius S., O'Brien P. J. Quinone toxicity in hepatocytes without oxidative stress. Arch Biochem Biophys. 1986 Nov 15;251(1):25–35. doi: 10.1016/0003-9861(86)90047-0. [DOI] [PubMed] [Google Scholar]
- Sies H., Brigelius R., Graf P. Hormones, glutathione status and protein S-thiolation. Adv Enzyme Regul. 1987;26:175–189. doi: 10.1016/0065-2571(87)90013-6. [DOI] [PubMed] [Google Scholar]
- Skoglund G., Cotgreave I., Rincon J., Patarroyo M., Ingelman-Sundberg M. H2O2 activates CD11b/CD18-dependent cell adhesion. Biochem Biophys Res Commun. 1988 Dec 15;157(2):443–449. doi: 10.1016/s0006-291x(88)80269-9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Miyake R., Kikkawa U., Nishizuka Y. Rapid assay of binding of tumor-promoting phorbol esters to protein kinase C1. J Biochem. 1986 Jan;99(1):257–261. doi: 10.1093/oxfordjournals.jbchem.a135467. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Troll W., Wiesner R. The role of oxygen radicals as a possible mechanism of tumor promotion. Annu Rev Pharmacol Toxicol. 1985;25:509–528. doi: 10.1146/annurev.pa.25.040185.002453. [DOI] [PubMed] [Google Scholar]
- Tschesche H., Macartney H. W. A new principle of regulation of enzymic activity. Activation and regulation of human polymorphonuclear leukocyte collagenase via disulfide-thiol exchange as catalysed by the glutathione cycle in a peroxidase-coupled reaction to glucose metabolism. Eur J Biochem. 1981 Nov;120(1):183–190. doi: 10.1111/j.1432-1033.1981.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]



