Abstract
Objective
Patients with onychomycosis may use nail polish to camouflage affected nails, despite potential interactions between nail polish use and topical onychomycosis treatments. Our objective was to review available data on nail polish use concurrent with topical efinaconazole 10% solution for the treatment of onychomycosis.
Methods
We conducted a PubMed search and narrative review of data on effects of nail polish on penetration of efinaconazole and clinical studies of efinaconazole in the treatment of toenail onychomycosis concurrent with nail polish use, including results of an investigator-initiated study of gel nail polish pedicures.
Results
In vitro, penetration of efinaconazole through cadaverous nails coated with traditional nail polish was similar to penetration through uncoated nails. In a 52-week clinical study, efinaconazole treatment was associated with similar improvements in onychomycosis severity and clear toenail growth between participants who used traditional nail polish and those who did not use nail polish. In a second clinical study, participants received efinaconazole treatment concurrent with monthly gel nail polish pedicures. After 6 months, 100% of participants tested negative for fungal infection and all experienced visible improvements in treated toenails. Efinaconazole application was associated with degradation of traditional nail polish texture/appearance. In contrast, efinaconazole did not affect the duration, quality, or texture of gel polish.
Limitations
Only four small studies have assessed nail penetration and efficacy of efinaconazole 10% solution with concurrent nail polish use.
Conclusion
Efinaconazole 10% solution demonstrated efficacy in the treatment of toenail onychomycosis among participants concurrently using toenail polish, with no visible impact on gel-polished nails.
Keywords: dermatophyte, efinaconazole, nail polish, onychomycosis, toenail, topical
Fungal infection of the toenail (onychomycosis) is the most common nail disorder seen in clinical practice, accounting for up to 90 percent of all toenail infections and affecting up to 14 percent of individuals in North America.1–3 Onychomycosis can result in pain and physical impairment and has profound negative impacts on quality of life.3,4 Though it rarely resolves without intervention, onychomycosis is often undertreated, providing a fungal reservoir that increases the risk of spread to other parts of the body as well as transmission of the fungal infection to others.1,5,6
Oral antifungals such as terbinafine, itraconazole, and fluconazole are the most often prescribed treatments for onychomycosis in the United States.7 However, topical treatments, including efinaconazole 10% solution, tavaborole 10% solution, and ciclopirox 8% lacquer, may be a better option for some patients due to safety concerns, disease severity, or individual preference.1,8 Efinaconazole 10% solution is the first and only topical azole antifungal approved for the treatment of onychomycosis in the United States and has demonstrated the highest rates of efficacy outcomes in clinical trials.9 Efinaconazole has also demonstrated efficacy in the treatment of onychomycosis regardless of disease severity/duration or patient comorbidities, age, ethnicity, or sex.9
Although onychomycosis prevalence may be lower in women than men, women accounted for almost 60 percent of outpatient visits among patients seeking help or treatment for infected nails in the United States between 1993 and 2010.10,11 Potential reasons for this include greater concerns over the cosmetic implications of onychomycosis and a greater impact on quality of life among women than men.12 As antifungal therapy typically does not improve the appearance of infected nails in the short term, and because women in particular report feelings of stigmatization and unattractiveness due to their nails,13 they may wish to camouflage affected nails with nail polish. However, data are limited on how nail polish and topical treatments for onychomycosis interact, as clinical studies prohibit nail polish use or pedicures during treatment.14
During a six-year period from June 2016 to May 2022, 53.5 percent of prescriptions for efinaconazole 10% solution were written for female patients,11 suggesting that there may be particular interest in the interaction between efinaconazole treatment and nail polish use. The purpose of this review is to provide an overview of available data on the use of nail polish concurrent with efinaconazole treatment, including results of an investigator-initiated study of gel nail polish manicures during efinaconazole treatment for toenail onychomycosis.
Efficacy of topical efinaconazole in the treatment of toenail onychomycosis: focus on female patients. In two Phase III clinical trials, participants with mild-to-moderate toenail onychomycosis applied efinaconazole 10% solution daily for 48 weeks, with posttreatment follow up at Week 52.15,16 At the Week 52 visit, rates of mycological cure (indicated by negative KOH microscopy and negative fungal culture) and complete cure (indicated by mycological cure and 0% involvement of the target toenail) were significantly greater with efinaconazole treatment than with vehicle (mycological cure: 56% vs. 17%; complete cure: 19% vs. 5%; P<0.001, both).16 The rate of complete cure with efinaconazole 10% solution was roughly 2 to 3 times greater than was observed with other FDA-approved topical antifungals for onychomycosis, and the rate of mycological cure was in the range of that achieved with oral antifungal therapy, though differences in study designs and participant populations must be considered.9
In post hoc analyses, efficacy of efinaconazole 10% solution was greater for females than males. Among efinaconazole-treated participants, a significantly greater percentage of females than males achieved complete cure at Week 52 (27.1% vs. 15.8%; P=0.001).16 In addition, compared to males, females achieved higher rates of mycological cure (64.8% vs. 53.7%), complete or almost complete cure (≤5% clinical involvement of the target toenail and mycological cure; 36.8% vs. 24.9%), and treatment success (≤10% clinical involvement; 59.1% vs. 43.5%), as well as lower target toenail involvement at follow-up (14.4% vs. 20.6%).17,18 Moreover, self-assessments of treatment satisfaction and improvements from baseline in quality of life were greater for females than males.18 Rates of adverse events in these studies were somewhat higher among females than males (71.3% vs. 63.5%), though most were of mild-to-moderate severity (96.7%) and unrelated to study drug (89.9%).18 Though it is unclear why efinaconazole 10% solution was generally more efficacious for females compared to males, it should be noted that the net benefit (efficacy of active treatment minus vehicle) was similar for both sexes.17
Effect of nail polish on nail penetration of efinaconazole and treatment of toenail onychomycosis. Clinical trials of topical antifungals for onychomycosis prohibit the use of nail polish during treatment.14 However, one ex vivo and two in vivo studies have been performed to better understand how nail polish affects nail penetration and treatment of onychomycosis with efinaconazole 10% solution (Table 1).
TABLE 1.
Studies evaluating effects of nail polish on efinaconazole nail penetration and onychomycosis treatment
| PUBLICATION | STUDY DESIGN | POPULATION | NAIL POLISH BRAND/COLOR | RESULTS |
|---|---|---|---|---|
| Zeichner 201412 | In vitro nail penetration: once-daily efinaconazole application on Days 1, 2, 3, 4, and 7 | Human cadaverous thumbnails (free of disease or pathology) | Dior (999 Red Royalty); Essie (488 Forever Yummy; Revlon (550 Cherry) | Drug permeation of efinaconazole was not affected by prior application of cosmetic nail polish |
| Canavan 201919 | Prospective, blinded, 52-week study | Females (N=13) aged 19-70 yearsa with moderate-to-severe onychomycosis | No restrictions on brand, but gel polish excluded; control group did not use nail polish | No apparent differences in efinaconazole efficacy between those who used or did not use nail polish |
| Pandit 202320 | Prospective, open-label, 6-month study | Females (N=12) aged 23-62 yearsb with mild-to-moderate toenail onychomycosis | Gel polish from 2 brands: OPI, Gelish >30 colors in total |
100% mycological cure; 88.9% clinical cure; 44.4% complete curec,d; decrease in target toenail involvement from 55.0% to 4.4%d; visible improvements in 100% of participants |
aInclusion criteria; actual participant ages were not provided;
bActual participant ages;
cMycological cure defined as negative mycology (assessed via periodic acid-Schiff and Grocott’s methenamine silver staining); clinical cure defined as ≤10% target toenail involvement; complete cure defined as negative mycology and 0% target toenail involvement;
dAmong 9 participants with onychomycosis mycologically confirmed at baseline via periodic acid-Schiff and Grocott’s methenamine silver staining.
Penetration of efinaconazole 10% solution through polished nails. Nail penetration was assessed in an ex vivo study using human cadaverous thumbnails.12 The nails were placed in Bronaugh cells prior to application of two coats of one of three brands of red nail polish; unpolished nails served as negative controls. The polish was allowed to dry over several days prior to application of radiolabeled efinaconazole 10% solution, covering the entire nail, on Days 1, 2, 3, 4, and 7. At all times, there were no significant differences between coated and uncoated nails in the cumulative percentage of the applied efinaconazole dose that had permeated through the nails (receptor phase levels). At Day 7, cumulative permeation of radiolabeled efinaconazole was 0.56 percent of the applied dose for uncoated nails versus 0.43 to 0.51 percent for coated nails. It is important to note that the nails used in this study were free of apparent disease, and thus extrapolation to the treatment of onychomycosis through polished nails is not possible. Nevertheless, this was the first study to demonstrate that nail penetration of efinaconazole 10% solution does not appear to be affected by prior nail polish application.
Treatment of toenail onychomycosis with efinaconazole 10% solution and concurrent traditional nail polish use. A prospective, blinded, 52-week study enrolled female participants (N=13; aged 19–70 years) with moderate-to-severe onychomycosis of at least one great toenail.19 Treatment consisted of applying efinaconazole 10% solution (to the nail plate, lateral and proximal nail folds, hyponychium, and undersurface of the nail) every night before bed for 48 weeks with a four-week post-treatment follow-up. Participants were separated into a control group (n=6) that abstained from nail polish use and a study group (n=7) of self-identified frequent nail polish users; these participants were allowed to apply any brand of nail polish and to touch up their nail polish without restriction. For both groups, improvements were observed in investigator-assessed onychomycosis severity score, nail thickness, and the amount of clear nail growth over the course of the study; there were no apparent differences between groups at any time point. Conclusions of this study are limited by small sample size, small range of polish brands/types, and inconsistent application of top and base coats among nail polish users. In addition, findings from mycological testing performed at baseline, end of treatment, and follow-up were not published. Regardless, the results are consistent with the previous in vitro study in that nail polish use did not appear to affect transungual penetration of efinaconazole 10% solution. Moreover, nail polish did not impact improvements in toenail onychomycosis over 48 weeks of efinaconazole treatment.
Treatment of toenail onychomycosis with efinaconazole 10% solution and concurrent gel nail polish use. Gel, or “no-chip,” nail polish pedicures have become increasingly popular due to faster polish drying time and increased durability compared to traditional nail polish. The impact of gel nail polish on efficacy of efinaconazole in the treatment of onychomycosis was assessed in a six-month prospective clinical study of 12 female participants (aged 23–62 years) with mild-to-moderate onychomycosis of at least one great toenail.20 Self-reported disease duration ranged from 6 to 84 months. Eight participants (75%) had a history of previous onychomycosis treatment (duration, range: 2–60 months), including over-the-counter and/or prescription antifungal use (e.g., topical ciclopirox and tavaborole; oral terbinafine), nail avulsion, and partial matrixectomy. Percent involvement of the target toenail ranged from 10 to 100 percent at baseline. Every month, participants received a pedicure plus no-chip/gel polish; they were able to choose from over 30 colors of OPI® or Gelish® brand polishes, and brand/color could change from month to month. Polish was cured via 90-second ultraviolet (UV) light exposure and was followed by application of efinaconazole 10% solution to affected toenails, including the hyponychium and undersurface of the nail. On all other days, participants applied efinaconazole 10% solution to affected nails once daily and refrained from using nail polish or nail treatments outside the monthly pedicures.
At baseline, onychomycosis was mycologically confirmed via periodic acid-Schiff plus Grocott’s methenamine silver staining (PAS+GMS) in nine participants. Among these, onychomycosis was rated as moderate for seven (77.8%) and mild for two (22.2%) using the Onychomycosis Severity Index.21 After six months of treatment with efinaconazole 10% solution, all nine achieved mycological cure (negative PAS+GMS), eight (88.9%) achieved clinical cure (≤10% nail involvement) and four (44.4%) achieved complete cure (mycological cure and 0% nail involvement; Figure 1). Mean percent target toenail involvement decreased from 55.0 percent at baseline to 4.4 percent at six months (range: 0–20%; paired, 2-tailed t-test, P<0.001; Figure 1), and all participants had visible improvements in target toenail appearance (Figure 2). There were no treatment-related adverse events. Early in the study, one participant’s nail fell off; this was considered by the investigator to be unrelated to efinaconazole treatment, as this nail had fallen off three times prior to study enrollment.
FIGURE 1.
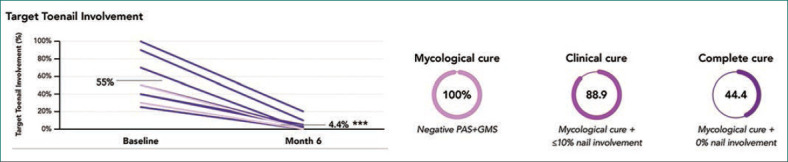
Efficacy at 6 Months With Efinaconazole 10% Solution and Gel-Polished Nails: Prospective, Open-Label, 6-Month Study20
***P<0.001 vs baseline. Data for participants with mycological confirmation of onychomycosis via PAS+GMS at baseline (n=9). Graph at left: each purple line represents one participant; grey lines indicate mean values. PAS+GMS, periodic acid-Schiff and Grocott’s methenamine silver staining.
FIGURE 2.
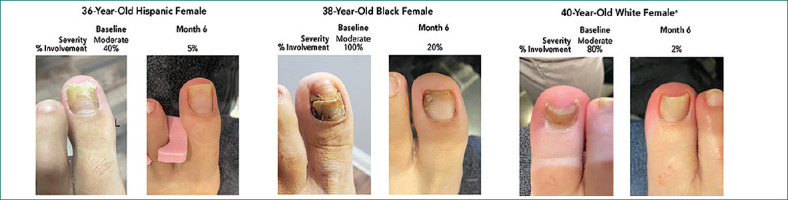
Onychomycosis Improvements With Efinaconazole 10% Solution: Prospective, Open-Label, 6-Month Study20
Individual results may vary.a At baseline, participant’s mycological testing was negative; onychomycosis was clinically diagnosed.
For the remaining three participants, histopathology was negative at baseline, though it should be noted that challenges to obtaining sufficient, high-quality samples often lead to false negative results with this technique.22 In these participants, onychomycosis was clinically diagnosed via subungual debris, lifting of the toenail, and superficial nail color changes. At Month 6, mycology results were negative for all three, two achieved clinical cure, and one achieved complete cure. Percent nail involvement for these participants decreased from 10 to 80 percent at baseline to 0 to 2 percent at end of study.
Conclusions from this study are limited by small sample size and small sample of gel nail polish brands used. Regardless, mycological cure and aesthetic improvements of participants’ nails were achieved with only six months of topical efinaconazole treatment, compared to 52 weeks of treatment in clinical trials.15,16 Collectively, data from these two in vivo clinical studies demonstrate that effectiveness of efinaconazole 10% solution in the treatment of toenail onychomycosis is not significantly impacted by concurrent nail polish use. To our knowledge, no similar in vivo trials have been conducted with any other FDA-approved topical antifungals to determine how nail polish use interacts with topical treatment of onychomycosis.
Effect of efinaconazole on the appearance of nail polish. Although nail polish does not appear to prevent efinaconazole 10% solution from reaching the site of toenail fungal infections, the impact of topical onychomycosis treatments on nail polish is another important consideration for patients. Two ex vivo and two in vivo studies have evaluated the effects of efinaconazole on the appearance of nail polish (Table 2).
TABLE 2.
Effects of efinaconazole on nail polish appearance
| PUBLICATION | STUDY DESIGN | POPULATION | NAIL POLISH BRAND/COLOR | RESULTS |
|---|---|---|---|---|
| Zeichner 201412 | In vitro: once-daily efinaconazole application on Days 1, 2, 3, 4, and 7 | Human cadaverous thumbnails (free of disease or pathology) | Dior (999 Red Royalty) Essie (488 Forever Yummy) Revlon (550 Cherry) |
Nail polish color transfer onto efinaconazole applicator; nail polish tackiness after efinaconazole application |
| Vlahovic 201623 | In vitro: once-daily efinaconazole application for 7 days | Human cadaverous fingernails | L’Oreal (420 Devil Wears Red) | Progressively worsening polish appearance and discoloration; nail polish color transfer to efinaconazole applicator and remaining solution |
| Canavan 201919 | Prospective, blinded, 52-week study | Females (N=13) aged 19-70 yearsa with moderate-to-severe onychomycosis | No restrictions on brand, but gel polish excluded; control group did not use nail polish | Nail polish quality was negatively affected by efinaconazole, though was partially mitigated with darker colors and use of top/base coats; all participants reported overall satisfaction with efinaconazole |
| Pandit 202320 | Prospective, open-label, 6-month study | Females (N=12) aged 23-62 yearsb with mild-to-moderate toenail onychomycosis | Gel polish from 2 brands: OPI, Gelish; >30 colors in total | No qualitative differences between gel-polished nails treated with efinaconazole vs untreated; no effect of efinaconazole on duration/quality/texture of gel polish |
aInclusion criteria; actual participant ages were not provided;
bActual participant ages.
An ex vivo study compared the effects of topical antifungals on the appearance of nail polish applied to cadaverous fingernails.23 Across seven daily applications of efinaconazole 10% solution to red-polished nails, appearance and discoloration of the polish progressively worsened. In addition, color transfer from the polish was observed on the efinaconazole applicator as well as to the unused solution remaining in the bottle. Neither effect was seen with application of topical tavaborole 5% solution. In the ex vivo nail penetration study described above, no deterioration of nail polish appearance was observed, though it should be noted that only five efinaconazole applications, as opposed to seven, were made over the course of that study.12 Polish texture was affected, however, as tackiness that persisted beyond efinaconazole drying was observed, as was color transfer to the applicator.
In the 52-week in vivo study described above, participants reported tackiness and decreased non-gel polish quality 60 percent of the time, with one participant reporting complete removal of the polish after application of efinaconazole 10% solution.19 Polish appearance and color were found to be more stable with efinaconazole application when darker colors as well as top and/or base coats were used, and all participants who applied polish reported overall satisfaction with efinaconazole.
In contrast, the aforementioned six-month study of gel-polished nails found no apparent differences in polish quality between nails that were treated with efinaconazole 10% solution versus untreated, regardless of polish color.20 Duration, quality, and texture of gel polish were not affected, perhaps due to UV-curing creating a hard polish surface that is less prone to disruption by efinaconazole application.
Despite limited data, results from these studies indicate that patients should be informed that the impact of efinaconazole on the appearance of nail polish may vary depending upon the type of polish used. Patients may prefer to use gel polish versus traditional nail polish, as efinaconazole did not affect the quality, texture, or duration of gel-polished nails. For those patients using traditional nail polish, darker colors and use of top and base coats may help improve the appearance of the polish.23–25
CONCLUSION
Topical treatments for onychomycosis must overcome several barriers to nail penetration to reach the site of infection. The nail plate itself provides the most significant of these barriers: nail hardness and thickness as well as densely packed keratin fibers are all known to impact transungual penetration.26 Considerably less is known about whether concurrent nail polish use serves as an additional penetration barrier. To our knowledge, the findings reviewed here represent all available data on nail polish and concurrent onychomycosis treatment with topical antifungals available in the United States (ciclopirox 8% lacquer, efinaconazole 10% solution; tavaborole 5% solution) or Europe (amorolfine 5% lacquer; terbinafine 10% lacquer).
This review focuses on efinaconazole 10% solution as it is the only topical antifungal for which there is clinical data demonstrating effectiveness against onychomycosis in polished toenails. The high nail permeability of efinaconazole results from low keratin affinity relative to other topical antifungals and low surface tension,27–30 which allows for access to the site of infection via diffusion through the subungual space.28,31 Subungual permeation may provide a pathway by which efinaconazole 10% solution can circumvent any barrier to penetration caused by coating nails with polish. In ex vivo and in vivo studies, nail penetration and treatment of toenail onychomycosis with efinaconazole 10% solution were not significantly impacted by concurrent application of traditional or gel nail polishes. Further, efinaconazole did not impact the appearance of UV-cured gel nail polish. These findings may be of particular importance when selecting treatment options for female patients with onychomycosis, as they are more likely than males to receive a prescription for topical efinaconazole 10% solution11 and also to use nail polish.
ACKNOWLEDGMENTS
Medical writing and editorial support were provided by Kevin Corcoran, PhD, from Prescott Medical Communications Group, a Citrus Health Group, Inc., company (Chicago, Illinois) with support from Ortho Dermatologics (Bridgewater, New Jersey). Ortho Dermatologics is a division of Bausch Health US, LLC.
REFERENCES
- Vlahovic TC, Joseph WS, Scher RK et al. Diagnosis and management of onychomycosis perspectives from a joint podiatric medicine-dermatology roundtable. J Am Podiatr Med Assoc. 2016;106(2):155–162. doi: 10.7547/14-170. [DOI] [PubMed] [Google Scholar]
- Lipner SR, Joseph WS, Vlahovic TC et al. Therapeutic recommendations for the treatment of toenail onychomycosis in the US. J Drugs Dermatol. 2021;20(10):1076–1084. doi: 10.36849/JDD.6291. [DOI] [PubMed] [Google Scholar]
- Albucker SJ, Falotico JM, Choo ZN et al. Risk factors and treatment trends for onychomycosis: a case-control study of onychomycosis patients in the All of Us research program. J Fungi (Basel). 2023;9(7) doi: 10.3390/jof9070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel RC. Onychomycosis: burden of disease and the role of topical antifungal treatment. J Drugs Dermatol. 2013;12(11):1263–1266. [PubMed] [Google Scholar]
- Rosen T, Friedlander SF, Kircik L. et al. Onychomycosis: epidemiology, diagnosis, and treatment in a changing landscape. J Drugs Dermatol. 2015. 14 3223-233 [PubMed] [Google Scholar]
- Gupta AK, Stec N, Summerbell RC et al. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020;34(9):1972–1990. doi: 10.1111/jdv.16394. [DOI] [PubMed] [Google Scholar]
- Gold JAW, Wu K, Jackson BR et al. Opportunities to improve guideline adherence for the diagnosis and treatment of onychomycosis: analysis of commercial insurance claims data, United States. J Am Acad Dermatol. 2023;88(3):683–686. doi: 10.1016/j.jaad.2022.06.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Venkataraman M, Renaud HJ et al. A paradigm shift in the treatment and management of onychomycosis. Skin Appendage Disord. 2021;7(5):351–358. doi: 10.1159/000516112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahovic TC, Gupta AK. Efinaconazole topical solution (10%) for the treatment of onychomycosis in adult and pediatric patients. Expert Rev Anti Infect Ther. 2022;20(1):3–15. doi: 10.1080/14787210.2021.1939011. [DOI] [PubMed] [Google Scholar]
- Taheri A, Davis SA, Huang KE et al. Onychomycosis treatment in the United States. Cutis. 2015;95(5):E15–E21. [PubMed] [Google Scholar]
- Lipner SR, Falotico JM, Konda A. On the basis of sex: impact and treatment of toenail onychomycosis in female patients. J Clin Aesthet Dermatol. 2023;16(10):52–57. [PMC free article] [PubMed] [Google Scholar]
- Zeichner JA, Stein Gold L, Korotzer A. Penetration of (14C)-efinaconazole topical solution, 10%, does not appear to be influenced by nail polish. J Clin Aesthet Dermatol. 2014;7(9):34–36. [PMC free article] [PubMed] [Google Scholar]
- Szepietowski JC, Reich A. National Quality of Life in Dermatology Group. Stigmatisation in onychomycosis patients: a population-based study. Mycoses. 2009;52(4):343–349. doi: 10.1111/j.1439-0507.2008.01618.x. [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ. Application of nail polish during topical management of onychomycosis: are data available to guide the clinician about what to tell their patients? J Clin Aesthet Dermatol. 2016;9(8):29–36. [PMC free article] [PubMed] [Google Scholar]
- Elewski BE, Rich P, Pollak R et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68(4):600–608. doi: 10.1016/j.jaad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Elewski BE, Sugarman JL et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13(7):815–820. [PubMed] [Google Scholar]
- Bhatia N. Managing assessments and expectations: patient responses following therapy with efinaconazole topical solution, 10%. J Drugs Dermatol. 2015;14(7):694–698. [PubMed] [Google Scholar]
- Rosen T. Evaluation of gender as a clinically relevant outcome variable in the treatment of onychomycosis with efinaconazole topical solution 10%. Cutis. 2015;96:197–201. [PubMed] [Google Scholar]
- Canavan TN, Bevans SL, Cantrell WC et al. Single-center, prospective, blinded study comparing the efficacy and compatibility of efinaconazole 10% solution in treating onychomycosis with and without concurrent nail polish use. Skin Appendage Disord. 2019;5(1):9–12. doi: 10.1159/000488369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data on file. Ortho Dermatologics.
- Carney C, Tosti A, Daniel R et al. A new classification system for grading the severity of onychomycosis: Onychomycosis severity index. Arch Dermatol. 2011;147(11):1277–1282. doi: 10.1001/archdermatol.2011.267. [DOI] [PubMed] [Google Scholar]
- Petrucelli MF, Abreu MH, Cantelli BAM et al. Epidemiology and diagnostic perspectives of dermatophytoses. J Fungi (Basel). 2020;6(4):310. doi: 10.3390/jof6040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahovic TC, Coronado D, Chanda S et al. Evaluation of the appearance of nail polish following daily treatment of ex vivo human fingernails with topical solutions of tavaborole or efinaconazole. J Drugs Dermatol. 2016;15(1):89–94. [PubMed] [Google Scholar]
- Penlac® (ciclopirox) topical solution, 8%. US prescribing information. Bridgewater, NJ: Sanofi-Aventis US, LLC. 2006.
- Loceryl® (amorolfine) topical lacquer, 5%. Package insert. London, England: Galderma (UK) Ltd. 2022.
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933–1957. doi: 10.2147/CCID.S362635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Sugimoto N, Hosaka S et al. The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment. Antimicrob Agents Chemother. 2014;58(7):3837–3842. doi: 10.1128/AAC.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircik LH. Enhancing transungual delivery and spreading of efinaconazole under the nail plate through a unique formulation approach. J Drugs Dermatol. 2014;13(12):1457–1461. [PubMed] [Google Scholar]
- Tachibana H, Kumagai N, Tatsumi Y. Fungicidal activity in the presence of keratin as an important factor contributing to in vivo efficacy: a comparison of efinaconazole, tavaborole, and ciclopirox. J Fungi (Basel). 2017;3(4):58. doi: 10.3390/jof3040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Sugiura K, Hashimoto T et al. Efficacy coefficients determined using nail permeability and antifungal activity in keratin-containing media are useful for predicting clinical efficacies of topical drugs for onychomycosis. PLoS One. 2016;11(7):e0159661. doi: 10.1371/journal.pone.0159661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewski BE, Pollak RA, Pillai R et al. Access of efinaconazole topical solution, 10%, to the infection site by spreading through the subungual space. J Drugs Dermatol. 2014;13(11):1394–1398. [PubMed] [Google Scholar]


