Abstract
This study investigated the epidemiology of gastrointestinal (GI) parasite infections among 42 meat goat herds in Khon Kaen, Northeast Thailand, based on 913 fecal samples. The predominant parasites identified in the herd were strongyle (100.0%, 42/42), Trichuris spp. (73.8%, 31/42), Eimeria spp. (66.7%, 28/42), Moniezia spp. (64.3%, 27/42), Strongyloides spp. (38.1%, 16/42), and Paramphistomum spp. (7.1%, 3/42). Coinfection with at least two GI parasites was observed in 90.4% of the herds. Molecular analysis confirmed Haemonchus contortus and Trichostrongylus spp. as the strongyle species. The study explored parasite prevalence among animals, finding significant correlations with season, sex, age, and breed. Notably, the wet season showed increased strongyle and Eimeria spp. infections. Female animals had higher odds of strongyle infection, while younger animals (less than 1 year) were more susceptible. Conversely, animals aged over 1 year were more likely to be positive for Trichuris spp., Moniezia spp., and Eimeria spp. infections. Female animals exhibited poor body condition scores (BCS) and anemia, as indicated by the FAMACHA score and packed cell volume (PCV) levels. Correlations between age, clinical signs, hematological parameters, biochemistry, and GI parasite burdens were investigated, revealing significant associations. These findings emphasize the need for tailored intervention strategies considering seasonal variations, age, and sex differences for effective GI parasite control in meat goats. Prioritizing animals exhibiting poor BCS and elevated FAMACHA score is imperative to mitigate the deleterious impacts of GI parasitic infections on health and productivity.
1. Introduction
Gastrointestinal (GI) parasite infections are a major health concern for small ruminants worldwide, causing significant losses in productivity, economy, and welfare [1–5]. Studies have estimated that helminth infections cost approximately €1.8 billion annually in Europe alone, with most of the losses attributed to decreased production [2]. GI parasites include nematodes, cestodes, trematodes, and protozoa, and they cause symptoms, such as reduced feed intake, slow growth, anemia, diarrhea, rough hair, and poor BCS, which can lead to higher mortality rates in herds. In Thailand, the small ruminant population, primarily consisting of 1.5 million goats and 0.1 million sheep, has been steadily increasing by approximately 28% per year [6, 7]. Farmers typically implement deworming programs every one to six months using drugs like albendazole, ivermectin, and levamisole. However, due to variations in GI parasite infections, tailored treatment, control, and prevention strategies are necessary. This study aims to document the epidemiology of GI parasite-infected meat goats in tropical zones like Khon Kaen, Thailand, and establish associations between factors and clinical signs based on laboratory findings. The information obtained from this study could help develop effective management programs to address challenges related to GI parasites.
2. Materials and Methods
2.1. Study Area and Design
The study was conducted from March to December 2021 in Khon Kaen province, situated at coordinates 16°26′48.16″N and 102°49′58.8″E with an altitude of 187 meters above sea level. The area experiences a range of annual temperatures, from 13.9°C to 39.6°C (minimum to maximum), and annual rainfall averaging between 0.0 and 9.38 mm. Relative humidity fluctuates between 21% and 99% [8]. The seasons are classified into two primary categories: the wet or monsoon season, which spans from May to October and is characterized by increased rainfall and higher humidity levels, and the dry season. The dry season is further subdivided into two distinct periods: the summer season, occurring from March to April, and the winter season, occurring from November to February. Both of these subseasons are marked by lower humidity levels.
A total of 42 meat goat herds across 8 districts in Khon Kaen province were included in the study, comprising Ban Fang (3), Ban Haet (1), Muang (2), Nam Phong (2), Nong Ruea (9), Phu Wiang (5), Si Chomphu (13), and Wiang Kao (7) districts (Figure 1). Additionally, 1,868 animals underwent physical examinations to determine their sex, breed, and age.
Figure 1.
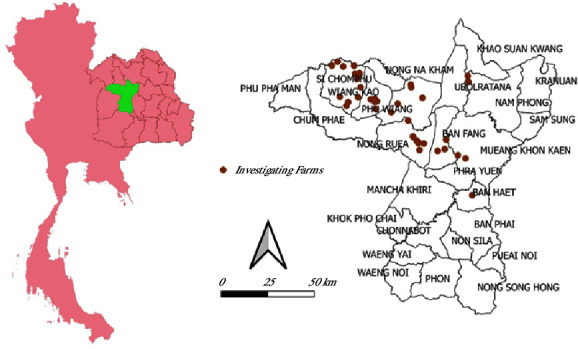
The geographic distribution of meat goat herds in Khon Kaen, Northeast Thailand.
The clinical examination included assessments using the FAMACHA score and Dag score. Age classification relied on the presence of permanent teeth, categorizing animals as adult (1, 2, 3, 4, and >4 years) or young (<1 year) if nonpermanent teeth were observed. BCS was applied following Detweiler et al. [9], assessing the lumbar and sternum regions for classifications, including obese (1), fat (2), average (3), thin (4), and emaciated (5). FAMACHA scores were assigned based on conjunctival coloration, indicating nonanemia (1), mild (2), moderate (3), severe (4), or very severe anemia (5) [10]. The Dag score assessed fecal staining severity on the tail and crutch, categorized as normal (1), light (2), moderate (3), severe (4), or very severe (5) [11, 12]. The presence of bottle jaw was recorded. A total of 913 animals were randomly sampled for blood and fecal collection to detect GI parasites. The number of animals categorized by age and clinical examination is shown in Table 1.
Table 1.
Distribution of animals categorized by age and clinical examination.
| Age (yr) | Total | BCS | FAMACHA score | Dag score | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2.5 | 3 | 3.5 | 4 | 4.5 | 5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| <1 | 274 | 1 | 1 | 130 | 101 | 39 | 2 | — | 18 | 1 | 108 | 1 | 130 | 5 | 8 | — | 3 | 254 | 14 | 3 | 3 | — |
| 1 | 168 | — | — | 42 | 82 | 41 | 3 | — | 4 | — | 53 | 1 | 103 | 3 | 2 | 1 | 1 | 159 | 8 | — | — | 1 |
| 2 | 92 | — | — | 21 | 42 | 26 | 2 | 1 | 2 | — | 26 | 1 | 59 | 2 | 1 | — | 1 | 83 | 8 | 1 | — | — |
| 3 | 139 | — | 1 | 41 | 50 | 41 | 6 | — | 1 | — | 32 | — | 97 | 4 | 5 | — | — | 134 | 4 | 1 | — | — |
| 4 | 118 | 1 | 4 | 24 | 47 | 34 | 8 | — | — | — | 14 | 1 | 94 | 4 | 3 | 2 | — | 108 | 9 | — | 1 | — |
| >4 | 122 | — | 2 | 12 | 36 | 57 | 13 | 2 | — | — | 18 | 1 | 85 | 8 | 8 | 1 | 1 | 112 | 9 | — | — | 1 |
|
| ||||||||||||||||||||||
| Total | 913 | 2 | 8 | 270 | 358 | 238 | 34 | 3 | 25 | 1 | 251 | 5 | 568 | 26 | 27 | 4 | 6 | 850 | 52 | 5 | 4 | 2 |
| (%) | 0.22 | 0.88 | 29.57 | 39.21 | 26.07 | 3.72 | 0.33 | 2.74 | 0.11 | 27.49 | 0.55 | 62.21 | 2.85 | 2.96 | 0.44 | 0.66 | 93.10 | 5.70 | 0.55 | 0.44 | 0.22 | |
N = number of animals.
2.2. Study Animals and Data Collection
The animals included in the study were over 1 month old and represented both sexes and various age groups. Prior to the investigation, all herds had refrained from anthelmintic treatment for at least 1 month. A total of 913 animals from 42 small-holder meat goat herds were included in the study. Each animal underwent blood and feces sample collection. A 5 ml blood sample was taken from the jugular vein and divided into 3 ml of serum and 2 ml of EDTA, which were stored in separate tubes. In addition, at least 10 g of fresh feces was collected from the rectum of each animal and placed in a zip-lock plastic bag. All samples were transported and stored at 4°C until laboratory diagnostics were conducted at the Faculty of Veterinary Medicine, Khon Kaen University. Data obtained from individual animals comprised signalment (including name/number, age, sex, and breed) along with findings from physical examinations (such as FAMACHA score, BCS, Dag score, and the presence of bottle jaw).
3. Laboratory Analysis
3.1. Blood Analysis
EDTA blood samples were processed to determine packed cell volume (PCV, %) by centrifuging EDTA in microhematocrit tubes at 3,000 rpm for 15 minutes. PCV (%) was calculated as the ratio of red blood cells to the total blood volume. Additionally, hemoglobin (Hb) levels (g/dL) were measured using the HemoCue (Hb 201+ System, manufactured by HemoCue AB, located at Kuvettgatan 1, SE-262 71, Ängelholm, Sweden).
Serum samples were assessed for the concentration of total serum protein (TP) (g/dL) using a hand refractometer (Erma, model D, Tokyo, Japan).
3.2. Fecal Analysis
3.2.1. Fecal Egg Count and Identification of Worm Eggs
Fecal egg counts and the identification of worm eggs were conducted according to the methodology outlined by Brummaier et al. [13]. The subsequent differentiation of worm eggs based on their morphological characteristics followed the established protocol delineated by Taylor et al. [14].
3.2.2. Coproculture and Larva Identification
Fecal samples from the same herd were pooled and cultured for larval identification following established protocols. At least 50 grams of fecal samples from each herd was combined in sterile plastic bags with an equal amount of sterile litter and moistened with distilled water. The pooled fecal samples were then incubated at an ambient temperature range of approximately 25–35°C for a period of 14 days within a dark environment. Following the incubation period, third-stage larvae were harvested using the modified Baermann technique over a 24-hour period. The larvae collected in a 15 ml conical tube were morphologically identified using a standard key. For identification purposes, hundreds of L3 larvae were fixed with 1–2% formalin and subjected to brief heating, facilitating morphological differentiation to determine the species or genus of gastrointestinal nematodes, as per the methodology described by Van Wyk et al. [15]. Notably, it should be acknowledged that certain nematode species, such as Trichuris spp., were not identified during this phase of the study.
3.2.3. DNA Extraction, Genus-Specific PCRs, Amplification, and Sequencing
To ascertain the species infected with GI strongyle, pooled larval solutions from 41 herds in Khon Kaen were subjected to molecular techniques for nematode count confirmation. DNA extraction from larval pools was conducted using the Thermo Scientific GeneJET Genomic DNA Purification Kit (Thermo Fisher) as per the manufacturer's guidelines. DNA concentration was measured using a BioDrop DUO spectrophotometer, and the extracted DNA was stored at −20°C until further use. Genus-specific PCRs were employed to identify strongyle worms of the Haemonchus and Trichostrongylus genera in L3 samples, following the protocols outlined by Demeler et al. [16] and Mohammedsalih et al. [17]. Haemonchus spp. and Trichostrongylus spp. were identified based on larval morphology. PCRs targeted the amplification of partial internal transcribed spacer 2 (ITS-2) using specific primers designed for Haemonchus spp. and Trichostrongylus spp.
The PCR consisted of 12.5 μl of DreamTaq Green PCR Master Mix (2X), 0.2 mM of each primer (Thermo Fisher Scientific, Waltham, USA), and 2 μl (20–50 ng) of template DNA. Reaction conditions included an initial denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, primer pair-specific annealing at the designated temperature for 30 s, and extension at 72°C for 30 s. A final elongation step at 72°C for 5 min was performed using a C1000 Touch Thermal Cycler (Bio-Rad, California, USA). Subsequently, PCR products were analyzed on 1.5% agarose gels stained with RedSafe (iNtRON Biotechnology, Korea). Purification of PCR products was carried out using the GF-1 AmbiClean Kit (Gel and PCR) (Vivantis, Malaysia), followed by submission to ATGC Co., Ltd (Pathum Thani, Thailand) for sequencing. Nucleotide sequences were compared against the NCBI database using the BLAST tool and aligned with published reference sequences using the BioEdit Sequence Alignment Editor. Preliminary sequencing was conducted on 10 PCR product samples.
3.3. Statistical Analysis
The data were analyzed using Microsoft Excel, Statistix software (version 8.0 for Windows), and MedCalc® version 22.021 (MedCalc Software Ltd., 2024). Descriptive statistics, including median, mean, standard deviation (SD), minimum (min), and maximum (max), were calculated for the parameters. Normal distribution was assessed using the Shapiro–Wilk test. Risk factors, including herd size, grazing practices, housing conditions, presence of other livestock, deworming programs, management protocols, and cleanliness of feed and water trays, were taken into consideration. A univariate analysis was performed to identify variables associated with positive herds and individual animals, with a significance threshold set at 0.05. Odds ratios, along with their corresponding 95% confidence intervals, were computed to gauge the strength of these associations.
Spearman rank correlations were employed to examine associations among age, BCS, FAMACHA score, Dag score, hematological parameters (PCV, Hb), biochemistry (total protein), and the number of GI parasite infections (EPG/OPG).
Statistical significance was defined as a p value less than 0.05 (∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ = p < 0.001). Most of the data did not conform to a normal distribution (p < 0.05, Shapiro–Wilk test), necessitating the use of nonparametric tests for statistical analysis.
3.4. Spatial Distribution
Quantum GIS was employed to generate a map illustrating the distribution of the cases.
4. Results
4.1. Prevalence and Spatial Distribution of Gastrointestinal Parasite Infections
4.1.1. Microscopic Examination
GI parasites such as strongyle, Strongyloides spp., Trichuris spp., Paramphistomum spp. (rumen fluke), Moniezia spp., and Eimeria spp. oocysts were discovered in the herds (Figure 2). The number of eggs or oocysts from gastrointestinal parasite infections, categorized by age, is shown in Table 2.
Figure 2.
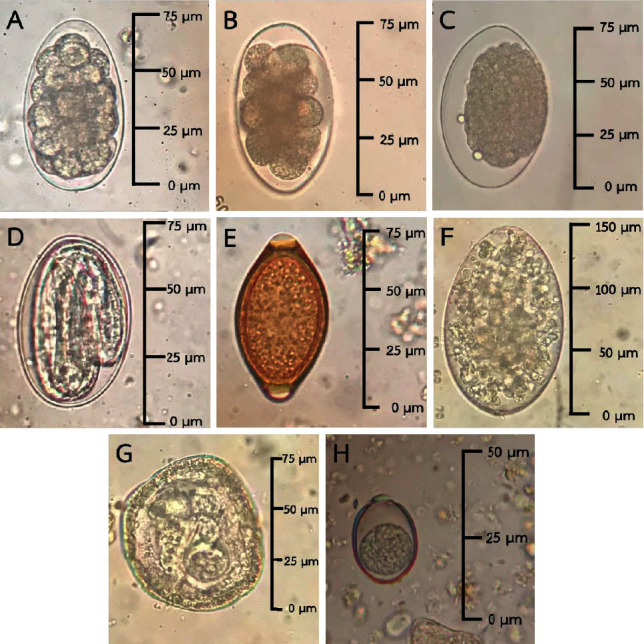
Morphology of GI parasite eggs or oocysts detected by microscopic examination. (A–C) Strongyle egg; (D) Strongyloides spp. (larvae egg); (E) Trichuris spp. egg; (F) Paramphistomum spp. egg; (G) Moniezia spp. egg; (H) Eimeria spp. oocyst (microscope image at 400x magnification).
Table 2.
The distribution of eggs or oocysts from gastrointestinal parasite infections, stratified by age.
| Age (yr) | N | Strongyle (EPG) | Strongyloides spp. (EPG) | Trichuris spp. (EPG) | Paramphistomum spp. (EPG) | Moniezia spp. (EPG) | Eimeria spp. (OPG) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| <1 | 274 | 75.5 ± 215.4 | 49.9–101.1 | 1.2 ± 9.9 | 0–2.4 | 3.6 ± 13.2 | 2.0–5.1 | 0.04 ± 0.7 | 0–0.1 | 30.9 ± 174.5 | 10.1–51.7 | 164.7 ± 1,731.0 | 0–370.6 |
| 1 | 168 | 124.1 ± 413.8 | 61.1–187.2 | 1.3 ± 7.1 | 0.2–2.4 | 5.9 ± 19.2 | 3.0–8.9 | — | — | 15.0 ± 121.2 | 0–33.4 | 7.1 ± 29.0 | 2.7–11.6 |
| 2 | 92 | 158.7 ± 480.0 | 59.2–258.2 | 2.2 ± 9.6 | 0.2–4.2 | 3.2 ± 10.9 | 0.9–5.4 | — | — | 2.4 ± 11.1 | 0.1–4.7 | 11.0 ± 46.5 | 1.4–20.7 |
| 3 | 139 | 53.97 ± 100.2 | 37.1–70.7 | 0.1 ± 1.2 | 0–0.3 | 1.1 ± 4.7 | 0.3–1.9 | — | — | 3.5 ± 26.4 | 0–8.0 | 26.0 ± 255.3 | 0–68.9 |
| 4 | 118 | 62.6 ± 91.6 | 45.9–79.3 | — | — | 2.7 ± 8.2 | 1.2–4.3 | 0.03 ± 0.3 | 0–0.1 | 1.5 ± 8.7 | 0–3.1 | 4.1 ± 27.8 | 0–9.2 |
| >4 | 122 | 114.1 ± 282.4 | 63.4–164.7 | 1.1 ± 4.4 | 0.3–1.9 | 1.4 ± 5.0 | 0.5–2.3 | 0.1 ± 0.9 | 0–0.3 | 2.8 ± 21.0 | 0–6.6 | 14.8 ± 111.4 | 0–34.8 |
4.1.2. Seasonal Variations in the Prevalence of Gastrointestinal Parasite Infections
The prevalence of gastrointestinal parasite infections focuses on seasonal variations across districts. The data show fluctuations in parasite prevalence, with seasonal variations being significant. The wet season is associated with higher infection rates for various parasite species, including Strongyle, Strongyloides spp., Paramphistomum spp., Moniezia spp., and Eimeria spp., in different districts (Table 3). The prevalence of gastrointestinal parasites includes both single and mixed infections. Strongyle infection is the most common single parasite, accounting for over half of the cases, followed by Trichuris spp., Moniezia spp., and Eimeria spp. Mixed infections, while less frequent, involve various combinations of nematodes, cestodes, trematodes, and protozoa (Table 4). Notably, combinations of nematodes with protozoa make up a significant portion of mixed infections.
Table 3.
Seasonal fluctuations in the prevalence of gastrointestinal parasite infections among meat goat herds in Khon Kaen, Thailand.
| District | Farm | Strongyle (%) | Strongyloides spp. (%) | Trichuris spp. (%) | Paramphistomum spp. (%) | Moniezia spp. (%) | Eimeria spp. (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | ||
| Ban Fang | A | 35.7 | 54.5 | 0.0 | 4.5 | 14.3 | 9.1 | 0.0 | 0.0 | 7.1 | 9.1 | 14.3 | 4.5 |
| B | 55.6 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | |
| C | 66.7 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 11.1 | ND | |
|
| |||||||||||||
| Ban Haet | D | 50.0 | 63.3 | 0.0 | 0.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.6 | 22.4 |
|
| |||||||||||||
| Muang | E | ND | 73.9 | ND | 0.0 | ND | 17.4 | ND | 0.0 | ND | 0.0 | ND | 8.7 |
| F | 94.4 | ND | 0.0 | ND | 44.4 | ND | 0.0 | ND | 11.1 | ND | 11.1 | ND | |
|
| |||||||||||||
| Nam Phong | G | 20.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND |
| H | 100.0 | ND | 9.5 | ND | 23.8 | ND | 0.0 | ND | 33.3 | ND | 4.8 | ND | |
|
| |||||||||||||
| Nong Ruea | I | 38.9 | ND | 0.0 | ND | 16.7 | ND | 0.0 | ND | 0.0 | ND | 5.6 | ND |
| J | 42.9 | ND | 0.0 | ND | 28.6 | ND | 0.0 | ND | 0.0 | ND | 28.6 | ND | |
| K | 88.9 | 82.4 | 0.0 | 0.0 | 22.2 | 5.9 | 0.0 | 0.0 | 11.1 | 5.9 | 11.1 | 5.9 | |
| L | 78.1 | 91.7 | 0.0 | 0.0 | 15.6 | 8.3 | 0.0 | 0.0 | 12.5 | 12.5 | 9.4 | 16.7 | |
| M | 57.1 | 83.3 | 14.3 | 8.3 | 42.9 | 25.0 | 0.0 | 0.0 | 0.0 | 0.0 | 28.6 | 8.3 | |
| N | 100.0 | ND | 0.0 | ND | 50.0 | ND | 0.0 | ND | 25.0 | ND | 0.0 | ND | |
| O | 22.2 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 11.1 | ND | 0.0 | ND | |
| P | 100.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | |
| Q | 75.0 | ND | 0.0 | ND | 37.5 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | |
|
| |||||||||||||
| Phu Wiang | R | 28.6 | ND | 14.3 | ND | 14.3 | ND | 0.0 | ND | 14.3 | ND | 0.0 | ND |
| S | 20.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | |
| T | 100.0 | 81.8 | 0.0 | 0.0 | 40.0 | 9.1 | 0.0 | 0.0 | 20.0 | 0.0 | 0.0 | 0.0 | |
| U | 46.7 | 20.0 | 0.0 | 0.0 | 26.7 | 20.0 | 0.0 | 0.0 | 0.0 | 6.7 | 13.3 | 26.7 | |
| V | 71.4 | 66.7 | 42.9 | 8.3 | 14.3 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 14.3 | 0.0 | |
|
| |||||||||||||
| Si Chomphu | W | ND | 90.0 | ND | 0.0 | ND | 5.0 | ND | 0.0 | ND | 30.0 | ND | 25.0 |
| AA | ND | 82.4 | ND | 0.0 | ND | 5.9 | ND | 5.9 | ND | 5.9 | ND | 0.0 | |
| AB | ND | 90.9 | ND | 36.4 | ND | 18.2 | ND | 0.0 | ND | 0.0 | ND | 0.0 | |
| AC | ND | 93.8 | ND | 0.0 | ND | 12.5 | ND | 0.0 | ND | 6.3 | ND | 0.0 | |
| AD | ND | 85.3 | ND | 17.6 | ND | 44.1 | ND | 0.0 | ND | 2.9 | ND | 26.5 | |
| AE | ND | 100.0 | ND | 18.2 | ND | 45.5 | ND | 0.0 | ND | 9.1 | ND | 72.7 | |
| AF | 83.3 | 85.7 | 41.7 | 32.1 | 16.7 | 60.7 | 0.0 | 0.0 | 50.0 | 0.0 | 83.3 | 32.1 | |
| AG | ND | 72.7 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 9.1 | ND | 0.0 | |
| AH | ND | 92.3 | ND | 0.0 | ND | 46.2 | ND | 0.0 | ND | 0.0 | ND | 7.7 | |
| AI | ND | 69.2 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 3.8 | ND | 84.6 | |
| AJ | ND | 70.0 | ND | 30.0 | ND | 30.0 | ND | 0.0 | ND | 10.0 | ND | 60.0 | |
| AK | ND | 89.3 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 7.1 | ND | 0.0 | |
| AL | ND | 84.6 | ND | 0.0 | ND | 15.4 | ND | 0.0 | ND | 3.8 | ND | 7.7 | |
|
| |||||||||||||
| Wiang Kao | AM | 57.1 | 84.0 | 0.0 | 4.0 | 14.3 | 28.0 | 0.0 | 0.0 | 14.3 | 16.0 | 0.0 | 12.0 |
| AN | 75.0 | 95.7 | 16.7 | 0.0 | 8.3 | 8.7 | 0.0 | 0.0 | 0.0 | 4.3 | 0.0 | 26.1 | |
| AO | 100.0 | 81.0 | 0.0 | 9.5 | 28.6 | 9.5 | 0.0 | 0.0 | 14.3 | 14.3 | 0.0 | 4.8 | |
| AP | 100.0 | ND | 9.1 | ND | 0.0 | ND | 27.3 | ND | 18.2 | ND | 9.1 | ND | |
| AQ | 100.0 | 96.3 | 0.0 | 7.4 | 0.0 | 11.1 | 0.0 | 3.7 | 9.1 | 11.1 | 9.1 | 51.9 | |
| AR | ND | 69.6 | ND | 0.0 | ND | 26.1 | ND | 0.0 | ND | 13.0 | ND | 8.7 | |
| AS | ND | 100.0 | ND | 9.1 | ND | 0.0 | ND | 0.0 | ND | 0.0 | ND | 36.4 | |
|
| |||||||||||||
| Total (%) | 42 | 27 (100.0) | 28 (100.0) | 7 (25.9) | 12 (42.8) | 19 (70.3) | 23 (82.1) | 1 (3.7) | 2 (7.1) | 14 (51.8) | 19 (67.8) | 15 (55.5) | 21 (75.0) |
ND = Not done.
Table 4.
Characteristics of gastrointestinal (GI) parasite infection as determined by the fecal egg count technique.
| Characteristic | Parasites | Number positive (%) |
|---|---|---|
| Single | Strongyle | 417 (55.67) |
| Strongyloides spp. | 1 (0.13) | |
| Trichuris spp. | 15 (2.00) | |
| Moniezia spp. | 7 (0.93) | |
| Eimeria spp. | 19 (2.54) | |
|
| ||
| Mixed | 2-Type nematode | |
| (i) Strongyle and Strongyloides spp. | 14 (1.87) | |
| (ii) Strongyle and Trichuris spp. | 81 (10.81) | |
| (iii) Strongyloides spp. and Trichuris spp. | 1 (0.13) | |
| 3-Type nematode | ||
| (i) Strongyle, Strongyloides spp., and Trichuris spp. | 10 (1.34) | |
| Nematode and Cestode | ||
| (i) Strongyle and Moniezia spp. | 32 | |
| (ii) Strongyle, Strongyloides spp., and Moniezia spp. | 1 (0.13) | |
| (iii) Strongyle, Trichuris spp., and Moniezia spp. | 10 (1.34) | |
| (iv) Trichuris spp. and Moniezia spp. | 2 (0.27) | |
| Nematode and Trematode | ||
| (i) Strongyle and Paramphistomum spp. | 3 (0.40) | |
| (ii) Strongyle, Strongyloides spp., and Paramphistomum spp. | 1 (0.13) | |
| Nematode and Protozoa | ||
| (i) Strongyle and Eimeria spp. | 83 (11.08) | |
| (ii) Trichuris spp. and Eimeria spp. | 2 (0.27) | |
| (iii) Strongyle, Strongyloides spp., and Eimeria spp. | 10 (1.34) | |
| (iv) Strongyle, Trichuris spp., and Eimeria spp. | 15 (2.00) | |
| (v) Strongyle, Strongyloides spp., Trichuris spp., and Eimeria spp. | 9 (1.20) | |
| Cestode and Protozoa | ||
| (i) Moniezia spp. and Eimeria spp. | 1 (0.13) | |
| Nematode, Cestode, and Protozoa | ||
| (i) Strongyle, Moniezia spp., and Eimeria spp. | 9 (1.20) | |
| (ii) Strongyloides spp., Moniezia spp., and Eimeria spp. | 1 (0.13) | |
| (iii) Strongyle, Trichuris spp., Moniezia spp., and Eimeria spp. | 3 (0.40) | |
| (iv) Strongyle, Strongyloides spp., Trichuris spp., Moniezia spp., and Eimeria spp. | 1 (0.13) | |
| Nematode, Cestode, and Trematode | ||
| (i) Strongyle, Moniezia spp., and Paramphistomum spp. | 1 (0.13) | |
| Nematode and Trematode | ||
| (i) Strongyle and Paramphistomum spp. | 3 (0.40) | |
| (ii) Strongyle and Strongyloides spp. and Paramphistomum spp. | 1 (0.13) | |
4.1.3. Coproculture to Identify Strongyle Larva
In this study, the proportion of Strongyle larvae in each herd comprised Haemonchus spp., Trichostrongylus spp., and Strongyloides spp., as illustrated in Figure 3.
Figure 3.
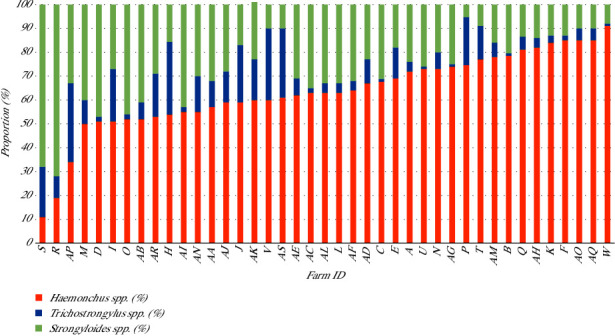
Proportion of strongyle larvae based on coproculture in 41 meat goat herds, Khon Kaen, Northeast Thailand.
4.1.4. Molecular Techniques for Identifying GI Strongyle Larva
Ten samples yielded positive PCR results, comprising five samples of Trichostrongylus spp. and five samples of Haemonchus spp. The restriction sites of the ITS-2 region are depicted in Figure 4. Subsequently, all positive samples underwent further characterization through gene sequencing. Sequencing revealed that the larvae belonged to H. contortus, exhibiting 100% sequence identity and query cover with the GenBank database. Similarly, Trichostrongylus spp. demonstrated identical sequence characteristics to the GenBank database.
Figure 4.
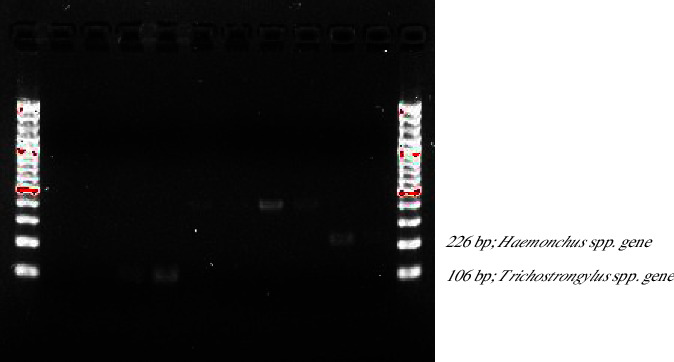
Restriction fragment of amplified ITS-2 regions of Trichostrongylus spp. (106 bp) and Haemonchus spp. (226 bp).
4.1.5. Risk Factors Associated with GI Parasite Infection
The data indicate that herd management factors do not consistently exhibit significant associations with parasite prevalence across various parasite types (Table 5). However, there is a slight association observed between the frequency of cleaning feed and water trays and parasite prevalence, although it is not statistically significant.
Table 5.
Univariate analysis of GI parasite infection in meat goat herds at the herd level.
| GI parasites | Factors | Category | OR | 95% CI | p value |
|---|---|---|---|---|---|
| Nematodes | Herd size | ≥33 | 0.7 | 0.01–36.25 | 0.9 |
| Trematodes | Herd size | ≥33 | 3.2 | 0.26–38.42 | 0.4 |
| Cestodes | Herd size | ≥33 | 2.6 | 0.64–10.05 | 0.2 |
| Protozoa | Herd size | ≥33 | 3.7 | 0.83–16.04 | 0.1 |
| Nematodes | Grazing | Communal | 0.1 | 0.002–6.64 | 0.3 |
| Trematodes | Grazing | Communal | 6.0 | 0.41–86.97 | 0.2 |
| Cestodes | Grazing | Communal | 5.9 | 0.29–118.20 | 0.2 |
| Protozoa | Grazing | Communal | 0.5 | 0.05–3.67 | 0.5 |
| Nematodes | Housing | Loose stalls | 0.2 | 0.003–11.51 | 0.4 |
| Trematodes | Housing | Loose stalls | 0.6 | 0.02–13.30 | 0.8 |
| Cestodes | Housing | Loose stalls | 0.7 | 0.13–3.62 | 0.7 |
| Protozoa | Housing | Loose stalls | 1.3 | 0.21–7.75 | 0.8 |
| Nematodes | Other livestock | Presence | 0.5 | 0.01–24.30 | 0.7 |
| Trematodes | Other livestock | Presence | 5.1 | 0.41–62.00 | 0.2 |
| Cestodes | Other livestock | Presence | 0.5 | 0.13–2.01 | 0.3 |
| Protozoa | Other livestock | Presence | 0.7 | 0.18–2.82 | 0.6 |
| Nematodes | Deworming program | Not have | 0.2 | 0.004–13.33 | 0.5 |
| Trematodes | Deworming program | Not have | 0.6 | 0.02–13.30 | 0.8 |
| Cestodes | Deworming program | Not have | 0.7 | 0.13–3.62 | 0.7 |
| Nematodes | Protocol deworming | Individual animal | 0.7 | 0.01–36.25 | 0.9 |
| Trematodes | Protocol deworming | Individual animal | 3.2 | 0.26–38.42 | 0.4 |
| Cestodes | Protocol deworming | Individual animal | 2.6 | 0.64–10.05 | 0.2 |
| Nematodes | Grouping of animals | Mixed age | 2.7 | 0.05–146.25 | 0.6 |
| Trematodes | Grouping of animals | Mixed age | 0.7 | 0.05–8.45 | 0.8 |
| Cestodes | Grouping of animals | Mixed age | 1.8 | 0.42–7.13 | 0.4 |
| Protozoa | Grouping of animals | Mixed age | 1.2 | 0.28–5.06 | 0.8 |
| Nematodes | Roughage management | Pasture paddock | 0.5 | 0.01–24.30 | 0.7 |
| Trematodes | Roughage management | Pasture paddock | 1.1 | 0.09–13.63 | 0.9 |
| Cestodes | Roughage management | Pasture paddock | 2.4 | 0.53–10.40 | 0.3 |
| Protozoa | Roughage management | Pasture paddock | 0.7 | 0.18–2.82 | 0.6 |
| Nematodes | Rotate pasture | No | 5.6 | 0.10–308.99 | 0.4 |
| Trematodes | Rotate pasture | No | 0.3 | 0.02–3.87 | 0.4 |
| Cestodes | Rotate pasture | No | 0.9 | 0.14–5.50 | 0.9 |
| Protozoa | Rotate pasture | No | 1.0 | 0.15–6.25 | 1.0 |
| Nematodes | Position conc. feed tray | Ground | 0.1 | 0.002–8.17 | 0.3 |
| Trematodes | Position conc. feed tray | Ground | 0.9 | 0.04–19.82 | 0.9 |
| Cestodes | Position conc. feed tray | Ground | 0.8 | 0.12–5.49 | 0.8 |
| Protozoa | Position conc. feed tray | Ground | 0.7 | 0.10–4.89 | 0.7 |
| Nematodes | Position roughage tray | Ground | 0.1 | 0.001–5.18 | 0.2 |
| Trematodes | Position roughage tray | Ground | 1.5 | 0.06–35.10 | 0.8 |
| Cestodes | Position roughage tray | Ground | 1.1 | 0.09–13.48 | 0.9 |
| Protozoa | Position roughage tray | Ground | 0.2 | 0.01–2.69 | 0.2 |
| Nematodes | Feces contaminated in feed or water tray | Found | 0.3 | 0.01–17.31 | 0.6 |
| Trematodes | Feces contaminated in feed or water tray | Found | 19.7 | 0.93–413.52 | 0.1 |
| Cestodes | Feces contaminated in feed or water tray | Found | 2.7 | 0.49–15.01 | 0.2 |
| Protozoa | Feces contaminated in feed or water tray | Found | 6.2 | 0.69–54.64 | 0.1 |
| Nematodes | Frequency of cleaning feed and water tray | 2–3 days/time | 0.6 | 0.01–29.83 | 0.8 |
| Trematodes | Frequency of cleaning feed and water tray | 2–3 days/time | 0.9 | 0.07–10.74 | 0.9 |
| Cestodes | Frequency of cleaning feed and water tray | 2–3 days/time | 0.8 | 0.20–2.77 | 0.7 |
| Protozoa | Frequency of cleaning feed and water tray | 2–3 days/time | 0.6 | 0.16–2.37 | 0.5 |
GI parasite infection at animal levels examines the associations between variables, such as sex, age, and breed. Female goats have a significantly higher risk of strongyle infection. Older goats (over one year old) are also more prone to Trichuris spp., Moniezia spp., and Eimeria spp. infections, indicating vulnerability related to age. However, breed (Mixed Anglo-Nubian) does not demonstrate a significant correlation with parasite prevalence. The data are presented in Table 6.
Table 6.
Univariate analysis of GI parasite infection at the animal level.
| GI parasites | Factors | Category | Number positive (%) | OR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Strongyle | Season | Wet | 700 (76.7) | 1.81 | 1.32–2.48 | <0.001 |
| Strongyloides spp. | Season | Wet | 49 (5.4) | 1.2 | 0.64–2.23 | 0.57 |
| Trichuris spp. | Season | Wet | 149 (16.3) | 1.05 | 0.72–1.51 | 0.81 |
| Paramphistomum spp. | Season | Wet | 5 (0.5) | 0.35 | 0.05–2.08 | 0.25 |
| Moniezia spp. | Season | Wet | 68 (7.4) | 0.64 | 0.38–1.05 | 0.08 |
| Eimeria spp. | Season | Wet | 153 (16.8) | 2.04 | 1.35–3.07 | <0.001 |
| Strongyle | Sex | Female | 700 (76.7) | 2.76 | 1.75–4.34 | <0.0001 |
| Strongyloides spp. | Sex | Female | 49 (5.4) | 2.63 | 0.62–11.02 | 0.19 |
| Trichuris spp. | Sex | Female | 149 (16.3) | 1.43 | 0.73–2.75 | 0.29 |
| Paramphistomum spp. | Sex | Female | 5 (0.5) | 1.2 | 0.06–21.90 | 0.9 |
| Moniezia spp. | Sex | Female | 68 (7.4) | 0.8 | 0.36–1.72 | 0.56 |
| Eimeria spp. | Sex | Female | 153 (16.8) | 0.77 | 0.44–1.33 | 0.36 |
| Strongyle | Age | <1 year | 700 (76.7) | 1.41 | 1.03–1.91 | <0.05 |
| Strongyloides spp. | Age | >1 year | 49 (5.4) | 1.22 | 0.68–2.16 | 0.5 |
| Trichuris spp. | Age | >1 year | 149 (16.3) | 1.91 | 1.33–2.73 | <0.001 |
| Paramphistomum spp. | Age | >1 year | 5 (0.5) | 0.26 | 0.02–2.37 | 0.24 |
| Moniezia spp. | Age | >1 year | 68 (7.4) | 1.92 | 1.15–3.20 | <0.05 |
| Eimeria spp. | Age | >1 year | 153 (16.8) | 1.95 | 1.36–2.78 | <0.001 |
| Strongyle | Breed | Mixed Anglo-Nubian | 700 (76.7) | 1.04 | 0.69–1.56 | 0.83 |
| Strongyloides spp. | Breed | Mixed Anglo-Nubian | 49 (5.4) | 0.63 | 0.26–1.50 | 0.3 |
| Trichuris spp. | Breed | Mixed Anglo-Nubian | 149 (16.3) | 0.72 | 0.43–1.18 | 0.19 |
| Paramphistomum spp. | Breed | Mixed Anglo-Nubian | 5 (0.5) | 1.15 | 0.12–10.36 | 0.9 |
| Moniezia spp. | Breed | Mixed Anglo-Nubian | 68 (7.4) | 0.59 | 0.27–1.26 | 0.18 |
| Eimeria spp. | Breed | Mixed Anglo-Nubian | 153 (16.8) | 0.93 | 0.58–1.47 | 0.76 |
Bold value shows that significant association was classified as p < 0.05.
4.1.6. Association between Factors and Clinical Signs according to Laboratory Results
In this investigation, clinical signs were assessed using BCS, FAMACHA score, and Dag score. However, no sign of bottle jaw was detected. The results revealed significant differences between genders in certain aspects. Specifically, female subjects exhibited distinct variations in BCS (p < 0.001) and FAMACHA score (p < 0.01) compared to males, suggesting potential gender-related differences in these clinical signs. Additionally, significant variations were observed in packed cell volume (PCV) (p < 0.01), a key hematological parameter, indicating gender-related discrepancies in blood composition. However, no significant differences were found in other hematological parameters, such as hemoglobin levels (p > 0.05). Furthermore, biochemical analysis showed no significant disparities in total protein levels (p > 0.05) between female and male subjects. Notably, significant gender-related variations were identified in GI parasite infection rates, particularly for strongyle infections (p < 0.001). However, no significant differences were observed in the infection rates of other GI parasite species (p > 0.05). All gender-related parameters are presented in Table 7.
Table 7.
Wilcoxon test results comparing BCS, FAMACHA score, Dag score, blood parameters, and fecal analysis outcomes between male and female groups (n = 913).
| Parameter | Female (mean (N)) | Male (mean (N)) | p value |
|---|---|---|---|
| Clinical sign | |||
| BCS | 476.9 (824) | 273.0 (89) | ∗∗∗ |
| FAMACHA score | 464.6 (824) | 387.0 (89) | ∗∗ |
| Dag score | 458.1 (824) | 446.8 (89) | NS |
| Hematology | |||
| PCV (%) | 411.4 (751) | 493.1 (88) | ∗∗ |
| Hemoglobin (g/dL) | 39.7 (69) | 50.8 (13) | NS |
| Biochemistry | |||
| Total protein (g/dL) | 426.1 (761) | 420.0 (89) | NS |
| GI parasites | |||
| Strongyle (EPG) | 469.4 (824) | 342.4 (89) | ∗∗∗ |
| Strongyloides spp. (EPG) | 458.5 (824) | 443.1 (89) | NS |
| Trichuris spp. (EPG) | 458.9 (824) | 439.1 (89) | NS |
| Paramphistomum spp. (EPG) | 457.3 (824) | 454.5 (89) | NS |
| Moniezia spp. (EPG) | 456.3 (824) | 463.6 (89) | NS |
| Eimeria spp. (OPG) | 454.8 (824) | 477.0 (89) | NS |
∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ = p < 0.001; NS = not significant.
The clinical signs indicate that goats with poor BCS are more prone to increased anemia and fecal staining severity. Positive correlations were found between BCS and both FAMACHA score (r = 0.3, p < 0.01) and Dag score (r = 0.4, p < 0.001). There is also a positive correlation between FAMACHA score and Dag score (r = 0.29, p < 0.01) (Table 8). However, age does not appear to have a significant correlation with fecal staining severity.
Table 8.
Spearman's correlation analysis for age, BCS, FAMACHA score, and Dag score in relation to clinical signs, blood parameters, and fecal analysis outcomes (n = 913).
| Parameter | Age | BCS | FAMACHA score | Dag score | ||||
|---|---|---|---|---|---|---|---|---|
| r= | p value | r= | p value | r= | p value | r= | p value | |
| Clinical sign | ||||||||
| BCS | 0.27 | ∗ | — | — | 0.30 | ∗∗ | 0.40 | ∗∗∗ |
| FAMACHA score | 0.55 | NS | 0.30 | ∗∗ | — | — | 0.29 | ∗∗ |
| Dag score | −0.08 | NS | 0.40 | ∗∗∗ | 0.29 | ∗∗ | — | — |
| Hematology | ||||||||
| PCV (%) | −0.32 | ∗∗ | −0.45 | ∗∗∗ | −0.48 | ∗∗∗ | −0.34 | ∗∗ |
| Hemoglobin (g/dL) | −0.23 | ∗ | −0.47 | ∗∗∗ | −0.55 | ∗∗∗ | −0.39 | ∗∗∗ |
| Biochemistry | ||||||||
| Total protein (g/dL) | 0.38 | ∗∗∗ | −0.05 | NS | −0.19 | NS | −0.21 | NS |
| GI parasites | ||||||||
| Strongyle (EPG) | 0.12 | NS | 0.30 | ∗∗ | 0.33 | ∗∗ | 0.26 | ∗ |
| Strongyloides spp. (EPG) | −0.17 | NS | 0.15 | NS | 0.23 | ∗ | 0.41 | ∗∗∗ |
| Trichuris spp. (EPG) | −0.09 | NS | 0.12 | NS | 0.05 | NS | 0.15 | NS |
| Moniezia spp. (EPG) | −0.15 | NS | 0.12 | NS | 0.07 | NS | 0.18 | NS |
| Eimeria spp. (OPG) | −0.21 | NS | −0.07 | NS | 0.28 | ∗∗ | 0.37 | ∗∗∗ |
∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ = p < 0.001; NS = not significant.
Age shows moderate negative correlations with hematological parameters. Older goats may have lower PCV (r = −0.32, p < 0.01) and hemoglobin levels (r = −0.23, p < 0.05). In contrast, BCS and FAMACHA score exhibit strong negative correlations with hematological parameters. Goats with poorer BCS and higher FAMACHA score tend to have lower PCV (r = −0.45 to −0.48, p < 0.001) and hemoglobin levels (r = −0.47 to −0.55, p < 0.001). The Dag score also displays moderate negative correlations with hematological parameters. Goats with more severe fecal staining may have lower PCV (r = −0.34, p < 0.01) and hemoglobin levels (r = −0.39, p < 0.001). Additionally, total protein shows a moderate positive correlation with age (r = 0.38, p < 0.001), indicating that older goats may have higher total protein levels.
In terms of GI parasite infections in meat goats, BCS shows a positive correlation with strongyle (r = 0.3, p < 0.01) and FAMACHA scores (r = 0.33, p < 0.01). Moreover, Strongyloides spp. displays a positive correlation with FAMACHA score (r = 0.23, p < 0.05) and Dag score (r = 0.41, p < 0.001). The Dag score also demonstrates positive correlations with strongyle and Strongyloides spp. Additionally, Eimeria spp. shows a positive correlation with FAMACHA score (r = 0.28, p < 0.01) and Dag score (r = 0.37, p < 0.001).
Regarding GI parasite infections, significant positive correlations were detected between strongyle infections and BCS (r = 0.30, p < 0.01) and FAMACHA score (r = 0.33, p < 0.01). Moreover, Strongyloides spp. infections exhibited significant positive correlations with age, FAMACHA score, and Dag score.
The correlation of various parameters, including PCV (packed cell volume), hemoglobin, and total protein, with different gastrointestinal (GI) parasite infections are presented in Table 9. PCV and hemoglobin exhibit significant negative correlations with strongyle EPG counts (r = −0.38, p < 0.001, and r = −0.44, p < 0.001, respectively). Additionally, hemoglobin and total protein display significant negative correlations with Strongyloides spp. EPG counts (r = −0.22, p < 0.05, and r = −0.25, p < 0.05, respectively). Furthermore, hemoglobin (r = −0.31, p < 0.01) and total protein (r = −0.38, p < 0.001) demonstrate a moderate negative correlation with Eimeria spp. counts.
Table 9.
Results of Spearman's correlation for PCV, hemoglobin, and total protein according to GI parasite infection (n = 913).
| Parameter | PCV | Hemoglobin | Total protein | |||
|---|---|---|---|---|---|---|
| r= | p value | r= | p value | r= | p value | |
| GI parasites | ||||||
| Strongyle (EPG) | −0.38 | ∗∗∗ | −0.44 | ∗∗∗ | −0.15 | NS |
| Strongyloides spp. (EPG) | −0.05 | NS | −0.22 | ∗ | −0.25 | ∗ |
| Trichuris spp. (EPG) | −0.15 | NS | −0.10 | NS | −0.05 | NS |
| Moniezia spp. (EPG) | 0.001 | NS | −0.14 | NS | −0.10 | NS |
| Eimeria spp. (OPG) | −0.08 | NS | −0.31 | ∗∗ | −0.38 | ∗∗∗ |
∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ = p < 0.001; NS = not significant.
5. Discussions
Infection by GI parasites was generally found in meat goat herds in Khon Kaen, Northeast Thailand. All herds had strongyle nematodes, with Trichuris spp., Moniezia spp., and Eimeria spp. occurring in approximately 60–70% of herds. Coinfection involving at least two types of nematodes, such as Strongyle and Strongyloides spp., Strongyle and Trichuris spp., or Strongyloides spp. and Trichuris spp., as well as cestodes, trematodes, or protozoa, was observed in 90% of herds. This finding is consistent with previous studies that showed a high prevalence of GI parasitic infections in herds and common coinfections of nematodes, protozoa, and cestodes in small ruminants [18–20]. In this study, a few herds showed trematode infections, such as Paramphistomum spp., which could be attributed to the geographical location of these herds near natural water resources, such as floating swamps, streams, flood plains, or rivers. These areas are habitats for living snails, which are intermediate hosts of trematodes [21]. Additionally, neighboring beef cattle herds that share pasture with meat goats may transmit trematodes to other species.
The spatial distribution of GI parasitic infections in meat goat herds can provide insights into the main helminth infections in this area. Haemonchus spp., Trichostrongylus spp., Trichuris spp., and Strongyloides spp. were dominant nematodes in meat goats. Molecular techniques confirmed that the dominant Haemonchus spp. was H. contortus, which was highly related to H. contortus strains in Kanchanaburi, Thailand (access no. MT294437.1) [22], Cameroon (no. MN708986.1) [19], and Nigeria (no. LC368075.1) [23] (100% sequence identity and 100% query cover to GenBank database). Trichostrongylus spp. were closely related to T. colubriformis strains in Kanchanaburi, Thailand (no. MT294439.1) [22] and Egypt (no. MK936884.1) [24], as well as T. axei strains in Europe (no. ON677955.1) [25] and Ghana (no. MH481571.1) [26] (100% sequence identity and 100% query cover to GenBank database). Similarly, cestode and protozoa infections were also common health problems in meat goats in this area.
This study revealed an association between GI strongyle and Eimeria spp. infections during the wet season, consistent with prior research indicating a propensity for elevated parasite infection rates in livestock during wet conditions, as evidenced in studies conducted in Senegal and India [27, 28]. Conversely, GI nematode burdens may surge during the dry season in the West Indian Islands [29], suggesting geographical variations in GI parasite prevalence among ruminants.
Laboratory analyses found that male animals had higher body condition scores (BCS) and FAMACHA score than females in terms of the association between factors and clinical signs. This supports previous studies, indicating that females are more susceptible to strongyle infections [5, 30]. Female goats, in particular, are vulnerable to parasitic infections due to their reproductive cycles, which affect the secretion of worm eggs. This increased susceptibility is most evident during the periparturient and postparturient periods, characterized by heightened physiological stress and compromised immunity, resulting in greater shedding of strongyle eggs [30–32].
Concerning age factors, young animals exhibited a stronger association with strongyle infection, whereas older animals displayed a higher prevalence of Trichuris spp., Moniezia spp., and Eimeria spp. infections, consistent with the previous literature [33, 34].
An escalation in strongyle eggs corresponded with clinical manifestations, such as anemia, diarrhea, emaciation, and bottle jaw, indicative of heightened adult worm infections. This corroborates earlier studies, demonstrating that nematodes, particularly H. contortus infections, can provoke progressive anemia and hypoproteinemia in small ruminants [10, 14, 35–37]. In critical scenarios, such as instances of poor BCS, elevated FAMACHA or Dag scores, or markedly low levels of PCV or Hb, progressive anemia can precipitate rapid mortality, especially when compounded by inadequate nutrition or stress [14, 38]. Additionally, poor BCS was identified as a risk factor for GI strongyle infections in livestock animals [39].
Based on our investigation, a notable prevalence of strongyle nematode infections was identified within meat goat herds. Premunition, characterized by early-life exposure leading to subsequent resistance to further infection, may manifest within these herds, with chronic infections persisting if the infecting strongyle remains within the host [38]. Notably, in cases where hosts do not exhibit clinical signs, such as anemia, progressive weight loss, diarrhea, or bottle jaw, treatment may not be warranted for this infective condition. To facilitate targeted selective treatments, farmers could employ the FAMACHA score to assess subclinical strongyle infections, with scores of 1–2 indicating no immediate requirement for anthelmintic treatment, while scores of 3 close to 4 or 4–5 signify an urgent need for intervention. In our area, BCS was not employed to guide treatment decisions due to prevalent undernutrition, with a majority of adult animals presenting as thin or emaciated. Moreover, animals experiencing malnutrition are predisposed to heightened risks of GI parasite infections owing to compromised immune responses [39–41]. We advocate for treating animals exhibiting poor BCS alongside FAMACHA scores of 3 to 4 and Dag scores of 2. However, it is crucial to acknowledge that the elimination of stable and established infections may lead to the loss of premunition, potentially resulting in rapid reinfection, with an increased parasite burden [38, 42]. The scope of this study was confined to 42 small-holder meat goat herds situated in an agricultural area known for its prevalence of meat goat farming in Khon Kaen, Thailand. While univariate analysis revealed significant variations in risk factors related to GI parasite infection, such as season, gender, and age, deeper exploration and intervention may be required to fully understand their implications. Future research initiatives are warranted to assess the effectiveness of pharmaceutical interventions and to evaluate the emergence of antiparasitic drug resistance in GI parasitic infections.
6. Conclusions
In conclusion, gastrointestinal (GI) parasite infections are common among meat goats in Khon Kaen, Northeast Thailand. Strongyle nematodes emerged as the predominant parasites in all herds, with coinfections involving multiple parasite types being commonplace, and seasonal variations, with wet seasons correlating with increased infections of strongyle and Eimeria spp. Moreover, gender and age were identified as significant factors influencing susceptibility to GI parasite infection. Clinical indicators such as anemia, diarrhea, and emaciation were found to be associated with strongyle infections. The findings underscore the necessity for implementing effective management strategies targeting animals with poor BCS and elevated FAMACHA scores to mitigate the impact of GI parasitic infections on meat goat health and productivity.
Acknowledgments
The authors would like to thank the farmers involved in the study for their cooperation. Special thanks are due to Ceva Animal Health (Thailand) LTD for supporting hands-on hemoglobin testing and cassettes (HemoCue Hb 201+). The authors would like to express our sincere gratitude to Assoc.Prof.Dr. Somboon Sangmaneedet for his invaluable advice and guidance throughout this study. His expertise and insights have significantly contributed to the success of this research. This research was supported by the Faculty of Veterinary Medicine, Khon Kaen University, under grant number KKU Vet. Res. VM022/2565, and by the Research and Graduate Studies (Research Program) of Khon Kaen University, project number RP66-9-001. The article processing charges (APC) were also funded by Khon Kaen University.
Data Availability
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.
Ethical Approval
The study procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Khon Kaen University (record no. IACUC-KKU-101/63, approved on December 17, 2020, and record no. IACUC-VM-KKU-02/63, approved in May 2019). All cases were anonymized and aggregated at the village level. No personal identifiers were presented, and the maps presented in this study did not identify respondents' precise addresses.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
SR conceptualized the study, was involved in data collection, laboratory analysis, and statistical analysis, administered the project, and wrote the draft. SL, RM, and AW were involved in the epidemiological analysis and statistical analysis. PK was involved in laboratory analysis. NPa, MV, PPa, NPi, PKr, and PS were involved in sampling collection and laboratory analysis. SC was involved in molecular analysis. All authors commented on and discussed the manuscript.
References
- 1.Rinaldi L., Hendrickx G., Cringoli G., et al. Mapping and modelling helminth infections in ruminants in Europe: Experience from GLOWORM. Geospatial Health . 2015;9(2):257–259. doi: 10.4081/gh.2015.347. [DOI] [PubMed] [Google Scholar]
- 2.Charlier J., Rinaldi L., Musella V., et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Preventive Veterinary Medicine . 2020;182 doi: 10.1016/j.prevetmed.2020.105103.105103 [DOI] [PubMed] [Google Scholar]
- 3.Zajac A. M., Garza J. Biology, Epidemiology, and control of gastrointestinal nematodes of small ruminants. Veterinary Clinics of North America: Food Animal Practice . 2020;36(1):73–87. doi: 10.1016/j.cvfa.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Suarez V. H., Martínez G. M., Viñabal A. E., Alfaro J. R. Epidemiology and effect of gastrointestinal nematodes on dairy goats in Argentina. Onderstepoort Journal of Veterinary Research . 2017;84(1):e1–e5. doi: 10.4102/ojvr.v84i1.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul B. T., Jesse F. F. A., Chung E. L. T., Che’Amat A., Mohd Lila M. A. Risk factors and severity of gastrointestinal parasites in selected small ruminants from Malaysia. Veterinary Sciences . 2020;7(4):208–214. doi: 10.3390/vetsci7040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ict D. L. D. Goat population in Thailand (divided into provinces) 2021. https://ict.dld.go.th/webnew/images/stories/stat_web/yearly/2564/province/T8-1-Goat.pdf .
- 7.Data Thai’s farmer in 2022. https://ict.dld.go.th/webnew/index.php/th/service-ict/report/396-report-thailand-livestock/reportservey2565/1660-2565-monthly .
- 8.Upper Northeastern Meteorological Center. Thai meteorological department data. 2021. https://www.khonkaen.tmd.go.th/opendata/data/C10.3.5/2564/khonkaen.pdf .
- 9.Detweiler G., Gipson T., Merkel R., Goetsch A., Sahlu T. Proceeding of 23rd Annual Goat Field Day . Langston, OK, USA: Langston University; 2008. Body condition scores in goats; pp. 127–133. [Google Scholar]
- 10.Van Wyk J. A., Bath G. F. The FAMACHA system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Veterinary Research . 2002;33(5):509–529. doi: 10.1051/vetres:2002036. [DOI] [PubMed] [Google Scholar]
- 11.Bath G. F., Van Wyk J. A. The Five Point Check© for targeted selective treatment of internal parasites in small ruminants. Small Ruminant Research . 2009;86(1-3):6–13. doi: 10.1016/j.smallrumres.2009.09.009. [DOI] [Google Scholar]
- 12.Larsen J. W. A., Anderson N., Vizard A. L., Anderson G. A., Hoste H. Diarrhoea in Merino ewes during winter: association with trichostrongylid larvae. Australian Veterinary Journal . 1994;71(11):365–372. doi: 10.1111/j.1751-0813.1994.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 13.Brummaier T., Archasuksan L., Watthanakulpanich D., et al. Improved detection of intestinal helminth infections with a formalin ethyl-acetate-based concentration technique compared to a crude formalin concentration technique. Tropical Medicine and Infectious Disease . 2021;6(2):p. 51. doi: 10.3390/tropicalmed6020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor M. A., Coop R. L., Wall R. L. Veterinary Parasitology . 4th. New Delhi, India: Wiley-Blackwell; 2016. [Google Scholar]
- 15.Van Wyk J. A., Cabaret J., Michael L. M. Morphological identification of nematode larvae of small ruminants and cattle simplified. Veterinary Parasitology . 2004;119(4):277–306. doi: 10.1016/j.vetpar.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Demeler J., Ramünke S., Wolken S., et al. Discrimination of gastrointestinal nematode eggs from crude fecal egg preparations by inhibitor-resistant conventional and real-time PCR. PLoS One . 2013;8(4) doi: 10.1371/journal.pone.0061285.e61285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammedsalih K. M., Krücken J., Khalafalla A., et al. New codon 198 β-tubulin polymorphisms in highly benzimidazole resistant Haemonchus contortus from goats in three different states in Sudan. Parasites & Vectors . 2020;13(1):p. 114. doi: 10.1186/s13071-020-3978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chikweto A., Tiwari K., Bhaiyat M. I., et al. Gastrointestinal parasites in small ruminants from Grenada, West Indies: A coprological survey and a review of necropsy cases. Veterinary Parasitology, Regional Studies and Reports . 2018;13:130–134. doi: 10.1016/j.vprsr.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Paguem A., Abanda B., Ngwasiri N. N., et al. Host specificity and phylogeny of Trichostrongylidae of domestic ruminants in the Guinea savannah of the Adamawa plateau in Cameroon. Veterinary Parasitology, Regional Studies and Reports . 2020;21 doi: 10.1016/j.vprsr.2020.100412.100412 [DOI] [PubMed] [Google Scholar]
- 20.Hatam-Nahavandi K., Carmena D., Rezaeian M., et al. Gastrointestinal parasites of domestic mammalian hosts in Southeastern Iran. Veterinary Sciences . 2023;10(4):p. 261. doi: 10.3390/vetsci10040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Warleta M., Lladosa S., Castro-Hermida J. A., et al. Bovine paramphistomosis in Galicia (Spain): Prevalence, intensity, aetiology and geospatial distribution of the infection. Veterinary Parasitology . 2013;191(3-4):252–263. doi: 10.1016/j.vetpar.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Income N., Tongshoob J., Taksinoros S., et al. Helminth infections in cattle and goats in Kanchanaburi, Thailand, with focus on strongyle nematode infections. Veterinary Sciences . 2021;8(12):p. 324. doi: 10.3390/vetsci8120324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawe S. M., Hikosaka K. Morpho-Molecular characterization of Haemonchus (Nematoda: trichostrongyloidea) from cattle, sheep and goats in Nigeria. GenBank LC . 368075(1) [Google Scholar]
- 24.El-Alfy E. S., Abu-Elwafa S., Abbas I., et al. Molecular screening approach to identify protozoan and trichostrongylid parasites infecting one-humped camels (Camelus dromedarius) Acta Tropica . 2019;197 doi: 10.1016/j.actatropica.2019.105060.105060 [DOI] [PubMed] [Google Scholar]
- 25.Buchmann K., Christiansen L. L., Kania P. W., Thamsborg S. M. Introduced European bison (Bison bonasus) in a confined forest district: A ten year parasitological survey. International Journal for Parasitology: Parasites and Wildlife . 2022;18:292–299. doi: 10.1016/j.ijppaw.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squire S. A., Yang R., Robertson I., Ayi I., Squire D. S., Ryan U. Gastrointestinal helminths in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Parasitology Research . 2018;117(10):3183–3194. doi: 10.1007/s00436-018-6017-1. [DOI] [PubMed] [Google Scholar]
- 27.Ndao M., Belot J., Zinsstag J., Pfister K. Epidémiologie des helminthoses gastro-intestinales des petits ruminants dans la zone sylvo-pastorale au Sénégal. [Epidemiology of gastrointestinal helminthiasis in small ruminants in the tree-cropping pasture zone in Senegal] Veterinary Research . 1995;26(2):132–139. [PubMed] [Google Scholar]
- 28.Khajuria J. K., Katoch R., Yadav A., Godara R., Gupta S. K., Singh A. Seasonal prevalence of gastrointestinal helminths in sheep and goats of middle agro-climatic zone of Jammu province. Journal of Parasitic Diseases: Official Organ of the Indian Society for Parasitology . 2013;37(1):21–25. doi: 10.1007/s12639-012-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieuchand S., Charles R., Caruth J., Basu A., Samson‐Himmelstjerna G., Georges K. A field study on the occurrence of gastrointestinal nematodes in sheep over the wet and dry seasons in two West Indian Islands. Transboundary and Emerging Diseases . 2020;67(S2):193–200. doi: 10.1111/tbed.13521. [DOI] [PubMed] [Google Scholar]
- 30.Khan M. N., Sajid M. S., Khan M. K., Iqbal Z., Hussain A. Gastrointestinal helminthiasis: prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitology Research . 2010;107(4):787–794. doi: 10.1007/s00436-010-1931-x. [DOI] [PubMed] [Google Scholar]
- 31.Dorny P., Symoens C., Jalila A., Vercruysse J., Sani R. Strongyle infections in sheep and goats under the traditional husbandry system in peninsular Malaysia. Veterinary Parasitology . 1995;56(1-3):121–136. doi: 10.1016/0304-4017(94)00657-x. [DOI] [PubMed] [Google Scholar]
- 32.Sylvester H. J., Griffith E. H., Jacob M. E., Foster D. M. Factors associated with strongyle infection in goats at the individual and farm level. Journal of the American Veterinary Medical Association . 2018;253(7):907–917. doi: 10.2460/javma.253.7.907. [DOI] [PubMed] [Google Scholar]
- 33.Vieira V. D., Feitosa T. F., Vilela V. L., et al. Prevalence and risk factors associated with goat gastrointestinal helminthiasis in the Sertão region of Paraíba State, Brazil. Tropical Animal Health and Production . 2014;46(2):355–361. doi: 10.1007/s11250-013-0496-y. [DOI] [PubMed] [Google Scholar]
- 34.Jabar Jasim H., Mijbas Mohammed Alomari M., Abbas Ali N., Khalid Alani Z., Abed S. M., Nasser Kadim A. Prevalence, haematological and molecular studies of Haemonchus contortus isolated from goat at AL-Muthanna province, Iraq. Arch Razi Inst . 2023;78(1):287–295. doi: 10.22092/ARI.2022.359004.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abosse J. S., Terefe G., Teshale B. M. Comparative study on pathological changes in sheep and goats experimentally infected with Haemonchus contortus. Surgical and Experimental Pathology . 2022;5:14–12. doi: 10.1186/s42047-022-00116-8. [DOI] [Google Scholar]
- 36.Diogenes P. V. A., Suassuna A. C. D., Ahid S. M. M., Blanco B. S. Serum protein electrophoretic profile of goats infected with Haemonchus contortus. Journal of Animal and Veterinary Advances . 2010;9(11):1603–1606. doi: 10.3923/javaa.2010.1603.1606. [DOI] [Google Scholar]
- 37.Ceï W., Salah N., Alexandre G., Bambou J. C., Archimède H. Impact of energy and protein on the gastro-intestinal parasitism of small ruminants: a meta-analysis. Livestock Science . 2018;212:34–44. doi: 10.1016/j.livsci.2018.03.015. [DOI] [Google Scholar]
- 38.Bowman D. D. Georgi’s Parasitology for Veterinarians . 9th. St Louis, MI, USA: Elsevier; 2009. [Google Scholar]
- 39.Terfa W., Kumsa B., Ayana D., Maurizio A., Tessarin C., Cassini R. Epidemiology of gastrointestinal parasites of cattle in three districts in Central Ethiopia. Animals . 2023;13(2):p. 285. doi: 10.3390/ani13020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villalobos-Cortés J., Martínez D. F., Delgado J. R. Nutrición y parasitismo gastrointestinal en producción de rumiantes. Archivos de Zootecnia . 2008;58(224):131–144. doi: 10.21071/az.v58i224.5079. [DOI] [Google Scholar]
- 41.Coop R. L., Holmes P. H. Nutrition and parasite interaction. International Journal for Parasitology . 1996;26(8-9):951–962. doi: 10.1016/S0020-7519(96)80070-1. [DOI] [PubMed] [Google Scholar]
- 42.Dearborn L., de Alvarez L. R., Torres-Acosta J. F. 51 determining the best indicators for targeted selective treatment against gastrointestinal nematodes in sheep and goats in Mississippi. Journal of Animal Science . 2022;100(Supplement_3):18–19. doi: 10.1093/jas/skac247.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.


