Abstract
Endonuclease assays of the H-N-H proteins encoded by two group I introns in the Chlamydomonas moewusii chloroplast psbA gene revealed that the CmpsbA·1 intron specifies a site-specific DNA endonuclease, designated I-CmoeI. Like most previously reported intron-encoded endonucleases, I-CmoeI generates a double-strand break near the insertion site of its encoding intron, leaving 3′ extensions of 4 nt. This enzyme was purified from Escherichia coli as a fusion protein with a His tag at its N-terminus. The recombinant protein (rI-CmoeI) requires a divalent alkaline earth cation for DNA cleavage (Mg2+ > Ca2+ > Sr2+ > Ba2+). It also requires a metal cofactor for DNA binding, a property shared with H-N-H colicins but not with the homing endonucleases characterized to date. rI-CmoeI binds its recognition sequence as a monomer, as revealed by gel retardation assays. Km and kcat values of 100 ± 40 pM and 0.26 ± 0.04 min–1, respectively, were determined. Replacement of the first histidine of the H-N-H motif by an alanine residue abolishes both rI-CmoeI activity and binding to its substrate. We propose that this conserved histidine residue plays a role in binding the metal cofactor and that such binding induces a structural modification of the enzyme which is required for DNA recognition.
INTRODUCTION
Group I introns can move efficiently from intron-containing alleles to intronless alleles of cognate genes (1–4). This process, referred to as homing, is promoted by intron-encoded DNA endonucleases (homing endonucleases) that recognize and specifically cleave the intronless alleles. Homing endonucleases can be classified into four families on the basis of the following conserved motifs in their amino acid sequences: LAGLIDADG, GIY-YIG, H-N-H and His-Cys box (3,5). Endonucleases from the four families recognize DNA sequences ranging from 15 to 40 bp in size, require the presence of a divalent cation to perform their cleavage reactions and most often generate double-strand breaks with 3′ extensions of 4 nt. Repair of these double-strand breaks through gene conversion leads to the propagation of intron sequences.
Although the LAGLIDADG, GIY-YIG and His-Cys box homing endonucleases have been characterized extensively at the biochemical and structural levels (6), no detailed characterization of an H-N-H homing endonuclease has been reported thus far. The DNA cleavage patterns of three H-N-H homing endonucleases (I-TevIII, I-HmuI and I-HmuII) have been determined, uncovering their unusual properties. The phage T4 enzyme I-TevIII is the only homing endonuclease known to generate a double-strand break with 5′ extensions (7), whereas the Bacillus subtilis phage enzymes I-HmuI and I-HmuII cleave only one strand of their DNA recognition sequences (8). H-N-H homing endonucleases are also unique in that their consensus motif has been identified in multifunctional proteins encoded by group II introns as well as proteins unrelated to homing endonucleases such as colicins, a family of non-specific DNA nucleases found in Escherichia coli (9–11).
The crystal structures of the DNase domains of colicins E7 and E9 have revealed that the 32 amino acid residues delimiting the H-N-H motif are likely to make up the active sites of these monomeric proteins (12,13). The residues of the H-N-H motif form a concave cleft that could accommodate double-stranded DNA. Some of these residues coordinate a divalent metal ion that is probably required for cleavage. It has recently been reported that the putative active site of colicin E9 is structurally similar to the active sites of the His-Cys box homing endonuclease from Physarum polycephalum (I-PpoI) and the non-specific His-Cys box endonuclease from Serratia marcescens (14–17). This observation suggests that H-N-H and His-Cys box nucleases form a single enzyme family (16). Despite this similarity, His-Cys box enzymes act as homodimers and contain two separate active sites (18,19), whereas H-N-H colicins act as monomers and presumably contain only one active site (12,20).
In the present study we have tested the hypothesis that the H-N-H proteins encoded by the two group I introns (CmpsbA·1 and CmpsbA·2) in the Chlamydomonas moewusii chloroplast psbA gene (9,11,21) are homing endonucleases. Evidence supporting this hypothesis comes from the observation of a unidirectional conversion event at the locus of the 21 kb extra sequence of C.moewusii (a sequence encompassing the two psbA introns) during reciprocal crosses between the interfertile green algae C.moewusii and Chlamydomonas eugametos (22). We report here that the CmpsbA·1 intron encodes a site-specific endonuclease that introduces a double-strand break in the intronless C.eugametos psbA gene. The biochemical properties of this homing endonuclease suggest that its active site is similar to those of H-N-H colicins, but significantly different from those of His-Cys box endonucleases.
MATERIALS AND METHODS
DNA substrate
In all experiments, except the stoichiometric analysis, the DNA substrate was prepared by PCR amplification of a 274 bp region of C.eugametos cpDNA with the primers 5′-GTTACTTCATCTTTAATCCGT-3′ (1010) and 5′-TTGAATCTACAACTGACTGGT-3′ (1011). To obtain a labeled substrate, the primers were end-labeled with T4 polynucleotide kinase in the presence of [γ-32P]ATP or [γ-33P]ATP before amplification or the PCR amplification was carried out in the presence of [α-33P]dCTP.
In vitro synthesis of proteins and assays of DNA endonuclease activity
The CmpsbA·1- and CmpsbA·2-encoded proteins were produced in vitro from rabbit reticulocyte lysates and tested for endonuclease activity as described previously for other intron-encoded proteins (23). Regions spanning the intron ORFs were PCR amplified from C.moewusii cpDNA using the following primers: for the CmpsbA·1 ORF, 5′-TAATACGACTCACTATAGGGAGAGTATTCATCAAATATAACAAT-3′ and 5′-ACGCAGGCCTGAACTTAATGT-3′; for the CmpsbA·2 ORF, 5′-TAATACGACTCACTATAGGGAGAGAAGTTATTATAAATTGGGAT-3′ and 5′-AGAAGAAGTGTAGTGAACGAG-3′.
Cloning of the I-CmoeI gene
The I-CmoeI gene was PCR amplified from C.moewusii cpDNA with the primers 5′-AATTAACAACAAATCCATGGCTCGTAAAAAAACTATAG-3′ (1012) and 5′-CCATCTGTAGCTTGGATCCGGATTGCCATATTA-3′ (1013). Primer 1012 contains the NcoI recognition sequence (underlined) at the initiation codon site (italics); the introduction of this sequence into the I-CmoeI gene resulted in an amino acid substitution (S2A). Primer 1013 contains a BamHI recognition sequence (underlined) downstream of the termination codon (italics). After digestion with NcoI and BamHI, the PCR products were cloned into the corresponding sites of the pET30a expression vector (Novagen, Madison, WI) using E.coli STBL2 as host. Plasmid DNA (pET-ICmoeI) from a recombinant clone was sequenced to ensure the absence of mutations in the I-CmoeI coding sequence. pET-ICmoeI was subsequently transferred to E.coli BL21(DE3).
Purification of rI-CmoeI
Escherichia coli BL21(DE3) cells harboring pET-ICmoeI were grown at 37°C in 1 l of LB broth. At mid exponential phase (0.6 OD600 nm) gene expression was induced by adding IPTG to a final concentration of 1 mM. Cells were further incubated for 2.5 h and harvested by centrifugation. All subsequent steps were performed at 4°C, unless noted otherwise. The cell pellet was resuspended in 15 ml of buffer A [50 mM sodium phosphate, pH 8.0, 0.3 M NaCl, 1 mM phenylmethylsulfonide fluoride (PMSF)] containing 10 mM imidazole and disrupted with a French press. The soluble fraction of the lysate was added to 4 ml of Ni–NTA resin (Qiagen, Mississauga, Ontario, Canada). After shaking for 1 h, the resin was transferred to a disposable column and washed with buffer A containing 20 mM imidazole. Bound proteins were eluted with buffer A containing 250 mM imidazole. This protein fraction was dialyzed against buffer B (50 mM HEPES–NaOH, pH 7.5, 1 mM PMSF) and applied to a 6.5 ml S-ceramic Hyper D column (BioSepra, Marlborough, MA) equilibrated with buffer B at room temperature. Bound proteins were eluted at a flow rate of 4 ml/min with a linear gradient of 150–500 mM NaCl made up in buffer B. Fractions of 0.5 ml were collected and analyzed by SDS–PAGE. Fractions containing the recombinant protein (rI-CmoeI) were pooled and used immediately or frozen in liquid nitrogen and stored at –70°C. The rI-CmoeI preparation is very stable at –70°C; 80% of the initial endonuclease activity was recovered after a 9 month period. Protein concentration was estimated using the MicroBCA kit (Pierce, Rockford, IL).
Endonuclease assays and steady-state kinetic analysis
All assays for monitoring rI-CmoeI activity, except those used to determine kinetic parameters, were performed under single turnover conditions. The purified enzyme (2.5 nM) was incubated at 37°C in standard reaction buffer (10 mM Taps–KOH, pH 8.5, 1 mM dithiothreitol, 10 µg/ml bovine serum albumin, 10 mM MgCl2) containing 25 pM DNA substrate uniformly labeled or end-labeled with 33P. Steady-state kinetic parameters were determined under the same conditions, except that the enzyme concentration was 1 pM and the DNA substrate concentration ranged from 10 to 200 pM. At appropriate times, aliquots were removed and the reaction was stopped by adding EDTA to 1 mM, SDS to 0.5% and proteinase K to 0.5 mg/ml, followed by incubation at 50°C for 1 h. DNA was ethanol precipitated in the presence of 20 µg glycogen and 0.75 M ammonium acetate and dissolved in 4 µl of loading buffer (2% Ficoll 400, 10 mM EDTA, 0.02% bromophenol blue). DNA samples were electrophoresed in a 5% polyacrylamide–1× TBE (90 mM Tris–borate, pH 8.0, 2 mM EDTA) gel. The gel was fixed in a solution containing 10% ethanol and 10% acetic acid, dried and exposed to an imaging plate (Fuji Photo System, Japan). The amounts of uncleaved substrate and of the two cleavage products were determined with a Fuji BAS1000 Bio Imaging Analyzer and MacBAS software. For the single turnover assays, the program KaleidaGraph (Synergy Software, Reading, PA) was used to determine a pseudo first order constant (k) by fit to the equation [S] = [S0] e–kt, where [S0] and [S] are the substrate concentrations at time 0 and time t, respectively. Kinetic parameters were determined by fit of initial rate determinations to the Michaelis–Menten equation with LEONORA (24).
Stoichiometric analysis
A 104 bp DNA region containing the CmpsbA·1 homing site was PCR amplified from C.eugametos cpDNA with the primers 5′-GGTTACCGTTTCGGACAAGAAG-3′ and 5′-GAACGAGAGTTGTTGAATGAAG-3′ which had been previously end-labeled with T4 polynucleotide kinase in the presence of [γ-33P]ATP. Equimolar concentrations (10 nM) of the resulting DNA substrate and of purified rI-CmoeI were incubated at room temperature for 15 min in 20 µl of standard reaction buffer containing 10% glycerol and 50 µg/ml poly(dI·dC). The latter component was used to prevent non-specific binding of the enzyme to the DNA substrate. Protein–DNA complexes were electrophoresed at 4°C in a series of 6.0, 6.5, 7.0, 7.5 and 8.0% polyacrylamide–0.25× TB (22.5 mM Tris–borate, pH 8.0) gels containing 10 mM MgCl2. Alongside this DNA sample, 5 µg of native protein molecular weight standards (Sigma-Aldrich, Oakville, Ontario, Canada) and the 33P-labeled substrate were electrophoresed. Each gel was stained with Coomassie brilliant blue, destained, dried and exposed to an imaging plate (Fuji). The stoichiometry of the enzyme–DNA complex was determined as described (25).
Production and analysis of mutant H154A
The H154A substitution was introduced into the I-CmoeI gene carried by pET-ICmoeI using the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The mutant protein was purified using the procedure described for the wild-type rI-CmoeI. Conditions for gel retardation assays were identical to those described for the wild-type protein, except that protein concentration ranged from 10 to 300 nM. Electrophoresis was carried out in a 5% polyacrylamide–0.25× TB gel containing 10 mM MgCl2.
RESULTS
DNA endonuclease assays of the CmpsbA·1- and CmpsbA·2-encoded proteins
Because some homing endonucleases are toxic when their genes are overexpressed in E.coli cells (23), we used an in vitro translation approach to produce the CmpsbA·1 and CmpsbA·2 intron-encoded proteins. For each protein the procedure can be summarized as follows. A PCR amplified DNA fragment containing the intron ORF downstream of a T7 promoter was first prepared. The corresponding RNA was obtained by T7 RNA polymerase-mediated transcription and translation was carried out in a rabbit reticulocyte lysate. Analysis of 35S-labeled translation products by SDS–PAGE disclosed major bands corresponding to the sizes predicted for the CmpsbA·1- and CmpsbA·2-encoded proteins (39 and 36 kDa, respectively); these products were observed specifically in assays in which exogenous RNA was added (data not shown).
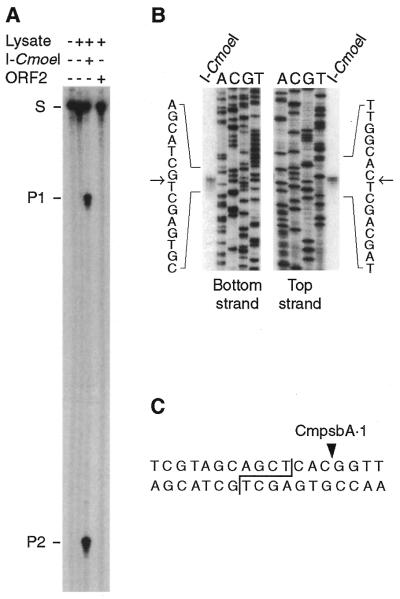
To test the endonuclease activity of the CmpsbA·1- and CmpsbA·2-encoded proteins, we incubated each translation product in the presence of a 32P-end-labeled DNA substrate originating from the intronless C.eugametos psbA gene and containing both the CmpsbA·1 and CmpsbA·2 homing sites. As shown in Figure 1A, only the CmpsbA·1-encoded protein cleaves the DNA substrate, yielding fragments of 196 and 78 nt. Further endonuclease assays for prolonged incubation periods failed to reveal any activity associated with the CmpsbA·2-encoded protein. According to the current nomenclature (5), the CmpsbA·1-encoded DNA endonuclease was named I-CmoeI. Its DNA cleavage pattern was determined as shown in Figure 1B. I-CmoeI introduces a double-strand break 3 bp upstream of the CmpsbA·1 intron insertion site, generating 3′ overhangs of 4 nt (Fig. 1C).
Figure 1.
I-CmoeI activity and cleavage pattern of this endonuclease. (A) DNA endonuclease assays with the CmpsbA·1 and CmpsbA·2 intron-encoded proteins. An unprogrammed rabbit reticulocyte lysate (Lysate +, I-CmoeI –, ORF2 –) and reticulocyte lysates containing in vitro synthesized RNAs derived from the CmpsbA·1 (Lysate +, I-CmoeI +, ORF2 –) and CmpsbA·2 (Lysate +, I-CmoeI –, ORF2 +) ORFs were incubated at 37°C for 1 h in 50 µl reaction mixtures containing 20 mM Tris–HCl, pH 8.0, 2.5 mM MgCl2, 1 mM dithiothreitol and a 32P-end-labeled DNA substrate (274 bp) originating from the intronless psbA gene of C.eugametos. DNA from these reaction mixtures was electrophoresed in a 5% polyacrylamide–9 M urea gel alongside the substrate (Lysate –, I-CmoeI –, ORF2 –). Partial degradation of the substrate and cleavage products is attributed to the residual micrococcal nuclease present in the rabbit reticulocyte lysate. S, substrate; P1 and P2, cleavage products. (B) Strategy used to determine the cleavage pattern of I-CmoeI. Dideoxy sequencing reactions of the DNA substrate were initiated with the 32P-end-labeled primers 1010 (bottom strand) and 1011 (top strand) (see Materials and Methods). The resulting ladders were electrophoresed in a 5% polyacrylamide–9 M urea gel alongside the 32P-end-labeled substrate cleaved by I-CmoeI. Arrows indicate the positions corresponding to the 3′-termini of the cleavage products. (C) Cleavage pattern of I-CmoeI (staggered line) and insertion site of the CmpsbA·1 intron (solid triangle).
Purification of rI-CmoeI
Using a recombinant pET-30a plasmid, we expressed the I-CmoeI gene in E.coli as a fusion protein carrying a His6 tag at the N-terminus. After a 2.5 h induction of I-CmoeI gene expression, a recombinant protein of expected size, representing ∼10% of total cellular proteins, was detected (Fig. 2). More than 95% of this recombinant protein, designated here rI-CmoeI, was recovered in the soluble fraction of the cell lysate. Two sequential chromatography steps were sufficient to purify rI-CmoeI to >95% homogeneity, as estimated by SDS–PAGE analysis (Fig. 2). The first step, chromatography on a Ni–NTA resin, yielded rI-CmoeI to ∼75–80% homogeneity. The second step, cation exchange chromatography in which rI-CmoeI eluted as a single peak at 0.2 M NaCl (data not shown), allowed us to recover rI-CmoeI with a yield of 4 mg protein/l bacterial culture.
Figure 2.
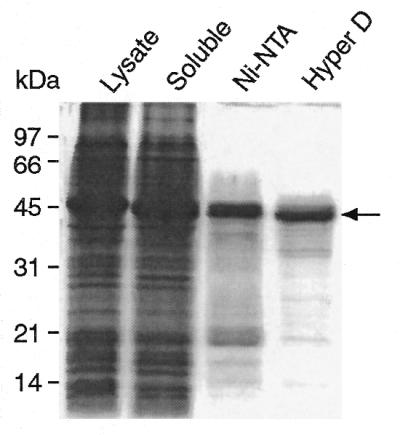
SDS–PAGE of fractions recovered during the purification of rI-CmoeI. These fractions consist of the lysate of cells induced with IPTG (Lysate), the soluble fraction of the lysate (Soluble), the fraction eluted from the Ni–NTA resin (Ni-NTA) and the pool of fractions eluted from the S-ceramic Hyper D column (Hyper D). Proteins were electrophoresed in a 12% polyacrylamide gel and stained with Coomassie brilliant blue. Proteins corresponding to 100 µl of bacterial culture were loaded in the first three lanes; the last lane contains 2.5 µg of purified protein. The arrowhead denotes the position of the rI-CmoeI protein.
Attempts to specifically remove the fusion partner from rI-CmoeI with enterokinase were unsuccessful. Treatment of rI-CmoeI with this protease yielded numerous peptide fragments even though the rI-CmoeI amino acid sequence predicted the presence of a unique site of cleavage by enterokinase. As no protease activity was detected in purified rI-CmoeI fractions (data not shown), it appears that rI-CmoeI features a number of cryptic enterokinase cleavage sites.
Optimization of conditions for DNA cleavage by rI-CmoeI
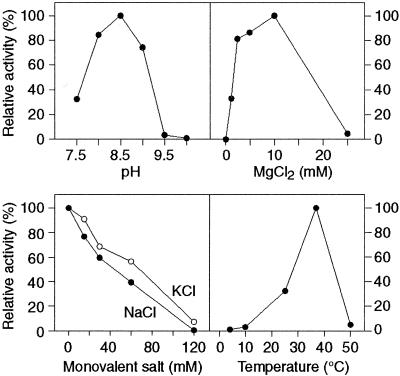
To determine the optimal cleavage conditions for rI-CmoeI activity, the influence of various factors such as pH, Mg2+ concentration, salts and temperature was measured in endonuclease assays. When the pH of the reaction mixture was varied from 7.5 to 11.0, the highest enzyme activity was observed at pH 8.5 (Fig. 3A). Maximal DNA cleavage was recorded in 5 mM Tris–HCl (pH 8.5) and 10 mM Taps–KOH (pH 8.5). At higher buffer concentrations rI-CmoeI activity diminished gradually to reach 30% of the maximal activity in 250 mM Taps–KOH (pH 8.5), with no detectable activity in 250 mM Tris–HCl (pH 8.5) (data not shown). To provide sufficient buffer strength, we employed 10 mM Taps–KOH (pH 8.5) in all subsequent cleavage assays, even though the enzyme activity is slightly higher in 5 mM Tris–HCl (pH 8.5). Assays with various concentrations of MgCl2 revealed that Mg2+ is absolutely required for rI-CmoeI activity and that its optimal concentration is 10 mM (Fig. 3B). Monovalent cations such as Na+ or K+ inhibit enzyme activity even at low concentrations (Fig. 3C). rI-CmoeI activity is also sensitive to the reaction temperature; incubation at temperatures lower or higher than 37°C (the optimal value recorded) leads to a reduction or a loss of endonuclease activity (Fig. 3D). Altogether, the aforementioned results allowed us to define an optimized reaction mixture, which was used in all remaining cleavage assays. rI-CmoeI is rather stable in this reaction mixture; its half-life is ∼4 h at 37°C in the absence of DNA substrate (data not shown).
Figure 3.
Influence of pH, temperature and various concentrations of MgCl2, NaCl and KCl on DNA cleavage by rI-CmoeI. Enzyme activity is expressed relative to the maximal value observed in the corresponding series of reactions in which a given reaction parameter was tested. All other reaction conditions are described in Materials and Methods.
Divalent cations preferred by rI-CmoeI
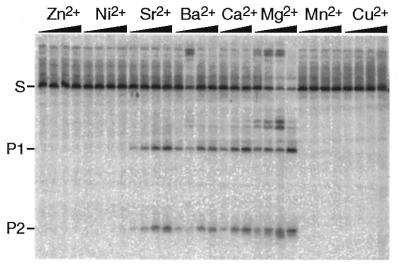
We tested whether Mg2+ can be substituted by another divalent cation in cleavage reactions. We found that rI-CmoeI cleaves its DNA substrate in the presence of Mg2+, Ca2+, Sr2+ and Ba2, but not in the presence of Mn2+, Zn2+, Ni2+, Cu2+ or Co2+. Figure 4 shows the results of cleavage assays in which the concentration of divalent cation was 1 mM. It can be seen that cleavage efficiency was maximal with Mg2+ and that rI-CmoeI activity in the presence of Ca2+, Sr2+ and Ba2+ represented 21, 12 and 8% of the activity observed with Mg2+, respectively. Similar results were obtained with a 10 mM concentration of divalent cation.
Figure 4.
Influence of divalent metal ions on DNA cleavage by rI-CmoeI. All reaction conditions were those described in Materials and Methods, except that Mg2+ was replaced by a 1 mM concentration of another divalent cation. In each cleavage assay aliquots were removed from the reaction mixture after 1, 2, 5 and 10 min. DNA was electrophoresed in a 5% polyacrylamide–1× TBE gel.
Kinetic properties of rI-CmoeI
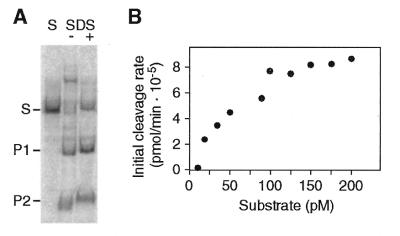
We determined the kinetic parameters of rI-CmoeI under Michaelis–Menten conditions. As some homing endonucleases cannot be analyzed under these conditions because they form very stable complexes with their cleavage products (such complexes must be dissociated by heat denaturation or treatment with SDS in order to visualize the cleavage products by gel electrophoresis) (26–28), we first ascertained that rI-CmoeI dissociates from its cleavage products. Figure 5A shows that the cleavage products generated by rI-CmoeI are visible in a non-denaturing polyacrylamide gel when the reaction sample is free of denaturing agent and was not heat denatured prior to electrophoresis. To determine the Km and kcat values of rI-CmoeI, endonuclease assays were carried out using various concentrations of substrate and the initial cleavage rates were measured by quantifying the cleavage products (Fig. 5B). The Km and kcat values derived from these experiments are 100 ± 40 pM and 0.26 ± 0.04 min–1, respectively.
Figure 5.
Kinetic analyses of rI-CmoeI. (A) Release of cleavage products from rI-CmoeI. Two cleavage reactions were carried out under standard conditions; SDS and proteinase K were added to one of these reactions (SDS +), but not to the other (SDS –). After ethanol precipitation, DNA was electrophoresed in a 5% polyacrylamide–1× TBE gel alongside the substrate (S). (B) Initial rates of DNA cleavage as a function of substrate concentration. Steady-state kinetic parameters were determined by curve fitting of these values to the Michaelis–Menten equation.
Stoichiometry of the rI-CmoeI–homing site interaction
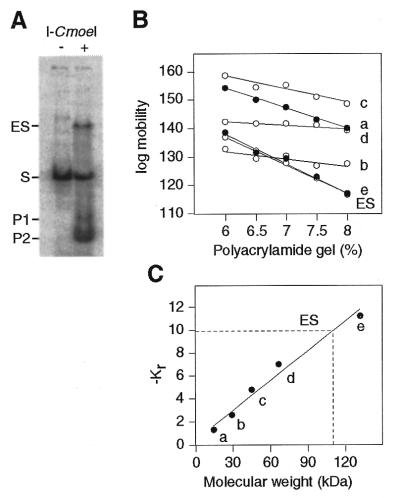
A gel retardation assay based on a Ferguson analysis (29) was carried out to determine the stoichiometry of the rI-CmoeI–homing site interaction. To favor formation of the enzyme–DNA complex, equimolar concentrations of rI-CmoeI and DNA substrate were incubated in the optimized reaction buffer containing 10% glycerol and 0.05 mg/ml poly(dI·dC). Although DNA cleavage occurred under the conditions used, a significant amount of the enzyme–DNA complex was observed (Fig. 6A). It should be noted here that rI-CmoeI cannot bind to its DNA substrate in the absence of a divalent cation; requirement of this cofactor for the formation of the enzyme–DNA complex was revealed by gel retardation assays conducted in the presence of 1 mM EDTA (data not shown). The electrophoretic mobilities of the enzyme–DNA complex were determined in a series of non-denaturing gels containing 6.0–8.0% polyacrylamide. The relative mobilities of protein standards were measured in the same gels and plotted as a function of gel concentration (Fig. 6B). The slopes of the straight lines obtained for the various protein standards and for the enzyme–DNA complex represent retardation coefficients (Kr) that are inversely related to molecular weights of the standards or the complex. These retardation coefficients were plotted as a function of the molecular weights of the protein standards (Fig. 6C), allowing us to determine that the molecular weight of the rI-CmoeI–DNA complex is 110.0 kDa. Considering that the theoretical molecular weight of the 104 bp DNA substrate is 68.6 kDa, the molecular weight of the bound protein was estimated to be 41.4 kDa. This value indicates that rI-CmoeI (theoretical molecular weight 43.4 kDa) binds its homing site as a monomer.
Figure 6.
Stoichiometry of the rI-CmoeI–homing site interaction. (A) Analysis of the rI-CmoeI–DNA complex (ES) in polyacrylamide gels. rI-CmoeI was incubated in the presence of a 33P-end-labeled DNA substrate (104 bp) containing the CmpsbA·1 homing site and the mixture was electrophoresed in gels containing 6.0, 6.5, 7.0, 7.5 and 8.0% polyacrylamide. After staining with Coomassie blue, the gels were exposed to a phosphorimaging plate. The image of the 6.0% polyacrylamide gel is shown. (B) Mobility of the ES and protein standards as a function of polyacrylamide concentration. a, α-lactalbumin (14.2 kDa); b, carbonic anhydrase (29.0 kDa); c, chicken egg albumin (45.0 kDa); d and e, bovine serum albumin monomer (66.0 kDa) and dimer (132.0 kDa), respectively. (C) Plot of –Kr values of protein standards as a function of molecular weight. Interpolation of the –Kr value of the ES reveals a molecular weight of 110 kDa.
Role of the first histidine residue in the H-N-H motif of rI-CmoeI
The first histidine residue in the H-N-H motif of rI-CmoeI (corresponding to position 154 in I-CmoeI) was substituted for an alanine by site-directed mutagenesis. The mutant enzyme [rI-CmoeI(H154A)] displayed no detectable endonuclease activity (data not shown). To determine whether H154 is required for binding of the enzyme to its DNA substrate, we assayed rI-CmoeI(H154A) in gel retardation experiments. As shown in Figure 7, the wild-type enzyme induces retardation of the substrate when these two components are present in equimolar concentrations, however, the mutant enzyme fails to induce any detectable retardation even when its concentration is 30-fold higher than that of the substrate.
Figure 7.

Effect of mutation H154A on DNA binding. Protein–DNA complexes were formed in reaction mixtures containing 10, 30, 100 and 300 nM wild-type I-CmoeI (W) or mutant H154A (M) and 10 nM 33P-labeled DNA substrate. As a control, a mixture containing only the DNA substrate was also electrophoresed in the 5% polyacrylamide gel (lane S).
DISCUSSION
Possible role of I-CmoeI unidirectional gene conversion
We have found that the CmpsbA·1 intron specifies an H-N-H homing endonuclease (I-CmoeI) that cleaves at the cognate site within the intronless C.eugametos psbA gene. Although the CmpsbA·2-encoded protein revealed no endonuclease activity, it is not known whether this H-N-H protein actually lacks any endonuclease activity or whether such activity was completely lost as a result of protein instability under the conditions we used for in vitro translation or cleavage reactions. I-CmoeI and/or the putative CmpsbA·2-encoded endonuclease may play a determinant role in unidirectional conversion of the 21 kb extra sequence of C.moewusii during interspecific C.moewusii × C.eugametos crosses (22). Considering that the C.moewusii psbA exon 2 and 3 sequences are repeated in a direct orientation at the right junction of the 21 kb sequence and that the copy at the latter junction shows higher sequence identity with the C.eugametos gene (C.Lemieux and M.Turmel, unpublished results), one or two double-strand breaks caused by homing endonucleases within C.eugametos psbA could initiate a conversion event involving the entire 21 kb sequence.
It has recently been reported that a protein homologous to I-CmoeI is encoded by the second group I intron (CrpsbA·4) in the Chlamydomonas reinhardtii psbA gene (30). This protein is most probably an endonuclease with properties identical or very similar to those reported here for I-CmoeI. The two Chlamydomonas proteins share 58% sequence identity and are encoded by similar introns that are inserted at exactly the same position in the psbA gene (30).
I-CmoeI shares several of the characteristics displayed by other homing endonucleases
Like most other homing endonucleases, I-CmoeI cleaves both strands of its DNA substrate very close to the insertion site of its encoding intron, generating 3′ overhangs of 4 nt. This DNA cleavage pattern contrasts with those reported for the H-N-H homing endonucleases I-TevIII, I-HmuI and I-HmuII (7,8), suggesting that substantial differences exist between the active sites of H-N-H enzymes.
rI-CmoeI also resembles several homing endonucleases with respect to optimal conditions for DNA cleavage. The requirement for Mg2+ is not surprising as all known endonucleases need a metal cofactor for catalysis and are active in the presence of Mg2+ (6,31). The lack of requirement for a monovalent salt and the observation of a maximal rate of DNA cleavage at alkaline pH are also usual characteristics of homing endonucleases. In contrast to I-CreI (28), I-CeuI (32) and homing endonucleases from hyperthermophyles (33–37), rI-CmoeI shows limited activity at temperatures >50°C.
Unlike I-SceI (26), PI-SceI (27) and I-CreI (28), rI-CmoeI dissociates from its cleavage products, thus allowing the determination of steady-state kinetic parameters. A comparison of Km values for different endonucleases tested under optimal conditions suggests that rI-CmoeI has a 5- to 36-fold higher affinity for its DNA substrate than the restriction endonucleases EcoRI, EcoRV and BamH1 and a 10-fold lower affinity than I-PpoI (38). However, the kcat of rI-CmoeI is 3- to 29-fold lower than those of these restriction enzymes and 5-fold higher than that of I-PpoI. In the case of I-PpoI, it has been shown that the limiting factor of reaction turnover is release of cleavage products (39).
rI-CmoeI is unusual in its specificity for alkaline earth cations
rI-CmoeI cleaves its DNA substrate exclusively in the presence of cations that are part of the family of alkaline earth elements, with a cleavage efficiency decreasing as the size of the cation increases (Mg2+ > Ca2+ > Sr2+ > Ba2+). Assuming that a divalent cation participates in the cleavage mechanism of I-CmoeI, the active site of this enzyme can accommodate cations that vary from 0.78 (Mg2+) to 1.43 Å (Ba2+) in ionic radius. Most of the nucleases characterized to date show no strict requirement for an alkaline earth cation; they cleave their DNA substrate in the presence of Mg2+ or a divalent cation belonging to a separate family (e.g. the transition metal cation Mn2+) (6,31). Compared to other nucleases, rI-CmoeI is also unusual in cleaving DNA in the presence of Ca2+. Only a few endonucleases, such as I-PpoI (38) and colicin E9 (40), exhibit activity in the presence of Ca2+.
Another unusual property of rI-CmoeI, which is shared with colicin E9 (41), is the requirement for a divalent cation for DNA binding. Restriction enzymes (6) and other homing endonucleases (31), including the His-Cys box enzymes (42), bind DNA at specific or non-specific sites in the absence of a metal cofactor. Considering that rI-CmoeI carries a His tag at its N-terminus, one may hypothesize that this 44 amino acid extension, which is a metal ion binding site, does not fold properly in the absence of Mg2+ and thereby interferes with proper folding of the enzyme, rendering it dependent upon a metal divalent cation for DNA binding. This hypothesis appears unlikely for the following two reasons. First, it is generally believed that the addition of a His tag to the terminus of a protein does not lead to a significant change in the protein structure. Second, and most importantly, another H-N-H endonuclease, colicin E9, has been shown to require a metal cation for DNA binding in the course of a study with a recombinant protein that lacked a His tag (41).
rI-CmoeI shares functional similarities with H-N-H colicins
It has been proposed that H-N-H and His-Cys box endonucleases form a single family of enzymes (16) following the finding that the active site of the DNase domain of colicin E9 is structurally similar to those of the His-Cys box endonuclease I-PpoI and the non-specific Serratia nuclease (14–17). As discussed below, our studies of the rI-CmoeI(H154A) mutant and of the stoichiometry of the rI-CmoeI–DNA interaction suggest that the active site of rI-CmoeI is similar to those of H-N-H colicins, but significantly different from those of His-Cys box endonucleases.
The H154A mutation abolishes not only rI-CmoeI activity, but also DNA binding. Although the histidine corresponding to H154 in I-PpoI (H98) acts as the general base that activates a water molecule for an in-line nucleophilic attack on the scissile phosphate (15,39,42), it is unlikely that the rI-CmoeI H154 residue shares the same function because the I-PpoI(H98A) mutant has the ability to bind its DNA substrate (42). We also dismiss the possibility that the rI-CmoeI H154 residue interacts directly with the DNA recognition sequence, as homing endonucleases contact DNA via multiple amino acids, thus making it improbable that mutation of only one of these residues leads to complete loss of protein–DNA interaction (6). Considering that a metal cofactor is required for formation of the rI-CmoeI–DNA complex, we propose that H154 plays a role in binding this cofactor and that such binding induces a structural modification of the enzyme which is essential for DNA recognition. This hypothesis is consistent with the observation that binding of a transition metal ion to the H-N-H motif of colicin E9 DNase induces a structural modification that allows the endonuclease domain to adopt a functional conformation (20). Although the latter result and the properties reported here for the rI-CmoeI(H154A) mutant suggest that the first histidine residue of the H-N-H motif plays an important structural role, there is evidence that other residues within this motif are essential for catalysis. In colicin E9 DNase it has been shown that H127 (a residue equivalent to H177 in I-CmoeI) participates in catalysis (41).
Our finding that rI-CmoeI binds DNA as a monomer suggests that I-CmoeI has a single active site. Surprisingly, the presence of a GIY-YIG motif has been reported in the sequences of I-CmoeI and its C.reinhardtii homolog (30), raising the possibility that two different active sites originating from unrelated homing endonucleases coexist in the same protein. As the observed GIY-YIG motif lacks a tyrosine that is critical for the activity of GIY-YIG enzymes as well as several adjacent residues that are usually conserved in these enzymes (43), the presence of this degenerate motif appears to be fortuitous. The colicin E9 DNase has been shown to be a monomer in solution (40) and monomeric structures have been observed for the colicin E9 and E7 DNases (12,13). In contrast, the structures of I-PpoI and the Serratia endonuclease have revealed that these His-Cys box enzymes are homodimers with two symmetry-related active sites (14,42).
Acknowledgments
ACKNOWLEDGEMENTS
We thank Lindsay Eltis and Nathalie Drouin for their help with protein purification. M.T. and C.L. are Associates in the Program in Evolutionary Biology of the Canadian Institute for Advanced Research. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (GP0003293 to M.T. and GP0002830 to C.L.).
REFERENCES
- 1.Lambowitz A.M. and Belfort,M. (1993) Annu. Rev. Biochem., 62, 587–622. [DOI] [PubMed] [Google Scholar]
- 2.Dujon B. (1989) Gene, 82, 91–114. [DOI] [PubMed] [Google Scholar]
- 3.Belfort M. and Perlman,P.S. (1995) J. Biol. Chem., 270, 30237–30240. [DOI] [PubMed] [Google Scholar]
- 4.Gimble F S. (2000) FEMS Microbiol. Lett., 185, 99–107. [DOI] [PubMed] [Google Scholar]
- 5.Belfort M. and Roberts,R.J. (1997) Nucleic Acids Res., 25, 3379–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurica M.S. and Stoddard,B.L. (1999) Cell. Mol. Life Sci., 55, 1304–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eddy S.R. and Gold,L. (1991) Genes Dev., 5, 1032–1041. [DOI] [PubMed] [Google Scholar]
- 8.Goodrich-Blair H. and Shub,D.A. (1996) Cell, 84, 211–221. [DOI] [PubMed] [Google Scholar]
- 9.Shub D.A., Goodrich-Blair,H. and Eddy,S.R. (1994) Trends Biochem. Sci., 19, 402–404. [DOI] [PubMed] [Google Scholar]
- 10.Dalgaard J.Z., Klar,A.J., Moser,M.J., Holley,W.R., Chatterjee,A. and Mian,I.S. (1997) Nucleic Acids Res., 25, 4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorbalenya A.E. (1994) Protein Sci., 3, 1117–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleanthous C., Kuhlmann,U.C., Pommer,A.J., Ferguson,N., Radford,S.E., Moore,G.R., James,R. and Hemmings,A.M. (1999) Nature Struct. Biol., 6, 243–252. [DOI] [PubMed] [Google Scholar]
- 13.Ko T.P., Liao,C.C., Ku,W.Y., Chak,K.F. and Yuan,H.S. (1999) Structure Fold Des., 7, 91–102. [DOI] [PubMed] [Google Scholar]
- 14.Friedhoff P., Franke,I., Meiss,G., Wende,W., Krause,K.L. and Pingoud,A. (1999) Nature Struct. Biol., 99, 102–103. [DOI] [PubMed] [Google Scholar]
- 15.Friedhoff P., Franke,I., Krause,K.L. and Pingoud,A. (1999) FEBS Lett., 443, 209–214. [DOI] [PubMed] [Google Scholar]
- 16.Kühlmann U.C., Moore,G.R., James,R., Kleanthous,C. and Hemmings,A.M. (1999) FEBS Lett., 463, 1–2. [DOI] [PubMed] [Google Scholar]
- 17.Miller M.D., Cai,J. and Krause,K.L. (1999) J. Mol. Biol., 288, 975–987. [DOI] [PubMed] [Google Scholar]
- 18.Miller M.D. and Krause,K.L. (1996) Protein Sci., 5, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flick K.E., Jurica,M.S., Monnat,R.J. and Stoddard,B.L. (1998) Nature, 394, 96–101. [DOI] [PubMed] [Google Scholar]
- 20.Pommer A.J., Kuhlmann,U.C., Cooper,A., Hemmings,A.M., Moore,G.R., James,R. and Kleanthous,C. (1999) J. Biol. Chem., 274, 27153–27160. [DOI] [PubMed] [Google Scholar]
- 21.Turmel M., Boulanger,J. and Lemieux,C. (1989) Nucleic Acids Res., 17, 3875–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussières J., Lemieux,C., Lee,R.W. and Turmel,M. (1997) Curr. Genet., 30, 356–365. [DOI] [PubMed] [Google Scholar]
- 23.Turmel M., Otis,C., Côté,V. and Lemieux,C. (1997) Nucleic Acids Res., 25, 2610–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornish-Bowden A. (1995) Analysis of Enzyme Kinetic Data. Oxford University Press, New York, NY.
- 25.Mueller J.E., Smith,D., Bryk,M. and Belfort,M. (1995) EMBO J., 14, 5724–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrin A., Buckle,M. and Dujon,B. (1993) EMBO J., 12, 2939–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimble F.S. and Stephens,B.W. (1995) J. Biol. Chem., 270, 5849–5856. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Kim,H.H., Yuan,X. and Herrin,D.L. (1997) Nucleic Acids Res., 25, 3767–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson A. (1964) Metabolism, 13, 985–1002. [DOI] [PubMed] [Google Scholar]
- 30.Holloway S.P., Deshpande,N.N. and Herrin,D.L. (1999) Curr. Genet., 36, 69–78. [DOI] [PubMed] [Google Scholar]
- 31.Pingoud A. and Jeltsch,A. (1997) Eur. J. Biochem., 97, 1–22. [DOI] [PubMed] [Google Scholar]
- 32.Marshall P., Davis,T.B. and Lemieux,C. (1994) Eur. J. Biochem., 220, 855–859. [DOI] [PubMed] [Google Scholar]
- 33.Dalgaard J.Z., Garrett,R.A. and Belfort,M. (1993) Proc. Natl Acad. Sci. USA, 90, 5414–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lykke-Andersen J., Thi-Ngoc,H.P. and Garrett,R.A. (1994) Nucleic Acids Res., 22, 4583–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishioka M., Fujiwara,S., Takagi,M. and Imanaka,T. (1998) Nucleic Acids Res., 26, 4409–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komori K., Fujita,N., Ichiyanagi,K., Shinagawa,H., Morikawa,K. and Ishino,Y. (1999) Nucleic Acids Res., 27, 4167–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saves I., Ozanne,V., Dietrich,J. and Masson,J.M. (2000) J. Biol. Chem., 275, 2335–2341. [DOI] [PubMed] [Google Scholar]
- 38.Wittmayer P.K. and Raines,R.T. (1996) Biochemistry, 35, 1076–1083. [DOI] [PubMed] [Google Scholar]
- 39.Mannino S.J., Jenkins,C.L. and Raines,R.T. (1999) Biochemistry, 38, 16178–16186. [DOI] [PubMed] [Google Scholar]
- 40.Pommer A J., Wallis,R., Moore,G.R., James,R. and Kleanthous,C. (1998) Biochem. J., 334, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garinot-Schneider C., Pommer,A.J., Moore,G.R., Kleanthous,C. and James,R. (1996) J. Mol. Biol., 260, 731–742. [DOI] [PubMed] [Google Scholar]
- 42.Galburt E.A., Chevalier,B., Tang,W., Jurica,M.S., Flick,K.E., Monnat,R.J. and Stoddard,B.L. (1999) Nature Struct. Biol., 6, 1096–1099. [DOI] [PubMed] [Google Scholar]
- 43.Kowalski J.C., Belfort,M., Stapleton,M.A., Holpert,M., Dansereau,J.T., Pietrokovski,S., Baxter,S.M. and Derbyshire,V. (1999) Nucleic Acids Res., 27, 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]