Abstract
Background
Conventional stented, rapid deployment and new-generation stented valves are now available for surgical aortic valve replacement (SAVR). New-generation devices feature advanced tissue treatment for theoretical prolonged durability and a new stent design able to expand in case of future transcatheter Valve-in-Valve. Aim of this retrospective, multicenter, propensity-weighted study was to compare early clinical and hemodynamic outcomes of these three different bioprostheses.
Methods
We analyzed data of 2589 patients from two national multicenter registries and one Institutional database. Study devices were Magna Ease, Intuity/Intuity Elite and Inspiris Resilia (Edwards Lifesciences, Irvine, CA, USA) and were implanted in 296 (11.4 %), 1688 (65.2 %) and 605 (23.4 %) patients, respectively. A propensity score weighting approach was employed.
Results
In isolated SAVR, aortic cross clamp (ACC) time was shorter for Intuity (Magna Ease: 87, Intuity: 55, Inspiris: 70 min; Magna Ease vs. Intuity: p < 0.001; Inspiris vs. Intuity: p < 0.001). Overall mortality was 2 %, 1.7 % and 0.5 % in Magna Ease, Intuity and Inspiris groups, respectively (Magna Ease vs. Intuity: p = 0.476; Inspiris vs. Intuity: p = 0.395); permanent pace-maker implantation rate was lower for Inspiris (Magna Ease: 6 %, Intuity: 6 %, Inspiris: 2 %; Magna Ease vs. Intuity: p = 0.679; Inspiris vs. Intuity: p < 0.001). Median mean gradients were 13, 10 and 10 mmHg for Magna Ease, Intuity and Inspiris, respectively (Magna Ease vs. Intuity: p < 0.001; Inspiris vs. Intuity: p = 0.13).
Conclusions
All study devices provide excellent early clinical and hemodynamic outcomes. Inspiris shows low rates of permanent pace-maker implantation and its transaortic gradients are similar to rapid-deployment valves and lower than Magna Ease.
Keywords: Aortic valve replacement, Bioprostheses, Rapid-deployment aortic bioprostheses, Stented aortic bioprosthesis
1. Introduction
The use of aortic bioprostheses has progressively and constantly increased during the last decades [1], [2], [3], [4] due to several reasons. First, technological evolution enabled physicians to choose among several different valve substitutes in terms of design (stented, stentless, rapid-deployment), materials (pericardium, bovine) and anti-calcification treatment. Second, the possibility to perform transcatheter valve-in-valve (ViV) in case of structural valve deterioration is encouraging surgeons to implant bioprostheses in younger patients [5]. Third, patients’ preferences are moving towards avoidance of lifelong anticoagulation [6]. An accurate knowledge of device characteristics (design, expected durability, possibility of future ViV, hemodynamics) is mandatory in order to choose the most appropriate device for every single patient. In this multicenter, retrospective, propensity weighted study we aimed at comparing early clinical and hemodynamic outcomes of three different aortic valve substitutes: conventional stented Magna Ease, rapid-deployment Intuity and new-generation stented Inspiris Resilia (all manufactured by Edwards Lifesciences, Irvine, CA, USA).
2. Methods
Patient-informed consent for treatment, data collection, and analysis for scientific purposes was always collected. Because this was a retrospective study on commercially available devices, protocol submission to the ethics committee has been waived; however, ethics permission was granted by the regional ethics committee in centers where it was deemed necessary. The Italian Registry of the Intuity Valve (INTU-ITA) and the Italian Registry of Inspiris Resilia valve (RES-ITA) were approved by the appropriate ethics committee (4352/AO/17 and PZ 52/2022).
We included patients who underwent surgical aortic valve replacement (SAVR) with the three study devices for aortic valve stenosis, isolated or combined with other procedures. Patients with aortic insufficiency and active endocarditis were excluded because these are contraindications for the Intuity valve. Data came from two national multicenter registries and one Institutional database. INTU-ITA is the Italian Registry of the Intuity valve. It is a real-world, all-comers, independent multicenter registry that includes all patients who underwent SAVR with the Intuity (and its evolution Intuity Elite) at 23 Italian cardiac surgery institutions starting from June 2012 to September 2019 [7]. The Italian Registry of Inspiris Resilia valve (RES-ITA) is a real-world, all-comers, independent multicenter registry that includes all patients who underwent SAVR with Inspiris Resilia at 13 Italian cardiac surgery centers and were collected starting from June 2017 to December 2020 [8]. Finally, data about patients who underwent SAVR with Magna Ease were collected from January 2017 to December 2020 at the Division of Cardiac Surgery of the University Hospital of Padova. Data were analyzed by the Unit of Biostatistics of the University of Padova. The choice of the device was left at surgeon’s discretion. Preoperative variables were defined according to European system for cardiac operative risk evaluation (EuroSCORE) definitions [9] and postoperative outcomes were defined according to the updated Valve Academic Research Consortium (VARC-2) definitions [10]. Echocardiographic and clinical assessment were performed before surgery and at discharge.
2.1. Study devices
2.1.1. Magna Ease
The Carpentier-Edwards Perimount Magna Ease is made of a cobalt-chromium stent and three bovine pericardial leaflets. Leaflets are treated using Thermafix tissue process. It is implanted in supra-annular position, and it can be used both for aortic valve stenosis and regurgitation.
2.1.2. Rapid-deployment Intuity
The Intuity (and its evolution Intuity Elite) valve is similar to the previously described Magna Ease (pericardial leaflets with Thermafix) with the adjunct of a balloon-expandable subannular skirt frame (inspired by transcatheter valve design) that serves both for anchoring and sealing. Intuity implantation technique has been extensively described elsewhere [11], [12]. Briefly, after native leaflets removal and annular decalcification, three single guiding sutures are placed at the nadir of each sinus. The valve is parachuted inside the aortic annulus and the balloon is inflated. The delivery system is then removed, and the three sutures are tied. The Intuity valve is indicated only in aortic valve stenosis and is contraindicated in aortic regurgitation and endocarditis.
2.1.3. Inspiris Resilia
The Inspiris Resilia valve is a new-generation stented bovine pericardial bioprosthesis that introduces two new features. First, pericardial leaflets are treated with a novel integrity preservation technology that eliminates free aldehydes that are involved in tissue calcification [13]. Second, the prosthesis features the V-Fit technology that enables stent uniform and controlled expansion during deployment of a transcatheter valve for ViV procedures. The Inspiris valve can be used both for aortic valve stenosis and regurgitation.
2.2. Statistical analysis
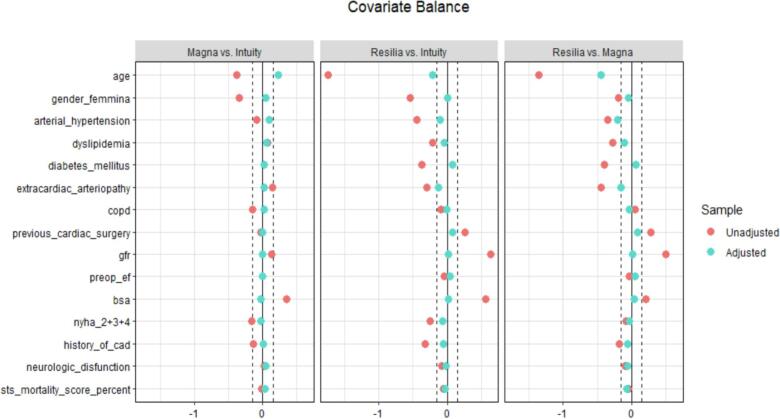
Descriptive statistics were reported as I quartile/median/III quartile for continuous variables, and absolute numbers (percentages) for categorical variables. Wilcoxon-Kruskal-Wallis and Pearson Chi-squared tests were performed to compare the distribution of continuous and categorical variables, respectively. A propensity score weighting approach was employed to account for potential confounding related to the non-random allocation of the patients to the three prostheses. Propensity scores were estimated using covariate balancing propensity score (CBPS) and a trimming of the weights was performed at 90° quantile. Propensity scores were estimated considering age, gender, arterial hypertension, dyslipidemia, diabetes mellitus, extracardiac arteriopathy, chronic obstructive pulmonary disease (COPD), previous cardiac surgery, Glomerular Filtration Rate (GFR), preoperative left ventricular ejection fraction (LVEF), body surface area (BSA), New York Heart Association (NYHA), previous history of coronary artery disease, neurological disfunction and Society of Thoracic Surgeon (STS) mortality score. To account for missing data in variables used for propensity score estimation, multiple imputation was employed. Covariate balance was evaluated using Standardized Mean Differences. Age was found to be not balanced after propensity score weighting procedure, so it was included in the weighted regression models to assess the effect of the prostheses type on the outcomes of interest to account for potential residual confounding. (Fig. 1). A weighted logistic regression approach was adopted for binary outcomes. For analysis of results, the Intuity valve was considered the level of reference. Results were reported as Odds Ratio (OR), 95 % Confidence Interval (CI), and p-value. A Gamma model was employed for continuous outcomes, given the non-normal distribution of all the continuous outcomes considered. The marginal effect was computed considering the partial derivatives of the marginal expectation. Results were reported as average marginal effect (AME), 95 % CI, and p-value. The models for postoperative hemodynamic parameters were adjusted for the baseline value of the parameter.
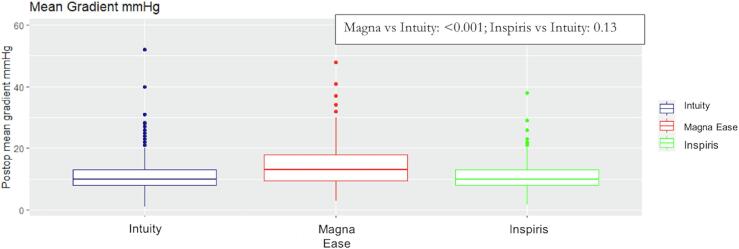
Fig. 1.
Boxplot of mean gradients at discharge of the study devices.
Analyses were performed using R software within the packages rms, CBPS and WeightIt for propensity score weighting procedure estimation, and margins for AME computation.
3. Results
3.1. Baseline
A total of 2589 patients who underwent isolated or combined SAVR for aortic valve stenosis were included in the analysis. Magna Ease, Intuity and Inspiris Resilia were implanted in 296 (11.4 %), 1688 (65.2 %) and 605 (23.4 %) patients, respectively. Overall median age was 72 years (IQR 64–78) and median STS score was 1.66 % (1.08–2.58). Females represented 40 % (1040 patients) of the overall population. Patients treated using Inspiris Resilia valve were younger than patients treated using Magna Ease and Intuity (60 years [IQR: 53–65] vs 71.5 years [IQR: 66–76] and 75 years [IQR: 70–79]; p < 0.001). Also, the STS score was lower in the Inspiris Resilia group compared to Magna Ease and Intuity groups (0.95 [IQR: 0.65–1.52] vs 1.3 [IQR: 0.95–2.08] and 1.9 [IQR: 1.32–2.82]; p < 0.001). Inspiris Resilia valve was also more used in redo procedures (10 % vs 4 % and 4 %; p < 0.001). There were no significant differences concerning preoperative left ventricular ejection fraction (LVEF) among groups (60 % [55–65] in the three groups; p = 0.359). Preoperative data used as variables for propensity score estimation are summarized in Table 1. After propensity score weighting procedure, covariate balance was satisfying, except for age which was included in all the weighted regression models to account for potential residual confounding (See supplementary figure S1).
Table 1.
Pre-operative characteristics. Continuous data are median (I quartile-III quartile), categorical data are absolute numbers (percentages).
| Variables | Intuity (N=1688) | Magna (N=296) | Inspiris (N=605) | Combined (N=2589) | P-value |
|---|---|---|---|---|---|
| Age (y) | 75 [70–79] | 71.5 [66–76] | 60 [53–65] | 72 [64–78] | < 0.001 |
| Female gender | 807 (47.8 %) | 94 (31.8 %) | 139 (23.0 %) | 1040 (40.2 %) | < 0.001 |
| Body surface area (m2) | 1.8 [1.7–1.9] | 1.9 [1.8–2.0] | 1.9 [1.8–2.0] | 1.8 [1.7–2.0] | < 0.001 |
| Dyslipidemia | 935 (55.4 %) | 176 (59.5 %) | 275 (45.5 %) | 1386 (53.6 %) | < 0.001 |
| Arterial hypertension | 1350 (80.0 %) | 228 (77.0 %) | 373 (61.7 %) | 1951 (75.4 %) | < 0.001 |
| Diabetes mellitus | 406 (24.1 %) | 74 (25.0 %) | 59 (9.8 %) | 539 (20.8 %) | < 0.001 |
| NYHA functional class | < 0.001 | ||||
| I | 134 (7.9 %) | 40 (13.5 %) | 98 (16.2 %) | 272 (10.5 %) | |
| ≥II | 1535 (91.6 %) | 256 (86.5 %) | 506 (83.8 %) | 2297 (88.7 %) | |
| Peripheral arterial disease | 269 (15.9 %) | 62 (20.9 %) | 35 (5.8 %) | 366 (14.1 %) | < 0.001 |
| COPD | 232 (13.8 %) | 27 (9.1 %) | 64 (10.6 %) | 323 (12.5 %) | 0.018 |
| Neurological dysfunction | 57 (3.4 %) | 11 (3.7 %) | 13 (2.1 %) | 81 (3.1 %) | 0.265 |
| GFR (mL/min/1.73 m2) | 70 [53.6–85.9] | 80 [63.2–90.5] | 87.7 [71.2–97.9] | 75.0 [58.7–91.0] | < 0.001 |
| Coronary artery disease | 532 (31.5 %) | 77 (26.0 %) | 110 (18.2 %) | 719 (27.8 %) | < 0.001 |
| Previous cardiac surgery | 73 (4.3 %) | 11 (3.7 %) | 62 (10.2 %) | 146 (5.6 %) | < 0.001 |
| STS Score (%) | 1.9 [1.3–2.8] | 1.3 [0.9–2.0] | 0.9 [0.6–1.5] | 1.6 [1.0–2.5] | < 0.001 |
| LVEF (%) | 60 [55–65] | 60 [55–65] | 60 [55–65] | 60 [55–65] | 0.359 |
Table legend: NYHA: New York Heart Association, COPD: Chronic obstructive pulmonary disease; GFR: Glomerular filtration rate; STS: Society of Thoracic Surgeons; LVEF: Left ventricular ejection fraction.
3.2. Intraoperative results
Table 2, Table 3 show intraoperative variables. Valve size distribution was as follows: Magna Ease 19, 21, 23, 25 and 27 mm were implanted in 26 (9 %), 80 (27 %), 116 (39 %), 57 (19 %) and 17 (6 %) patients, respectively; Intuity 19, 21 23, 25, and 27 mm were implanted in 207 (12 %), 495 (29 %), 526 (31 %), 347 (21 %), and 113 (7 %) patients, respectively; Inspiris Resilia 19, 21, 23, 25, 27 and 29 mm were implanted in 19 (3 %), 90 (15 %), 189 (31 %), 167 (28 %), 109 (8 %) and 31 (5 %) patients, respectively. In both isolated SAVR and combined procedures, ACC time was shorter for Intuity if compared to Magna Ease and Inspiris Resilia (median ACC isolated/combined, Magna Ease: 87/131, Intuity: 55/83, Inspiris Resilia: 70/101.5 min; Magna Ease vs. Intuity: p < 0.001; Inspiris Resilia vs. Intuity: p < 0.001). Intuity shows shorter CPB time if compared to Magna Ease both in isolated SAVR and combined procedure (median CPB isolated/combined, Magna Ease: 110/168.5, Intuity: 82.5/120, Inspiris Resilia: 90/130; Magna Ease vs. Intuity: p < 0.001; Inspiris Resilia vs. Intuity: p < 0.001).
Table 2.
Procedural characteristics. Data are absolute numbers (percentages).
| Variables | Intuity (N=1688) | Magna (N=296) | Inspiris (N=605) | Combined (N=2589) |
|---|---|---|---|---|
| Surgical approach | ||||
| Full Sternotomy | 965 (57.2 %) | 289 (97.6 %) | 431 (71.2 %) | 1685 (65.1 %) |
| Mini Sternotomy | 638 (37.8 %) | 7 (2.4 %) | 145 (24.0 %) | 790 (30.5 %) |
| Mini Thoracotomy | 64 (3.8 %) | 0 (0.0 %) | 25 (4.1 %) | 89 (3.4 %) |
| Surgical Procedure | ||||
| Isolated SAVR | 1136 (67.3 %) | 139 (47.0 %) | 306 (50.6 %) | 1581 (61.1 %) |
| Combined procedure | 552 (32.7 %) | 157 (53.0 %) | 299 (49.4 %) | 1008 (38.9 %) |
| Prosthesis size | ||||
| 19 | 206 (12.2 %) | 26 (8.8 %) | 19 (3.1 %) | 251 (9.7 %) |
| 21 | 495 (29.3 %) | 80 (27.0 %) | 90 (14.9 %) | 665 (25.7 %) |
| 23 | 526 (31.2 %) | 116 (39.2 %) | 189 (31.2 %) | 831 (32.1 %) |
| 25 | 347 (20.6 %) | 57 (19.3 %) | 167 (27.6 %) | 571 (22.1 %) |
| 27 | 113 (6.7 %) | 17 (5.7 %) | 109 (18.0 %) | 239 (9.2 %) |
| 29 | 1 (0.1 %) | 0 (0.0 %) | 31 (5.1 %) | 32 (1.2 %) |
Table legend: SAVR: Surgical aortic valve replacement.
Table 3.
A: Distribution of aortic cross-clamp time and cardiopulmonary bypass time according to valve type in the study population overall, and in isolated and combined procedures; data are median (I quartile-III quartile). B: Results of the weighted Gamma models evaluating the association with valve type. Gamma models’ results are reported as Average Marginal Effect (AME) for Magna Ease vs. Intuity and Intuity vs. Inspiris, lower and upper bound of the 95 % Confidence Interval, and p-value.
|
A | ||||
|---|---|---|---|---|
| Variables | Intuity (N=1688) | Magna (N=296) | Inspiris (N=605) | Combined (N=2589) |
| Cardiopulmonary bypass time (CPB) (min) | ||||
| Isolated SAVR | 82.5 [65–101] | 110 [95–123] | 90 [77.5–111] | 87 [68.25–107] |
| Combined procedure | 120 [95–147.5] | 168.5 [145–194.25] | 130 [104–150] | 130 [102–162] |
| Overall | 91 [71–118] | 135 [109–175] | 107 [86–134] | 99.5 [77–130] |
| Aortic Cross Clamp time (ACC) (min) | ||||
| Isolated SAVR | 55 [45–70] | 87 [75–98] | 70 [60–85] | 60.5 [48–77] |
| Combined procedure | 83 [67–106] | 131 [110–149] | 101.5 [80–118] | 96 [73–122] |
| Overall | 62 [48–81] | 105 [85–134] | 82 [66–107] | 71 [53–95] |
|
B | ||||
|---|---|---|---|---|
| Device comparison | AME | Lower | Upper | P-value |
| ME vs Intuity | ||||
| CPB Isolated | 23.37 | 19.13 | 27.61 | < 0.001 |
| CPB Combined | 46.38 | 38.56 | 54.19 | < 0.001 |
| ACC Isolated | 27.44 | 24.3 | 30.58 | < 0.001 |
| ACC combined | 42.85 | 37.38 | 48.31 | < 0.001 |
| Inspiris vs Intuity | ||||
| CPB Isolated | 3.33 | −1.003 | 7.67 | 0.132 |
| CPB Combined | 7.68 | −0.55 | 15.91 | 0.067 |
| ACC Isolated | 10.56 | 7.34 | 13.78 | < 0.001 |
| ACC combined | 11.18 | 5.51 | 16.84 | < 0.001 |
Table legend: CPB: Cardiopulmonary bypass; SAVR: Surgical aortic valve replacement; ACC: Aortic cross-clamp; ME: Magna Ease.
3.3. Postoperative results – Clinical
Postoperative clinical outcomes are shown in Table 4. Device success (defined according to Valve Academic Research Consortium – VARC) at 30-day was similar for the three valves (96 % overall, Magna Ease vs. Intuity: p = 0.885; Inspiris Resilia vs. Intuity: p = 0.663); 30-day overall mortality was 2 %, 1.7 % and 0.5 % in Magna Ease, Intuity and Inspiris Resilia groups, respectively (Magna Ease vs. Intuity: p = 0.476; Inspiris Resilia vs. Intuity: p = 0.395). In patients undergoing isolated SAVR, 30-day mortality was absent in both Magna Ease and Inspiris groups and 1 % (11 patients) in the Intuity group (Magna vs Intuity: p = 0.062; Inspiris Resilia vs Intuity: p = 0.719).
Table 4.
A: Distribution of postoperative variables according to valve type in the study population overall, and in the three groups; continuous data are median (I quartile-III quartile), categorical data are absolute numbers (percentages). B: Results of the univariable weighted logistic regression models evaluating the association with valve type. Models’ results are reported as Odds Ratio (OR) for ME vs. Intuity and Inspiris vs. Intuity with lower and upper bound of the 95% Confidence Interval.
| A | ||||
|---|---|---|---|---|
| Variables | Intuity (N=1688) | Magna (N=296) | Inspiris (N=605) | Combined (N=2589) |
| VARC device success | 1610 (95.4 %) | 283 (95.6 %) | 581 (96.0 %) | 2475 (95.6 %) |
| New permanent PM implantation | 104 (6.2 %) | 17 (5.7 %) | 9 (1.5 %) | 130 (5.0 %) |
| VARC all-cause mortality | 30 (1.8 %) | 7 (2.4 %) | 3 (0.5 %) | 40 (1.5 %) |
| − Isolated SAVR | 11 (0.7 %) | 0 (0.0 %) | 0 (0.0 %) | 11 (0.4 %) |
| VARC CV mortality | 23 (1.4 %) | 5 (1.7 %) | 3 (0.5 %) | 31 (1.2 %) |
| VARC bleeding | 95 (5.6 %) | 14 (4.7 %) | 9 (1.5 %) | 118 (4.6 %) |
| VARC AMI | 11 (0.7 %) | 3 (1.0 %) | 2 (0.3 %) | 16 (0.6 %) |
| VARC stroke | 40 (2.4 %) | 9 (3.0 %) | 7 (1.2 %) | 56 (2.2 %) |
|
B | ||||
|---|---|---|---|---|
| Device comparison | Odds Ratio | Lower | Upper | P-value |
| ME vs Intuity | ||||
| VARC device success | 0.98 | 0.69 | 1.36 | 0.89 |
| New permanent PM implantation | 1.06 | 0.81 | 1.38 | 0.68 |
| VARC all-cause mortality | 2.61 | 1.7 | 3.99 | 0.48 |
| VARC CV mortality | 2.78 | 1.71 | 4.49 | 0.48 |
| VARC bleeding | 0.8 | 0.61 | 1.06 | 0.12 |
| VARC AMI | 1.15 | 0.57 | 2.32 | 0.69 |
| VARC stroke | 1.31 | 0.91 | 1.89 | 0.15 |
| Inspiris vs Intuity | ||||
| VARC device success | 0.92 | 0.64 | 1.33 | 0.66 |
| New permanent PM implantation | 0.41 | 0.28 | 0.61 | < 0.001 |
| VARC all-cause mortality | 1.32 | 0.69 | 2.51 | 0.39 |
| VARC CV mortality | 1.93 | 0.98 | 3.79 | 0.06 |
| VARC bleeding | 0.37 | 0.25 | 0.54 | < 0.001 |
| VARC AMI | 0.19 | 0.05 | 0.69 | 0.01 |
| VARC stroke | 0.54 | 0.33 | 0.91 | 0.02 |
Table legend: VARC: Valve academic research consortium, PM: pace-maker; SAVR: Surgical aortic valve replacement; CV: Cardiovascular; AMI: Acute myocardial infarction.
Permanent pace-maker implantation rate was lower for Inspiris Resilia (Magna Ease: 6 %, Intuity: 6 %, Inspiris Resilia: 2 %; Magna Ease vs. Intuity: p = 0.679; Inspiris Resilia vs. Intuity: p < 0.001). Intensive care unit (ICU) stay was similar between the three groups (median ICU stay: Magna Ease, 30.5, Intuity, 48, Inspiris Resilia, 38 h; Magna Ease vs. Intuity: p = 0.39; Inspiris Resilia vs. Intuity: p = 0.314).
3.4. Echocardiographic data
Hemodynamic performance of the study devices is shown in Fig. 1. Mean gradients were 13, 10 and 10 mmHg for Magna Ease, Intuity and Inspiris Resilia, respectively (Magna Ease vs. Intuity: p < 0.001; Inspiris vs. Intuity: p = 0.13) (Table 5). When analyzed by size, peak and mean gradients were lower in Intuity than Magna Ease in sizes 19, 21, 23 and 25 mm (p < 0.001) while they were similar between Intuity and Inspiris Resilia in all sizes (Table 6). For the 29 mm valves it was not possible to estimate any type of models due to the low amount of data.
Table 5.
A: Hemodynamics characteristics. Continuous data are median (I quartile-III quartile), categorical data are absolute numbers (percentages). AVAi: Aortic Valve Area indexed. B: The table also reports results of the weighted Gamma models evaluating the association with valve type. Gamma models’ results are reported as Average Marginal Effect (AME) for ME vs. Intuity and Intuity vs. Inspiris, lower and upper bound of the 95% Confidence Interval, and p-value.
| A | ||||
|---|---|---|---|---|
| Variables | Intuity (N=1688) | Magna (N=296) | Inspiris (N=605) | Combined (N=2589) |
| LVEF (%) | 58 [55–62] | 57 [53–62] | 57.75 [52–60.4] | 58 [53.2–62] |
| AVAi (cm2/m2) | 1.09 [0.91–1.31] | 1.04 [0.86–1.27] | 1.01 [0.81–1.27] | 1.06 [0.89–1.29] |
| Mean Gradient (mmHg) | 10 [8], [9], [10], [11], [12], [13] | 13 [9.5–18] | 10 [8], [9], [10], [11], [12], [13] | 10 [8], [9], [10], [11], [12], [13], [14] |
| Peak Gradient (mmHg) | 18 [14–23] | 21 [16–29] | 18 [14–24] | 19 [14–24] |
| Aortic Regurgitation (>mild) | 9 (0.5 %) | 2 (0.7 %) | 1 (0.2 %) | 12 (0.5 %) |
|
B | ||||
|---|---|---|---|---|
| Device comparison | AME | Lower | Upper | P-value |
| ME vs Intuity | ||||
| Mean Gradient (mmHg) | 3.49 | 2.96 | 4.03 | < 0.001 |
| Peak Gradient (mmHg) | 4.41 | 3.46 | 5.35 | < 0.001 |
| Inspiris vs Intuity | ||||
| Mean Gradient (mmHg) | 0.41 | −0.12 | 0.95 | 0.13 |
| Peak Gradient (mmHg) | 1.29 | 0.28 | 2.29 | 0.012 |
Table legend: LVEF: Left ventricular outflow tract; AVAi: Aortic valve area index; ME: Magna Ease.
Table 6.
The table reports results of the weighted Gamma models evaluating the association with valve type. Gamma models’ results are reported as Average Marginal Effect (AME) for ME vs. Intuity and Intuity vs. Inspiris, lower and upper bound of the 95 % Confidence Interval, and p-value.
| Prosthesis 19 | AME | Lower | Upper | P-value |
|---|---|---|---|---|
| ME vs Intuity | ||||
| Mean Gradient (mmHg) | 5.45 | 3.61 | 7.29 | < 0.001 |
| Peak Gradient (mmHg) | 7.05 | 3.68 | 10.41 | < 0.001 |
| Inspiris vs Intuity | ||||
| Mean Gradient (mmHg) | −1.003 | −2.65 | 0.65 | 0.234 |
| Peak Gradient (mmHg) | 1.33 | −2.01 | 4.67 | 0.44 |
| Prosthesis 21 | ||||
| ME vs Intuity | ||||
| Mean Gradient (mmHg) | 4.8 | 3.75 | 5.85 | < 0.001 |
| Peak Gradient (mmHg) | 6.08 | 4.17 | 7.99 | < 0.001 |
| Inspiris vs Intuity | ||||
| Mean Gradient (mmHg) | 0.5 | −0.54 | 1.55 | 0.341 |
| Peak Gradient (mmHg) | 1.19 | −0.93 | 3.31 | 0.271 |
| Prosthesis 23 | ||||
| ME vs Intuity | ||||
| Mean Gradient (mmHg) | 2.72 | 1.92 | 3.52 | < 0.001 |
| Peak Gradient (mmHg) | 3.67 | 2.19 | 5.15 | < 0.001 |
| Inspiris vs Intuity | ||||
| Mean Gradient (mmHg) | 0.84 | −0.06 | 1.73 | 0.066 |
| Peak Gradient (mmHg) | 2.24 | 0.48 | 4.01 | 0.013 |
| Prosthesis 25 | ||||
| ME vs Intuity | ||||
| Mean Gradient (mmHg) | 2.08 | 1.22 | 2.93 | < 0.001 |
| Peak Gradient (mmHg) | 2.11 | 0.68 | 3.54 | 0.004 |
| Inspiris vs Intuity | ||||
| Mean Gradient (mmHg) | −0.33 | −1.11 | 0.44 | 0.4 |
| Peak Gradient (mmHg) | −0.22 | −1.59 | 1.16 | 0.755 |
| Prosthesis 27 | ||||
| ME vs Intuity | ||||
| Mean Gradient (mmHg) | −0.7 | −2.35 | 0.94 | 0.403 |
| Peak Gradient (mmHg) | −1.42 | −4.14 | 1.29 | 0.304 |
Table legend. ME: Magna Ease.
4. Discussion
The main findings of our study are that Magna Ease, Intuity and Inspiris Resilia provide good early clinical and hemodynamic outcomes. In particular, Inspiris Resilia and Intuity valve have similar transaortic gradients while Intuity shows better hemodynamic performance if compared to Magna Ease. Inspiris Resilia is implanted in younger patients with lower risk scores, and it shows lower rates of permanent pace-maker implantation than Intuity. Furthermore, there are no significant differences between the three devices in terms of ICU stay. Patients undergoing both isolated and combined SAVR with the Intuity valve have shorter CPB and ACC times than those receiving Magna Ease and Inspiris Resilia. In particular Intuity valve have 25 and 10 min shorter ACC time in isolated SAVR than Magna Ease and Inspiris Resilia, respectively. In patients undergoing cardiac surgery surgical times, both CPB and ACC times, have already been demonstrated to have an impact on postoperative outcomes. Al-Sarraf and colleagues showed that prolonged ACC time significantly correlated with major morbidity (low cardiac output, prolonged ventilation, renal complication, blood transfusion, prolonged hospital stay) and mortality in both high-risk and low-risk patients [14]. Furthermore, Iino and colleagues showed that operative mortality rates and major morbidity (reoperation for bleeding, stroke, sternal infection, prolonged ventilation > 24 h and new required dialysis) are increased when ACC is 90 min or more [15]. Finally, Swinkels and colleagues showed that prolonged ACC time was independently associated with decreased late survival in a cohort of 456 consecutive patients with severe aortic stenosis during a mean follow-up of 25.3 ± 2.7 years [16]. In our study, we found that, in the overall population, Intuity has significantly better hemodynamic properties than Magna Ease in terms of transaortic gradients. No differences were found when compared to Inspiris Resilia. This aspect could be justified by the new design of the stent and the new Resilia tissue used in the Inspiris valve. Although we haven’t directly compared Inspiris and Magna Ease, the former seems to have better hemodynamic performance than Magna Ease and this confirms what found by Shala and colleague [17].
Valve size distribution analysis showed that the hemodynamic advantages of Intuity compared to Magna Ease are more evident in small sizes. This can be explained by the absence of pledgets and by the subannular skirt that opens the left ventricular outflow tract and optimizes blood flow through the valve. However, the balloon-expandable subanular skirt may damage the conduction system. In fact, one of the major concerns related to Intuity implantation valve is the occurrence of postoperative conduction disorders and permanent pacemaker implantation rate. Postoperative conduction disorders occur in approximately one third of treated patients [18] and the incidence of permanent pacemaker after Intuity implantation ranges between 5 % and 11 % [19]. Since in our preliminary experience [20], like in other similar studies, it has been highlighted that conduction disorders and pacemaker implantation are strongly influenced by the presence of baseline conduction anomalies, in particular right bundle branch block [21], the Intuity valve should be implanted with caution is such patients.
The choice among the three study devices must be done according to specific clinical and anatomical patient characteristics. In case of aortic regurgitation, the Intuity valve is not indicated due to the lack of annular calcifications that serve as anchoring tissue. Consequently, a conventional stented valve should be selected: Magna Ease can be used in elderly patients while Inspiris can be chosen in younger patients due to its long expected durability as shown by in vitro testing [22] although long-term follow up in the clinical scenario is still not available and no definitive recommendations can be provided. In patients whose life expectancy exceeds valve durability (at least with conventional valves) the use of Inspiris may have two advantages: long expected durability and the possibility of stent expansion in case of ViV. However, it’s important to highlight that these advantages are purely theoretical since, as previously reported, there is no clinical evidence available yet. Furhtermore, ViV is also feasible with Magna Ease and with Intuity. Intuity may be selected in case of minimally invasive surgery, in case of combined procedure (in order to reduce surgical times) and in patients without preoperative conduction disorders in order to reduce the risk of pacemaker implantation and to reduce surgical times. Also, Intuity may be beneficial in patients with small aortic annulus due to its good hemodynamic performance.
Another important aspect to consider is the cost differences among these valves. In Italy the average price of the Intuity valve is 6000 euros, the Inspiris valve is 3650 euros, the Magna Ease valve is 2800 euros. Due to these significant cost differences, the choice of valve should also take into consideration the financial impact. The higher costs associated with certain valves, such as Intuity, should be justified by advantages in terms of hemodynamics, postoperative complications and rehospitalizations for heart failure. By carefully weighing these factors, clinicians can optimize both clinical outcomes and cost-effectiveness, ensuring the best possible care for patients based on their individual anatomies and characteristics.
4.1. Limitations
This study has several limitations that should be acknowledged. Firstly, data for the Magna Ease valve were collected from only one center, while data for the Resilia and Intuity valves were sourced from two multicenter registries. This introduces a potential bias for the Magna Ease group. However, the center using Magna Ease was also included in the Resilia and Intuity groups, which helps to mitigate this bias.
Additionally, while our study focuses on early outcomes with a particular emphasis on hemodynamics, it does not include long-term follow-up data. This is due to the heterogeneous nature of follow-up periods across the three valve types, which would render long-term comparisons less reliable. Furthermore, long-term outcomes for these valves are already well documented in the literature.
The rationale for selecting only these three valves from Edwards was to reduce confounding variables associated with different manufacturers, such as varying sizing/labeling criteria and tissue types. The three valves included in this study represent different concepts: traditional stented, rapid deployment with a balloon-expandable skirt on the outflow tract, and a new generation with the same stent but new tissue treatment. This choice enhances the reliability and accuracy of our comparisons but limits the generalizability of our findings to other valves on the market. Another potential bias could be represented by the choice of the bioprostheses that is left to surgeon’s preference. However, the optimal balancing of covariates with propensity weighting, allows overcoming this bias. Echocardiographic exams were performed by different echocardiographic labs using different machines and this could lead to inter-operator differences. Another limitation is represented by the lack of direct comparison between Inspiris and Magna Ease. This is due to the three-treatment level of the analysis that requires to choose a level of reference, Intuity in our case.
5. Conclusions
Our analysis shows that conventional stented (Magna Ease), rapid-deployment (Intuity) and new-generation (Inspiris Resilia) aortic valve substitutes provide good early clinical and hemodynamic outcomes. Each device has its own peculiar characteristics as well as advantages and disadvantages when compared to the others. An optimal knowledge of these characteristics is necessary to choose the most appropriate device for every single patient in order to optimize clinical and hemodynamic outcomes and to plan a lifetime strategy for patients requiring surgery for aortic valve stenosis.
CRediT authorship contribution statement
Augusto D’Onofrio: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Giorgia Cibin: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Chiara Tessari: Writing – review & editing, Methodology, Investigation, Data curation, Conceptualization. Giulia Lorenzoni: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Giampaolo Luzi: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Data curation, Conceptualization. Erica Manzan: Writing – review & editing, Methodology, Investigation, Data curation. Dario Gregori: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Formal analysis, Data curation. Gino Gerosa: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Augusto D’Onofrio reports a relationship with Edwards that includes: speaking and lecture fees and travel reimbursement. Giorgia Cibin reports a relationship with Edwards Lifesciences Corporation that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Dr Cibin’s PhD doctoral fellowship is supported by a grant by Edwards Lifesciences.
Prof. D’Onofrio received travel grants and speaking fees from Edwards Lifesciences.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101487.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Figure 1.
References
- 1.Isaacs A.J., Shuhaiber J., Salemi A., Isom O.W., Sedrakyan A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J. Thorac. Cardiovasc. Surg. 2015;149:1262–1269. doi: 10.1016/j.jtcvs.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 2.Fujita B., Ensminger S., Bauer T., Möllmann H., Beckmann A., Bekeredjian R., et al. Trends in practice and outcomes from 2011 to 2015 for surgical aortic valve replacement: an update from the German Aortic Valve Registry on 42 776 patients. Eur. J. Cardiothorac. Surg. 2018;53:552–559. doi: 10.1093/ejcts/ezx408. [DOI] [PubMed] [Google Scholar]
- 3.Siregar S., de Heer F., Groenwold R.H., Versteegh M.I., Bekkers J.A., Brinkman E.S., et al. Trends and outcomes of valve surgery: 16-year results of Netherlands Cardiac Surgery National Database. Eur. J. Cardiothorac. Surg. 2014;46:386–397. doi: 10.1093/ejcts/ezu017. [DOI] [PubMed] [Google Scholar]
- 4.Dunning J., Gao H., Chambers J., Moat N., Murphy G., Pagano D., et al. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use–an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database. J. Thorac. Cardiovasc. Surg. 2011;142:776–782. doi: 10.1016/j.jtcvs.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 5.Schnittman S.R., Adams D.H., Itagaki S., Toyoda N., Egorova N.N., Chikwe J. Bioprosthetic aortic valve replacement: Revisiting prosthesis choice in patients younger than 50 years old. J. Thorac. Cardiovasc. Surg. 2018;155:539–547. doi: 10.1016/j.jtcvs.2017.08.121. [DOI] [PubMed] [Google Scholar]
- 6.Korteland NM, Bras FJ, van Hout FM, Kluin J, Klautz RJ, Bogers AJ, et al. Prosthetic aortic valve selection: current patient experience, preferences and knowledge. Open Heart. doi: 10.1136/openhrt-2015-000237. [DOI] [PMC free article] [PubMed]
- 7.D'Onofrio A., Tessari C., Cibin G., Lorenzoni G., Martinelli G.L., Solinas M., et al. Clinical and hemodynamic outcomes of rapid-deployment aortic bioprostheses. Semin. Thorac. Cardiovasc. Surg. 2022;34:453–461. doi: 10.1053/j.semtcvs.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Manzan E, Prestipino F, D’Ascoli R, Matera A, Musumeci F, Di credico G, et al. RESILIA aortic tissue valve Italian registry (RES-ITA): Early and Mid-term results. Structural heart. 2020;51:142-43.
- 9.Nashef S.A., Roques F., Sharples L.D., Nilsson J., Smith C., Goldstone A.R., et al. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012;41:734–744. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 10.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H., et al. Valve Academic Research Consortium (VARC)-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2) Eur. J. Cardiothorac. Surg. 2012;42:S45–S60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 11.Borger MA, Dohmen P, Misfeld M, Mohr FW. Minimal invasive implantation of an EDWARDS INTUITY rapid deployment aortic valve. Multimed Man Cardiothorac Surg. doi: 10.1093/mmcts/mmt011. [DOI] [PubMed]
- 12.Glauber M., Miceli A., Di Bacco L. Sutureless and rapid deployment valves: implantation technique from A to Z-the INTUITY Elite valve. Ann. Cardiothorac. Surg. 2020;9:417–423. doi: 10.21037/acs-2020-surd-23-intuity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flameng W., Hermans H., Verbeken E., Meuris B. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J. Thorac. Cardiovasc. Surg. 2015;149:340–345. doi: 10.1016/j.jtcvs.2014.09.062. [DOI] [PubMed] [Google Scholar]
- 14.Al-Sarraf N., Thalib L., Hughes A., Houlihan M., Tolan M., Young V., et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int. J. Surg. 2011;9:104–109. doi: 10.1016/j.ijsu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Iino K., Miyata H., Motomura N., Watanabe G., Tomita S., Takemura H., et al. Prolonged cross-clamping during aortic valve replacement is an independent predictor of postoperative morbidity and mortality: Analysis of the Japan Cardiovascular Surgery Database. Ann. Thorac. Surg. 2017;103:602–609. doi: 10.1016/j.athoracsur.2016.06.060. [DOI] [PubMed] [Google Scholar]
- 16.Swinkels B.M., Ten Berg J.M., Kelder J.C., Vermeulen F.E., Van Boven W.J., de Mol B.A. Effect of aortic cross-clamp time on late survival after isolated aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2021;32:222–228. doi: 10.1093/icvts/ivaa244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shala M., Niclauss L. Early results of the Resilia Inspiris aortic valve in the old age patients - A retrospective comparison with the Carpentier Edwards Magna Ease. J. Cardiovasc. Thorac. Res. 2020;12:222–226. doi: 10.34172/jcvtr.2020.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ensminger S., Fujita B., Bauer T., Mollmann H., Beckmann A., Bekeredjian R., et al. Rapid deployment versus conventional bioprosthetic valve replacement for aortic stenosis. J. Am. Coll. Cardiol. 2018;71:1417–1428. doi: 10.1016/j.jacc.2018.01.065. [DOI] [PubMed] [Google Scholar]
- 19.D'Onofrio A., Tessari C., Bagozzi L., Migliore F., Filippini C., Cibin G., et al. Conduction disorders after aortic valve replacement with rapid-deployment bioprostheses: Early occurrence and one-year evolution. Ann. Cardiothorac. Surg. 2020 doi: 10.21037/acs-2020-surd-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Onofrio A., Bagozzi L., Tessari C., Francescato A., Cibin G., Besola L., et al. Evaluation of conduction disorders after aortic valve replacement with rapid deployment bioprostheses. Innovations (Phila) 2017;13:356–360. doi: 10.1097/IMI.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 21.Coti I., Schukro C., Drevinja F., Haberl T., Kaider A., Kocher A., et al. Conduction disturbances following surgical aortic valve replacement with a rapid-deployment bioprosthesis. J. Thorac. Cardiovasc. Surg. 2021;162:803–811. doi: 10.1016/j.jtcvs.2020.01.083. [DOI] [PubMed] [Google Scholar]
- 22.Sadri V., Trusty P.M., Madukauwa-David I.D., Yoganathan A.P. Long-term durability of a new surgical aortic valve: A 1 billion cycle in vitro study. JTCVS Open. 2021;9:59–69. doi: 10.1016/j.xjon.2021.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]