Abstract
The SARS-CoV-2 virus responsible for the COVID-19 pandemic has profoundly impacted global health, economics, and society. This review seeks to encompass an overview of current knowledge on COVID-19, including its transmission, pathogenesis, and clinical presentation related to various systems within the human body. COVID-19 is a highly contagious illness that has rapidly spread worldwide. As of August 4, 2023, the WHO reported over 570 million confirmed cases of COVID-19 and over 6.3 million deaths. Although the virus is most common in adults, children can also be infected. Respiratory droplets that are produced when an infected person coughs or sneezes are the primary transmission mode for COVID-19. Additionally, the virus can be disseminated via contact with contaminated surfaces or objects, as it can remain viable for several hours or days. SARS-CoV-2 is a respiratory virus that enters cells by bonding with the angiotensin-converting enzyme 2 (ACE2) receptor. Once inside the cell, the virus replicates and produces new particles that can infect other cells. Interestingly, the effects of post-acute sequelae of SARS-CoV-2 infection (PASC) encompass more than just respiratory system. The findings presented in the data suggest that PASC significantly impacts multiple organs and their respective physiological processes. In light of these observations, we aim to provide a detailed discussion of the relevant findings in this paper. Through our review, we hope to provide healthcare professionals with a deeper understanding of the effects of PASC on the human body, which could ultimately lead to improved patient outcomes and treatment strategies.
Keywords: Long COVID-19, Systemic effects, Inflammation, Thrombosis
Introduction
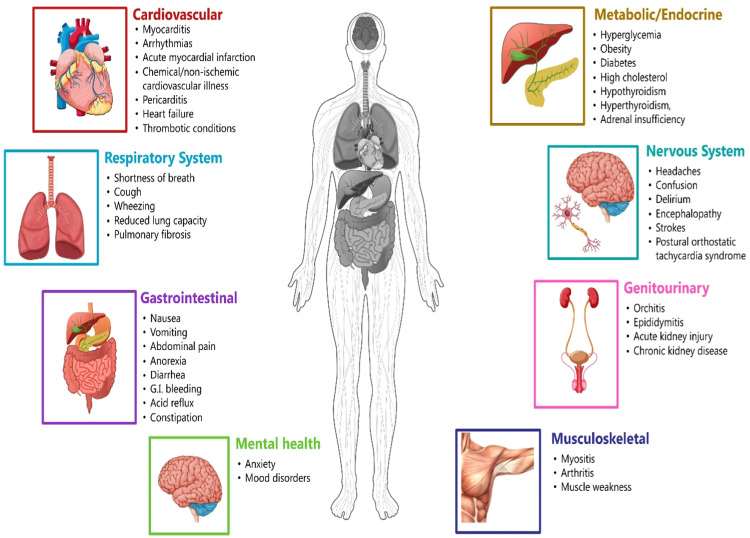
Post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID-19, is a complex and poorly understood condition that can affect people that can last for weeks, months, or even years after their initial COVID-19 infection. PASC can manifest with a wide range of symptoms, including fatigue, dyspnea, cognitive dysfunction, chest pain, headache, muscle aches, joint pain, insomnia, depression and anxiety, anosmia, ageusia, dizziness, palpitations, and dermatological problems [Fig. 1]. The exact prevalence of PASC is unknown, but estimates show that 10-30% of people have had COVID-19. PASC is more common in people who have a severe case of COVID-19, but it can also occur in people who have mild or asymptomatic infections. Patients with various comorbidities, such as chronic lung disease, heart disease, diabetes mellitus, and cancer, are at increased risk of developing PASC.
Fig. 1.
Effects of Long COVID-19 on major systems.
The pathophysiology of PASC is not fully understood, but it is thought to be due to a combination of factors, including viral persistence, organ damage, inflammation, and immune dysregulation. Viral persistence has been demonstrated in various tissues, including the lungs, heart, and brain, and may contribute to ongoing symptoms. Organ damage may occur due to the direct cytopathic effects of the virus, as well as from inflammatory and immune-mediated mechanisms. Inflammation is a hallmark of PASC and has been implicated in the development of various symptoms, including fatigue, cognitive dysfunction, and pain. Immune dysregulation may also play a role in PASC, as evidenced by autoantibodies and elevated levels of inflammatory cytokines in some patients. There is no cure for PASC, and treatment is primarily focused on symptom management. Patients with PASC may benefit from a multidisciplinary approach involving pulmonology, cardiology, neurology, psychiatry, and rehabilitation specialists. Treatment may include medications, physiotherapy, occupational therapy, cognitive behavioral therapy, and other interventions. PASC is a significant public health challenge, and further research is needed to understand its pathophysiology better and develop more effective treatment protocols.1
PASC AND ITS EFFECTS ON THE MAJOR SYSTEMS
Cardiovascular System
The pathophysiology of COVID-19 and the cardiovascular system needs to be simplified and better understood. However, several mechanisms have been proposed to explain the cardiovascular complications of COVID-19. The SARS-CoV-2 virus can directly infect the heart muscle, leading to myocarditis. Viral particles have been detected in the heart muscle of patients who have died from COVID-19. Additionally, studies have shown that the SARS-CoV-2 virus can replicate in cardiomyocytes. COVID-19 infection often triggers a systemic inflammatory response, which can damage the cardiovascular system. Inflammatory cytokines can cause damage to the myocytes, blood vessels, and endothelium. Additionally, COVID-19 infection can lead to immune dysregulation, contributing to cardiovascular injury.
Endothelial dysfunction is a hallmark of COVID-19 and is thought to play a central role in the development of cardiovascular complications. Endothelium regulates blood flow, blood pressure, and inflammation. Endothelial dysfunction can lead to vasoconstriction, increased blood pressure, and inflammation. COVID-19 infection can also lead to microvascular thrombosis. This can occur due to inflammation, endothelial dysfunction, and platelet activation. Microvascular thrombosis can damage the myocytes and other organs. COVID-19 Infection can also lead to coagulation abnormalities. This can increase the risk of thrombosis forming in the arteries and veins. Thrombus/Emboli can block blood flow to the heart and other organs, leading to myocardial ischemia, stroke, and other serious systemic complications. The exact mechanisms underlying the long-term cardiovascular complications of COVID-19 are not fully understood. However, they may be related to persistent inflammation, immune dysregulation, and microvascular damage.
Additionally, COVID-19 infection may accelerate the progression of underlying cardiovascular disease. Direct viral infection, systemic inflammation and immune dysregulation, endothelial dysfunction, microvascular thrombosis, and coagulation abnormalities are documented in the pathogenesis of cardiovascular complications.1, 2 In COVID-19 patients, arrhythmias can be caused by a variety of factors, such as metabolic imbalances, hypoxia, acidosis, imbalances in intravascular volume, and neurohormonal and catecholaminergic stress. Sepsis, on the other hand, is characterized by a systemic environment that involves inflammatory cytokines and autonomic dysfunction.3, 4 Atrial fibrillation (AF) can develop when these maladaptive pathophysiologies are present. It is worth noting that AF is a known occurrence in critically ill patients, with almost 10% of ICU patients experiencing it. Additionally, studies show that new-onset AF can lead to worse outcomes than non-AF patients. Therefore, restoring sinus rhythm is crucial to improving the patient's hemodynamics. AF can impede cardiac output by hindering left ventricular filling, mainly when there is a rapid ventricular response.5
Numerous retrospective studies have aimed to establish the absolute and relative risks associated with symptomatic or asymptomatic SARS-CoV-2 infections for late cardiovascular events and all-cause mortality. Several of these studies have discovered a heightened risk for late cardiovascular outcomes and all-cause mortality in individuals with both asymptomatic and symptomatic SARS infections during the late post-COVID-19 periods.6 Myocardial damage has been documented during the acute phases of COVID-19 illness in various studies, with most patients having elevated cardiac inflammatory biomarkers and LV dysfunction.7 SARS-CoV-2 infection can lead to subacute or chronic myocarditis, as evidenced in studies evaluating myocarditis in patients more than a month after acute COVID-19 illness.8 The cardiovascular symptom most frequently observed following acute Covid 19 illness is tachycardia, which has been identified as a potential sub-syndrome of long-term COVID-19, including Inappropriate Sinus Tachycardia (IST) and Postural Orthostatic Tachycardia Syndrome (POTS) after SARS. Mechanisms of injury leading to re-entrant arrhythmias have been suggested to involve myocardial fibrosis and cardiomyopathy. Additionally, injuries and microvascular disease have been observed in patients during both the acute and sequel phases of COVID-19 illness.9
It has been concluded that patients suffering from COVID-19 have a significantly higher risk of experiencing myocardial infarction. The underlying cause is believed to be a hypercoagulable state triggered by SARS-CoV-2, which can lead to endothelial damage and plaque rupture. These factors combine to increase the likelihood of myocardial infarction and coronary thrombus, making it imperative to closely monitor and manage any potential cardiovascular complications in COVID-19 patients.10,11
Suggested pathogenesis involves increased inflammatory molecules, reduced contractility due to myocardial damage, and increased right ventricular afterload.12,13 Another poorly understood sequela of COVID-19 is hypertension. New onset hypertension has been seen worryingly among young and previously healthy patients post-acute COVID-19 illness.14 It has been noted that several young patients who had no hypertension risk factors have experienced this phenomenon. Furthermore, an observational study is being conducted to examine blood pressure readings for more than a year following SARS-COV2 infection. Most of these cardiovascular effects are chronic and worsen the individual quality of life. As such, it is crucial that clinicians and patients who have previously suffered from COVID-19, particularly those with severe illness, remain vigilant and proactive in terms of their cardiovascular health. This will enable them to detect and address any potential complications early on.
Respiratory System
The study of COVID-19 and its impact on the respiratory system is a complex field that continues to evolve. While much research remains elusive, scientists have proposed several mechanisms to explain how the SARS-CoV-2 virus infects and complicates the lung functions. The virus enters alveoli by binding to the Angiotensin-converting enzyme -2 (ACE2) receptor, a transmembrane protein expressed on the surface of various cells, including lung epithelial cells, alveolar macrophages, and type II pneumocytes. Once the virus has bound to ACE2, it undergoes endocytosis and begins to replicate. The newly replicated virus particles then exit the cell and can infect other cells. This viral replication can cause cellular damage and lysis, releasing inflammatory mediators and cellular debris that can further harm the lung parenchyma. Additionally, the virus can directly damage the alveolar epithelium and microvascular endothelium, leading to pulmonary edema and alveolar-capillary leak.12
The immune system's response plays a crucial role in eliminating SARS-CoV-2 and promoting recovery, but it can also cause damage to the lungs. Early in the infection, cells like alveolar macrophages and neutrophils are summoned to the lungs and release cytokines like IL-6, IL-1β, and TNF-α to combat the virus. Unfortunately, these same cytokines can also harm the lungs. In certain pathological conditions, the immune system can be triggered to mount an excessive and uncontrolled immune response, leading to an overproduction of pro-inflammatory cytokines, a phenomenon commonly known as the cytokine storm. This aberrant immune response can lead to widespread inflammation, tissue damage, and organ failure and is associated with a range of acute and chronic inflammatory disorders, including viral infections, autoimmune diseases, and cancer. The cytokine storm can have severe consequences in terms of morbidity and mortality, highlighting the importance of understanding the underlying mechanisms and developing effective therapeutic strategies to modulate the immune response and to prevent or treat cytokine storm-related complications. Cytokine storm can lead to severe lung injury, multiorgan failure, and death. Acute Respiratory Distress Syndrome (ARDS) is a serious respiratory condition characterized by diffuse alveolar damage, pulmonary edema, and respiratory collapse. ARDS is a common complication of COVID-19 and is associated with high mortality. The pathophysiology of ARDS in COVID-19 is complex and likely involves a combination of direct viral injury, the host immune response, and cytokine storm. In addition to ARDS, COVID-19 can also cause other respiratory complications, such as pneumonia, bronchitis, acute lung injury, pulmonary embolism, and multisystem inflammatory syndrome in children (MIS-C).
Some patients who recover from COVID-19 may experience long-term respiratory problems, such as dyspnea, cough, wheezing, reduced lung capacity, and pulmonary fibrosis. The mechanisms underlying these long-term complications are not fully understood, but they may be related to persistent inflammation, microvascular damage, and parenchymal injury.
Direct viral infection, viral replication, lysis, the immune response, cytokine storms, and ARDS all play a role in developing respiratory complications from COVID-19. Further research is needed to understand the pathophysiology of COVID-19 lung injury better and develop effective therapies to prevent and treat these complications. When harmful microorganisms like viruses invade our bodies, our immune system must identify and respond to them. This recognition happens by detecting antigenic structures in virus-infected cells, facilitated by pattern recognition receptors (PRRs). There are different types of PRRs, including toll-like receptors (TLR), RIG-I-like receptors (RLR), NOD-like receptors (NLR), C-type lectin-like receptors (CLmin), and cytoplasmic DNA receptors like cGAS, IFI16, STING, and DAI. Once these receptors identify the virus, they trigger antiviral responses, including the production of cytokines and adaptive and inflammatory immunity responses. Interferons are a key cytokine produced after virus infection, initiating the immune response and subsequent adaptation.14
After macrophages bind and digest the antigenic parts of the coronavirus, they present the parts of the COVID antigen to T lymphocyte cells. This step activates and detects T cells, producing cytokines associated with various subsets of T cells (eg. Th17), followed by the release of cytokines to enhance the immune response. However, in patients with weaker immune systems, the number of T lymphocytes may decrease due to inaccurate virus identification, causing an increase in cytokine production and inflammatory response. The ongoing release of inflammatory mediators from the virus can hinder the activation of NK and CD8 T cells, which play a crucial role in clearing COVID-19. Fortunately, CD8 T cells can produce highly effective mediators to combat the virus. Recent COVID-19 research has revealed that the production of interferons is vital in increasing the release of antiviral proteins. However, COVID-19′s non-structural proteins can interfere with TLR-3 signaling during pneumonia, which can prevent TLR-3 activation and hinder the immune response. TLR-4, on the other hand, is likely to detect the S protein, leading to the activation of proinflammatory cytokines via the MyD88-dependent signaling pathway. The virus and cell interaction can trigger excessive production of immune mediators, including chemokines and cytokines like IL-1, IL-6, IL-8, IL-21, TNF-β, and MCP-1, released in response to COVID-19 infection in affected cells. Specifically, individuals with more robust immune systems tend to have faster and more effective T lymphocyte responses, which helps to eliminate the pathogen. Conversely, patients with SARS, a virus genetically similar to the current novel coronavirus, have experienced a notable decrease in their T cell count. This suggests a critical role for lymphocytes in providing specific immunity and underscores the close connection between pneumonia and immune cells.
Gastrointestinal System
The development of Gastrointestinal (GI) symptoms in COVID-19 patients is a complex process that involves various factors. The virus can directly infect cells in the GI tract, causing damage to the GI barrier and leading to malabsorption and other complications. Additionally, inflammation in the body can damage the GI tract and contribute to developing symptoms. An imbalance in the gut microbiome has also been observed in COVID-19 patients and may play a role in developing GI symptoms. In some cases, patients may require more intensive treatment for issues like acute abdominal pain or GI bleeding. Despite these challenges, supportive care remains the primary focus of therapy for COVID-19 patients experiencing GI symptoms.15 The enduring complications of COVID-19, once termed ``long COVID,'' now recognized as ``post-COVID-19 condition,'' impact individuals for extended durations after initial infection.16
The SARS-COV-2 virus, beyond its well-known respiratory effects, also impacts multiple organs, including the gastrointestinal GI tract, resulting in sequelae such as nausea, vomiting, abdominal pain, anorexia, diarrhea, GI bleeding, acid reflux, and constipation.17,18 This connection was first noted in Wuhan, where patients with GI issues faced more extended hospital stays.19 Studies in the U.S.A and the U.K. reported G.I. symptoms in approximately 60% of patients and atypical appendicitis in children, respectively.20 Moreover, SARS-CoV-2 RNA has been identified in over 50% of patient stool samples, with concentrations peaking 2-3 weeks post-symptom onset, explaining the involvement of the GI tract. Recent research indicates that many patients still report persistent G.I. problems post-recovery.21,22 However, the specifics surrounding gastrointestinal complications in the aftermath of COVID-19, their occurrence rate, and underlying causes are still unclear.
Several mechanisms are speculated behind the emergence of post-COVID-19- condition-related GI symptoms. The pulmonary and gastrointestinal systems stem from a shared embryological precursor, the primordial foregut. Postnatally, they function as critical mucosal barriers, with the lungs mediating alveolar gas exchange and the gut overseeing enteric nutrient absorption. Neonatally, both systems undergo analogous microbial colonization, but with progression, there is a diversification in their microbiota composition and density.23 Recent research highlights the gut-lung axis, emphasizing mutual communication between the microbiota of both systems. Studies have shown that shifts in gut microbiota can influence pulmonary conditions and vice versa, emphasizing their interconnectedness in health and disease.24,25 Such microbial interrelationships severely impact systemic immune responses.26 The intricate feedback between these systems might heighten the propensity for gastrointestinal complications, underscoring the importance of their interactive communication. Their combined role in health and disease pathways further emphasizes the importance of understanding this connection.
The gut barrier serves the essential function of allowing beneficial nutrients to be absorbed into the bloodstream while blocking the entry of harmful pathogens and toxins. Once inside the cell, the viral replication damages it, inducing multiorgan implications, potentially leading to conditions like sepsis.27,28 Severe COVID-19 infections often manifest elevated serum levels of proinflammatory markers like interleukin-6 (IL-6) and interleukin-10 (IL-10), which can lead to dysbiosis and a subsequent ``leaky gut'' syndrome, facilitating bacterial product and toxin entry, further increasing inflammation.29, 30, 31 Notably, in COVID-19 patients, increased fecal calprotectin levels, indicative of GI mucosal inflammation, and increased plasma markers of gut permeability, such as FABP2, PGN, and LPS, highlight this phenomenon.32,33 The persistence of gastrointestinal symptoms post-recovery from the acute phase of COVID-19 is becoming increasingly documented. While some patients recover completely, others continue to report issues like recurring nausea, chronic episodes of diarrhea, and unexplained abdominal pain.34,35 Such persistent symptoms can severely impact an individual's quality of life, making it vital to understand their cause and develop targeted treatments.36
The gut microbiome, a complex ecosystem of beneficial bacteria, plays a pivotal role in numerous functions, including digestion, immunity, and mood regulation.37 This disruption can manifest as gastrointestinal symptoms and potentially have broader impacts. For example, imbalanced gut microbiota can influence immune responses, potentially exacerbate inflammation, and can also impact neurotransmitter production, affecting mental health.38,39
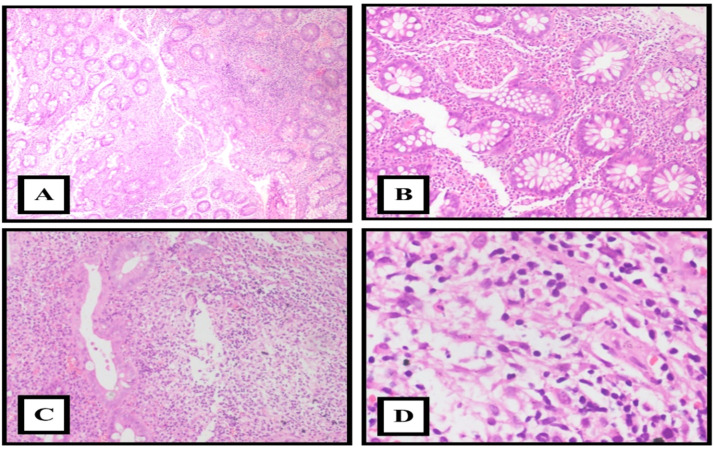
Beyond the immediate aftermath of the infection, there is a concern that the gastrointestinal system might face prolonged challenges.40 Drawing parallels with other diseases, some COVID-19 patients might develop conditions like post-infectious irritable bowel syndrome (PI-IBS) or functional dyspepsia, which could have roots in their initial COVID-19 infection.41, 42, 43 Individuals with pre-existing gastrointestinal conditions, such as inflammatory bowel disease (IBD), might experience a different interaction with the SARS-CoV-2 virus and produce a variety of histomorphological features [Fig. 2]. On the contrary, specific protective mechanisms, such as elevated levels of soluble ACE2, might protect these patients against severe disease manifestations.44, 45, 46, 47
Fig. 2.
Microscopic images of Inflammatory bowel disease appearance in a patient with post COVID-19. A: Crypt architectural distortion and branching (H&E x10).. B. Cryptitis (H&E x40). C and D: Dense basal lymphoplasmacytic infiltrate in the lamina propria (H&E x100).
Endocrine System
The endocrine system is susceptible to SARS-CoV-2. The SARS-CoV-2, infiltrates human cells via the ACE2 receptor. The virus's surface features a homotrimeric spike glycoprotein comprising S1 and S2 subunits, crucial for attaching to ACE2. When the S1 subunit binds to ACE2, the ACE2 receptor dissociates, thanks to transmembrane serine protease 2 (TMPRSS2). TMPRSS2 is known to drive oncogenic transcription in prostate cancer and is controlled by androgens. Interestingly, androgen deprivation or antagonism can reduce SARS-CoV-2′s ability to enter human cells in vitro by attenuating S-mediated cellular entry. This results in a conformational change, allowing the S2 subunit's membrane fusion with increased stability. Creating a bond with the ACE2 receptor is essential for SARS-CoV-2 cellular entry. This has been demonstrated in vitro studies, where the SARS-CoV-2 virus could not infect HeLa cells that did not express ACE2 proteins. Additionally, raising antiserum to human ACE2 prevented cellular access by SARS-CoV-2. Moreover, unlike other coronaviruses, SARS-CoV-2 does not appear to use other receptors for cellular access, such as dipeptidyl peptidase four or aminopeptidase N.
ACE2 mRNA is expressed in several human endocrine glands, including the pancreas, thyroid gland, ovaries, and testes. Crucially, TMPRSS2 mRNA is also expressed in these endocrine glands. TMPRSS2 is a serine protease necessary for SARS-CoV-2 spike protein priming, a critical step in viral entry. In summary, there is cumulative evidence that the endocrine system is particularly vulnerable to both destruction and alteration in function due to COVID-19. Clinical observations of endocrine complications in COVID-19 patients, such as hyperglycemia, hypothyroidism, hyperthyroidism, and adrenal insufficiency, support this.48
The pathophysiology of COVID-19 leading to diabetes mellitus is not fully understood, but several mechanisms have been proposed. The SARS-CoV-2 virus can directly infect pancreatic beta cells responsible for producing insulin. This can lead to beta necrosis and disruption of insulin production. COVID-19 infection can trigger a systemic inflammatory response, damaging pancreatic beta cells. Inflammatory cytokines released by immune cells and other cells throughout the body can cause damage to beta cells, leading to insulin deficiency. COVID-19 infection can also lead to immune dysregulation, manifesting in various ways, including autoimmunity. Autoimmune reactions can target pancreatic beta cells and disrupt insulin production. There are a few factors that may contribute to the development of diabetes mellitus in COVID-19 patients, including stress, medications, and hospitalization. Stress can activate the hypothalamic-pituitary-adrenal (HPA) axis and increase cortisol secretion, a stress hormone that can impair insulin sensitivity. Some medicines used to treat COVID-19, such as corticosteroids, can also have side effects on insulin sensitivity and glucose metabolism. COVID-19 patients who are hospitalized are at increased risk of developing diabetes mellitus due to a combination of stress, hyperglycemia, and medications that can impair insulin sensitivity.
The pathophysiology of COVID-19 leading to diabetes mellitus is complex and not fully understood. It is important to note that COVID-19 does not always lead to diabetes mellitus. However, COVID-19 patients should be monitored for hyperglycemia and other signs and symptoms of diabetes mellitus. Early diagnosis and treatment of diabetes mellitus can help to prevent complications and improve quality of life.49
Central Nervous System
The SARS-CoV-2 virus can directly infect CNS cells, including neurons, astrocytes, and microglia, through binding to the ACE2 receptor. Viral replication in CNS cells can lead to neuronal death and disruption of CNS function. COVID-19 infection can trigger a systemic inflammatory response and damage the CNS. Inflammatory cytokines released by immune cells and other cells throughout the body can cause damage to the blood-brain barrier (BBB), allowing the virus and other inflammatory cells to enter the CNS. Additionally, inflammatory cytokines can directly damage CNS cells. COVID-19 infection can also lead to immune dysregulation, manifesting in various ways, including autoimmunity. Autoimmune reactions can target CNS cells and damage the CNS. COVID-19 can cause multiple complications in the CNS, which may be attributed to various factors. These include hypoxia, which can result in reduced oxygen levels in the blood and damage to CNS cells, leading to neurological issues. Coagulopathy, or blood clotting disorders, can also occur due to COVID-19 infection, resulting in blood clots in the brain that can block blood flow and cause strokes. Additionally, some medications used to treat COVID-19, such as corticosteroids, may have side effects on the CNS. CNS complications of COVID-19 may manifest clinically in the form of headache, confusion, delirium, encephalopathy, or stroke.
POTS is a complex multisystem disorder characterized by orthostatic intolerance, defined as an excessive and sustained increase in heart rate upon standing or head-up tilt in the absence of hypotension. POTS is associated with a variety of symptoms, including lightheadedness, dizziness, syncope, palpitations, fatigue, and cognitive impairment. Recent reports suggest that individuals who have recovered from COVID-19 may face a higher risk of developing POTS. While the exact mechanisms behind post-COVID-19 POTS are not fully understood, several theories have been proposed. These include autoimmunity triggered by SARS-CoV-2 infection, direct damage to autonomic nerve fibers by the virus, toxic injury caused by SARS-CoV-2, and invasion of the CNS by the virus. With this increased risk in mind, healthcare providers should remain vigilant and actively screen for POTS in patients experiencing post-acute COVID-19 syndrome symptoms.
Once a diagnosis of POTS has been confirmed, it is essential to focus on managing the condition through a combination of lifestyle changes and medication. Some lifestyle modifications that may benefit POTS patients include increasing salt and fluid intake, using graduated compression stockings, pacing activities, and avoiding triggers such as dehydration, heat, and prolonged standing. Medication options for POTS include heart rate-lowering drugs like beta-blockers, ivabradine, and fludrocortisone, as well as midodrine and droxidopa, which can help raise blood pressure, and pyridostigmine, which improves autonomic function. In more severe cases of POTS, hospitalization may be necessary for intravenous fluids and medications.50
Genitourinary System
The SARS-CoV-2 virus can directly infect genitourinary epithelial cells, including those in the bladder, urethra, kidneys, and testes. Once infected, genitourinary epithelial cells can undergo viral replication and lysis, leading to cell death and disruption of the genitourinary barrier. COVID-19 infection can trigger a systemic inflammatory response, damaging the genitourinary system. Inflammatory cytokines released by immune cells and other cells throughout the body can cause damage to the genitourinary epithelium, vasculature, and nerves. COVID-19 infection can also lead to immune dysregulation, contributing to genitourinary problems. Immune dysregulation can manifest in various ways, such as overproduction of inflammatory cytokines, autoimmunity, and impaired T cell function. Immune dysregulation can damage the genitourinary epithelium and contribute to the development of inflammatory diseases of the genitourinary system, such as orchitis and epididymitis.
A couple of factors may contribute to the genitourinary complications associated with COVID-19. One is the dysregulation of the ACE2 receptor, a protein responsible for regulating blood pressure and inflammation. ACE2 is found in many cells throughout the body, including those in the genitourinary system. When someone is infected with SARS-CoV-2, their ACE2 expression may become downregulated or disrupted, potentially leading to problems like hematuria and proteinuria. Additionally, some medications used to treat COVID-19 (such as antibiotics and corticosteroids) can have side effects on the genitourinary system. The COVID-19 pandemic has led to the emergence of various genitourinary complications that have been reported in patients infected with the virus. These complications may arise due to direct viral invasion or due to the systemic effects of the disease. The most reported complications include acute kidney injury, proteinuria, and hematuria. Additionally, there have been reports of viral shedding in the urine of infected individuals, which has raised concerns about the potential for transmission through the genitourinary tract. It is essential for healthcare providers to be aware of these complications and to monitor patients for signs and symptoms of genitourinary involvement to provide appropriate management and prevent further complications.
Although the pathophysiology behind AKI caused by COVID-19 infection is not yet fully understood, three potential mechanisms are believed to be involved. These include cytokine release syndrome (CRS), organ crosstalk, and systemic metabolic alterations. CRS is characterized by the release of proinflammatory cytokines, including interleukin-6 (IL-6), which can cause intrarenal inflammation, increased vascular permeability, volume depletion, and cardiomyopathy, all of which can contribute to AKI. Organ crosstalk, particularly between the lungs and kidneys, can also play a role in COVID-19-associated AKI. Increased serum levels of IL-6 and other proinflammatory cytokines can damage tubular cells and lead to AKI. Finally, systemic metabolic alterations, such as fluid overload, sepsis, and rhabdomyolysis, can further destabilize the patient's hemodynamics and worsen AKI. It is important to note that these three mechanisms are likely interconnected. For example, CRS can lead to organ crosstalk, and organ crosstalk can lead to systemic metabolic alterations. Additionally, the relative importance of each mechanism in developing COVID-19-associated AKI may vary depending on the patient's clinical characteristics. Further research is needed to understand the pathophysiology of COVID-19-associated AKI better and develop more effective therapies for this condition.51
Musculoskeletal System
The SARS-CoV-2 virus can directly infect musculoskeletal cells, including muscle cells, osteocytes, and chondrocytes. Once infected, musculoskeletal cells can undergo viral replication and lysis, leading to cell death and disruption of musculoskeletal function. COVID-19 infection can trigger a systemic inflammatory response, damaging the musculoskeletal system. Inflammatory cytokines released by immune cells and other cells throughout the body can cause damage to the muscles, bones, and joints. COVID-19 infection can also lead to immune dysregulation, contributing to musculoskeletal problems. Immune dysregulation can manifest in various ways, such as overproduction of inflammatory cytokines, autoimmunity, and impaired T cell function. Immune dysregulation can damage the muscles, bones, and joints and contribute to the development of inflammatory diseases of the musculoskeletal system, such as myositis and arthritis.
COVID-19 may contribute to musculoskeletal complications through various mechanisms, such as extended immobilization, medication side effects, and critical illness. Prolonged bed rest during hospitalization can result in muscle atrophy, weakness, and joint stiffness. Certain medications used to treat COVID-19, such as corticosteroids, may also affect the musculoskeletal system. Critically ill patients with COVID-19 are at higher risk of developing critical illness myopathy (CIM), a muscle disorder characterized by muscle weakness and wasting that may be caused by inflammation, immobilization, and malnutrition. The range of musculoskeletal complications that COVID-19 can cause includes myalgia, arthralgia, muscle weakness, muscle atrophy, rhabdomyolysis, myositis, arthritis, CIM, osteoporosis, and fractures.
Some people who recover from COVID-19 may experience long-term musculoskeletal problems, such as post-COVID-19 myalgia and fatigue. The mechanisms underlying these long-term musculoskeletal complications are not fully understood. Still, they may be related to persistent inflammation, immune dysregulation, and direct viral damage to the musculoskeletal system.
The COVID-19 pandemic caused by the SARS-CoV-2 virus is affecting daily life worldwide. The symptoms of the virus can vary greatly, from no symptoms to critical illness. While COVID-19 is primarily a respiratory disease, studies have shown that it can also cause musculoskeletal symptoms such as myalgias, arthralgias, vasulitis and neuropathies/myopathies [Fig. 3]. One study found that 15.5% of patients experienced myalgia and arthralgia. Therefore, clinicians must investigate these symptoms further in COVID-19 patients.
Fig. 3.
Microscopic images demonstrating vasculitis in a patient with musculoskeletal features. A. Orthokeratosis, hyperkeratosis (H&E x10x). B and C: Leukocytoclastic vasculitis (H&E x40x and x100). D. Vessel elastic tissue damage causing emigration of neutrophils into the surrounding dermis (H&E x100).
Furthermore, it is essential to understand how COVID-19 impacts the musculoskeletal system. Previous studies have shown that the virus induces a proinflammatory state in patients, which can have systemic effects. While most studies have focused on the respiratory system, the impact of inflammation on other organ systems, specifically the musculoskeletal system, is less understood. Inflammation has been linked to bone and joint pathology, skeletal muscle damage, and disease. Therefore, investigating the potential impact of COVID-19-induced inflammation on musculoskeletal health is essential. Lastly, it is crucial to consider the possible side effects of current COVID-19 therapies on the musculoskeletal system. Medications such as chloroquine, hydroxychloroquine, colchicine, specific antivirals, and corticosteroids have been used to treat COVID-19, but many of them are associated with toxic myopathies, arthralgias, and other side effects. Clinicians need to understand these potential side effects when treating COVID-19 patients.52
Long-Term Psychological Impact of COVID-19
We shall now explore the persistent psychological ramifications of COVID-19, encompassing both adverse and favourable facets. While negative consequences such as Posttraumatic Stress Disorder (PTSD), cognitive deficits, sleep disruptions, substance dependency, social alienation, economic strain, and stigmatization are evident, the review highlights positive dimensions like resilience, posttraumatic growth, and augmented mental health awareness. Moreover, it underscores the accelerated adoption of telemedicine and digital mental health resources, offering novel avenues for mental health care.53,54
The enduring psychological repercussions of COVID-19 constitute a multifaceted domain demanding a meticulous examination. Initially, the focus was on immediate psychological distress, but recent research unveils the persisting nature of these effects, necessitating a comprehensive review to capture the current understanding. This review aims to provide a detailed analysis of the enduring psychological outcomes of the COVID-19 pandemic, encapsulating its detrimental and constructive dimensions.55,56 The enduring psychological toll of the pandemic is palpable in the heightened prevalence of anxiety and depression among survivors. Notably, recent literature illuminated a significantly elevated risk of psychiatric disorders, including anxiety and mood disorders, in the post-recovery phase. Factors such as the specter of reinfection, the loss of loved ones, and pervasive uncertainty have collectively contributed to these lingering mental health afflictions, underscoring the enduring nature of the pandemic's psychological impact.57
The stringent lockdowns and consequential social isolation measures have precipitated an alarming surge in alcohol and drug consumption. This escalating pattern of substance misuse exacerbates existing mental health concerns, posing an acute challenge for healthcare practitioners.58 Economic adversities stemming from the pandemic have augmented the psychological burden, with individuals grappling with job loss, financial precariousness, and associated stressors. Simultaneously, the enforced social isolation resulting from quarantine measures has engendered sentiments of solitude, further compounding the mental health predicament.59 Concurrently, stigmatization and discrimination have emerged as pernicious psychological repercussions of the pandemic. Specific cohorts, particularly healthcare workers and marginalized populations have been subjected to prejudice and societal ostracization due to their perceived links with the virus.60 Despite the manifold challenges posed by COVID-19, resilience has surfaced as a noteworthy positive outcome. Many survivors have reported an intensified appreciation for life, enhanced interpersonal relationships, and a rejuvenated sense of purpose, (2021).61
The phenomenon of posttraumatic growth, signifying the capacity of individuals to discover meaning and personal development amidst adversity, has been manifested by certain COVID-19 survivors. This underlines the inherent human potential to adapt and flourish in hardship.62 A notable growth in mental health support has been the accelerated embrace of telemedicine and digital mental health resources. The effectiveness of virtual mental health services in ameliorating symptoms of depression and anxiety. This trend is anticipated to endure post-pandemic, substantially augmenting access to mental healthcare.63 The enduring psychological consequences of COVID-19 encompass an intricate interplay of detrimental and advantageous outcomes. Furthermore, the widespread adoption of telemedicine and digital mental health resources represents a promising evolution in the landscape of mental health care. A thorough comprehension of these psychological ramifications is imperative for addressing the protracted impact of COVID-19 on mental well-being and steering future research and interventions.
Conclusion
The emergence of Long COVID, or PASC, is a growing concern for public health globally. Through a comprehensive systemic review, we have shed light on the diverse and debilitating effects of this complex condition. Our review highlights some key findings. Firstly, Long COVID affects a large proportion of individuals, with estimates suggesting that up to 80% experience lasting sequelae beyond the acute phase of infection. Secondly, the symptoms of Long COVID are more widespread and varied than initially recognized, encompassing physical, mental, and cognitive domains. Fatigue, breathlessness, neurological impairments, and mental health issues are some of the most reported effects. Thirdly, the duration and severity of Long-term COVID symptoms can vary widely, with some individuals experiencing gradual improvement while others face persistent, debilitating symptoms for months or even years. Despite ongoing research, the underlying mechanisms of Long COVID remain largely elusive. There are theories that viral persistence, autoimmune responses, and multi-organ damage may contribute to the condition, but further investigation is crucial. One potential area for further research and exploration is the investigation of the impact of emerging technologies on various industries. Another promising avenue for future study is the examination of the effectiveness of different educational models and approaches in promoting student learning and engagement. Additionally, there is a need for continued exploration of the intersection between social issues and technology, particularly in terms of how technology can be leveraged to address pressing societal challenges. These and other future directions hold great promise for advancing our understanding and application of key concepts in a range of academic fields.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication
Not required.
CRediT authorship contribution statement
Srikanth Umakanthan: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Arun Rabindra Katwaroo: Writing – review & editing, Writing – original draft, Resources, Formal analysis, Data curation. Maryann Bukelo: Writing – review & editing, Writing – original draft, Investigation. Shashidhar BG: . Prashanth Boralingaiah: Writing – review & editing, Writing – original draft, Investigation. Anu V Ranade: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation. Pallavi Rangan: Writing – review & editing, Formal analysis. Shabnam Shashidhar: Writing – review & editing, Writing – original draft. Jyoti Ramanath Kini: Writing – review & editing, Visualization, Data curation. Gayathri Kini: Visualization.
Declaration of competing interest
We acknowledge that we have read the journal's policy statement and agree to the same. We declare that we do not have an conflicts of interest and declare that this manuscript is not submitted elsewhere nor considered for publication in any other journals. Hence, we submit the manuscript entitled “Post-Acute Sequelae of Covid-19: A System-wise Approach on the Effects of Long-Covid-19” for full consideration in your esteemed journal.
References
- 1.Horberg M.A., et al. Post-acute sequelae of SARS-CoV-2 with clinical condition definitions and comparison in a matched cohort. Nature communications. 2022;13(1):5822. doi: 10.1038/s41467-022-33573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seecheran R., et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. Journal of Investigative Medicine High Impact Case Reports. 2020;8 doi: 10.1177/2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus, W., Dashboard. WHO Coronavirus (COVID-19) dashboard with vaccination data; 2021. Internet Available from: https://covid19.who.int/[cited 2021 Dec 13], 2021.
- 4.Xie Y., et al. Long-term cardiovascular outcomes of COVID-19. Nature medicine. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization, W.H. World Health Organization; 2021. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. [Google Scholar]
- 6.Tereshchenko L.G., et al. Risk of cardiovascular events after COVID-19. The American Journal of Cardiology. 2022;179:102–109. doi: 10.1016/j.amjcard.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tereshchenko L.G., et al. Risk of Cardiovascular Events After COVID-19. Am J Cardiol. 2022;179:102–109. doi: 10.1016/j.amjcard.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho J.S., et al. Coronavirus-induced myocarditis: a meta-summary of cases. Heart & Lung. 2020;49(6):681–685. doi: 10.1016/j.hrtlng.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blagova O., et al. Chronic biopsy proven post-COVID myoendocarditis with SARS-Cov-2 persistence and high level of antiheart antibodies. Clinical Cardiology. 2022;45(9):952–959. doi: 10.1002/clc.23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ståhlberg M., et al. Post-COVID-19 tachycardia syndrome: a distinct phenotype of post-acute COVID-19 syndrome. The American journal of medicine. 2021;134(12):1451–1456. doi: 10.1016/j.amjmed.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar N., et al. Acute myocardial infarction in COVID-19 patients. A review of cases in the literature. Archives of Medical Science-Atherosclerotic Diseases. 2021;6(1):169–175. doi: 10.5114/amsad.2021.109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou-Ismail M.Y., et al. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thrombosis research. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akkaya F., et al. Long term effects of mild severity COVID‑19 on right ventricular functions. The International Journal of Cardiovascular Imaging. 2021;37(12):3451–3457. doi: 10.1007/s10554-021-02340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szeghy R.E., et al. Long-Term Recovery from SARS-CoV-2 (COVID-19): Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. Journal of Applied Physiology. 2022;132(5):1297. doi: 10.1152/japplphysiol.00793.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lip S., et al. Protocol: Rationale and Design for the LOnger-term effects of SARS-CoV-2 INfection on blood Vessels And blood pRessure (LOCHINVAR): an observational phenotyping study. Open Heart. 2022;9(1) doi: 10.1136/openhrt-2022-002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 17.Lin L., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 18.Cipriano M., Ruberti E., Giacalone A. Gastrointestinal infection could be new focus for coronavirus diagnosis. Cureus. 2020;12(3) doi: 10.7759/cureus.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norouzi Masir M., Shirvaliloo M. Symptomatology and microbiology of the gastrointestinal tract in post-COVID conditions. JGH Open. 2022;6(10):667–676. doi: 10.1002/jgh3.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan L., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. The American journal of gastroenterology. 2020;115 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redd W., et al. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS-CoV-2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159(2):765–767. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tullie L., et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. The Lancet Child & Adolescent Health. 2020;4(7):e19–e20. doi: 10.1016/S2352-4642(20)30165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang L., et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. Journal of Korean medical science. 2020;35(47) doi: 10.3346/jkms.2020.35.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen M.S., et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clinical Infectious Diseases. 2021;73(11):e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nature immunology. 2019;20(10):1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 26.Keely S., Talley N.J., Hansbro P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal immunology. 2012;5(1):7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai H.-C., et al. Irritable bowel syndrome increases the risk of chronic obstructive pulmonary disease: a retrospective cohort study. Scientific Reports. 2020;10(1):10008. doi: 10.1038/s41598-020-66707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barcik W., et al. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52(2):241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingula R., et al. Desired turbulence? Gut-lung axis, immunity, and lung cancer. Journal of oncology. 2017;2017 doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamming I., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomao R., et al. Sepsis of Patients Infected by SARS-CoV-2: Real-World Experience From the International. Sepsis and COVID-19: Cross-Talk in Signalling Pathways and in Therapeutic Perspectives. 2022 [Google Scholar]
- 32.Baig A.M., et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS chemical neuroscience. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 33.Han H., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging microbes & infections. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Effenberger M., et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-321388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto T., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng S.C., Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye Gut: first published as 10.1136/gutjnl-2020-321195 on 9 April 2020. Gut Month. 2020:0. doi: 10.1136/gutjnl-2020-321195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H., et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69(6):1010–1018. [Google Scholar]
- 38.Zhou J., et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nature medicine. 2020;26(7):1077–1083. doi: 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- 39.Ye Q., et al. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2020;319(2):G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yong SJ, T T., Chew J, Lim WL. Antidepressive mechanisms of probiotics and their therapeutic potential. Frontiers in neuroscience. 2020;14(13):1361. doi: 10.3389/fnins.2019.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weng J., et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6(5):344–346. doi: 10.1016/S2468-1253(21)00076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vodnar D.C., et al. Coronavirus Disease (COVID-19) Caused by (SARS-CoV-2) Infections: A Real Challenge for Human Gut Microbiota. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.575559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuo T., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955. doi: 10.1053/j.gastro.2020.05.048. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowak J.K., et al. Age, inflammation, and disease location are critical determinants of intestinal expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 2020;159(3):1151–1154. doi: 10.1053/j.gastro.2020.05.030. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bangma A., Voskuil M.D., Weersma R.K. TNFα-antagonist use and mucosal inflammation are associated with increased intestinal expression of SARS-CoV-2 host protease TMPRSS2 in patients with inflammatory bowel disease. Gastroenterology. 2021;160(7):2621–2622. doi: 10.1053/j.gastro.2020.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambrose P.A., Goodman W.A. Impact of COVID-19 on patients with inflammatory bowel disease. Journal of exploratory research in pharmacology. 2022;7(1):37–44. doi: 10.14218/jerp.2021.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahimi B., Vesal A., Edalatifard M. Coronavirus and Its effect on the respiratory system: Is there any association between pneumonia and immune cells. Journal of Family Medicine and Primary Care. 2020;9(9):4729. doi: 10.4103/jfmpc.jfmpc_763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kariyawasam J.C., et al. Gastrointestinal manifestations in COVID-19. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2021;115(12):1362–1388. doi: 10.1093/trstmh/trab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke S.A., Abbara A., Dhillo W.S. Impact of COVID-19 on the Endocrine System: A Mini-review. Endocrinology. 2022;163(1):bqab203. doi: 10.1210/endocr/bqab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anonymous, How COVID-19 Can Lead to Diabetes. 2021. June 8, .
- 52.Ormiston C.K., Świątkiewicz I., Taub P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Heart Rhythm. 2022;19(11):1880–1889. doi: 10.1016/j.hrthm.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallak J., et al. SARS-CoV-2 and its relationship with the genitourinary tract: Implications for male reproductive health in the context of COVID-19 pandemic. Andrology. 2021;9(1):73–79. doi: 10.1111/andr.12896. [DOI] [PubMed] [Google Scholar]
- 54.Hasan L.K., et al. Effects of COVID-19 on the musculoskeletal system: Clinician's guide. Orthopedic Research and Reviews. 2021:141–150. doi: 10.2147/ORR.S321884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taquet M., et al. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ettman C.K., et al. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA network open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19686. -e2019686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nalbandian A., et al. Post-acute COVID-19 syndrome. Nature medicine. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cellini N., et al. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. Journal of sleep research. 2020;29(4):e13074. doi: 10.1111/jsr.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volkow N.D. American College of Physicians; 2020. Collision of the COVID-19 and addiction epidemics; pp. 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shear M. Complicated Grief New England Journal of Medicine. Mass Med Soc. 2015;372:153–160. doi: 10.1056/NEJMcp1315618. [DOI] [PubMed] [Google Scholar]
- 61.Stangl A.L., et al. The Health Stigma and Discrimination Framework: a global, crosscutting framework to inform research, intervention development, and policy on health-related stigmas. BMC medicine. 2019;17:1–13. doi: 10.1186/s12916-019-1271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tedeschi R.G., Calhoun L.G. Posttraumatic growth: conceptual foundations and empirical evidence. Psychological inquiry. 2004;15(1):1–18. [Google Scholar]
- 63.Torous J., et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR mental health. 2020;7(3):e18848. doi: 10.2196/18848. [DOI] [PMC free article] [PubMed] [Google Scholar]