Abstract
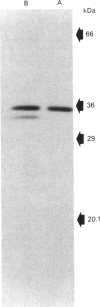
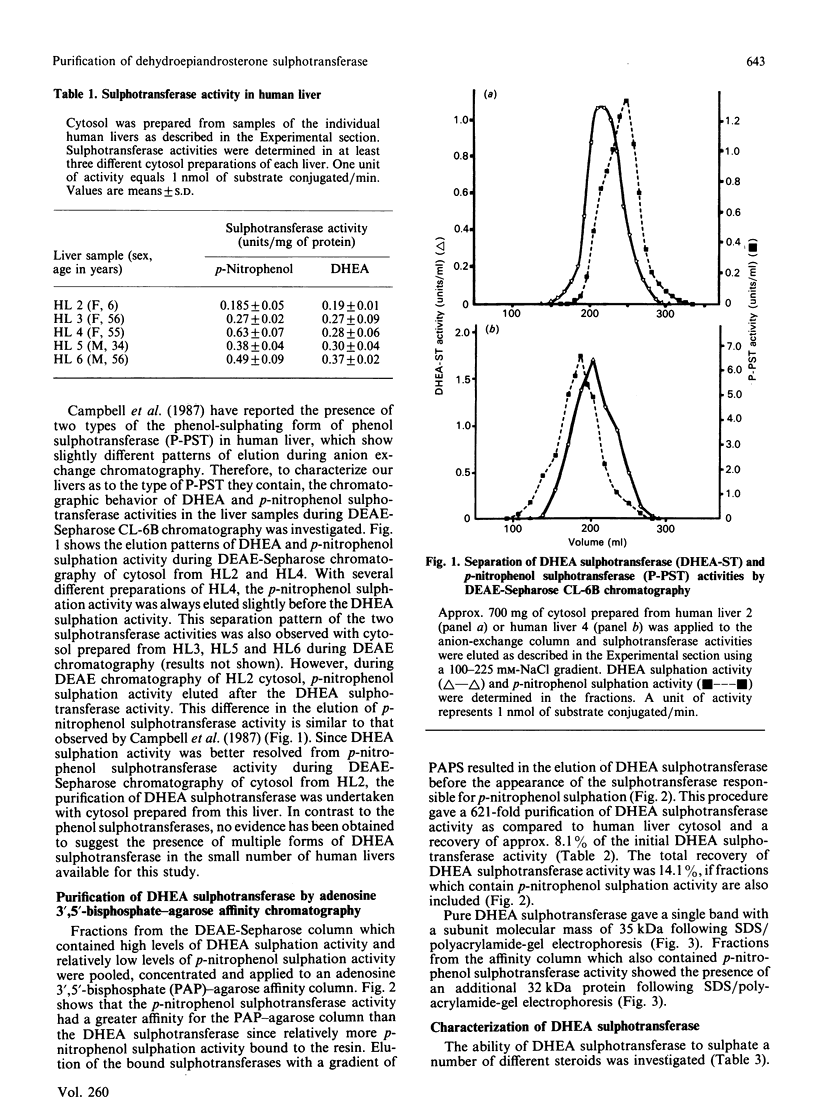
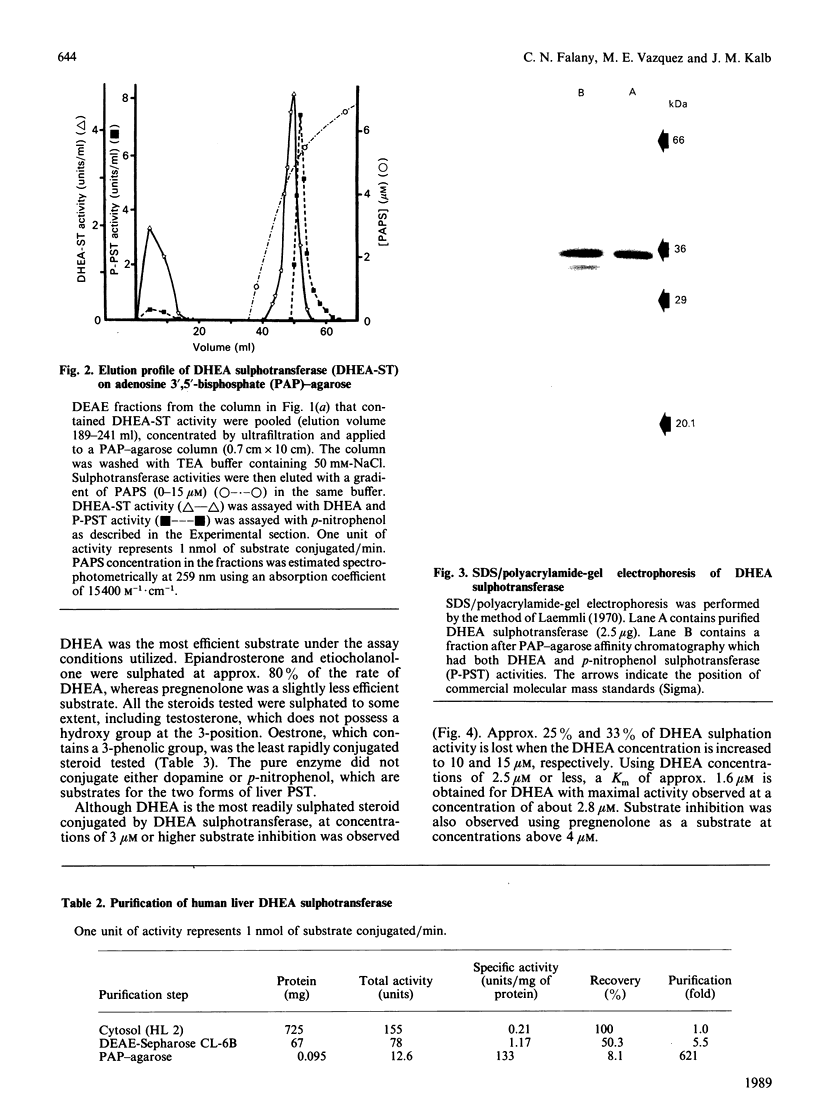
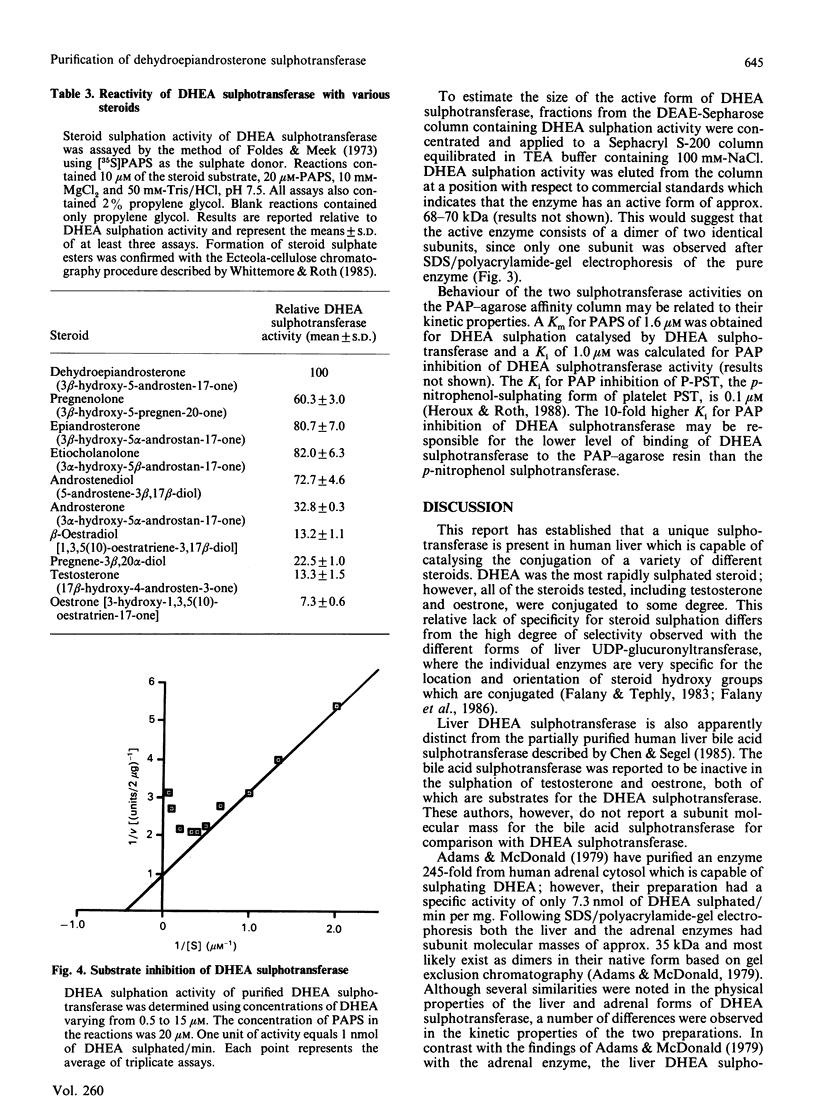
A form of sulphotransferase capable of sulphating dehydroepiandrosterone and other steroids was purified from cytosol prepared from human liver. Dehydroepiandrosterone sulphotransferase was purified 621-fold when compared with the activity in cytosol using DEAE-Sepharose CL-6B and adenosine 3',5'-bisphosphate-agarose affinity chromatography. During affinity chromatography, dehydroepiandrosterone sulphation activity could be resolved from p-nitrophenol sulphation activity catalysed by phenol sulphotransferase by using a gradient of adenosine 3'-phosphate 5'-phosphosulphate. The purified enzyme was most active towards dehydroepiandrosterone but was capable of conjugating a number of other steroids, including pregnenolone, androsterone and beta-oestradiol. No activity towards p-nitrophenol or dopamine, substrates for the phenol sulphotransferase, was observed with the pure enzyme. A single band with a subunit molecular mass of 35 kDa was observed by Coomassie Blue staining following SDS/polyacrylamide-gel electrophoresis of the purified enzyme. A molecular mass of 68-70 kDa was calculated for the active form of the enzyme by chromatography on Sephacryl S-200, suggesting that the active form of the enzyme is a dimer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. B., McDonald D. Enzymic synthesis of steroid sulphates. XII. Isolation of dehydroepiandrosterone sulphotransferase from human adrenals by affinity chromatography. Biochim Biophys Acta. 1979 Mar 16;567(1):144–153. doi: 10.1016/0005-2744(79)90181-5. [DOI] [PubMed] [Google Scholar]
- Adams J. B., McDonald D. Enzymic synthesis of steroid sulphates. XIII. Isolation and properties of dehydroepiandrosterone sulphotransferase from human foetal adrenals. Biochim Biophys Acta. 1980 Sep 9;615(1):275–278. doi: 10.1016/0005-2744(80)90031-5. [DOI] [PubMed] [Google Scholar]
- Adams J. B., McDonald D. Enzymic synthesis of steroid sulphates. XIV. Properties of human adrenal steroid alcohol sulphotransferase. Biochim Biophys Acta. 1981 Jun 23;664(3):460–468. doi: 10.1016/0005-2760(81)90124-7. [DOI] [PubMed] [Google Scholar]
- Barańczyk-Kuźma A. Phenol sulfotransferase in human lung. Biochem Med Metab Biol. 1986 Feb;35(1):18–30. doi: 10.1016/0885-4505(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell N. R., Van Loon J. A., Weinshilboum R. M. Human liver phenol sulfotransferase: assay conditions, biochemical properties and partial purification of isozymes of the thermostable form. Biochem Pharmacol. 1987 May 1;36(9):1435–1446. doi: 10.1016/0006-2952(87)90108-0. [DOI] [PubMed] [Google Scholar]
- Chen L. J., Segel I. H. Purification and characterization of bile salt sulfotransferase from human liver. Arch Biochem Biophys. 1985 Sep;241(2):371–379. doi: 10.1016/0003-9861(85)90559-4. [DOI] [PubMed] [Google Scholar]
- Falany C. N., Green M. D., Swain E., Tephly T. R. Substrate specificity and characterization of rat liver p-nitrophenol, 3 alpha-hydroxysteroid and 17 beta-hydroxysteroid UDP-glucuronosyltransferases. Biochem J. 1986 Aug 15;238(1):65–73. doi: 10.1042/bj2380065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falany C. N., Tephly T. R. Separation, purification and characterization of three isoenzymes of UDP-glucuronyltransferase from rat liver microsomes. Arch Biochem Biophys. 1983 Nov;227(1):248–258. doi: 10.1016/0003-9861(83)90368-5. [DOI] [PubMed] [Google Scholar]
- Foldes A., Meek J. L. Rat brain phenolsulfotransferase: partial purification and some properties. Biochim Biophys Acta. 1973 Dec 19;327(2):365–374. doi: 10.1016/0005-2744(73)90419-1. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Shantz L. M., Talalay P. Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv Enzyme Regul. 1987;26:355–382. doi: 10.1016/0065-2571(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Gugler R., Rao G. S., Breuer H. Reinigung und Charakterisierung einer 3'-Phosphoadenylylsulfat: Steroid-Sulfotransferase aus der Leber des Menschen. Biochim Biophys Acta. 1970 Oct 14;220(1):69–84. doi: 10.1016/0005-2744(70)90230-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcus C. J., Sekura R. D., Jakoby W. B. A hydroxysteroid sulfotransferase from rat liver. Anal Biochem. 1980 Sep 15;107(2):296–304. doi: 10.1016/0003-2697(80)90386-3. [DOI] [PubMed] [Google Scholar]
- Reiter C., Mwaluko G., Dunnette J., Van Loon J., Weinshilboum R. Thermolabile and thermostable human platelet phenol sulfotransferase. Substrate specificity and physical separation. Naunyn Schmiedebergs Arch Pharmacol. 1983 Sep;324(2):140–147. doi: 10.1007/BF00497020. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Duffel M. W., Jakoby W. B. Aryl sulfotransferases. Methods Enzymol. 1981;77:197–206. doi: 10.1016/s0076-6879(81)77026-5. [DOI] [PubMed] [Google Scholar]
- Singer S. S., Giera D., Johnson J., Sylvester S. Enzymatic sulfation of steroids: I. The enzymatic basis for the sex difference in cortisol sulfation by rat liver preparations. Endocrinology. 1976 Apr;98(4):963–974. doi: 10.1210/endo-98-4-963. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Stolz A., Sugimoto M., Kuhlenkamp J., Yamada T., Kaplowitz N. Identification and partial purification of a unique phenolic steroid sulphotransferase in rat liver cytosol. Biochem J. 1984 Dec 15;224(3):947–953. doi: 10.1042/bj2240947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore R. M., Roth J. A. A modified Ecteola cellulose assay for M and P phenol sulfotransferase. Biochem Pharmacol. 1985 May 15;34(10):1647–1652. doi: 10.1016/0006-2952(85)90629-x. [DOI] [PubMed] [Google Scholar]