Abstract
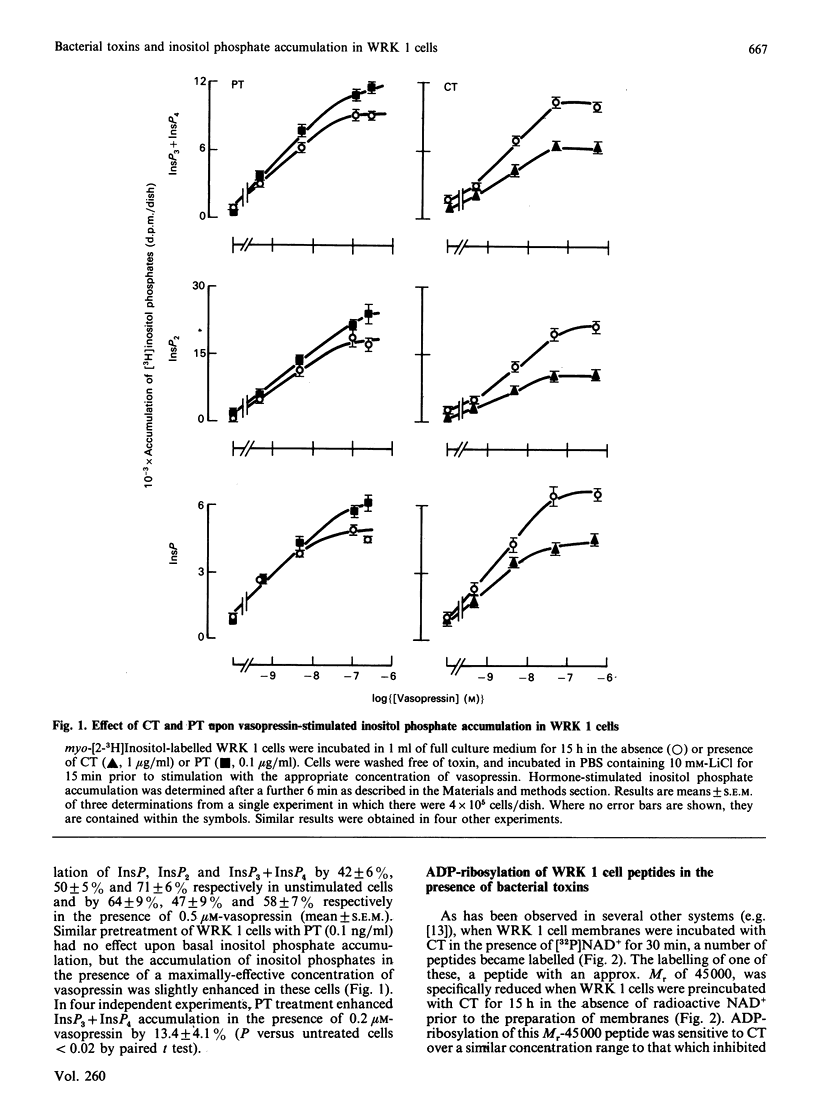
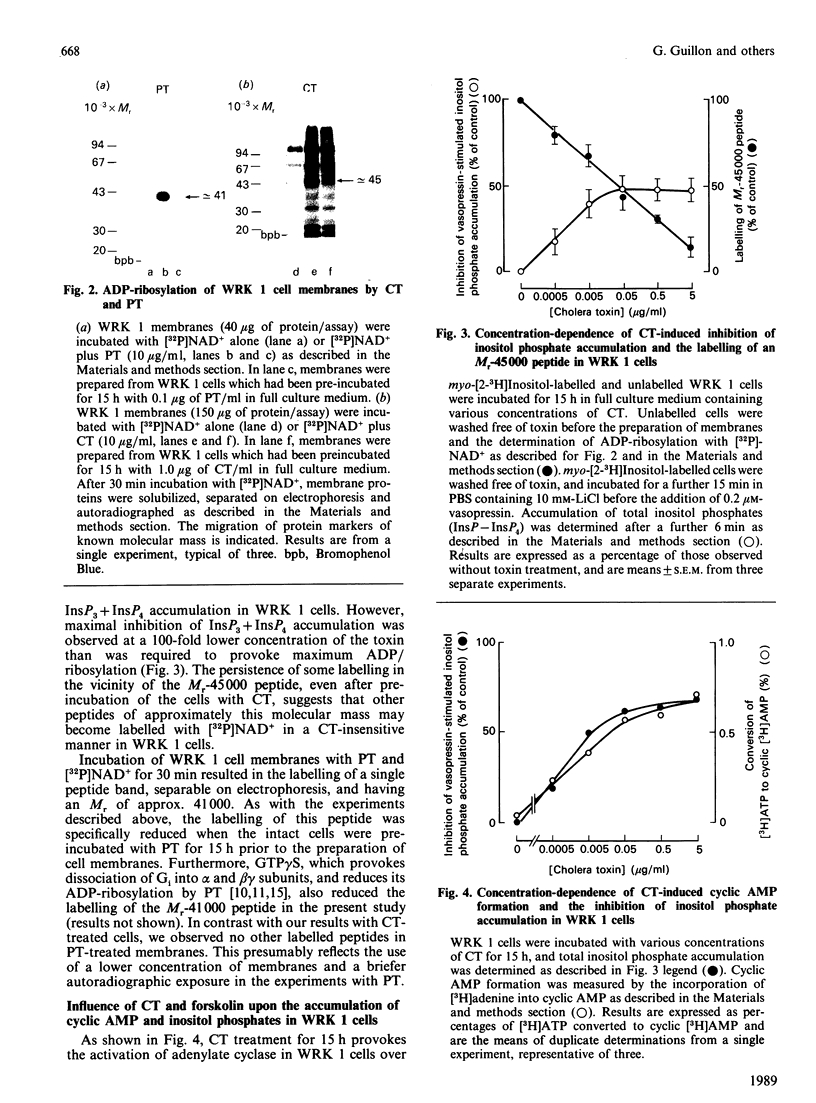
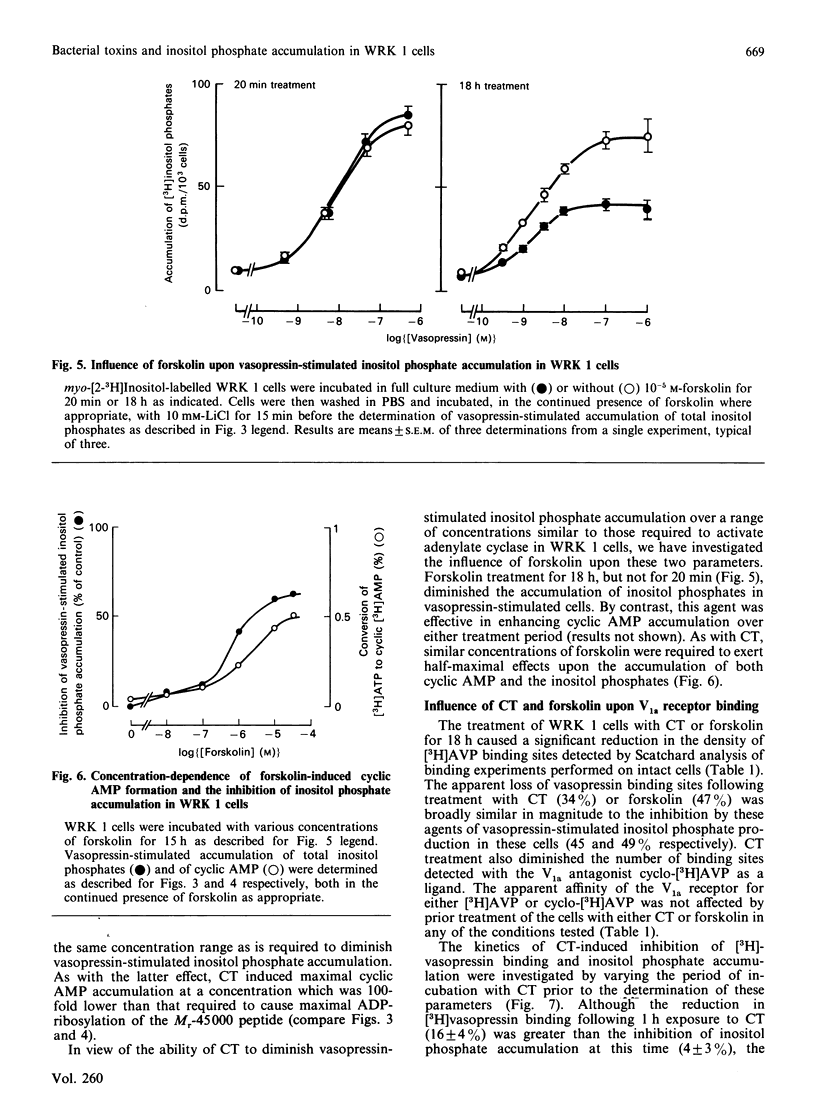
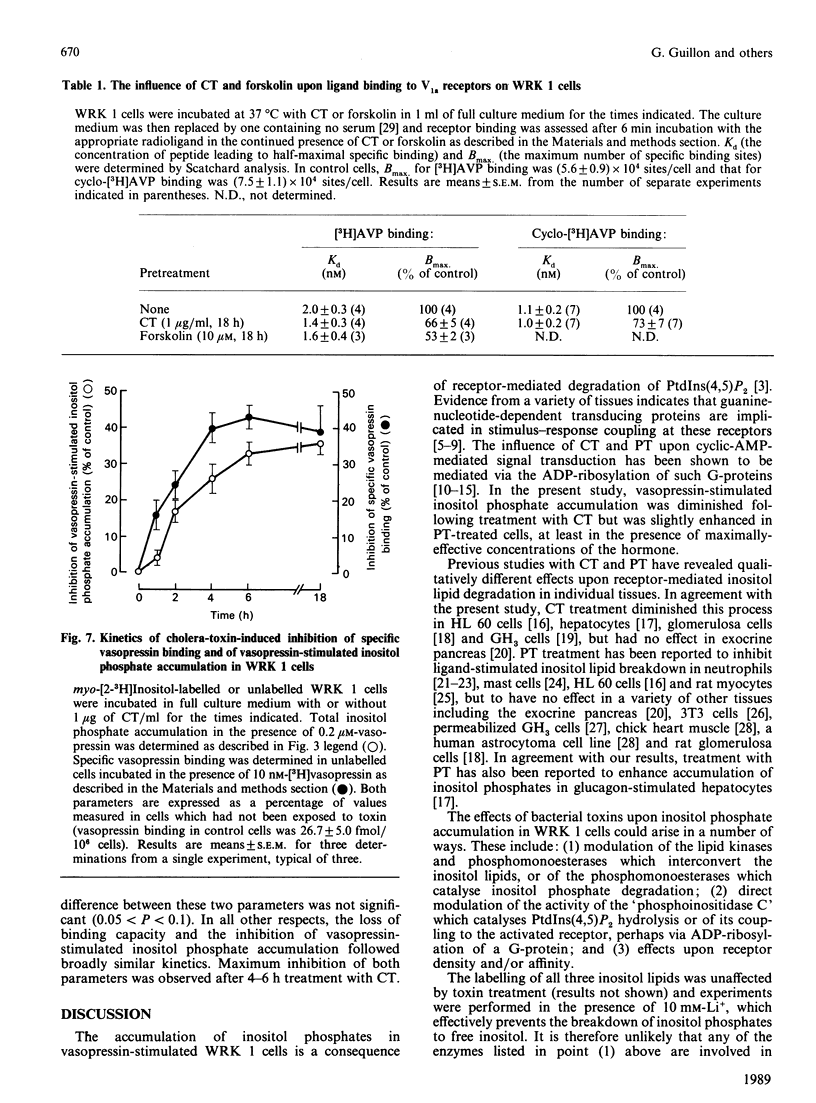
The accumulation of inositol phosphates in WRK 1 cells, stimulated with a range of vasopressin concentrations, was diminished by prior exposure to cholera toxin or forskolin, whilst that observed in the presence of maximal concentrations of the hormone was enhanced in pertussis-toxin-treated cells. In the presence of [32P]NAD+, both cholera toxin and pertussis toxin provoked the labelling of peptides with approximate Mrs of 45,000 and 41,000 respectively in the membranes of WRK 1 cells. Exposure to cholera toxin or forskolin for 15-18 h enhanced cyclic AMP accumulation in these cells. The concentrations of these agents which provoked half-maximal cyclic AMP accumulation were similar to those required to diminish receptor-mediated inositol phosphate accumulation by 50%. In contrast, half-maximal ADP-ribosylation of the 45,000Mr peptide needed 100-fold greater concentrations of the toxin than were effective in provoking half-maximal inhibition of inositol phosphate accumulation. Cholera toxin or forskolin also reduced the maximal specific binding, to intact WRK 1 cells, of both [3H][Arg8]vasopressin and the V1a antagonist [3H][beta-mercapto-beta,beta-cyclopentamethylenepropionic acid,O-methyl-Tyr2, Arg8]vasopressin. The kinetics for the loss of this binding capacity following cholera-toxin treatment were very similar to those describing the diminution of vasopressin-stimulated inositol phosphate accumulation in the same cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. Rapid accumulation of inositol phosphates in isolated rat superior cervical sympathetic ganglia exposed to V1-vasopressin and muscarinic cholinergic stimuli. Biochem J. 1984 Aug 1;221(3):803–811. doi: 10.1042/bj2210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R. GTP-binding proteins. One molecular machine can transduce diverse signals. 1986 Jun 26-Jul 2Nature. 321(6073):814–816. doi: 10.1038/321814a0. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Guillon G., Balestre M. N., Mouillac B., Berrada R., Kirk C. J. Mechanisms of phospholipase C activation: a comparison with the adenylate cyclase system. Biochimie. 1987 Apr;69(4):351–363. doi: 10.1016/0300-9084(87)90026-5. [DOI] [PubMed] [Google Scholar]
- Guillon G., Balestre M. N., Mouillac B., Devilliers G. Activation of membrane phospholipase C by vasopressin. A requirement for guanyl nucleotides. FEBS Lett. 1986 Feb 3;196(1):155–159. doi: 10.1016/0014-5793(86)80232-0. [DOI] [PubMed] [Google Scholar]
- Guillon G., Gallo-Payet N., Balestre M. N., Lombard C. Cholera-toxin and corticotropin modulation of inositol phosphate accumulation induced by vasopressin and angiotensin II in rat glomerulosa cells. Biochem J. 1988 Aug 1;253(3):765–775. doi: 10.1042/bj2530765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden T. K., Stephens L., Hawkins P. T., Downes C. P. Turkey erythrocyte membranes as a model for regulation of phospholipase C by guanine nucleotides. J Biol Chem. 1987 Jul 5;262(19):9057–9061. [PubMed] [Google Scholar]
- Hildebrandt J. D., Sekura R. D., Codina J., Iyengar R., Manclark C. R., Birnbaumer L. Stimulation and inhibition of adenylyl cyclases mediated by distinct regulatory proteins. Nature. 1983 Apr 21;302(5910):706–709. doi: 10.1038/302706a0. [DOI] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Evidence for tight coupling of thyrotropin-releasing hormone receptors to stimulated inositol trisphosphate formation in rat pituitary cells. J Biol Chem. 1985 Sep 5;260(19):10536–10540. [PubMed] [Google Scholar]
- Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984 Mar 25;259(6):3578–3585. [PubMed] [Google Scholar]
- Kirk C. J., Guillon G., Balestre M. N., Jard S. Stimulation, by vasopressin and other agonists, of inositol-lipid breakdown and inositol phosphate accumulation in WRK 1 cells. Biochem J. 1986 Nov 15;240(1):197–204. doi: 10.1042/bj2400197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Guillon G., Balestre M. N., Jard S. Stimulation, by vasopressin and other agonists, of inositol-lipid breakdown and inositol phosphate accumulation in WRK 1 cells. Biochem J. 1986 Nov 15;240(1):197–204. doi: 10.1042/bj2400197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Masters S. B., Martin M. W., Harden T. K., Brown J. H. Pertussis toxin does not inhibit muscarinic-receptor-mediated phosphoinositide hydrolysis or calcium mobilization. Biochem J. 1985 May 1;227(3):933–937. doi: 10.1042/bj2270933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Monaco M. E. Inositol metabolism in WRK-1 cells. Relationship of hormone-sensitive to -insensitive pools of phosphoinositides. J Biol Chem. 1987 Sep 25;262(27):13001–13006. [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Neer E. J., Wolf L. G., Gill D. M. The stimulatory guanine-nucleotide regulatory unit of adenylate cyclase from bovine cerebral cortex. ADP-ribosylation and purification. Biochem J. 1987 Jan 15;241(2):325–336. doi: 10.1042/bj2410325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron Y., Straub R. E., Traktman P., Gershengorn M. C. Decreased TRH receptor mRNA activity precedes homologous downregulation: assay in oocytes. Science. 1987 Dec 4;238(4832):1406–1408. doi: 10.1126/science.2825350. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefcyk J., Yassin R., Volpi M., Molski T. F., Naccache P. H., Munoz J. J., Becker E. L., Feinstein M. B., Sha'afi R. I. Pertussis but not cholera toxin inhibits the stimulated increase in actin association with the cytoskeleton in rabbit neutrophils: role of the "G proteins" in stimulus-response coupling. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1174–1181. doi: 10.1016/0006-291x(85)90309-2. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F., Chow Y. K., Robinson R. B., Bilezikian J. P. A pertussis toxin substrate regulates alpha 1-adrenergic dependent phosphatidylinositol hydrolysis in cultured rat myocytes. Endocrinology. 1987 May;120(5):1889–1895. doi: 10.1210/endo-120-5-1889. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Weiss S., Sebben M., Bockaert J. Corticotropin-peptide regulation of intracellular cyclic AMP production in cortical neurons in primary culture. J Neurochem. 1985 Sep;45(3):869–874. doi: 10.1111/j.1471-4159.1985.tb04074.x. [DOI] [PubMed] [Google Scholar]