Abstract
毛母细胞瘤(trichoblastoma,TB)是一种罕见的毛发生殖细胞皮肤附件肿瘤,罕见的皮肤滤泡肿瘤,分化于毛胚上皮,常被视为良性皮肤肿瘤,但局限性差且具有局部浸润性生长模式。TB好发于头部和颈部区域,尤其是面部,临床表现为生长缓慢、边界清晰及隆起的结节。TB常规以手术治疗为主,由于目前缺乏普遍接受的治疗指南或方案,术后复发率高,为临床治愈增加了一定的难度。本研究报道1例65岁女性患者,发现右侧外耳道口及耳甲腔肿物并反复破溃流脓,最初误诊为外耳道炎,以常规抗感染治疗,症状未缓解并逐渐加重后来我院就诊。该病例所展现的病症极为罕见,因此,及时且准确的诊断与治疗对此类疫病的诊疗和预后至关重要。
Keywords: 毛母细胞瘤, 毛源性肿瘤, 耳甲腔良性肿瘤, 诊断, 治疗
Abstract
Trichoblastoma(TB) is a rare germ cell skin adnexal tumor of the hair, and it is a rare follicular tumor of the skin that differentiates from the hair germ epithelium and is often regarded as a benign skin tumorHowever, it is poorly confined and has a local infiltrative growth pattern. tb occurs in the head and neck region, especially in the face, and presents clinically as a slow growing, well-defined and elevated nodule. TB is routinely treated surgically. Due to the lack of universally accepted treatment guidelines or protocols, the recurrence rate after surgery is high, which makes clinical cure more difficult. In this study, a 65-year-old female patient was found to have a swelling with recurrent rupture and pus flow from the right external auditory canal opening and the auricular cavity. After initial misdiagnosis as otitis externa, she was treated with conventional anti-infective therapy, but her symptoms did not resolve and gradually worsened before coming to our hospital. The condition presented in this case is relativelyrare, therepre, timely and accurate diagnosis and treatment are crucial for prognosis improvement of such diseases.
Keywords: trichoblastoma, hair-derived tumor, benign tumor of the ear nail cavity, diagnosis, treatment
本研究介绍了1例罕见的耳甲腔毛母细胞瘤(trichoblastoma,TB)。TB是毛源性上皮瘤的一种,是一种罕见的、生长缓慢的良性皮肤肿瘤,可为原发性,也可由皮脂腺痣演变而来[1]。其流行病学数据仍不完整。TB最常见于50~60岁成人[2-3],性别差异无统计学意义。TB通常表现为生长缓慢的色素沉着或黑色素丘疹,与基底细胞癌(basal cell carcinoma,BCC)鉴别困难。一般以临床表现、皮肤镜和病理结果作为主要诊断手段[4]。本例患者发现耳甲腔肿物,经组织学检查发现为TB同时伴有活跃的癌细胞存在,这在临床上较罕见。研究表明,大多数TB为良性,容易切除。但该疾病具有侵袭性特征,如不能及时发现并治疗,待病情恶变进展为毛母细胞癌时,其潜在的攻击性行为远高于TB,增加了治疗难度的同时降低了患者的生活质量[5]。因此,早期发现和治疗对于预防良恶性肿瘤进展和改善患者预后至关重要。
1. 病例介绍
患者,女,65岁。自诉3年前无明显诱因出现右侧外耳道肿物,反复破溃并间断性流脓,伴瘙痒、耳闷,与周围界限不清,无耳鸣、听力下降,就诊于当地医院,予以药物治疗后(具体不详),症状未见明显缓解。近1周来感上述症状加重,来我院就诊,门诊检查后以“外耳道肿物(右)”收入院(图 1)。自发病以来,患者无面瘫,精神可,饮食、睡眠佳,大小便正常,体重无明显变化。
图 1.
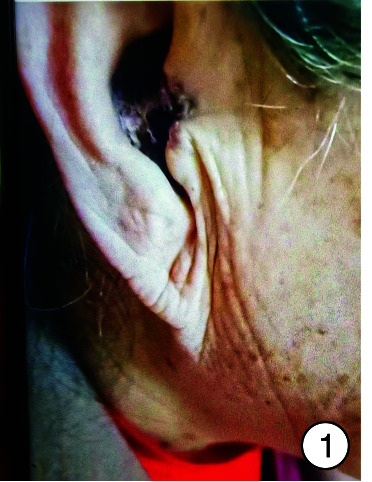
术前耳甲腔图
入院时实验室检查显示感染指标轻度增高,头颈部肿瘤2项结果阴性,内耳CT见图 2。予以右耳耳甲腔局部肿物活检送病检,结果见图 3。多倾向于良性肿瘤,遂采取手术治疗,排除手术禁忌后,全身麻醉下行“右侧外耳道肿物切除术+皮瓣移植修复术+外耳道成型术”。手术过程:患者右耳朝上仰卧,全身麻醉后消毒铺巾,术中见耳甲腔表面破溃流脓,基底部与软骨相连,与周围组织无明显粘连,切开基底部皮肤深达软骨,完整剥离肿物送检,耳后取约4 cm×4 cm大小游离皮瓣,铺于耳甲腔及外耳道创面,间断缝合皮瓣、切口周围皮肤及耳后切口(图 4),皮瓣表面放置碘仿纱条加压包扎。术后给与抗感染、补液及护胃等对症支持治疗,术区规律换药,并加压包扎,术后1周取出耳甲腔填塞物,并予以间断拆线,待伤口完全闭合后将术区缝线完全拆除,患者术区移植皮瓣生长良好,伤口愈合后出院,出院后继续术区换药1周,进行右侧外耳道红光治疗。1周后来我院门诊复查,伤口愈合佳,移植皮瓣生长良好(图 5、6)。术后1个月复查见伤口移植皮瓣生长良好,表面仅见少量干痂附着,外耳道口及外耳道通畅,术区干燥无渗出(图 7)。标本送病检结果毛源性肿瘤,高度倾向TB(图 8)。
图 2.
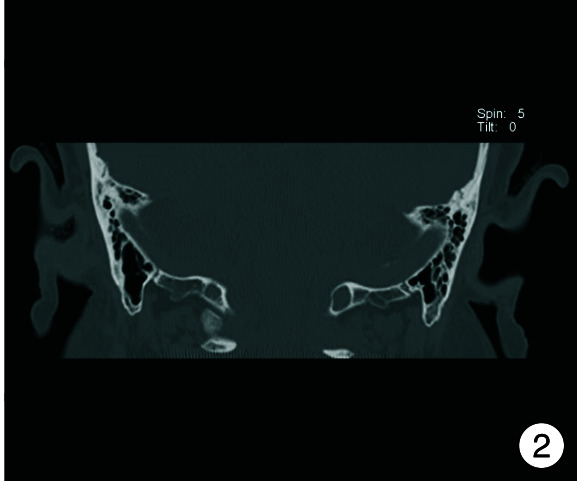
术前内耳CT图
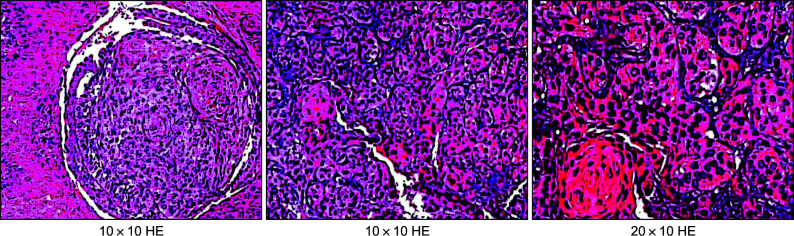
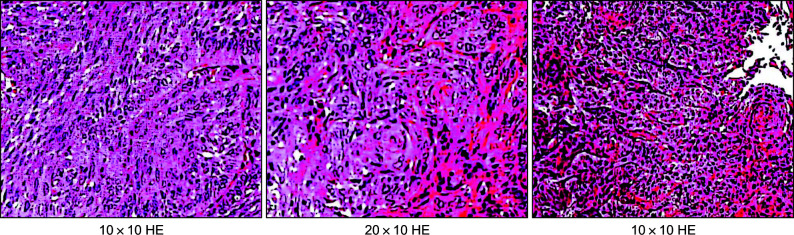
图 3.
耳甲腔病损局部活检病理切片
图 4.
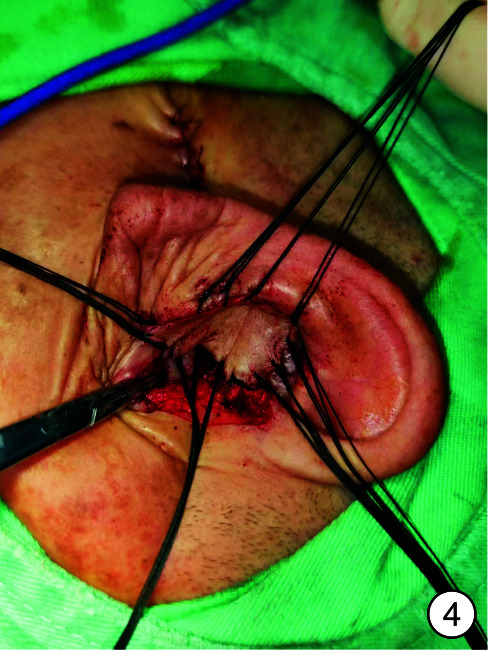
术中皮瓣移植
图 5.
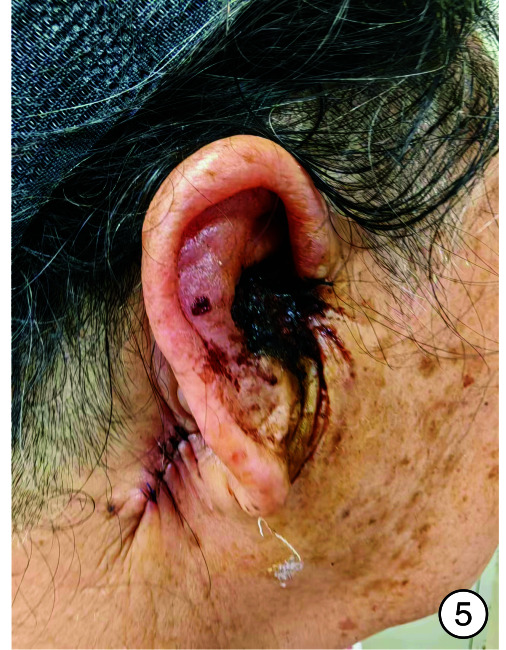
术后2 d
图 6.
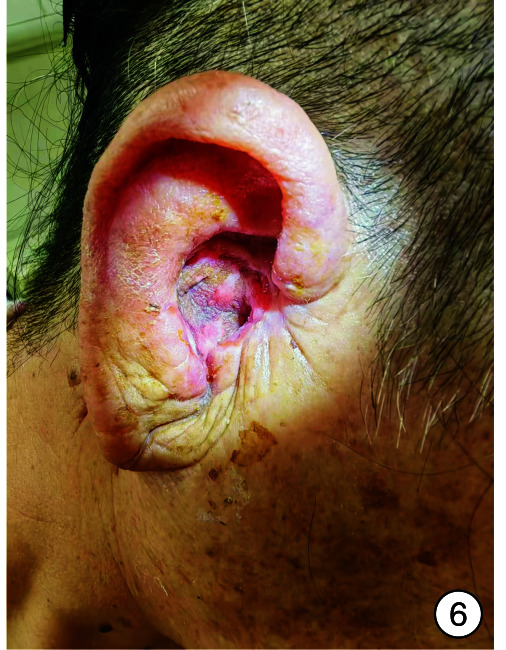
术后1周
图 7.
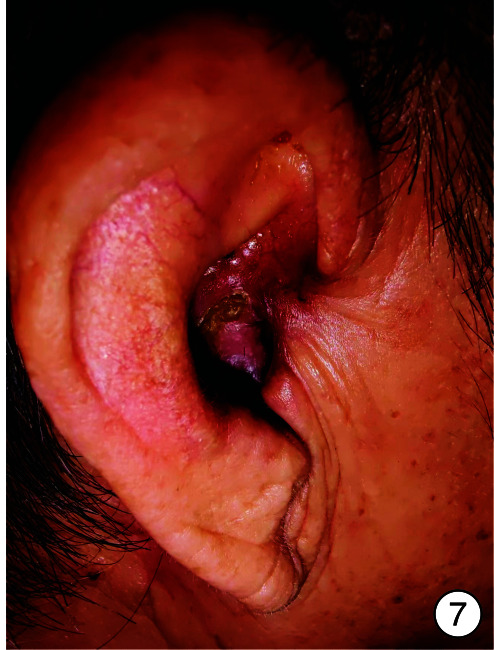
术后1个月
图 8.
术后病理切片
2. 讨论
该病例为耳甲腔毛源性肿瘤,在耳甲腔病变中极为罕见。该病属良性毛发胚芽肿瘤,头皮优先发生[6-9],罕见情况下,可见于四肢近端、躯干、肛周和生殖器区域[10]。可有上覆的表皮脱落且色素沉着,或有浅表毛细血管扩张并出现溃疡。其发病机制至今尚未明确,近几十年来,在几种汗腺和毛囊肿瘤实体中检测到复发性基因融合和(或)点突变,有研究指出SMO和PTCH1的基因突变可能是TB病变的驱动因素[11],并确定了TB中FOXK1::GRHL2 / 2和GPS1::GRHL3 / 22的复发基因融合。也有研究指出TB的家族遗传性[12],如9q22.3基因的突变或去泛素化酶CYLD的功能丧失[13-14],并表明独特的复发性遗传改变可能是大量附属肿瘤发生的原因[15]。鉴于TB皮肤镜下表现出与基底细胞癌相似的特征[16],又有学者提出了紫外线辐射或电离辐射对于TB的驱动作用。但目前对于以上发病机制的研究仍缺乏说服力,未来还需要更深入的研究来验证上述理论的可行性。
TB的诊断主要以临床特征、皮肤镜检查及术后病理结果为主。临床表现为单个孤立、生长缓慢的真皮或皮下结节,无任何临床症状,也可表现为不规则的黑色素沉着,糜烂或溃疡。本患者皮肤镜检查以树化血管和辐轮区域为主,相对较为典型[4]。病理特征是紧密混合的上皮细胞和间充质细胞的双相增殖,在组织学上,TB由对称的椭圆形细胞组成,通过尖锐的边界与环境隔开,包含具有很少有丝分裂活性的单形细胞[17]。根据间充质和上皮成分的生长模式和结构,TB分为8种类型:小结节型、大结节型、网状型(巨型孤立TB)、筛状(典型TB)、外消旋型(非经典TB)、柱状(结缔组织增生性TB)和金刚烷素样(皮肤淋巴腺瘤)[18]。目前报告相对较多的类型是筛状(典型TB)。本患者属色素性TB的一种变体,为TB的组织学变异,及其少见TB的诊断金标准是病理活检,依据活检结果进行下一步诊疗,误诊可导致不必要的切除或延迟转移性疾病的发生和治疗[19]。TB被认为是一种良性肿瘤,很少发生转移,部分肿瘤常表现为斑块变异型,也称毛母细胞纤维瘤,代表了一种更具侵袭性的肿瘤形式[20]。TB也可以转化为成毛母细胞癌(trichoblastoma cancer,TBC)从而发生局部浸润、复发和转移[11]。因此,对于这种具有可能侵袭性生长的肿瘤,不应低估这种罕见但可能未被诊断的双相实体。术前应充分澄清肿瘤性质,包括活检和影像学检查,是最重要的,还要评估放疗必要性[21]。
TBC的鉴别诊断也很重要,因为不同的肿瘤其生长特征、计划手术切缘和复发风险方面的差异[2],导致不同的结局与预后。主要鉴别疾病为基底细胞癌(basal cell carcinoma, BCC),BCC是全球最常见的恶性肿瘤,占所有皮肤恶性肿瘤的75%,是一种具有侵袭性但很少发生转移的癌症[22-23]。BCC通常发生于暴露在阳光下的皮肤区域如头面部,临床表现为日晒区珍珠粉红色结节伴毛细血管扩张,它具有与TB难以鉴别的组织学、形态学、免疫组织化学、临床表现和皮肤镜等特征,其可能原因是2种肿瘤都起源于毛囊,都由基底细胞组成[17]。在皮肤镜下,两者之间的显著差异之一是蓝灰色卵形巢和蓝灰色小球的存在。尽管这一特征具有高度提示性,但在鉴别肿瘤方面似乎价值有限[4]。据报道,免疫组织化学生物标志物,如CK20、雄激素受体(AR)和PHLDA1(一种滤泡干细胞标志物)、CD34(基质)、BCL-2、CK8、CK15、P53、前列素和脂肪因子METRNL阳性及黏蛋白沉积14都可用于帮助鉴别TB和BCC[9, 18, 24-30]。与TB比较,BCC显示出生物标志物的更高表达,这可能与BCC的侵袭性肿瘤行为有关。但目前研究还存在局限性,不足以作为鉴别诊断的主要依据。相关研究报告提出,TB中存在Merkel细胞,在42%~60%的TB中含量丰富,特别是CK20染色中突出显示了Merkel细胞的存在,可作为区分TB和BCC的依据[31-32]。但其鉴别意义仍不及组织病理学和免疫组织化学测定。在组织学上,TB通常由大量的基质细胞和排列在外围形成不规则小梁的肿瘤细胞构成。BCC的组织中存在光学空裂,称为伪影,可发现较少的间充质结构[33]。除与BCC鉴别外,TB还需与BCC变异型粉状纤维上皮瘤(fibroepithelial tumor,FepT)和基底样滤泡错构瘤(basaloid follicular hamartoma,BFH)鉴别。FepT类似于上皮源性肿瘤,组织学表现为基底层上皮肿瘤细胞呈栅栏状,并伴有明显的真皮纤维化,常见基质裂隙与肿瘤细胞回缩征象。BFH是一种罕见的良性附件肿瘤,由位于染色体9q23上的PTCH突变引起,BFH通常以毛囊为中心,局限于浅表真皮,疾病早期,可以看到基底细胞的分支线与毛囊单位相连,在长期疾病的情况下,肿瘤细胞可能完全取代它们。
迄今为止,文献中尚未报道TB的特异治疗方式[34]。诊断明确时,予以临床监测或手术治疗,诊断不明确时,予以活检确诊后手术切除。大多数报道的病例均表现出良性的生物学行为,并且在完全手术切除后未发生复发或转移。由于恶性肿瘤的风险很小但潜在,完全切除肿瘤仍是首选的治疗方法,并建议切除具有阴性切缘的TB[19]。莫氏显微手术应被视为皮下TB的首选治疗方法,以避免复发和不必要的皮肤牺牲在美容敏感区域[28]。
TB是一种毛源性的良性皮肤附件肿瘤,好发于成人头面部。本例患者病变位于耳甲腔,属临床罕见病例,至今尚未报道。也由于本病的罕见性,给临床环境带来了多重挑战,由于无足够的研究来确定恰当的管理过程而导致误诊,造成不必要的切除或延迟转移性疾病的治疗。因此,未来需要进一步的研究来发现合理且有效的治疗和管理方法,减少误诊率,为临床提供更有利的建议和指南,增加良性肿瘤治愈率,减少恶性转移,改善患者预后。
Footnotes
利益冲突 所有作者均声明不存在利益冲突
References
- 1.Cao Z, Liu Y, Wei G, et al. Basal Cell Carcinoma or Trichoblastoma? Two Cases of Nasal Nodules Difficult to Distinguish. Indian J Dermatol. 2022;67(4):448–450. doi: 10.4103/ijd.ijd_197_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sławińska M, Płaszczyńska A, Lakomy J, et al. Significance of Dermoscopy in Association with Clinical Features in Differentiation of Basal Cell Carcinoma and Benign Trichoblastic Tumours. Cancers(Basel) 2022;14(16):3964. doi: 10.3390/cancers14163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolm I, Kastnerova L, Konstantinova AM, et al. Trichoblastoma: A Consecutive Series of 349 Sporadic Cases Analyzed by Ackerman Subtypes. Am J Dermatopathol. 2021;43(12):887–897. doi: 10.1097/DAD.0000000000001895. [DOI] [PubMed] [Google Scholar]
- 4.Pampena R, Peccerillo F, Marghoob NG, et al. Peritumoural clefting as a key feature in differentiating basal cell carcinoma from trichoblastoma through in vivo reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2019;33(5):e201–e203. doi: 10.1111/jdv.15467. [DOI] [PubMed] [Google Scholar]
- 5.Boettler MA, Shahwan KT, Abidi NY, et al. Trichoblastic carcinoma: a comprehensive review of the literature. Arch Dermatol Res. 2022;314(5):399–403. doi: 10.1007/s00403-021-02241-y. [DOI] [PubMed] [Google Scholar]
- 6.Zeller KA, Billmire DF. Trichoblastoma: management of a rare skin lesion. J Pediatr Surg. 2012;47(1):250–252. doi: 10.1016/j.jpedsurg.2011.10.068. [DOI] [PubMed] [Google Scholar]
- 7.Pitarch G, Botella-Estrada R. Dermoscopic Findings in Trichoblastoma. Actas Dermosifiliogr. 2015;106(9):e45–e48. doi: 10.1016/j.ad.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Loh SH, Lew BL, Sim WY. Composite Tumor Associating Trichoblastoma and Seborrheic Keratosis. Ann Dermatol. 2015;27(5):601–604. doi: 10.5021/ad.2015.27.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HC, Sohng SH, Shin DH, et al. Immunohistochemical expression of cytokeratin 15, cytokeratin 19, follistatin, and Bmi-1 in basal cell carcinoma. Int J Dermatol. 2016;55(1):36–44. doi: 10.1111/ijd.12771. [DOI] [PubMed] [Google Scholar]
- 10.Umbert P, Muñoz JF. False-negative tumor-free margins following mohs surgery for aggressive trichoblastoma. Am J Dermatopathol. 2012;34(3):255–258. doi: 10.1097/DAD.0b013e318213f80d. [DOI] [PubMed] [Google Scholar]
- 11.Giang J, Mooyaart AL, Martens-de Kemp SR, et al. Hedgehog pathway mutations are involved in the pathogenesis of plaque-type "trichoblastoma": A report of two cases. J Cutan Pathol. 2023;50(7):674–680. doi: 10.1111/cup.14389. [DOI] [PubMed] [Google Scholar]
- 12.Rashid M, van der Horst M, Mentzel T, et al. ALPK1 hotspot mutation as a driver of human spiradenoma and spiradenocarcinoma. Nat Commun. 2019;10(1):2213. doi: 10.1038/s41467-019-09979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20(24):6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.South AP, Purdie KJ, Watt SA, et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol. 2014;134(10):2630–2638. doi: 10.1038/jid.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kervarrec T, Pissaloux D, Poilane J, et al. Recurrent FOXK1:: GRHL and GPS2:: GRHL fusions in trichogerminoma. J Pathol. 2022;257(1):96–108. doi: 10.1002/path.5872. [DOI] [PubMed] [Google Scholar]
- 16.Pogorzelska-Antkowiak A, Antkowiak R, Poloczek R. Trichoblastoma in reflectance confocal microscopy. Australas J Dermatol. 2023;64(1):e89–e90. doi: 10.1111/ajd.13970. [DOI] [PubMed] [Google Scholar]
- 17.Kocaman N, Yuksel EI, Demir B, et al. Two Novel Biomarker Candidates for Differentiating Basal Cell Carcinoma from Trichoblastoma; Asprosin and Meteorine Like Peptide. Tissue Cell. 2022;76:101752. doi: 10.1016/j.tice.2022.101752. [DOI] [PubMed] [Google Scholar]
- 18.Patel P, Nawrocki S, Hinther K, et al. Trichoblastomas Mimicking Basal Cell Carcinoma: The Importance of Identification and Differentiation. Cureus. 2020;12(5):e8272. doi: 10.7759/cureus.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demant M, Saltvig I, Trøstrup H, et al. Don't Judge a Tumor by Its Biopsy! Case Rep Dermatol. 2020;12(3):266–274. doi: 10.1159/000509764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Requena C, Requena L, Kazakov DV, et al. Multiple facial plaque variant of trichoblastoma. J Cutan Pathol. 2019;46(4):285–289. doi: 10.1111/cup.13416. [DOI] [PubMed] [Google Scholar]
- 21.Weiland T, Sadoghi B, Pondorfer P, et al. Trichoblastic carcinosarcoma of the neck: Case report and molecular analysis of a seldom, relapsing entity and a review of the literature. J Dermatol. 2023;50(5):700–704. doi: 10.1111/1346-8138.16716. [DOI] [PubMed] [Google Scholar]
- 22.Vega Memije ME, Luna EM, de Almeida OP, et al. Immunohistochemistry panel for differential diagnosis of Basal cell carcinoma and trichoblastoma. Int J Trichology. 2014;6(2):40–44. doi: 10.4103/0974-7753.138583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.夏 鹏, 吴 发印, 黄 志权, et al. 巨大结节与角化型面部基底细胞癌1例. 临床耳鼻咽喉头颈外科杂志. 2022;36(4):307–309. doi: 10.13201/j.issn.2096-7993.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi Y, Shi X, Chen D, et al. CD133, but Not CD44, May Serve as a Novel Biomarker for Differential Diagnosis Between Basal Cell Carcinoma and Trichoblastomas. Clin Cosmet Investig Dermatol. 2022;15:1517–1526. doi: 10.2147/CCID.S373331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZC, Hu J, Kong YY, et al. [Application of immunohistochemical staining of bcl-2, Ber-EP4, CD10, CK20, and Ki-67 in differential diagnosis between trichoblastoma and basal cell carcinoma] Zhonghua Bing Li Xue Za Zhi. 2021;50(4):376–381. doi: 10.3760/cma.j.cn112151-20200722-00587. [DOI] [PubMed] [Google Scholar]
- 26.Stanoszek LM, Wang GY, Harms PW. Histologic Mimics of Basal Cell Carcinoma. Arch Pathol Lab Med. 2017;141(11):1490–1502. doi: 10.5858/arpa.2017-0222-RA. [DOI] [PubMed] [Google Scholar]
- 27.Battistella M, Peltre B, Cribier B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: a case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol. 2014;36(8):643–650. doi: 10.1097/DAD.0b013e31828a31ae. [DOI] [PubMed] [Google Scholar]
- 28.Owen JL, Liu W, Croitoru A, et al. Aggressive subcutaneous trichoblastomas successfully treated with Mohs micrographic surgery. Australas J Dermatol. 2019;60(3):e249–e251. doi: 10.1111/ajd.12983. [DOI] [PubMed] [Google Scholar]
- 29.Fusumae T, Tanese K, Takeuchi A, et al. High-grade trichoblastic carcinoma arising through malignant transformation of trichoblastoma: Immunohistochemical analysis and the expression of p53 and phosphorylated AKT. J Dermatol. 2019;46(1):57–60. doi: 10.1111/1346-8138.14686. [DOI] [PubMed] [Google Scholar]
- 30.Leblebici C, Bambul Sıǧırcı B, Kelten Talu C, et al. CD10, TDAG51, CK20, AR, INSM1, and Nestin Expression in the Differential Diagnosis of Trichoblastoma and Basal Cell Carcinoma. Int J Surg Pathol. 2019;27(1):19–27. doi: 10.1177/1066896918781719. [DOI] [PubMed] [Google Scholar]
- 31.Pozo L, Diaz-Cano SJ. Trichogerminoma: further evidence to support a specific follicular neoplasm. Histopathology. 2005;46(1):108–110. doi: 10.1111/j.1365-2559.2005.01955.x. [DOI] [PubMed] [Google Scholar]
- 32.Leeman A, de Cuba E, Jaspars LH, et al. A Low-Grade Trichoblastic Carcinoma Treated with Mohs Micrographic Surgery. Case Rep Dermatol. 2021;13(1):129–133. doi: 10.1159/000512871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Mushcab N, Husain R, Al Subaiei M, et al. Trichoblastoma mimicking basal cell carcinoma and the approach to its management: Case report. Int J Surg Case Rep. 2021;86:106318. doi: 10.1016/j.ijscr.2021.106318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tellechea O, Cardoso JC, Reis JP, et al. Benign follicular tumors. An Bras Dermatol. 2015;90(6):780–796. doi: 10.1590/abd1806-4841.20154114. [DOI] [PMC free article] [PubMed] [Google Scholar]