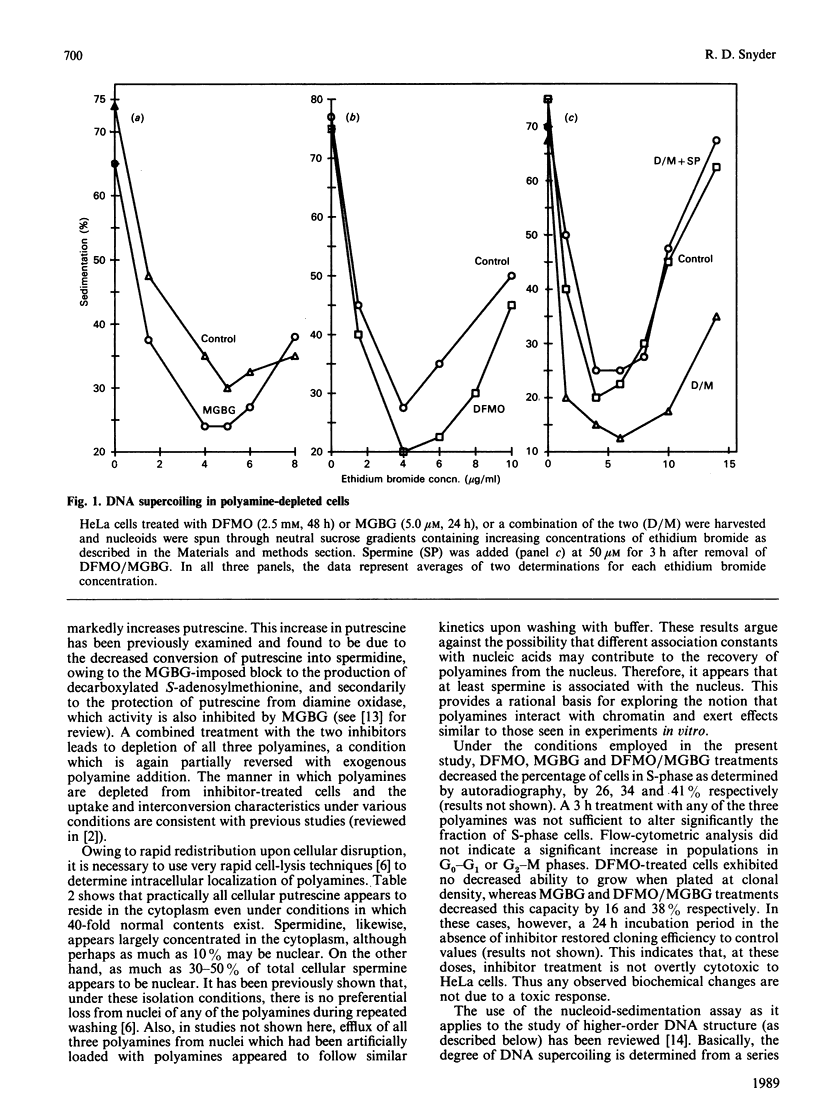
Abstract
HeLa cells depleted of polyamines by treatment with alpha-difluoromethylornithine (DFMO), methylglyoxal bis(guanylhydrazone) (MGBG) or a combination of the two, were examined for sensitivity to micrococcal nuclease, DNAase I and DNAase II. The degrees of chromatin accessibility to DNAase I and II appeared enhanced somewhat in all three treatment groups, and the released digestion products differed from those in non-depleted cells. DNA released from MGBG- and DFMO/MGBG-treated cells by DNAase II digestion was enriched 4-7-fold for Mg2+-soluble species relative to controls. DNA released by micrococcal nuclease digestion from all three treatment groups was characterized as consisting of higher-order nucleosomal structure than was DNA released from untreated cells. At least some of the altered chromatin properties were abolished by a brief treatment of cells with polyamines, notably spermine. These studies provide the first demonstration in vivo of altered chromatin structure in cells treated with inhibitors of polyamine biosynthesis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bowlin T. L., McKown B. J., Sunkara P. S. Ornithine decarboxylase induction and polyamine biosynthesis are required for the growth of interleukin-2- and interleukin-3-dependent cell lines. Cell Immunol. 1986 Apr 1;98(2):341–350. doi: 10.1016/0008-8749(86)90294-7. [DOI] [PubMed] [Google Scholar]
- Castro C. E., Armstrong-Major J., Ramirez M. E. Diet-mediated alteration of chromatin structure. Fed Proc. 1986 Aug;45(9):2394–2398. [PubMed] [Google Scholar]
- Crapper D. R., Quittkat S., de Boni U. Altered chromatin conformation in Alzheimer's disease. Brain. 1979 Sep;102(3):483–495. doi: 10.1093/brain/102.3.483. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Xue S. B., Melamed M. R. Effect of n-butyrate on cell cycle progression and in situ chromatin structure of L1210 cells. Exp Cell Res. 1981 Dec;136(2):279–293. doi: 10.1016/0014-4827(81)90006-9. [DOI] [PubMed] [Google Scholar]
- Hougaard D. M., Bolund L., Fujiwara K., Larsson L. I. Endogenous polyamines are intimately associated with highly condensed chromatin in vivo. A fluorescence cytochemical and immunocytochemical study of spermine and spermidine during the cell cycle and in reactivated nuclei. Eur J Cell Biol. 1987 Aug;44(1):151–155. [PubMed] [Google Scholar]
- Hung D. T., Marton L. J., Deen D. F., Shafer R. H. Depletion of intracellular polyamines may alter DNA conformation in 9L rat brain tumor cells. Science. 1983 Jul 22;221(4608):368–370. doi: 10.1126/science.6408733. [DOI] [PubMed] [Google Scholar]
- Kleppe K., Osland A., Fosse V., Male R., Lossius I., Helland D., Lillehaug J. R., Raae A. J., Kleppe R. K., Nes I. F. Effect of polyamines on enzymes involved in DNA repair. Med Biol. 1981 Dec;59(5-6):374–380. [PubMed] [Google Scholar]
- Kootstra A. Isolation of high mobility group-containing mononucleosomes from avian erythrocyte nuclei and their sensitivity to DNase I. J Biol Chem. 1982 Nov 10;257(21):13088–13094. [PubMed] [Google Scholar]
- Levitt A., Axel R., Cedar H. Nick translation of active genes in intact nuclei. Dev Biol. 1979 Apr;69(2):496–505. doi: 10.1016/0012-1606(79)90307-5. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Lukiw W. J., De Boni U., McLachlan D. R. Changes in chromatin structure associated with Alzheimer's disease. J Neurochem. 1981 Nov;37(5):1193–1202. doi: 10.1111/j.1471-4159.1981.tb04670.x. [DOI] [PubMed] [Google Scholar]
- Lipetz P. D., Galsky A. G., Stephens R. E. Relationship of DNA tertiary and quaternary structure to carcinogenic processes. Adv Cancer Res. 1982;36:165–210. doi: 10.1016/s0065-230x(08)60425-x. [DOI] [PubMed] [Google Scholar]
- Mach M., Ebert P., Popp R., Ogilvie A. Compartmentalization of polyamines in mammalian cells. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1327–1334. doi: 10.1016/0006-291x(82)91395-x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Analysis of methylglyoxal bis(guanylhydrazone)-induced alterations of hamster tumor mitochondria by correlated studies of selective rhodamine binding, ultrastructural damage, DNA replication, and reversibility. Cancer Res. 1984 Jun;44(6):2677–2688. [PubMed] [Google Scholar]
- Palvimo J., Pohjanpelto P., Linnala-Kankkunen A., Mäenpä P. H. Alterations in amounts and covalent modifications of low-molecular-weight chromosomal proteins in Chinese hamster ovary cells during polyamine depletion. Biochim Biophys Acta. 1987 Jun 6;909(1):21–29. doi: 10.1016/0167-4781(87)90042-x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P., Hölttä E., Jänne O. A., Knuutila S., Alitalo K. Amplification of ornithine decarboxylase gene in response to polyamine deprivation in Chinese hamster ovary cells. J Biol Chem. 1985 Jul 15;260(14):8532–8537. [PubMed] [Google Scholar]
- Pohjanpelto P., Knuutila S. Polyamine deprivation causes major chromosome aberrations in a polyamine-dependent Chinese hamster ovary cell line. Exp Cell Res. 1982 Oct;141(2):333–339. doi: 10.1016/0014-4827(82)90221-x. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986 Jul-Aug;6(4):525–542. [PubMed] [Google Scholar]
- Smith P. J. n-Butyrate alters chromatin accessibility to DNA repair enzymes. Carcinogenesis. 1986 Mar;7(3):423–429. doi: 10.1093/carcin/7.3.423. [DOI] [PubMed] [Google Scholar]
- Sunkara P. S., Rao P. N., Nishioka K., Brinkley B. R. Role of polyamines in cytokinesis of mammalian cells. Exp Cell Res. 1979 Mar 1;119(1):63–68. doi: 10.1016/0014-4827(79)90335-5. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Sikorska M. Modulation of the sensitivity of chromatin to exogenous nucleases: implications for the apparent increased sensitivity of transcriptionally active genes. Biochemistry. 1986 Jul 1;25(13):3839–3845. doi: 10.1021/bi00361a015. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Seidenfeld J. Aspects of the biochemical pharmacology of methyl glyoxal bis(guanylhydrazone). Biochem Pharmacol. 1986 Apr 15;35(8):1217–1225. doi: 10.1016/0006-2952(86)90263-7. [DOI] [PubMed] [Google Scholar]


