Abstract
Background and aim
This study applied Six Sigma metrics to facilitate the quality control (QC) review for hospital glucose meters.
Materials and methods
QC data from a period of six months on all hospital glucose meters were extracted from the data management system. Sigma values for each meter at two QC levels were calculated and evaluated each month by combining the imprecision, the absolute bias between the meter mean and all-meter mean, and the standards from ISO 15179:2013. The effectiveness of using Sigma values in identifying meters with possible quality problems for further Levey-Jennings QC chart review was assessed.
Results
More than 80 % of the meter's Sigma values within the six months were greater than 4 at either QC level. At the high QC level, twice as many Sigma values were below 4 than the low QC level. Including Sigma values 4, 3.5 or 3 in the criteria for the QC review reduced the number of chart review to 32.8 %, 11.2 % or 3.5 %, respectively.
Conclusions
The majority of the glucose meters examined in this study demonstrated optimal Sigma values. The Sigma metrics-based approach could be a valuable tool to guide an effective QC review of glucose meters for quality improvement.
Keywords: Glucose meter, Point-of-care testing, Quality control, Quality improvement, Six Sigma metrics
1. Introduction
Glucose meters are a common type of point-of-care testing (POCT) device used to monitor patient glucose concentrations for glycemic control. Errors related to operator competency, strip storage, or device stability can occur during the testing process and affect the measurement of glucose meters in service [[1], [2], [3]]. After glucose meters have been fully validated by the POCT program and implemented into clinical units, a quality control (QC) strategy must be established to regularly monitor the stability of their analytical performance [4,5].
A well-planned QC strategy for glucose meters should be similar to that for core laboratory tests. Two or more levels of internal QC materials, containing different concentrations of glucose are tested on the meters at a defined frequency. Meter operators are trained to review the results and troubleshoot the failures as needed. Patient samples should not be tested when a QC result falls outside of the acceptance range [6,7]. Additionally, QC performance of all glucose meters should be regularly reviewed and monitored (e.g. monthly) to ensure the long-term stability of their measurement. During this review, QC statistics, including imprecision and bias of QC results, are reviewed alongside meters’ Levey-Jennings QC charts plotted with daily QC results. Increased imprecision and/or bias beyond the fixed standard deviation (SD), as well as specific QC patterns in the Levey-Jennings QC charts, may indicate poor analytical performance, possible measurement changes, or other quality problems, requiring further investigation [6,7]. A mini-validation using patient samples or reference materials may be conducted on these meters. The types of errors identified may necessitate additional operator training, physician notification, or returning the meters to the manufacturer for further evaluation.
Although this QC strategy has been proven to be successful for core laboratory tests, its application to POCT glucose meters poses challenges. In current practice, the total number of meters used across all clinical units in a tertiary hospital can reach hundreds, making it difficult for POCT specialists to determine meter specific acceptance ranges for Levey-Jennings QC charts as is done for core laboratory tests. Instead, the acceptance ranges established by the manufacturer are commonly relied to set the low, high limits, as well as the fixed SD to determine whether the QC results pass or fail during daily QC check. However, the manufacturer's acceptance ranges are often broad and could be 6–13 times larger than the meter's actual running SD [8], leading to the overlooking of problematic meters in monitoring and investigation. Meanwhile, POCT specialists are unable to assess the analytical performance of meters and identify the meters with quality problems when the imprecision or bias on these meters fall within the manufacturer's SD. Moreover, reviewing QC charts for hundreds of meters in a limited timeframe is unfeasible for POCT Specialists, resulting in incomplete quality reviews of glucose meters. Currently, there are no recommendations for establishing QC acceptance ranges on hundreds of glucose meters using meters' running SDs, nor for conducting a monthly QC review for glucose meters, as is available for core laboratory instruments. Our literature search has not yielded any studies addressing the facilitation of QC review for glucose meters.
For safe glucose control, it is essential to establish an effective approach to overcome the existing deficiencies in current QC settings and QC review of glucose meters. Implementing a QC review based on Sigma metrics may serve as an effective approach to address these deficiencies. The Six Sigma metrics offer a promising tool for evaluating the quality of testing processes. This approach has been used to assess the analytical performance of core laboratory tests by combining the total allowable error (TEA), imprecision, and bias of QC data [9,10]. It has been also used to evaluate the analytical performance of POCT devices, such as blood gas analyzers and glucose meters [11,12]. According to the established standards, tests with a Sigma value above 6, 5, 4, and 3 are considered to demonstrate world-class, excellent, good, or minimal acceptable quality, respectively [9,10]. Hospital-used POCT glucose meters are typically connected to a data management system (DMS) that records various data related to patients, operators, samples, reagents, testing, and results from glucose measurements [13,14]. QC data from each meter, including the mean, imprecision, and bias can be easily extracted from the DMS on a monthly basis and used to calculate Sigma values. During regular QC reviews, these Sigma values may allow POCT specialists to assess the analytical performance of each glucose meter by considering TEAs, the imprecision, and the bias, rather than relying solely on the manufacturer's wide QC acceptance ranges.
In this study, we extracted QC results generated on more than one hundred glucose meters over a six month period from the DMS. Sigma values at two QC levels (low and high levels) were evaluated for each glucose meter each month. Additionally, we investigated the effectiveness of using Sigma values to identify the meters with poor analytical performance during the QC review. The Sigma metrics-based approach was established in this study for regular QC review of glucose meters to ensure the quality of glucose meters.
2. Materials and Methods
2.1. POC glucose meters and QC material
The hospital glucose meters (Nova StatStrip, Nova Biomedical, Mississauga, ON, Canada) were implemented under the quality management of the POCT program. The meters use electrochemistry methodology and are monitored by testing low and high levels of liquid quality control material from the manufacturer every 24 h. In current service, the manufacturer's QC acceptable ranges are employed in the QC settings. The typical assigned values are 3.4 mmol/L (SD 0.4 mmol/L) for the low level of QC material and 16.65 mmol/L (SD 1.38 mmol/L) for the high level of QC material. The acceptable ranges are 2.6–4.2 mmol/L and 13.93–19.40 mmol/L for the low and high QC materials when QC rule is 12S, respectively. The QC check fails if a result falls outside the QC acceptance range. The operators address the outliers following laboratory standardized procedures: verifying the level, quality, and expiration of QC material, reviewing the QC testing procedure, performing a retest with fresh material if necessary, and reporting unresolved outliers to the POCT specialists.
2.2. Data management system and data collection
Each hospital glucose meter is connected to the DMS (Telcor QML, Telcor Inc., Lincoln, NE, USA) for documentation. QC data from February 2022 to July 2022 was extracted for all meters by the POCT specialist and Clinical Biochemist, including the meter's serial number, location, and number of QC results. The mean, SD, and coefficient of variation (CV) of QC results were calculated by the DMS at two QC levels for each meter in each month. In addition, Levey-Jennings QC charts were generated by the DMS for all glucose meters, displaying QC results, fixed SD and running SD.
In this study, the majority of the meters performed QC testing every 24 h and accumulated more than 20 QC results per month for review and monitoring. A small number of meters had fewer than 20 QC results due to occasional change in the lot of QC material (usually once a year) or infrequent use. The statistical QC data was calculated by DMS for each meter by the lot number. To obtain reasonable quality of statistic QC data and monitor as many glucose meters as possible, only glucose meters with 15 or more QC points per month, using test strips from the same lot, were included in the data analysis.
2.3. Total allowable errors for glucose meters
The TEAs for the glucose meters were derived from the recommendations provided by the International Organization for Standardization (ISO) 15197:2013 [15], which allows for a variation of ±0.83 mmol/L when glucose concentration is less than 5.6 mmol/L or ±15 % when glucose concentration is greater than or equal to 5.6 mmol/L.
2.4. Calculation of Sigma values of glucose meters
A monthly Sigma value was calculated for each glucose meter using the following formula: Sigma value = (TEA-bias)/SD for QC low level, and (TEA% - bias%)/CV for QC high level. Here, SD of each meter was calculated by the DMS for each month. Bias was determined as the absolute difference between the QC mean of each meter in the particular month and the QC mean of all meters over the six months. Bias percentage was calculated relative to the mean of all meters.
2.5. Statistical analysis
Data sorting was performed in Excel (Microsoft, 2010). The meter's unique identification numbers were used to track them across the six months. The comparison of the mean of Sigma values and the comparison of categorical difference of Sigma values among multiple groups were conducted by one-way ANOVA and Chi-Square tests, respectively, in SPSS (IBM SPSS Software, version 28). Linear regression analysis between the Sigma values and the variables was also performed using SPSS. Differences were significant if p < 0.05. Sensitivity analysis was performed using what-if analysis in Excel to optimize meters' imprecision and bias and to achieve the best Six Sigma values. The data table was created by all-meter imprecision from mean-3SD to mean+3SD and all-meter bias from the 5th to 95th percentile.
3. Results
3.1. Overall QC performance and Sigma values of glucose meters in six months
Table 1 shows the overall QC performance and Sigma values of the meters within the six-month period. The QC mean in each month consistently fell below the manufacturer's assigned value at both QC levels. The average imprecision (SD for the low QC level, CV for the high QC level), average bias (value for low QC level, percentage for high QC level), and the median of absolute bias at both QC levels remained stable across the six months. The average Sigma value of all included meters throughout the study period at the low QC level was 5.61, which exceeded that of the high QC level at 4.91. The average Sigma values per month at both QC levels had no significant difference across the six months.
Table 1.
Overall QC performance and Sigma values of the glucose meters in the six months.
| Low QC level (mmol/L) |
High QC level (mmol/L) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | Number of Meters | Mean | SD | Bias | Absolute bias | Sigma value | Number of Meters | Mean | CV | Bias% | Absolute Bias% | Sigma Value |
| 1 | 103 | 3.22 ± 0.05 | 0.15 ± 0.04 | 0.00 ± 0.05 | 0.04 | 5.83 ± 1.79 | 101 | 15.71 ± 0.18 | 2.94 ± 0.65 | 0.03 ± 1.17 | 0.85 | 5.04 ± 1.24 |
| 2 | 102 | 3.21 ± 0.05 | 0.15 ± 0.03 | 0.00 ± 0.05 | 0.04 | 5.69 ± 1.39 | 104 | 15.68 ± 0.19 | 3.01 ± 0.69 | −0.03 ± 1.20 | 0.75 | 4.88 ± 0.96 |
| 3 | 98 | 3.23 ± 0.05 | 0.15 ± 0.03 | 0.00 ± 0.05 | 0.03 | 5.74 ± 1.49 | 97 | 15.73 ± 0.21 | 3.08 ± 0.91 | 0.02 ± 1.29 | 0.80 | 4.85 ± 0.93 |
| 4 | 102 | 3.25 ± 0.06 | 0.15 ± 0.03 | 0.00 ± 0.05 | 0.03 | 5.40 ± 1.23 | 105 | 15.79 ± 0.20 | 3.01 ± 0.70 | −0.00 ± 1.25 | 0.81 | 4.92 ± 1.24 |
| 5 | 101 | 3.25 ± 0.06 | 0.16 ± 0.04 | 0.00 ± 0.06 | 0.04 | 5.38 ± 1.39 | 108 | 15.77 ± 0.20 | 3.01 ± 0.63 | −0.03 ± 1.25 | 0.81 | 4.86 ± 1.05 |
| 6 | 88 | 3.27 ± 0.05 | 0.15 ± 0.03 | 0.00 ± 0.05 | 0.04 | 5.57 ± 1.40 | 96 | 15.75 ± 0.20 | 2.98 ± 0.56 | −0.00 ± 1.25 | 0.78 | 4.88 ± 1.00 |
Data were expressed as mean ± SD, except that absolute bias was expressed as median. Manufacturer's assigned value: low QC level 3.40 mmol/L, high QC level 16.65 mmol/L. QC; quality control. SD; Standard deviation. CV; correlation coefficient.
3.2. Sigma values at low and high QC levels
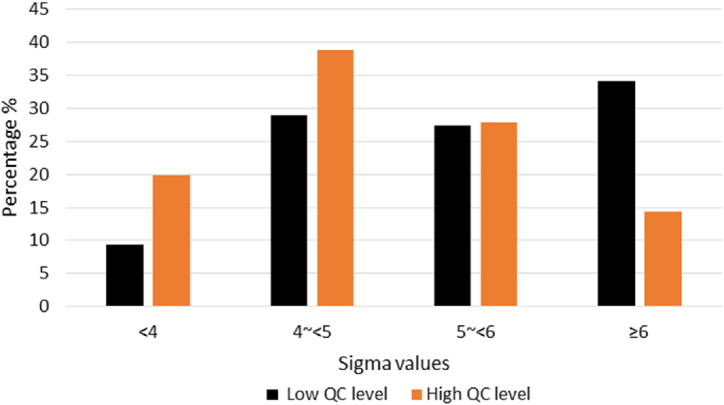
Sigma values of the glucose meters ranged from 2.75 to 12.92 at the low QC level and 1.86 to 10.16 at the high QC level across the six-month period. Fig. 1 shows the distribution of Sigma values throughout the study period. 90.5 % of Sigma values at the low QC level and 80.1 % at the high QC level were greater than 4. Furthermore, at the high QC level, there were half the amount of Sigma values observed greater than 6 (14.3 % vs. 34.1 %), and double the amount of Sigma values observed less than 4 (18.8 % vs. 9.4 %) compared to the low QC level. These differences were found to be statistically significant (p < 0.001).
Fig. 1.
Distribution of Sigma values of the glucose meters at the low and high QC levels in the six month period. QC; quality control.
3.3. Variation of Sigma values on the same glucose meter in six months
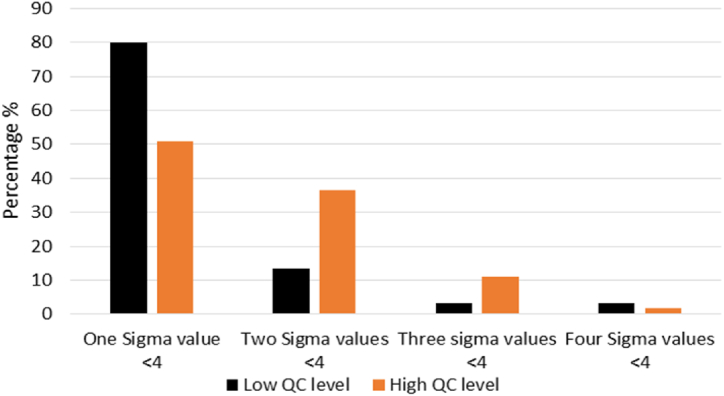
There were 64 meters at low QC level and 74 meters at high QC level that had more than 15 data points in all six months. None of the meters consistently had Sigma values ≥ 6 across the six months at either QC level. Only 6.3 % of the meters maintained Sigma values ≥ 5 across the six months at the low QC level. Additionally, 53.1 % (low QC level) and 25.7 % (high QC level) of the meters had consistent Sigma values ≥ 4 across the six months. The majority of the meters (95.3 % and 87.8 % at the low QC level and the high QC level, respectively) had Sigma values that varied greatly between 3 and ≥6 across the six months. It seems that low Sigma values could occur randomly in any month on the meters, as most of the meters (93.3 % at the low QC level and 87.3 % at the high QC level) had Sigma value < 4 only in one or two out of six months (Fig. 2). Several meters (2 meters at the low QC level and 7 meters at the high QC level) had Sigma values < 4 in three or four months. Furthermore, only 3 meters had Sigma values < 4 in two or more months at both the low and high QC levels.
Fig. 2.
Sigma values on the same glucose meter across the six months. QC; quality control.
3.4. Sigma values and meter QC performance
Table 2 illustrates the distribution of meters' imprecision and the bias compared to the six-month aggregate meter SD at different Sigma values. Meter SD/all-meter SD describes the difference between a meter's imprecision and the all-meter imprecision, while meter bias/all-meter SD, as the number of standard deviations from the overall mean, displays how far a meter's mean is from the all-meter mean. For the group of Sigma values ≥ 4, the imprecision in 60.9 % and 70.9 % of the meters fell within the all-meter SD at the low and high QC level, respectively. The imprecision of the remaining meters was within 1.5 times the all-meter SD. No meter with imprecision greater than 1.5 times the all-meter SD was found in the group of Sigma values ≥ 4. Most of the meters with Sigma values < 3 exhibited imprecision greater than 1.5 times the all-meter SD at both levels (4 out of 4 at the low QC level, 10 out of 12 at the high QC level). However, the majority of meters (99.2 % at the low QC level and 98.2 % at the high QC level) had their absolute bias within the all-meter SD at both QC levels. Only 16 meters included in both QC levels, had an absolute bias of 1–1.5 times the all-meter SD and were distributed in different groups of Sigma values. No meter's absolute bias exceeded 1.5 times the all-meter SD.
Table 2.
Glucose meters' QC performance at different Sigma values.
| Number of meter | Sigma value ∼<3 | Sigma value ∼<3.5 | Sigma value ∼<4 | Sigma value ≥ 4 | ||
|---|---|---|---|---|---|---|
| QC low level, meter SD/all-meter SD | ∼ ≤ 1 | 327 | 0 | 0 | 0 | 327 |
| ∼ ≤ 1.5 | 248 | 0 | 4 | 34 | 210 | |
| >1.5 | 19 | 4 | 12 | 3 | 0 | |
| QC low level, meter Bias/all-meter SD | ∼ ≤ 1 | 589 | 4 | 16 | 35 | 534 |
| ∼ ≤ 1.5 | 5 | 0 | 0 | 2 | 3 | |
| >1.5 | 0 | 0 | 0 | 0 | 0 | |
| QC high level, meter SD/all-meter SD | ∼ ≤ 1 | 350 | 0 | 0 | 0 | 350 |
| ∼ ≤ 1.5 | 247 | 2 | 22 | 78 | 145 | |
| >1.5 | 13 | 10 | 3 | 0 | 0 | |
| QC high level, meter Bias/all-meter SD | ∼ ≤ 1 | 599 | 7 | 25 | 80 | 487 |
| ∼ ≤ 1.5 | 11 | 3 | 1 | 0 | 7 | |
| >1.5 | 0 | 0 | 0 | 0 | 0 | |
All-meter SD of the six months at the low QC level was 0.15 mmo/L, at the high QC level 0.47 mmol/L. QC; quality control. SD; Standard deviation.
3.5. The use of Sigma values to identify meters with possible quality problems for Levey-Jennings QC chart review
In our current monthly QC review, following the local regulatory requirements [7], if the meter's imprecision exceeds the all-meter SD of the overall mean and/or the meter's absolute bias (mean shifts up or down) exceeds the all-meter SD of the overall mean, the meter's Levey-Jennings QC charts for that month need to be thoroughly reviewed for the causes [7]. From Table 2, although only 16 meters had an absolute bias greater than the all-meter SD, more than 40 % of the meters at either QC level had imprecision greater than the all-meter SD. Therefore, up to 543 QC charts during the study period needed to be reviewed if all-meter SD instead of the manufacturer's SD was used in QC review. When the criteria included Sigma value less than 4, 3.5 or 3, the number of QC chart requiring review in both QC levels greatly reduced to 32.8 %, 11.2 %, and 3.5 %, respectively (Table 3).
Table 3.
Number of Levey-Jennings QC charts needed to be reviewed for the investigation.
| Criteria for QC review | QC level | Number of QC chart | Total |
|---|---|---|---|
| Bias > all-meter SD | Low | 5 | 543 |
| high | 11 | ||
| Imprecision > all-meter SD | Low | 267 | |
| high | 260 | ||
| Bias > all-meter SD + Sigma value < 4 | Low | 2 | 178 |
| high | 4 | ||
| Imprecision > all-meter SD + Sigma value < 4 | Low | 57 | |
| high | 115 | ||
| Bias > all-meter SD + Sigma value < 3.5 | Low | 0 | 61 |
| high | 4 | ||
| Imprecision > all-meter SD + Sigma value < 3.5 | Low | 20 | |
| high | 37 | ||
| Bias > all-meter SD + Sigma value < 3 | Low | 0 | 19 |
| high | 3 | ||
| Imprecision > all-meter SD + Sigma value < 3 | Low | 4 | |
| high | 12 |
QC; Quality control. SD; standard deviation.
3.6. Sigma values and associated QC patterns in Levey-Jennings QC chart review
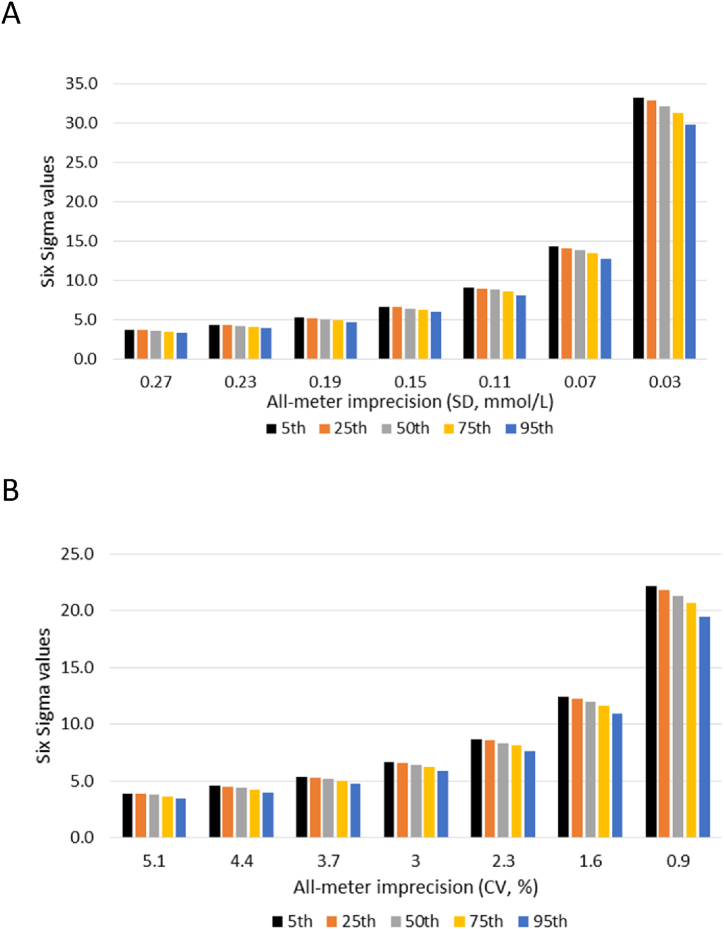
A total of 172 Levey-Jennings QC charts with Sigma values less than 4 were reviewed to understand the QC patterns that might be associated with poor Sigma values (Table 4). A common pattern observed in the QC chart review was 1–3 outliers per month at either QC levels. 75–80 % of the outliers were located above or below ±4SD of the QC charts when the meters' Sigma values were below 3. With an increased number of charts without outliers (from 25 % to 62 %), and more QC outliers located between ±3SD and 4SD than above or below ±4SD (from 25 % to 83 %), the meters' Sigma values increased. The decrease in the number of outliers and the decrease in the number of extreme outliers (above or below ±4SD) led to the reduction in the meters' imprecision from 1.95 to 1.21 (average of meter SD/all-meter SD). However, similar bias among the different Sigma values was observed. The significant association (p < 0.001) of Sigma values with the meters' imprecision was confirmed by linear regression analysis, but not with the meters' bias. Furthermore, in the sensitivity study (Fig. 3), we found that as long as the meters' imprecision did not exceed +2SD of the all-meters’ mean imprecision, the meters' bias, ranging from the 5th to 95th percentile of the all-meter bias did not prevent the meters’ Sigma values from reaching 4.
Table 4.
QC outliers on the glucose meters with Sigma values less than 4 in the QC chart review.
| Sigma value | QC level | Number of QC chart | Chart without outlier (%) | Outlier between ±3SD and 4SD (%) | Outlier above or below ±4SD(%) | Average of meter SD/all-meter SD | Average of meter bias/all-meter SD |
|---|---|---|---|---|---|---|---|
| ∼<3 | Low | 4 | 25 | 25 | 75 | 1.81 | 0.39 |
| High | 12 | 27 | 20 | 80 | 1.95 | 0.68 | |
| ∼<3.5 | Low | 16 | 31 | 67 | 33 | 1.56 | 0.45 |
| High | 25 | 22 | 30 | 70 | 1.42 | 0.39 | |
| ∼<4 | Low | 37 | 57 | 68 | 32 | 1.37 | 0.38 |
| High | 78 | 62 | 83 | 17 | 1.21 | 0.45 |
Absolute bias was used in the calculation of average meter bias/all-meter SD to eliminate the effect of the direction of bias. QC; quality control. SD; standard deviation.
A.
Fig. 3.
Sensitivity analysis for optimizing meters' imprecision and bias. A. Low QC level. B. High QC level. The all-meter imprecision on the X-axis from left to right represented mean+3SD, mean+2SD, mean+1SD, mean, mean-1SD, mean-2SD, and mean-3SD. The different bins represented the 5th, 25th, 50th, 75th, and 95th percentiles of all-meter absolute bias, respectively. The biases were 0.00, 0.01, 0.04, 0.06, and 0.11 mmo/L for the low QC level; 0.08, 0.37, 0.80, 1.38, and 2.47 % for the high QC level. QC; quality control. SD; standard deviation. CV; correlation coefficient.
4. Discussion
This study comprehensively evaluated the Sigma values of the glucose meters used in our hospitals during a six-month period and assessed the effectiveness of Sigma metrics-based approach in meter QC review. The majority of the Sigma values on the glucose meters were greater than 4 when ISO15197:2013 standards were used as TEAs. Sigma values varied between the two QC levels, and they also varied randomly from 3 to >6 on the same meter across the six-month period. When Sigma values were included in the criteria for QC review, the number of QC charts requiring further investigation was greatly reduced to a manageable level. Additionally, high imprecision resulted from extreme outliers might contribute to the meters’ poor Sigma values in our hospitals.
In our study, more than 80 % of the Sigma values on the glucose meters were greater than 4 at both QC levels, respectively, aligning with the improved analytical performance of the new generation glucose meters [3,17]. The Sigma values appear to be better than those reported in two studies that used different glucose meters from a similar generation [12,16]. The primary reason could be the application of different total allowable errors that have not been standardized for glucose meters [10]. The ranges of total allowable errors in recently published international guidelines and the local accreditation agency are from 0.67 to 1.0 mmol/L when glucose is below 5.6 mmol/L and 12 %–20 % when glucose is greater than or equal to 5.6 mmol/L, [15,18,19]. We used the standards from ISO 15197:2013 as did Fei's study, which is used globally [12]. The second factor that frequently affects Sigma values is the determination of bias [9]. Previous studies used assigned values of different testing material as the targets [12,16]. Our glucose meters showed consistent negative bias at both QC levels when compared to the QC assigned values from the manufacturer (Table 1). When we used the assigned values as the targets, the Sigma values could be falsely increased if the QC results were shifted up from the meter mean to the assigned value. Therefore, we used the group mean of all meters in the six months at each QC level as the target to calculate the bias for every meter as this would better reflect the shift or bias of the glucose meters in our study [6]. Additionally, in Fei's study the imprecision data was collected from meters used in various hospitals participating in a Proficiency Testing Program, so the imprecision might be affected greatly by inter-lab variation [12].
The average Sigma value at the high QC level was generally lower than the low QC level (4.91 vs 5.61). Additionally, at the high QC level, twice as many Sigma values were below 4, and half as many Sigma values were above 6 compared to the low QC level. The difference of Sigma values among analyte concentrations have been also observed when Sigma metrics was used to evaluate core laboratory chemistry tests [20]. It is likely attributed to the glucose measurement at different concentrations (3.24 at the low QC level vs. 15.74 mmol/L at the high QC level) and the use of different total allowable errors (0.83 mmol/L vs. 15 %) [16,20].
Sigma values also exhibited a large variation on the same glucose meter across the six months in this study. The number of meters that consistently had Sigma values ≥ 4 in all six months were limited. The Sigma values on most of meters (95.3 % for the low QC level and 87.8 % for the high QC level) varied from 3 to >6 over the six months. This variation seems to follow a random pattern due to outliers, as most meters had Sigma value below 4 in only one or two out of six months at one of the QC levels. With more QC points included in the calculation of Sigma values during long-term monitoring, the variation may be reduced.
Sigma values can be used to evaluate QC performance quantitatively by considering the total allowable error, meters’ imprecision, and the bias. Even if imprecision or bias is slightly increased, the overall analytical performance of the meters may still be acceptable as long as the Sigma values are kept at an acceptable level. If our current QC review criteria were followed, up to 543 QC charts in the period of study may require QC chart review for an investigation, which is impossible to complete with the limited time and staff. After we included Sigma values in the criteria for QC review, the number of QC chart needing review for both QC levels was greatly reduced to 32.8 % (Sigma 4), 11.2 % (Sigma 3.5) and 3.5 % (Sigma 3). These QC charts were able to be reviewed by our POCT specialists in a timely manner.
A thorough QC chart review allows us to identify the QC patterns that relate to types of errors in the testing process [21,22]. In this study, the Levey-Jennings QC charts of all meters with Sigma values less than 4 were reviewed, which provided us more details about the causes of poor Sigma values. It is worth noting that the glucose meters used in this study were a newer generation, resulting in remarkably small biases when compared to the group mean of all meters. The majority of meters had the mean shift within the SD of all-meter mean at both QC levels, which might have minor effect on the Sigma values in our study. However, more than 40 % of meters showed increased imprecision at both QC levels, which might be mainly associated with the number and the level of outliers. With a reduction in the number and level of outliers, meters' Sigma values were increased (Table 4). Moreover, from the sensitivity analysis, if the meters' imprecision did not exceed +2SD of the all-meter mean imprecision, the meters' bias, ranging from the 5th to 95th percentile of the all meter bias did not prevent the meters’ Sigma values from reaching 4. These information indicated that random operator errors in QC sample preparation or testing process might be an important cause of poor meter Sigma values in our hospitals [2,3]. Therefore, improving imprecision of glucose meters should be a focus for quality improvement. More details about meter locations or operators can be identified for planning retraining and audits [16].
Applying the Sigma metric-based approach to the QC review of glucose meters could be easy, robust, and applicable to other laboratories or POCT Programs. The approach can be included as a part of standardized QC review procedures. However, we should note that using different total allowable errors and different sources of bias make the Sigma values incomparable between the studies or hospitals. In addition, laboratories may have different criteria or procedures for the QC review of glucose meters based on meters' analytical performance, common types of errors in the testing process, capacity of quality management, and risk to patient safety. Therefore, implementing the Sigma metrics-based approach in glucose meter QC review should be optimized to fit the quality targets, special needs, and operation procedures of individual laboratories. When interpreting meters' Sigma values, a meter with a Sigma value of 4 or greater doesn't mean there are no errors, but it indicates better analytical performance than a meter with a Sigma value of 3 or less. Meanwhile, the error risk would be lower on the meters with a Sigma value of 4 or greater compared to those with a Sigma value of 3 or less. Nevertheless, if a meter shows unusual QC pattern, it must be investigated regardless of the Sigma value. Moreover, data sorting for the large amount of QC results cannot be completed manually; the POCT specialists may require training to obtain proficient Excel skills.
The Sigma metrics-based approach established in this study does not solve the issues in the QC settings of glucose meters, but it facilitates the QC review for glucose meters to identify the meters with possible quality problems. This study was conducted based on one brand of glucose meters used in a single healthcare center, therefore, more studies using different meters in different POCT programs are warranted. Additionally, the Sigma values were calculated with monthly QC data points, which may be inadequate to reflect meters’ stable performance. This may result in the variation of Six Sigma values observed on the same meter during the six-month period.
To improve the QC monitoring for glucose meters in the future, we would expect to set up hospital- or meter-specific QC acceptance ranges and QC rules to glucose meters, similar to those for core laboratory tests. This requires an appropriate data management system or middleware due to the large number of meters in service. We should forward these demands to the manufacturers so that these features can be integrated to the existing middleware to support comprehensive QC processes. Additionally, we could consider establishing long-term Six Sigma performance metrics for each glucose meter, possibly on an annual basis. This approach may help in interpreting the monthly Sigma values of the meters and reduce confusion caused by monthly variations.
5. Conclusions
The majority of the glucose meters in our hospitals displayed optimal Sigma values. Including Sigma metrics in the QC review of glucose meters allows for better identification of meters with possible quality problems out of hundreds of hospital meters and enable the completion of QC chart review to investigate the causes of poor performance in a timely manner so that corrective actions can be established accordingly. Therefore, this approach would enhance quality monitoring of glucose meters with imperfect QC settings, especially in the situations with limited availability of POCT Specialists, to meet the requirements of quality management for POCT.
Data availability
Data associated with the study has not been deposited into a publicly available repository. Data will be made available on reasonable request.
Ethics declarations
Review or approval by an ethics committee was not required for this study because this study only used existing data from quality control material and did not involve any studies using patient samples.
CRediT authorship contribution statement
Yun Huang: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Formal analysis, Conceptualization. Callie Loveday: Writing – review & editing, Methodology, Formal analysis, Data curation. Anne Vincent: Writing – review & editing, Resources, Methodology, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Nichols J.H. Blood glucose testing in the hospital: error sources and risk management. J. Diabetes Sci. Technol. 2011;5:173–177. doi: 10.1177/193229681100500124. https://doi-org.proxy.queensu.ca/10.1177/193229681100500124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freckmann G., Baumstark A., Jendrike N., Rittmeyer D., Pleus S., Haug C. Accuracy Evaluation of four blood glucose monitoring systems in the hands of intended users and trained personnel based on ISO 15197 requirements. Diabetes Technol. Ther. 2017;19:246–254. doi: 10.1089/dia.2016.0341. https://doi-org.proxy.queensu.ca/10.1089/dia.2016.0341 [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Campbell E., Colbourne B., Power J., Randell E. User competency is still a major factor affecting analytical performance of glucose meters in patient service. Clin. Biochem. 2019;63:66–71. doi: 10.1016/j.clinbiochem.2018.11.008. https://doi-org.proxy.queensu.ca/10.1016/j.clinbiochem.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 4.Venner A.A., Beach L.A., Shea J.L., Knauer M.J., Huang Y., Fung A.W.S., Dalton J., Provencal M., Shaw J.L.V. Quality assurance practices for point of care testing programs: recommendations by the Canadian society of clinical chemists point of care testing interest group. Clin. Biochem. 2021;88:11–17. doi: 10.1016/j.clinbiochem.2020.11.008. https://doi-org.proxy.queensu.ca/10.1016/j.clinbiochem.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 5.Accreditation Canada Diagnostics Medical laboratory accreditation requirements. Accreditation Canada Diagnostics. 2023:148–161. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institution; Wayne, PA: 2016. Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions; Approved Guideline (C24-A4) [Google Scholar]
- 7.Institute for quality management in healthcare, review – internal quality control practice. 2023. https://qview.ca/qview/FileView.aspx?resourceid=816189
- 8.Lim C.Y., Badrick T., Loh T.P. Patient-based quality control for glucometers: using the moving sum of positive patient results and moving average. Biochem. Med. 2020;30 doi: 10.11613/BM.2020.020709. https://doi-org.proxy.queensu.ca/10.11613/BM.2020.020709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westgard S., Bayat H., Westgard J.O. Analytical Sigma metrics: a review of Six Sigma implementation tools for medical laboratories. Biochem. Med. 2018;28 doi: 10.11613/BM.2018.020502. https://doi-org.proxy.queensu.ca/10.11613/BM.2018.020502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westgard S., Bayat H., Westgard J.O. Special issue on Six Sigma metrics - experiences and recommendations. Biochem. Med. 2018;28 doi: 10.11613/BM.2018.020301. https://doi-org.proxy.queensu.ca/10.11613/BM.2018.020301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols J.H., Cambridge T., Sanchez N., Marshall D. Clinical validation of a novel quality management system for blood gas, electrolytes, metabolites, and CO-Oximetry. J. Appl. Lab. Med. 2021;6:1396–1408. doi: 10.1093/jalm/jfab053. https://doi-org.proxy.queensu.ca/10.1093/jalm/jfab053 [DOI] [PubMed] [Google Scholar]
- 12.Fei Y., Wang W., He F., Zhong K., Wang Z. Evaluating laboratory performance on point-of-care glucose testing with six Sigma metric for 151 institutions in China, Diabetes Technol. Ther. 2015;17:745–754. doi: 10.1089/dia.2014.0421. https://doi-org.proxy.queensu.ca/10.1089/dia.2014.0421 [DOI] [PubMed] [Google Scholar]
- 13.Fung A.W.S. Utilizing connectivity and data management system for effective quality management and regulatory compliance in point of care testing. Pract. Lab. Med. 2020;22 doi: 10.1016/j.plabm.2020.e00187. https://doi-org.proxy.queensu.ca/10.1016/j.plabm.2020.e00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erasmus R., Sahni S., El-Sharkawy R. Connectivity strategies in managing a POCT service. EJIFCC. 2021;32:190–194. [PMC free article] [PubMed] [Google Scholar]
- 15.International Organization for Standardization . International Organization for Standardization; Geneva: 2013. In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. [Google Scholar]
- 16.Park S., Lee W., Jeong T.D., Chung H.S., Hong K.S. Application of six Sigma metrics to improve quality control for point-of-care glucose testing. Ewha. Med. J. 2020;43:43–48. [Google Scholar]
- 17.Chen H., Yao Q., Dong Y., Tang Z., Li R., Cai B., Wang R., Chen Q. The accuracy evaluation of four blood glucose monitoring systems according to ISO 15197:2003 and ISO 15197:2013 criteria, Prim. Care. Diabetes. 2019;13:252–258. doi: 10.1016/j.pcd.2018.12.010. https://doi-org.proxy.queensu.ca/10.1016/j.pcd.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 18.Bowman C.F., Nichols J.H. Comparison of accuracy guidelines for hospital glucose meters. J. Diabetes Sci. Technol. 2020;14:546–552. doi: 10.1177/1932296819898277. https://doi-org.proxy.queensu.ca/10.1177/1932296819898277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute for Quality Management in Healthcare Review - Proficiency testing precision goals and allowable performance limits for chemistry and hematology. https://qview.ca/qview/FileView.aspx?resourceid=700721
- 20.Liu Q., Bian G., Chen X., Han J., Chen Y., Wang M., Yang F. Application of a six sigma model to evaluate the analytical performance of urinary biochemical analytes and design a risk-based statistical quality control strategy for these assays: a multicenter study. J. Clin. Lab. Anal. 2021;35 doi: 10.1002/jcla.24059. https://doi-org.proxy.queensu.ca/10.1002/jcla.24059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson Y.J., Troxler R.G., Wease D.F., Fuchs R.J., Petit M. Three-pool analysis for quality control: a method for daily differentiation of analytic error from random variability. Am. J. Med. Technol. 1981;47:321–326. [PubMed] [Google Scholar]
- 22.Kelly D.T., Kelly M.E. Proposals and current practices in quality control. Am. J. Med. Technol. 1981;47:957–964. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository. Data will be made available on reasonable request.