Highlights
-
•
Neural connectivity of treatment-resistance vs sensitivity in depression.
-
•
Anterior-posterior cingulate hyperconnectivity characterizes treatment-resistance.
-
•
Inter-network connectivity differences in treatment resistance and response.
Keywords: Treatment-Resistant Depression, Functional Connectivity, Default-Mode Network, Anterior Cingulate Cortex
Abstract
Understanding why some patients with depression remain resistant to antidepressant medication could be elucidated by investigating their associated neural features. Although research has consistently demonstrated abnormalities in the anterior cingulate cortex (ACC) – a region that is part of the default mode network (DMN) − in treatment-resistant depression (TRD), a considerable research gap exists in discerning how these neural networks distinguish TRD from treatment-sensitive depression (TSD). We aimed to evaluate the resting-state functional connectivity (rsFC) of the ACC with other regions of the DMN to better understand the role of this structure in the pathophysiology of TRD. 35 TRD patients, 35 TSD patients, and 38 healthy controls (HC) underwent a resting-state functional MRI protocol. Seed-based functional connectivity analyses were performed, comparing the three groups for the connectivity between two subregions of the ACC (the subgenual ACC (sgACC) and the rostral ACC (rACC)) and the DMN (p < 0.05 FWE corrected). Furthermore, inter-network connectivity of the DMN with other neural networks was explored by independent component (ICA) analyses (p < 0.01, FDR corrected). The results demonstrated hyperconnectivity between the rACC and the posterior cingulate cortex in TRD relative to TSD and HC (F(2,105) = 5.335, p < 0.05). ICA found DMN connectivity to regions of the visual network (TRD<TSD) and a parietal region of the DMN (TRD>TSD), differentiating the two clinical groups. These results provide confirmatory evidence of DMN hyperconnectivity and preliminary evidence for its interactions with other neural networks as key neural mechanisms underlying treatment non-responsiveness.
1. Introduction
Major Depressive Disorder (MDD) is a prevalent psychiatric disorder resulting in significant negative outcomes. Although several therapeutic interventions are accessible for its management, attaining remission has proven challenging (Kverno and Mangano, 2021). Over 30 % of the patients with MDD fail to achieve complete remission after different levels of successive pharmacological treatment (Taylor et al., 2019), with decreasing likelihood of response at each subsequent antidepressant medication treatment trial. This is referred to as treatment-resistant depression (TRD). Alternative brain stimulation therapies are promising for patients who do not respond to standard treatment options (Wu et al., 2019), as they offer potential treatment avenues in the context of TRD, where the mechanisms are currently unknown and ideal therapeutic targets remain elusive.
Functional magnetic resonance imaging (fMRI) has been used to understand the neural mechanisms that underlie MDD, and research suggests that resting-state fMRI may elucidate neural mechanisms that can help predict the response to various forms of treatment (Ge et al., 2017). Functional connectivity (FC) is a widely used fMRI technique that enables the investigation of inter- and intra-brain network interactions and dependencies (Andreescu et al., 2013). FC measures the synchronised fluctuations of activity between brain regions across time. During rest, brain regions exhibit slow, correlated fluctuations which reflect the intrinsic architecture of the brain exposing how the primary functional networks of the brain are contributing to undirected thought patterns (Andreescu et al., 2013). Recent work has shown that it is possible to non-invasively modulate resting FC (Taylor et al., 2022) and to use it as a treatment intervention in psychiatric disorders such as TRD (Taylor et al., 2022) or post-traumatic stress disorder (PTSD) (Lieberman et al., 2023).
Depression is associated with altered FC within the default mode network (DMN) − a functional network consisting of several brain regions such as the posterior cingulate cortex, medial prefrontal cortex, medial temporal lobe, and inferior parietal lobe (Andreescu et al., 2013). In individuals with depression, there is often increased connectivity between anterior regions, such as the rostral anterior cingulate cortex (rACC) or the subgenual cingulate cortex (sgACC) and other DMN regions, such as the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) (Kaiser et al., 2015). This enhanced connectivity is thought to be related to excessive rumination and a heightened focus on negative self-referential thoughts, contributing to the persistent negative emotions characteristic of depression (Hamilton et al., 2015).
The DMN is not an isolated network; it interacts with various other networks, such as the cognitive control network (CCN) and somatomotor network, among others (Korgaonkar et al., 2014). The CCN is responsible for cognitive control processes like attention, working memory, and task execution, which are often impaired in depression (Korgaonkar et al., 2019). The interaction between the DMN and CCN is intriguing because they tend to exhibit an anti-correlation relationship, where one becomes more active as the other becomes less active (Whitton et al., 2018). This dynamic balance is crucial for shifting attention from internal thoughts (DMN) to external tasks (CCN). Disruptions in this balance could contribute to the cognitive inflexibility and difficulty disengaging from ruminative thoughts seen in depression (Whitton et al., 2018).
TRD is characterized by persistent rumination, a form of repetitive thought fixated on negative content, often from the past or present, leading to emotional distress (Machino et al., 2014). The DMN is implicated in these self-referential processes and is significantly altered in TRD patients (Hamilton et al., 2015, Machino et al., 2014, Li et al., 2013). Within the DMN, the ACC serves as a critical hub, showing increased activity during rest and self-referential tasks. Altered functional connectivity between the ACC and other DMN regions, such as the mPFC and PCC, has been observed in depression (Whitton et al., 2018). This altered relationship is theorized to play a role in regulating emotional processing and affective behavior, directly underpinning the persistent rumination seen in TRD (Fox & Raichle, 2007). Increased connectivity between the rACC and other DMN regions has been associated with excessive rumination and a heightened focus on negative self-referential thoughts, contributing to the maintenance of negative emotional states in depression (Greicius et al., 2007). There is evidence for a direct neural connection between the ACC and specific brain regions involved in regulating mood, depression, and the antidepressant response (Goldstein-Piekarski et al., 2018, Korgaonkar et al., 2014). Specifically, it has been suggested that the rACC has potential utility in identifying patients who may respond better to antidepressant treatments, irrespective of treatment modality (Mayberg et al., 1997; Pizzagalli, 2011; Fox et al., 2014). Increased pre-treatment rACC physiological activity represents a promising and nonspecific prognostic marker of treatment outcome in depression (Pizzagalli et al., 2018). We recently demonstrated that rACC activity was specifically related to level of treatment resistance using four large samples ranging from psychotherapy, to transcranial magnetic stimulation (TMS) and electroconvulsive therapy (ECT) (Prentice et al., 2023), possibly suggesting prior reports on rACC activation and treatment response could be mediated by treatment-resistance. There is also evidence for a direct association between the sgACC and treatment response in depression (Korgaonkar et al., 2014). For instance, deep brain stimulation targeting the sgACC is shown to be effective in reducing depressive symptoms, particularly anhedonia, in patients with TRD (Riva-Posse et al., 2014).
In summary, there is strong evidence in the literature suggesting abnormalities in connectivity related to specific subregions of the ACC – the rACC and the sgACC – and particularly that to other DMN regions in depression. Whether this neural circuitry affects response to treatment is still unclear, and there is a need to fully understand the significance of rACC and sgACC functional connectivity as a predictor of treatment response. The aim of this study is to investigate how functional connectivity of the rACC and sgACC with the DMN distinguishes treatment sensitive and treatment resistant depression, using resting-state fMRI. We hypothesized that TRD patients will have higher functional connectivity of the rACC and the sgACC with the DMN when compared to patients with depression who responded to treatment. First, we examined the functional connectivity of the rACC and sgACC with other regions of the DMN. Subsequently, we extended our analysis to investigate the inter-network connectivity between the DMN and the other brain regions, hypothesizing that TRD patients will show lower connectivity between the DMN and other networks of the brain, compared to treatment-sensitive depression (TSD) patients.
2. Materials & methods
2.1. Participants
Initially, 39 individuals with TRD and 35 individuals with TSD were recruited through a local network of specialist psychiatrists and clinics. Thirty-nine individuals with TRD were recruited, but only 35 were used in the final sample due to four participants being excluded for excessive head motion during the scan (see section 2.3 for details). TRD and TSD individuals met DSM-5 criteria for primary diagnosis of MDD, assessed through the Structured Clinical Interview for the DSM-5 (SCID-5) (APA, 2013). The inclusion criteria for TRD were: no remission of symptoms with at least two adequate trials (in terms of dosage, duration – 6 weeks for each trial) of antidepressant of different pharmacologic classes, as well as the presence of moderate to severe depressive symptoms (assessed by a rating greater or equal to 16 in the 21-item Hamilton Depression Rating Scale – HAMD-21) (Hamilton, 1960). Participants in the TSD group were identified as patients who had achieved complete remission of symptoms for at least two weeks (characterized by a HAMD-21 score of less than or equal to 9). The control group (HC) comprised of 38 healthy individuals recruited through community advertisements with no psychiatric illnesses, assessed using the SCID-5. All participants were aged between 18 and 65 years old.
For both patient groups, indices of illness severity and chronicity were assessed. These indices included age of onset, number of inpatient hospitalizations, length of remission period since last episode, number of previous depressive episodes, history of suicidal ideation and behaviour, and history of suicide attempt. Information on past and current medication and other forms of treatment (e.g. ECT, or TMS) was also collected. Level of functioning was assessed by the Social and Occupational Functioning Assessment Scale (SOFAS) (Goldman et al., 1992).
HC and patient groups (TRD and TSD) were matched for sex, age, and education status. Exclusion criteria for all participants included a) inability to provide consent, b) insufficient English proficiency, c) current primary diagnosis of eating disorder, psychosis, personality disorder or primary PTSD, d) substance dependence for the past 3 months, e) pregnancy, f) history or current neurological disorder or prior brain injury, g) ECT or TMS in the last 6 months, h) contraindication to MRI.
Data collection was conducted at Westmead Hospital, Department of Radiology and at the Brain Dynamics Centre, The Westmead Institute for Medical Research, in Sydney, Australia. The research protocol was approved by the Western Sydney Local Health District Human Research Ethics Committee and all participants provided written consent.
2.2. MRI data collection
MRI scanning was carried out with a 3 Tesla MRI Scanner running VE11C software (Prisma, Siemens Medical Solutions, Germany), with a 64-channel head/neck array head coil.
Participants underwent an 8-minutes resting-state protocol, in which they were instructed to remain still, to relax and let their mind wander while looking at a fixation cross projected onto the screen. Functional T2*-weighted echo-planar images were acquired (repetition time (TR) = 1 500 ms, echo-time (TE) = 33.0 ms, field of view = 255 mm; flip angle = 90°, phase encoding direction = A≫P, excitation = standard, 60 axial slices resulting in isotropic voxels of 2.5 mm3 encompassing the whole brain). A three-dimensional T1-weighted structural dataset was also acquired (TR/TE=2 400 ms/2.21 ms, field of view = 256 mm, flip angle = 8°, inversion time = 900 ms, phase encoding direction = A≫P, 192 sagittal slices resulting in isotropic voxels of 0.89 mm3 encompassing the whole brain).
2.3. MRI data processing
MRI data were processed and analysed using Matlab R2018b (The Mathworks inc, Natick, Massachusetts), SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK), CONN functional connectivity toolbox v16b. Details of pre-processing steps are presented in our previous study (Barreiros et al., 2022). To reiterate, anatomical images were segmented into grey matter, white matter, and cerebrospinal fluid. Pre-processing of the functional images included realignment, unwrapping, motion correction, co-registration to native space structural data, smoothing with a 6-mm FWHM Gaussian kernel, and normalization to Montreal Neurological Institute (MNI) space. To eliminate the influence of residual noise components in the blood-oxygen-level-dependent (BOLD) signal, the data were also subject to a denoising process, using the default pipeline for denoising (including anatomical component-based noise correction procedure and default bandpass filtering [0.01, 0.1] Hz) and functional outlier detection tools in CONN (ART-based scrubbing). Scrubbing correction outputs were analysed to detect datasets with high-motion volumes of BOLD data (we excluded any participant who had more than 25 % of their volumes flagged as outliers), and subjects with a volume-to-volume index of head motion (head displacement from previous frame) higher than 1 mm were considered outliers. Four TRD participant datasets were excluded for excessive head motion during the scan.
2.4. Demographic and clinical characteristics
Demographic variables, age and gender, were compared for differences across all groups using a one-way ANOVA and chi square test, respectively. The TRD and TSD groups were compared for age of onset, age of first episode, depression severity (HAMD-21 score), functionality (SOFAS score), severity of worst depressive episode, number of previous depressive episodes, using student t-tests. The groups were also compared for history of hospitalizations, suicidal attempts and suicidal ideation, using chi square tests of independence.
2.5. Functional connectivity analyses
FC analyses on the resting-state fMRI data were performed, through a seed-based FC approach. In this approach, regions of interest (ROIs) are selected a priori. For this study, we selected the rACC and the sgACC as the seeds and investigated the FC between these seeds and other regions of the DMN (DMN intra-network connectivity).
The selection of rACC ROI anatomic landmark derived from the voxels reported by Pizzagalli and colleagues (2001), and also used in our prior studies on rACC (Prentice et al., 2023, Arns et al., 2015). Each functional rACC ROI was a 10 × 10 × 10 mm cube placed around the central MNI coordinate in this region (−10 45––5). FC values were calculated from the rACC voxel-wise to the rest of the brain for each participant in the dataset as bivariate Fisher’s z-transformed correlation coefficients. This measures the association between the rACC seed BOLD timeseries and each voxel of the whole brain BOLD timeseries using generalized linear model (GLM).
The selection of sgACC ROI anatomic landmark derived from PET imaging studies localizing this region as Brodmann Area 25 (BA25) (Riva-Posse et al., 2014). The ROI for BA25 was generated from the AAL atlas (Rolls et al., 2020).
The rACC was selected based on its well-documented involvement in emotion regulation and its potential as a biomarker for treatment response (Mayberg et al., 1997; Pizzagalli, 2011). In contrast, the sgACC was chosen due to its significant role in the pathophysiology of depression and its connections with other brain regions implicated in mood regulation. This approach allowed us to capture the comprehensive network dynamics involved in TRD and TSD.
The ROIs for the DMN mask used for this analysis were selected based on published literature (Laird et al., 2009). Using a combination of activation likelihood estimation, Laird and collaborators (2009) assessed statistically significant convergence of neuroimaging results and identified core regions in the DMN. Through this meta-analytic work, they identified 9 anatomical regions as part of the DMN (coordinates listed in Talairach space): the precuneus cortex (−4, −58, 44), the posterior cingulate (−4, −52, 22), the ventral anterior cingulate (2, 43, −8), the right inferior parietal lobe (52, −28, 24), the medial prefrontal cortex (−2, 50, 18), the right middle temporal gyrus (46, −66, 16), the left middle frontal gyrus (−26, −36, 28), the left inferior parietal lobule (−56, −36, 28), and the left middle temporal gyrus (−42, −66, 18). We created an image mask that consisted of 9 sphere ROIs of 3-mm radius each, centered in the coordinates described for each DMN anatomical region described above, transformed into the MNI space. Pearson correlation coefficients between the average time series of the rACC and the time series of all voxels of the DMN mask were calculated.
Given our hypothesis regarding the DMN, we then explored the FC between the rACC and the sgACC and the DMN compared between the two patient groups (TRD and TSD) for our primary analysis of interest. The analysis of rACC and sgACC to DMN connectivity between the groups was performed in a step-wise manner focusing on comparing the two clinical groups using two-sample t-tests (TRD vs TSD). To further unpack connectivity differences between the two clinical groups, as a secondary analysis, each of the clinical groups was contrasted with the HC group (TRD vs HC, HC vs TSD) for significant connectivity measures from the primary analysis.
The statistical parametric maps threshold was set p < 0.05 at the cluster-level family-wise error (FWE)-corrected for multiple comparisons, and an initial whole-brain voxel-wise p < 0.001. The minimum cluster size necessary to be considered relevant was 20.
2.6. Independent component analyses
Subsequently, independent component analyses (ICA) were performed, in order to investigate differences in connectivity between the DMN and other brain networks, between the groups. ICA were performed on the functional data of individual subjects and extract subject-specific component maps, using the group-ICA in the CONN toolbox. This data-driven approach enabled the identification of functionally independent networks within each participant and facilitated the examination of the variability in these networks across subjects.
Prior to conducting ICA, we estimated the number of components in the dataset using the Group ICA of fMRI Toolbox (GIFT) v3.0b. For each subject, we estimated the components using minimum description length criteria and then calculated the average number of components across the dataset using the mean of the individual subject results. The group-ICA analysis was conducted using default settings for CONN 18b, with GICA3 back-projection and G1 FastICA with dimensionality reduction set to 64. We determined that there were 23 independent components (ICs) based on the component estimation.
Then, we matched the 23 ICs to a spatial template of neural networks provided by the CONN functional network atlas. The correlation coefficient values indicated the predominant network regions for each component. To evaluate connectivity differences related to each intrinsic connectivity network (ICN), we performed group comparisons. The statistical threshold was set to voxel-wise p < 0.001 at an uncorrected level to define the voxel size. Then, we applied a cluster-wise correction at a threshold of false discovery rate p < 0.05 to determine significant clusters.
2.7. Clinical factors
In order to analyse the associations between the FC measures and treatment resistance in the TRD group, we computed a variable to reflect chronicity of the disorder, by calculating the number of years from age of onset to current age, which is referred to as “number of years since onset”. The relationships between the mean FC values for the significant ROIs and different clinical factors (depression severity, functionality, severity of worst depressive episode, number of previous depressive episodes, and number of years since onset) in the two patient groups separately were analysed using Pearson correlation coefficients.
All statistical tests were corrected for multiple comparisons, using a Bonferroni adjustment. All effects were considered significant at the p < 0.05 significance level. Statistical analyses were performed using SPSS software version 21 (IBM Corp, 2012).
3. Results
3.1. Demographic and clinical characteristics
Demographic and clinical data for the final sample are summarized in Table 1. All three groups were comparable for age and gender. As expected, groups were significantly different in some clinical variables, reflecting a more severe clinical profile for TRD patients; although the groups were not different for the number of past depressive episodes, the TRD group had higher rates of history of hospitalizations, history of ECT, and history of suicidal attempts.
Table 1.
Summary of demographic and clinical characteristics of the sample.
| TRD (35) | TSD (35) | HC (38) | F/t/X^2 | sig | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, Mean ± SD [Min-Max] | 42.3 ± 14.1 [18.1–64.3] | 37.2 ± 11.0 [20.0–57.4] | 47.1 ± 14.3 [18.9–66.0] | n.s. | n.s. |
| Gender (M), N (%) | 14 (40 %) | 17 (48.6 %) | 17 (44.7 %) | n.s. | n.s. |
| Clinical Profile | |||||
| Age of onset, Mean ± SD [Min-Max] | 26.97 ± 13.13 [8–53] | 21.66 ± 9.26 [8–50] | n.a | n.s. | n.s. |
| Number of previous MDE, Mean ± SD [Min-Max] | 8 ± 11 [1–40] | 6 ± 6 [1–30] | n.a | n.s. | n.s. |
| Severity of worst MDE, Mean ± SD [Min-Max] | 6.77 ± 0.49 [5–7] | 5.21 ± 1.36 [3–7] | n.a | 6.346 | 0.000 |
| Length of current episode, days, Mean ± SD [Min-Max] | 117.1 ± 117.2 [14–365] | n.a. | n.a | n.a. | n.a. |
| HAM-D-21 score, Mean ± SD [Min-Max] | 25.23 ± 6.46 [16–41] | 4.18 ± 3.10 [0–9] | n.a | 15.819 | 0.000 |
| SOFAS score, Mean ± SD [Min-Max] | 73.91 ± 16.18 [40–100] | 89.58 ± 5.47 [78–95] | n.a | −5.275 | 0.000 |
| Antidepressant medication treatment past, N (%) | 31 (88.6 %) | 30 (85.7 %) | n.a. | 76.152 | < 0.001 |
| Antidepressant medication treatment current, N (%) | 19 (54.3 %) | 22 (62.9 %) | n.a. | 37.393 | < 0.001 |
| Length of time on antidepressant medication, years, Mean ± SD [Min-Max] | 4.44 ± 3.70 [0.17 – 12.0] | 4.18 ± 4.16 [0.12 – 17.0] | n.a. | n.s. | n.s. |
| History of Hospitalizations, N (%) | 25 (71.4 %) | 6 (17.1 %) | n.a | 20.902 | 0.000 |
| History of ECT, N (%) | 10 (28.6 %) | 0 (0) | n.a | 11.667 | 0.001 |
| History of TMS, N (%) | 3 (8.6 %) | 0 (0) | n.a | n.a. | n.a. |
| History of Suicidal Ideation, N (%) | 28 (80 %) | 26 (74.3 %) | n.a | n.s. | n.s. |
| History of Suicidal Attempt, N (%) | 19 (54.3 %) | 5 (14.3 %) | n.a | 13.938 | 0.000 |
n.a. – not applicable, n.s. – not significant; SD – Standard Deviation; M – male; MDE – Major Depressive Episode; HAMD-21 – Hamilton Depression Rating Scale, 21 items; SOFAS – Social and Occupational Functioning Assessment Scale; CGI-S – Clinical Global Impression, Severity; ECT – Electroconvulsive Therapy; TMS – Transcranial Magnetic Stimulation; N – total number.
There were no significant differences between groups for motion during the scan, after the exclusion of the four TRD participants for excessive motion.
3.2. DMN intra-network connectivity
3.2.1. rACC
Results showed significant differences between the TRD and the TSD groups for the connectivity of rACC with the PCC (t = 3.83, p = 0.004, k = 40, −2––54 20, family-wise error corrected; large effect size d = 0.861) (See Table 2). Subsequent post-hoc cluster analyses revealed significantly lower connectivity of the rACC and PCC in the TSD when compared to the TRD. When compared to the HC group, the TSD group showed significantly lower connectivity between the rACC and the PCC (t(71) = -2.649, p = 0.010, with a moderate effect size d = -0.621). There were no significant differences between the TRD and the HC, for the connectivity between the rACC and the other regions of the DMN (Table 2).
Table 2.
Summary of the main findings from the seed-DMN functional connectivity analyses.
| Source seed | DMN region | Contrast | Cluster size (voxels)* | Cluster P value** |
Peak voxel P value (unc.)*** |
Cluster analyses | Post-hoc | ||
| F/t | p | η2/d | |||||||
| sgACC | PCC | ANOVA | 81 | ns | 0.009 | 3.607 | 0.031 | 0.064 | TRD, HC>TSD |
| TRD vs TSD | 81 | ns | 0.002 | ||||||
| TRD vs HC | ns | ||||||||
| HC vs TSD | 81 | ns | < 0.001 | ||||||
| rACC | PCC | ANOVA | 116 | ns | 0.001 | 6.497 | 0.002 | 0.110 | TRD, HC>TSD |
| TRD vs TSD | 40 | 0.004 | < 0.001 | 3.601 | < 0.001 | 0.861 | |||
| TRD vs HC | ns | ||||||||
| HC vs TSD | ns | ||||||||
*considered only k > 10.
**p value threshold < 0.05, Family-wise error (FWE) corrected.
***uncorrected p values − did not survive family-wise error correction for multiple comparisons.
TRD – treatment-resistant depressive patients, TSD – treatment-sensitive depressive patients, HC – healthy controlsns – not significant.
DMN Mask includes 8 regions: precuneus, posterior cingulate cortex (PCC), ventral anterior cingulate, right (R) inferior parietal lobe, medial prefrontal cortex, R middle temporal gyrus, left (L) middle frontal gyrus, L inferior parietal lobule, L middle temporal gyrus.
3.2.2. sgACC
Results showed significant connectivity differences between the TRD and the TSD groups for the sgACC with the PCC (p < 0.002, k = 75, −2––48 20, uncorrected). Subsequent post-hoc cluster analyses revealed differences between the two patient groups (TRD and TSD) on the connectivity between sgACC and DMN (t(68) = 2.457, p = 0.017, with a moderate effect size d = 0.587), with the TSD group showing significantly lower connectivity when compared to the TRD. When compared to the HC group, the TSD group showed significantly lower connectivity between the sgACC and the PCC (t(71) = -2.703, p = 0.009, with a moderate effect size d = -0.633). There were no significant differences between the TRD and the HC, for the connectivity between the sgACC and the DMN.
3.3. DMN inter-network connectivity – Independent component analyses
In this study, ICA was used to identify functionally independent networks within each participant based on their fMRI data. An independent component (IC) represents a spatial pattern of brain activity that is statistically independent from other components in the analysis. Each IC is characterized by a spatial map showing regions of the brain that exhibit synchronous activity and a corresponding time course reflecting the fluctuations in activity over time. In our analysis, we identified 23 ICs, two of which were highly associated with the DMN. These ICs, labelled IC#6 and IC#15, were found to have correlations (r) of 0.33 and 0.42, respectively, with the DMN. The correlation coefficient indicates the strength of association between each IC and the DMN, with higher values indicating a stronger association. Whole brain voxel analyses were conducted to identify significant connectivity patterns related to each DMN component. A cluster size threshold greater than 20 was applied with voxel-level p < 0.001, cluster-level p < 0.05 FDR corrected, leading to the identification of clusters for the best-matching DMN component.
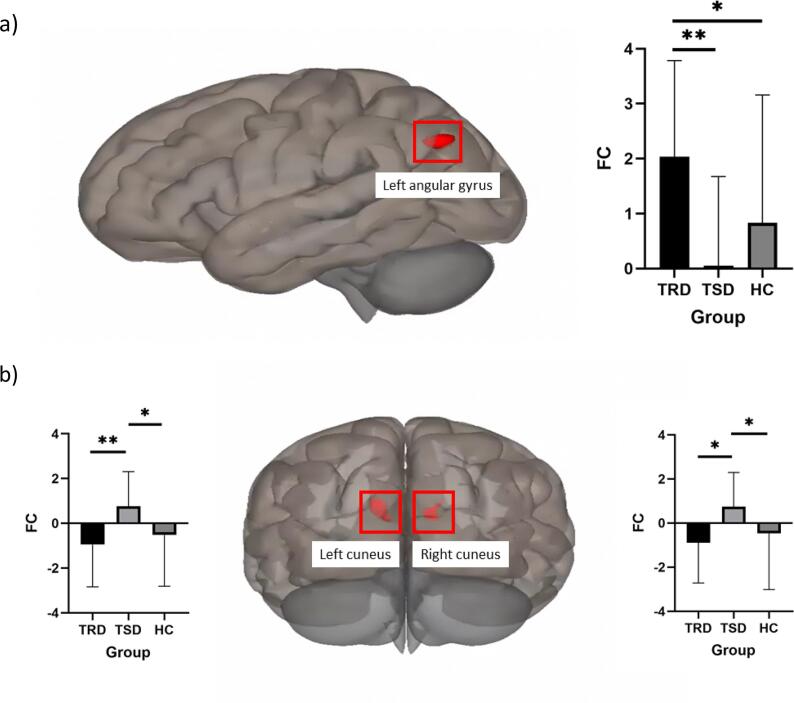
No significant group differences were observed for ICA#6, indicating that this component did not show robust connectivity differences that met the predefined threshold criteria. For ICA#15, the best match component (see Supplementary Figure 1), we identified 3 clusters showing significant connectivity patterns differentiating the two clinical groups (Fig. 1). These clusters included regions of the visual network (left (k = 144) and right (k = 90) cuneus), showing higher FC in the TSD group compared to the TRD group, and a parietal region of the DMN network (left angular gyrus (k = 117), indicating higher FC in the TRD group compared to the TSD group.
Fig. 1.
Inter-network connectivity differences between groups.
Post hoc comparisons indicated that the TRD group showed significantly higher connectivity between the best-matching DMN component and the left angular gyrus compared to both the TSD and HC groups with no differences between TSD and HC; and the TSD group showed higher connectivity compared with both the TRD and the HC groups between the DMN component and the left and right cuneus cortex (see summary in Table 3) with no differences between TRD and HC. No significant results were found for whole brain voxel wise comparisons for TRD with HC and TSD with HC.
Table 3.
Summary of the main findings from Independent Component Analyses (ICA), for differences between the three groups on functional connectivity between the best matching DMN component (ICA#15) and other brain regions.
| Contrast | MNI coordinates (x y z) | Voxels | Region | Neural network | F value | P value | Post-hoc |
|---|---|---|---|---|---|---|---|
| TSD>TRD | −10––92 26 | 144 | Left Cuneus Cortex | Visual | 7.430 | < 0.001 | TSD>TRD, HC |
| 10––84 20 | 90 | Right Cuneus Cortex | Visual | 6.112 | 0.003 | TSD>TRD, HC | |
| TRD>TSD | −44––72 38 | 117 | Left Angular Gyrus | Default Mode Network | 9.302 | < 0.001 | TRD>TSD, HC |
| TRD>HC | − | − | − | − | ns | ns | − |
| HC>TRD | − | − | − | − | ns | ns | − |
| TSD>HC | − | − | − | − | ns | ns | − |
| HC>TSD | − | − | − | − | ns | ns | − |
Effects significant at cluster-size False Discovery Rate p < 0.05; cluster size k > 20.
TRD – treatment-resistant depression group, TSD – treatment-sensitive depression group, HC – healthy controls groupns – not significant.
This illustration offers a comprehensive visualization of the different clusters that contribute to the resting-state functional connectivity differences between the three groups (Treatment-Resistant Depression (TRD) and Treatment-Sensitive Depression (TSD) on the averaged connectivity of ICA#15 (the best-match default mode network component) with the rest of the brain. Image a) represents the cluster showing TRD>TSD FC (left lateral view), and image b) represents the clusters showing TSD>TRD FC (posterior view).
3.4. Clinical factors
There were no significant effects of clinical variables and the rACC result or the DMN ICAs.
Chronicity of the disorder was significantly anti-correlated with the sgACC-PCC FC result, in the TRD group (r = -0.370, p < 0.05). When looking at the TSD group only, sgACC-PCC FC result is positively correlated with length of time on ADM (r = 0.433, p < 0.05). There were no other significant effects of other clinical variables on the sgACC result.
4. Discussion
This study aimed to investigate how the functional connectivity of the default mode network differentiates TRD from TSD, using resting-state fMRI. We focused on the functional connectivity of the rACC and the sgACC, two regions that have previously been identified as key regions in TRD, with other regions of the DMN. We explored whether the functional connectivity of these regions differentiates between treatment-response and treatment-resistance. We also used a data driven approach to investigate the inter-network connectivity between the DMN and other brain regions. The results evidenced a pattern of hyperconnectivity for the rACC and the sgACC with the PCC, hyperconnectivity of the angular gyrus with the rest of the DMN, and hypoconnectivity of the DMN with the visual network in the TRD group relative to TSD.
Consistent with previous research (Hamilton et al., 2015, Machino et al., 2014, Li et al., 2013), we observed altered functional connectivity between the rACC and the PCC, differentiating between TRD and TSD patients. This connectivity difference was found between the TSD and both TRD and HC groups, but not between TRD and HC, suggesting this as a marker of treatment response specifically, rather than depression severity. For the sgACC, results showed a similar trend, at an uncorrected level. This finding is consistent with pharmacological treatment findings from previous studies, showing that individuals with greater baseline rACC-PCC connectivity are more likely to show a positive response to antidepressant treatment (Dichter et al., 2015, Korgaonkar et al., 2014). However, the lack of significance at a corrected level may be attributed to several factors, including the exploratory nature of this analysis, the relatively small sample size, and the complexity of fMRI data. Correcting for multiple comparisons in fMRI studies is essential to minimize the risk of false positives, but it can also increase the likelihood of false negatives, particularly in studies with limited statistical power or subtle effects. Therefore, while the uncorrected trend in sgACC connectivity is intriguing and aligns with previous research, further studies with larger sample sizes are needed to confirm these findings and elucidate their clinical implications.
Despite the well-established theory of hyper-DMN connectivity and excessive rumination in TRD, our study did not find significant differences in connectivity between the sgACC and rACC with other DMN regions in the TRD group compared to the HC group. Several potential explanations for this discrepancy can be considered.
First, it is possible that the hyperconnectivity within the DMN in TRD is not uniformly present across all patients, but rather in a subset of individuals. This variability could result in non-significant findings when analyzing the entire TRD group. Additionally, the connectivity alterations in TRD may involve more complex, dynamic patterns that are not fully captured by static resting-state fMRI measures. For instance, fluctuations in connectivity over time or context-dependent connectivity changes may play a role in the pathology of TRD, which would require more advanced analytic techniques to detect.
Moreover, the role of other networks and regions outside the traditional DMN in TRD should be considered. For example, the interaction between the DMN and networks such as the salience network and the cognitive control network might be more critical in driving the symptoms of TRD. Disruptions in these inter-network dynamics could contribute to the persistence of depressive symptoms and rumination, even if intra-DMN connectivity differences are not pronounced.
Finally, it is important to note that the mechanisms underlying TRD are likely multifactorial and involve a complex interplay of various neural circuits. The absence of significant findings in the sgACC and rACC connectivity does not negate the theory of hyper-DMN connectivity but suggests that our understanding of the neural correlates of TRD needs to be nuanced and expanded to include broader network interactions and individual variability.
The anomalous neural activity implicated in TRD may not change in response to pharmacological treatments and has made non-pharmacological neuromodulation interventions increasingly attractive (Hitti et al., 2020). In line with the main findings of this study, fMRI studies have demonstrated activity of sgACC as a predictor of response to electroconvulsive therapy (ECT), a brain stimulation technique effective in TRD.
Clinical research investigating the underpinnings of resistance to the different types of treatment could provide additional insights into the concept of treatment-resistance. For instance, research on different DBS targets has the potential to enhance our understanding of treatment response mechanisms. In recent years, various brain stimulation locations have been studied for their potential efficacy, including the subcallosal cingulate (SCG) white matter (Mayberg et al., 2005), the ventral capsule/ventral striatum, the nucleus accumbens, the lateral habenula, the inferior thalamic peduncle, and the medial forebrain bundle (Riva-Posse et al., 2014, Zhou et al., 2018, Drobisz et al., 2019, Hitti et al., 2020). The ACC is the portion of the cingulum that lies ventral to the corpus callosum. The rACC/sgACC and PCC sit on opposing ends of the cingulum white matter bundle, which has been previously linked to treatment response (Korgaonkar et al., 2014, Bracht et al., 2015). The cingulum bundle is a significant white matter tract that connects frontal, parietal, and medial temporal regions, as well as linking subcortical nuclei to the cingulate gyrus. The relevance of the cingulum bundle to the limbic system was emphasized by Papez (1937) in his influential model of emotion, which highlighted its association with the cingulate gyrus. Disruptions in this pathway have been associated with altered functional connectivity patterns, such as hyperconnectivity of the rACC and sgACC with the PCC observed in TRD (Bubb et al., 2018). Nonetheless, efforts to integrate functional and anatomical knowledge of this pathway remain scarce, and this study represents a significant step in comprehending this highly complex pathway.
Chronicity, or the duration of depressive symptoms, is a key factor in understanding the progression and treatment response of depression. In this study, higher chronicity was associated with lower sgACC-PCC connectivity in the TRD group. However, the finding of lower sgACC-PCC connectivity in the TSD group compared to both the TRD and HC groups suggests that this specific connectivity pattern may be a marker of treatment sensitivity rather than chronicity alone. Important to note that there were no differences between TRD and HC which supports this theory. It's possible that while chronicity plays a role in connectivity alterations, other factors, such as the specific neural circuitry involved in treatment response and resistance, may override the effect of chronicity alone. Additionally, the impact of chronicity on sgACC-PCC connectivity may be nonlinear or influenced by other variables not measured in this study, contributing to the observed discrepancy. Future research incorporating longitudinal assessments and more comprehensive clinical and neuroimaging measures may help elucidate the complex relationship between chronicity, sgACC-PCC connectivity, and treatment response in depression.
The results from the ICA analyses emphasize the critical role of DMN connectivity with other brain networks in understanding the neural underpinnings of TRD. Our findings indicate that the patterns of connectivity between the DMN and other networks, such as the visual network are crucial in distinguishing individuals with TRD from those with TSD. In our study, we identified significant connectivity patterns in three clusters that differentiated the clinical groups: left and right cuneus, and the left angular gyrus. Notably, the TSD group exhibited higher functional connectivity between the DMN and the cuneus regions compared to the TRD group, while the TRD group showed higher FC between the DMN and the left angular gyrus compared to the TSD group.
The cuneus cortex, part of the visual network, is involved in mid-level visual processing and is also influenced by extraretinal effects such as attention, working memory, and reward expectation (Zhang et al., 2013; Fischer et al., 2019; Liu et al., 2022, Cechetto and Topolovec, 2002). The cuneus has been shown to be associated with reward attainment, with a positive correlation between cuneus activation and clinical symptoms of depression and anxiety (Liu et al., 2022). This finding aligns with the notion that reward processing is a core feature of major depressive disorder (Fischer et al., 2019). Specifically, in adolescent depression, dysfunctional reward processing is a significant concern, with resilient adolescents exhibiting different activation patterns in the cuneus during reward processing compared to those who have remitted from depression (Fischer et al., 2019).
Additionally, a meta-analysis of fMRI studies on reward-related processing in MDD has shown that the cuneus, along with other brain regions, preferentially responds to positive stimuli (Zhang et al., 2013). This increased activation in cortical regions, including the cuneus, during reward processing in MDD highlights its potential role in the neural circuitry of reward expectation and response to visual stimuli (Zhang et al., 2013). The higher FC observed in the TSD group between the DMN and the cuneus regions suggests that individuals with TSD may have enhanced reward processing capabilities. This enhanced connectivity might contribute to their responsiveness to treatment by supporting reward-related processes that are often impaired in depression. Additionally, the fact that there were no significant differences between the TRD and HC for this cluster suggests an effect of response to treatment in TSD, which should be evaluated in treatment studies with pre- and post-treatment imaging data.
Conversely, the TRD group's higher FC between the DMN component and the left angular gyrus was observed. The angular gyrus is traditionally considered a part of the DMN, which is involved in various higher-order cognitive functions such as memory retrieval, semantic processing, and social cognition (Kang et al., 2023). The identified higher connectivity between the DMN component and the left angular gyrus in TRD patients highlights the potential dysregulation within the DMN itself, reflecting an altered functional integration of this region within the network. This can be seen as an indication of the angular gyrus's role in the broader context of TRD pathology. The angular gyrus, especially in the left hemisphere, is crucial for recollection and the retrieval of detailed episodic memories (Bellana et al., 2023). Neuroimaging studies have shown that activity in the left angular gyrus is strongly associated with how well a memory representation matches the original encoded stimulus (Bellana et al., 2023). In TRD, the hyperconnectivity between the DMN and the left angular gyrus could reflect an over-reliance on or dysfunction in memory retrieval processes, potentially leading to persistent negative ruminations and cognitive rigidity seen in TRD (Bellana et al., 2023). Additionally, the angular gyrus's involvement in visual-spatial attention, memory retrieval, and semantic processing suggests that its altered connectivity in TRD might contribute to the deficits in these cognitive functions, further exacerbating depressive symptoms (Kang et al., 2023).
Moreover, the angular gyrus has also been implicated in the response to antidepressant treatments (particularly electroconvulsive therapy − ECT) in MDD patients. Studies indicate that ECT can modulate the functional connectivity between the habenula and the left angular gyrus, with changes in this circuit correlating with clinical improvements (Gao et al., 2021). This underscores the angular gyrus's role in the broader neural circuitry underlying depression and its treatment.
The hyperconnectivity observed between the DMN and the left angular gyrus in TRD patients indicates a potential compensatory or maladaptive mechanism within the DMN. This could signify an attempt by the brain to manage or integrate negative emotional and cognitive states through the angular gyrus, which is intricately involved in memory and semantic processing. However, this increased connectivity may also represent a failure of the DMN to appropriately regulate its internal processes, leading to persistent depressive symptoms. The absence of significant differences between TSD and HC groups further supports the notion that the left angular gyrus connectivity alterations are specific to TRD, providing a neural correlate for the persistent and treatment-resistant nature of the disorder.
However, we acknowledge certain limitations in this study. It is important to highlight that the results from the analyses using the sgACC as a seed were not significant after correcting for cluster level family wise error for multiple comparisons and should be interpreted with caution. The cross-sectional design limits our ability to establish causality between altered functional connectivity and treatment resistance. Future longitudinal studies, particularly those incorporating pre- and post-treatment scans, are warranted to explore the dynamic changes in connectivity patterns and their associations with treatment response.
The sample size, although carefully matched, is still relatively small, necessitating caution when generalizing the results. Also, the heterogeneity of the sample constitutes a major limitation in the study design. This study only focused on the symptoms featured on the HAMD-21 and did not explore in-depth symptoms of rumination, a feature that is particularly relevant given its functional role in the DMN. There is a potential confounding effect of depression severity on our results. Ideally, comparing the TRD group with an MDD group currently experiencing depression but not yet categorized as treatment-resistant would provide clearer insights. However, predicting which MDD patients will develop treatment resistance remains challenging. Additionally, the observed effects might be influenced by the multiple treatments received by the TRD group. Future longitudinal studies that track MDD patients from the onset of treatment through multiple interventions are necessary to better understand the features underlying treatment resistance. A lack of longitudinal study design also limits us from drawing definitive conclusions regarding trait versus state markers. Whether the identified connectivity patterns could be predictive markers for treatment resistance and guide early intervention remains to be tested. Future research could also benefit from evaluating dynamic functional connectivity of the DMN. Thirdly, there is a potential confounding effect of depression severity on our results. Ideally, comparing the TRD group with an MDD group currently experiencing depression but not yet categorized as treatment-resistant would provide clearer insights. However, predicting which MDD patients will develop treatment resistance remains challenging. Additionally, the observed effects might be influenced by the multiple treatments received by the TRD group. Future longitudinal studies that track MDD patients from the onset of treatment through multiple interventions are necessary to better understand the features underlying treatment resistance.
Finally, our study design primarily captures the neural correlates associated with treatment resistance at a single time point, preventing us from drawing definitive conclusions regarding trait versus state markers. Further longitudinal investigations are warranted to explore the temporal stability of these connectivity patterns and their potential utility as predictive markers for early intervention in depression treatment. This limitation underscores the need for future research endeavors to delve deeper into the dynamic nature of DMN connectivity and its interaction with other neural networks, shedding light on the potential for trait-specific markers and, consequently, more personalized and timely treatment strategies for individuals with depression.
5. Conclusions
In conclusion, this study contributes to the growing body of research investigating the neurobiological underpinnings of treatment-resistant depression. We demonstrate distinct patterns of functional connectivity in the cingulate cortex that differentiate TRD from TSD. Specifically, hyperconnectivity within the DMN, particularly involving the anterior to posterior cingulate cortex connections, may be a key characteristic of treatment response. These findings highlight the relevance of targeting the DMN in the development of novel treatment strategies for TRD. Furthermore, this study revealed a pattern of hypoconnectivity between the DMN and other key brain networks, particularly the visual network. By elucidating the neural circuits that underlie treatment resistance, our findings may pave the way for the development of targeted interventions, such as DBS, that can modulate these aberrant connectivity patterns and potentially improve treatment outcomes for TRD patients (Clark et al., 2020, Riva-Posse et al., 2014).
CRediT authorship contribution statement
Ana Rita Barreiros: Writing – review & editing, Writing – original draft, Visualization, Resources, Methodology, Investigation, Formal analysis, Data curation. Isabella A. Breukelaar: Writing – review & editing, Methodology, Formal analysis. Amourie Prentice: Writing – review & editing, Conceptualization. Prashanth Mayur: Writing – review & editing, Resources. Yoshiro Tomimatsu: Methodology, Conceptualization. Kenta Funayama: Methodology, Conceptualization. Sheryl Foster: Writing – review & editing, Resources. Gin S. Malhi: Writing – review & editing. Martijn Arns: Writing – review & editing, Conceptualization. Anthony Harris: Writing – review & editing, Resources, Project administration, Data curation, Conceptualization. Mayuresh S. Korgaonkar: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: Anthony Harris received funding from Takeda Pharmaceutical Company for this project. There are no other financial disclosures related to the work. Mayuresh Korgaonkar received funding from Takeda Pharmaceutical Company for this project. There are no other financial disclosures related to the work. And all other authors have declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by Takeda Pharmaceutical Company Limited, Japan - COCKPI-T program (Co-Create Knowledge for Pharma Innovation with Takeda) Research Grant [to Mayuresh Korgaonkar]), the National Health and Medical Research Council (NHMRC, Australia) (Grant No. APP1087560 [to Mayuresh Korgaonkar]), and The Stephen and Barbara Penfold PhD Scholarship, University of Sydney, Australia (to Ana Rita Barreiros). None of the Takeda members had any specific role in the design and execution of the clinical study itself, but supported the conceptualization, data analysis and its interpretation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103656.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- American Psychiatric Association . 5th ed. American Psychiatric Publishing; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Andreescu C., Tudorascu D.L., Butters M.A., Reynolds C.F., Aizenstein H.J. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 2013;214(3):319–325. doi: 10.1016/j.pscychresns.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arns M., Etkin A., Hegerl U., Williams L.M., DeBattista C., Palmer D.M. Frontal and rostral anterior cingulate (rACC) theta EEG in depression: Implications for treatment outcome? Eur Neuropsychopharmacol. 2015;25:1190–1200. doi: 10.1016/j.euroneuro.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Barreiros A.R., Breukelaar I., Mayur P., Foster S., Harris A., Williams L.M. Abnormal habenula functional connectivity characterizes treatment-resistant depression. Neuroimage Clin. 2022;34 doi: 10.1016/j.nicl.2022.102990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellana B., Ladyka-Wojcik N., Lahan S., Moscovitch M., Grady C.L. Recollection and prior knowledge recruit the left angular gyrus during recognition. Brain Struct Funct. 2023;228(1):197–217. doi: 10.1007/s00429-022-02597-5. [DOI] [PubMed] [Google Scholar]
- Bracht T., Linden D., Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J Affect Disord. 2015;187:45–53. doi: 10.1016/j.jad.2015.06.041. [DOI] [PubMed] [Google Scholar]
- Bubb E.J., Metzler-Baddeley C., Aggleton J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018;92:104–127. doi: 10.1016/j.neubiorev.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto, D. F., & Topolovec, J. C. (2002). Cerebral Cortex. In Encyclopedia of the Human Brain.
- Clark D.L., Johnson K.A., Butson C.R., Bejjani K.P., Hartlein J., Lozano A.M. Tract-based analysis of target engagement by subcallosal cingulate deep brain stimulation for treatment resistant depression. Brain Stimul. 2020;13:1094–1101. doi: 10.1016/j.brs.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Gibbs D., Smoski M.J., Riccardi C., Sibille E. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gao J., Li Y., Wei Q., Li X., Wang K., Tian Y., Wang J. Habenula and left angular gyrus circuit contributes to response of electroconvulsive therapy in major depressive disorder. Brain Imaging Behav. 2021;15(5):2246–2253. doi: 10.1007/s11682-020-00418-z. [DOI] [PubMed] [Google Scholar]
- Ge R., Blumberger D.M., Downar J., Daskalakis Z.J., Vila-Rodriguez F., Thorpe K.E. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment-resistant depression: A pilot study. J Affect Disord. 2017;128:75–81. doi: 10.1016/j.jad.2017.04.060. [DOI] [PubMed] [Google Scholar]
- Goldman H.H., Skodol A.E., Lave T.R. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149(9):1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- Goldstein-Piekarski A.N., Staveland B.R., Ball T.M., Yesavage J., Williams L.M. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl Psychiatry. 2018;8(1):57. doi: 10.1038/s41398-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti F.L., Yand A.I., Cristancho M.A., et al. Deep Brain Stimulation is effective for Treatment-Resistant Depression: A Meta-Analysis and Meta-Regression. J Clin Med. 2020;9:2796. doi: 10.3390/jcm9092796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015 Jun;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L., Wang W., Zhang N., Yao L., Tu N., Feng H., Zong X., Bai H., Li R., Wang G., Bu L., Wang F., Liu Z. Anhedonia and dysregulation of an angular gyrus-centred and dynamic functional network in adolescent-onset depression. J Affect Disord. 2023;324:82–91. doi: 10.1016/j.jad.2022.12.057. [DOI] [PubMed] [Google Scholar]
- Kverno K.S., Mangano E. Treatment-Resistant Depression - Approaches to Treatment. J Psychosoc Nurs. 2021;59(9):7–11. doi: 10.3928/02793695-20210816-01. [DOI] [PubMed] [Google Scholar]
- Laird A.R., Eickhoff S.B., Li K., et al. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Liu L., Friston K.J., et al. A Treatment-Resistant Default Mode Subnetwork in Major Depression. Biol Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Lieberman J.M., Rabellino D., Densmore M., et al. Posterior cingulate cortex targeted real-time fMRI neurofeedback recalibrates functional connectivity with the amygdala, posterior insula, and default-mode network in PTSD. Brain Behav. 2023;13(3):e2883. doi: 10.1002/brb3.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Ely B.A., Stern E.R., Xu J., Kim J.W., Pick D.G., Alonso C.M., Gabbay V. Neural function underlying reward expectancy and attainment in adolescents with diverse psychiatric symptoms. Neuroimage Clin. 2022;36 doi: 10.1016/j.nicl.2022.103258. Epub 2022 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machino A., Kunisato Y., Matsumoto T., et al. Possible involvement of rumination in gray matter abnormalities in persistent symptoms of major depression: An exploratory magnetic resonance imaging voxel-based morphometry study. J Affect Disord. 2014;168:229–235. doi: 10.1016/j.jad.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Mayberg, H.S., Lozano, A.M., Voon, V., et al., 2005. Deep brain stimulation for treatment-resistant depression. Neuron. 45(5), 651-60.Papez, J.W., 1937. A proposed mechanism of emotion. J Neuropsychiatry Clin Neurosci. 1994; 7(1), 103-12.
- Pizzagalli D., Oakes T.R., Davidson R.J., et al. Anterior Cingulate Activity as a Predictor of Degree of Treatment Response in Major Depression: Evidence From Brain Electrical Tomography Analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D., Webb C.A., Dillon D.G., et al. Pretreatment Rostral Anterior Cingulate Cortex Theta Activity in Relation to Symptom Improvement in Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(6):547–554. doi: 10.1001/jamapsychiatry.2018.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A., Barreiros A.R., van der Vinne N., et al. Rostral Anterior Cingulate Cortex Oscillatory Power Indexes Treatment-Resistance to Multiple Therapies in Major Depressive Disorder. Neuropsychobiology. 2023;82(6):373–383. doi: 10.1159/000533853. [DOI] [PubMed] [Google Scholar]
- Riva-Posse P., Choi K.S., Holtzheimer P.E., et al. Defining Critical White Matter Pathways Mediating Successful Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Biol Psychiatry. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T., Huang C.C., Lin C.P., et al. Automated anatomical labelling atlas 3. Neuroimage. 2020;206 doi: 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed] [Google Scholar]
- Taylor R.W., Marwood L., Greer B., et al. Predictors of response to augmentation treatment in patients with treatment-resistant depression: A systematic review. J Psychopharmacol. 2019;33(11):1323–1339. doi: 10.1177/0269881119872194. [DOI] [PubMed] [Google Scholar]
- Taylor J.E., Yamada T., Kawashima T., et al. Depressive symptoms reduce when dorsolateral prefrontal cortex-precuneus connectivity normalizes after functional connectivity neurofeedback. Sci Rep. 2022;12:2581. doi: 10.1038/s41598-022-05860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton A.E., Deccy S., Ironside M.L., et al. Electroencephalography Source Functional Connectivity Reveals Abnormal High-Frequency Communication Among Large-Scale Functional Networks in Depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(1):50–58. doi: 10.1016/j.bpsc.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.-R., Wang X., Baeken C. Baseline functional connectivity may predict placebo responses to accelerated rTMS treatment in major depression. Hum Brain Mapp. 2019;41:632–639. doi: 10.1002/hbm.24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Zhang H., Qin Y., et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:224–232. doi: 10.1016/j.pnpbp.2017.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.