Abstract
Background
Older candidates for transcatheter aortic valve replacement (TAVR) frequently present with both cardiac and noncardiac comorbidities. There are few risk scores that evaluate a wide range of comorbidities.
Methods
Patients who underwent TAVR for severe aortic stenosis were retrospectively evaluated. A new prediction model (Cardiac and nonCardiac Comorbidities risk score: 3C score) was determined based on coefficient in the multivariate Cox regression analysis for two-year all-cause mortality. C-statistics were assessed to compare the predictive abilities of the 3C score, the Charlson Comorbidities Index (CCI) score, the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II, and the Model for End-stage Liver Disease eXcluding International normalized ratio (MELD-XI) score.
Results
The present study included 226 patients (age, 86 ± 5 years; males, 38 %). The values of the CCI score, EuroSCORE II, and MELD-XI score were 2 (1–3), 3.36 (2.12–4.58), and 5.35 (3.05–8.55), respectively. Multivariate Cox regression analysis identified two cardiac (left ventricular ejection fraction [LVEF] <40 % [2 points]; pulmonary hypertension [1 point]) and three noncardiac comorbidities (hepatobiliary system impairment [3 points]; estimated glomerular filtration rate <30 ml/min/1.73 m2 [1 point]; cachexia [1 point]). The C-statistics of the 3C score, EuroSCORE II, MELD-XI score, and CCI score were 0.767 (0.666–0.867), 0.610 (0.491–0.729), 0.580 (0.465–0.696), and 0.476 (0.356–0.596), respectively (p < 0.001).
Conclusions
Among cardiac and noncardiac comorbidities, special attention should be given to hepatobiliary system impairment and reduced LVEF in older patients following TAVR. The 3C score may contribute to the risk stratification.
Keywords: Transcatheter aortic valve replacement, Comorbidity, Liver impairment, Renal impairment, Pulmonary hypertension
1. Introduction
Severe aortic stenosis (AS) can lead to high rates of mortality and hospitalization for heart failure, syncope, and chest pain. Its prevalence increased with age, varying from 0.2 % in the 50–59 year cohort, 1.3 % in the 60–69 year cohort, 3.9 % in the 70–79 year cohort and 9.8 % in the 80–89 year cohort [1]. Transcatheter aortic valve replacement (TAVR) has emerged as an alternative intervention for older patients at high or prohibitive surgical risk in the past two decades. TAVR reduced one-year all-cause mortality compared to medical therapy or balloon aortic valvuloplasty in the PARTNER trial [2]. Previous randomized controlled studies demonstrated comparable benefits of surgical intervention and TAVR in terms of all-cause and cardiac mortality, even in such fragile patients [3,4]. As a result of a decreased incidence of cardiac mortality, the majority of patients has been reported to undergo noncardiac death following the procedure [5]. It is important to evaluate noncardiac comorbidities appropriately to identify patients at high mortality risk after TAVR, considering the many older candidates and the high cost of the procedure. The Charlson Comorbidities Index (CCI) score is a representative prediction model that includes a wide range of comorbidities [6], and it has been well validated for the prediction of mid- or long-term mortality in various diseases [7,8]. However, along with medical progress, the prognostic impact of each variable has changed dramatically. Acquired immunodeficiency syndrome (AIDS) is no longer a lethal infection [9]. Recent breakthroughs in the field of oncology have led to improved survival rates [10]. Therefore, a new prediction model is necessary to identify patients at high mortality risk after TAVR and propose novel treatment options. Many studies have reported associations of certain comorbidities, such as liver impairment, with mortality, e.g. the Model for End-stage Liver Disease eXcluding International normalized ratio (MELD–XI) score, which can determine a treatment strategy in the setting of liver cirrhosis, has been reported to predict short-term mortality in patients following TAVR. However, its predictive ability is as low as 0.67, which indicates rather weak prediction [11]. Although the Combined Comorbidity Score includes various comorbidities and predicts short- and mid-term mortality [12], each variable in this score is not weighted on the basis of coefficients. Thus, the current study aimed to construct a new prediction model that includes cardiac and noncardiac comorbidities based on the weighting of each coefficient to avoid futile procedures.
2. Methods
2.1. Study design and population
This retrospective cohort study included consecutive patients following TAVR for severe AS at Showa University hospital from 2015 to 2022. A multidisciplinary heart team made recommendations for TAVR, based on the severity of AS, cardiac function, anatomical findings, clinical manifestations, clinical conditions, exercise capacity, and prognosis. Patients without information regarding left ventricular ejection fraction (LVEF, n = 3) or hepatobiliary system parameters such as total bilirubin (TB), alkaline phosphatase (ALP), and gamma glutamyl transferase (GGT) (n = 10) were excluded from the current evaluation.
2.2. Data collection
Information on patient backgrounds such as age, sex, body mass index (BMI), medical history, cardiac- and noncardiac comorbidities, the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II, laboratory data, echocardiographic findings, medications prescribed at discharge, and two-year outcomes (including all-cause, cardiac, and noncardiac mortality) were collected. Ultrasound cardiography was evaluated by highly experienced cardiologists or clinical technologists within three months prior to the procedure. Left ventricular ejection fraction (LVEF) was measured using the modified Simpson's method. Causes of death were determined by two or more cardiologists or cardiac surgeons on the basis of pathologic autopsies or medical charts.
2.3. Study outcome and follow-up
The present study assessed two-year all-cause mortality. The follow-up was completed on the earliest of the last medical interview date, the last examination date, or the date when the outcome was recorded.
2.4. Definitions
The CCI score is the sum of nineteen weighted comorbidities. Each comorbidity is assigned a weight from 1 to 6: (1 point: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease (COPD), connective tissue disease, ulcer disease, mild liver disease, diabetes; 2 points: hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, any tumor, leukemia, lymphoma; 3 points: moderate or severe liver disease; 6 points: metastatic solid tumor, AIDS) [6]. According to the CCI criteria, mild increases in liver enzymes indicating chronic hepatitis or cirrhosis without portal hypertension was regarded as mild liver disease. Moderate or severe liver disease included patients who had cirrhosis with portal hypertension, ascites, chronic jaundice, or a history of variceal bleeding or liver transplantation. Moderate or severe renal disease included patients with a serum creatinine of >3 mg/dl with and without uremia, dialysis, or a history of transplantation. Reduced LVEF was categorized as an LVEF below 40 %. Pulmonary hypertension (PH) was diagnosed if the systolic pulmonary arterial pressure (sPAP) was 38 mmHg or higher, as assessed by transthoracic echocardiography [13]. Baseline laboratory evaluation of hepatobiliary function included TB, ALP, and GGT. TB was regarded as abnormal if it was above 1.2 mg/dL, regardless of sex. For ALP and GGT, we used sex-specific cutoff values: ALP was considered elevated if above 130 U/L for males or 105 U/L for females, and GGT was regarded as elevated if above 59 U/L for males or 39 U/L for females. Hepatobiliary system impairment was determined by an increase in two or more of the following three parameters: TB, ALP, and GGT [14]. Chronic kidney disease (CKD) stages 4–5 were diagnosed if the estimated glomerular filtration rate was below 30 ml/min/1.73 m2. Cachexia was defined as the combination of BMI <20 kg/m2 and at least one of the following biochemical abnormalities: C-reactive protein >5 mg/L, hemoglobin <120 g/L, or albumin <32 g/L [15]. MELD-XI was calculated from a formula: 5.11 × ln (TB in mg/dl) + 11.76 × ln (creatinine in mg/dl) + 9.44 [16].
2.5. Ethics statement
The study protocol adhered to the ethical guidelines established in the 1975 Declaration of Helsinki and followed the Ethical Guidelines for Epidemiological Research issued by the Japanese government. The current study was approved by the Ethics Committee of Showa University on July 11, 2022 (the approval number: 22-087-B). The study met the criteria for waiving the requirement of written informed consent from participants. This waiver was approved by the ethics committee.
2.6. Statistical analysis
Continuous variables are reported as mean ± standard deviation for normally distributed data. For non-normally distributed data, variables are presented as medians with interquartile ranges. Categorical variables are reported as absolute numbers and percentages. Continuous variables were analyzed using either unpaired Student's t-tests or Mann–Whitney U tests, while categorical variables were evaluated using either Fisher's exact test or the chi-squared test, as appropriate. The risk of all-cause mortality at 2 years was evaluated using Cox regression analysis and expressed as a hazard ratio (HR) with the 95 % confidence interval (CI). Multivariate Cox regression analysis was performed using forward-backward stepwise selection. Variables with a p value less than 0.25 in the univariate Cox regression analysis were considered for inclusion in the multivariate Cox regression analysis [17]. Multivariate models were constructed using at least five outcomes for each predictor [18]. The relative quality of multivariate models was assessed by the Akaike information criterion and Bayesian Information Criterion. The ability to predict the clinical outcome was firstly evaluated by the EuroSCORE II, MELD-XI score, and CCI score. Secondly, a new prediction model (Cardiac and nonCardiac Comorbidities risk score: 3C score) was constructed on the basis of the results of multivariate Cox regression analysis. Points were determined by dividing each variable's coefficient by the total sum of coefficients, multiplying the result by 8, and then rounding to the nearest integer. The 3C score was assessed by a receiver operating characteristic (ROC) curve, and the cutoff point was acquired based the Youden index. Statistical significance was set as a p value < 0.05. All statistical analyses were conducted using Stata version 14 (StataCorp, College Station, TX, USA).
3. Results
3.1. Patient backgrounds
Among 239 patients, 226 (age, 86 ± 5 years; males, 38 %) were included after exclusion of missing data regarding TB, ALP, GGT, or LVEF. Patient characteristics and echocardiographic parameters are displayed in Table 1. A history of myocardial infarction, cerebrovascular disease, and concurrent congestive heart failure were noted in 19 (8 %), 27 (12 %), and 113 (50 %), respectively. LVEF was 58 ± 11 %, and sPAP was 32 ± 10 %. Reduced LVEF, PH, CKD stages 4–5, hepatobiliary system impairment, and cachexia were identified in 18 (8 %), 54 (24 %), 20 (9 %), 20 (9 %), and 49 (22 %), respectively. The EuroSCORE II, MELD–XI, and CCI score were 3.36 (2.12–4.58), 5.35 (3.05–8.55), and 2 (1–3), respectively.
Table 1.
Patient characteristics.
| All (n = 226) | |
|---|---|
| Age, years | 86 ± 5 |
| Male, n (%) | 72 (32) |
| Body mass index, kg/m2 | 22.4 ± 3.8 |
| Diabetes, n (%) | 56 (25) |
| Dyslipidemia, n (%) | 120 (53) |
| Hypertension, n (%) | 160 (71) |
| Chronic kidney disease stages 4–5, n (%) | 20 (9) |
| Hepatobiliary system impairment, n (%) | 20 (9) |
| Cachexia, n (%) | 49 (22) |
| Clinical frailty scale | 4 (3–5) |
| CCI score related variables | |
| Previous myocardial infarction, n (%) | 19 (8) |
| Congestive heart failure, n (%) | 113 (50) |
| Peripheral vascular disease, n (%) | 55 (24) |
| Cerebrovascular disease, n (%) | 27 (12) |
| Dementia, n (%) | 25 (11) |
| Chronic obstructive pulmonary disease, n (%) | 32 (14) |
| Connective tissue disease, n (%) | 16 (7) |
| Ulcer disease, n (%) | 4 (2) |
| Mild liver disease, n (%) | 20 (9) |
| Diabetes without end organ damage, n (%) | 46 (20) |
| Hemiplegia, n (%) | 3 (1) |
| Moderate or severe renal disease, n (%) | 4 (2) |
| Diabetes with end organ damage, n (%) | 10 (4) |
| Any tumor, n (%) | 33 (15) |
| Leukemia, n (%) | 1 (0) |
| Lymphoma, n (%) | 2 (1) |
| Moderate or severe liver disease, n (%) | 1 (0) |
| Metastatic solid tumor, n (%) | 0 (0) |
| Acquired immunodeficiency syndrome, n (%) | 0 (0) |
| CCI score | 2 (1–3) |
| EuroSCORE II | 3.36 (2.12–4.58) |
| MELD-XI score | 5.35 (3.05–8.55) |
| Medication | |
| Renin-angiotensin system inhibitors, n (%) | 112 (50) |
| Beta blockers, n (%) | 103 (46) |
| Mineralocorticoid receptor antagonists, n (%) | 149 (66) |
| Loop diuretics, n (%) | 175 (77) |
| Statin, n (%) | 113 (50) |
| Laboratory data | |
| Hemoglobin, g/dL | 11.1 ± 1.7 |
| Albumin, g/dL | 3.7 ± 0.5 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) |
| Estimated glomerular filtration rate, ml/min/1.73m2 | 52 ± 19 |
| Total bilirubin, mg/dL | 0.7 ± 0.3 |
| Alkaline phosphatase, IU/L | 167 (80–235) |
| Gamma glutamyl transferase, IU/L | 20 (14–31) |
| B-type natriuretic peptide, pg/mL | 173 (81–354) |
| C-reactive protein, mg/dl | 0.1 (0.1–0.5) |
| Echocardiography | |
| Aortic valve maximum pressure gradient, mmHg | 78 ± 24 |
| Aortic valve mean pressure gradient, mmHg | 46 ± 16 |
| Aortic valve area, cm2 | 0.7 (0.5–0.8) |
| Left ventricular end-diastolic volume, ml | 78 ± 30 |
| Left ventricular end-systolic volume, ml | 34 ± 19 |
| Left ventricular ejection fraction, % | 58 ± 11 |
| Left ventricular ejection fraction <40 %, n (%) | 18 (8) |
| Systolic pulmonary arterial pressure, mmHg | 32 ± 10 |
| Systolic pulmonary arterial pressure ≥38 mmHg, n (%) | 54 (24) |
| Mitral valve regurgitation severity ≥2+, n (%) | 26 (12) |
| Tricuspid valve regurgitation severity ≥2+, n (%) | 49 (22) |
CCI, Charlson Comorbidities Index; MELD–XI, Model for End-stage Liver Disease eXcluding International normalized ratio.
3.2. Risk assessment of two-year all-cause mortality
Two-year all-cause and cardiac mortality were observed in 34 (15 %) and 7 (3 %) patients. The follow-up duration was 676 (365–1027) days. The results of univariate Cox regression analysis for two-year all-cause mortality are disclosed in Table 2. It is noteworthy that the MELD–XI and CCI score did not predict the clinical outcome (HR for MELD–XI, 1.07; 95 % CI, 1.00–1.14; p = 0.064; HR for CCI score, 0.97; 95 % CI, 0.78–1.22; p = 0.825), whereas EuroSCORE II did (HR, 1.17; 95 % CI, 1.02–1.33; p = 0.021). HRs for reduced LVEF and PH were 1.78 (95 % CI, 0.85–3.71; p = 0.127) and 2.29 (1.16–4.53; p = 0.017). Noncardiac comorbidities, such as CKD stages 4–5, hepatobiliary system impairment, and cachexia, were strong predictors (HR for CKD stages 4–5, 2.69; 95 % CI, 1.10–6.53; p = 0.029; HR for hepatobiliary system impairment, 7.19; 95 % CI, 3.48–14.86; p < 0.001; HR for cachexia, 2.72; 95 % CI, 1.37–5.38; p = 0.004).
Table 2.
Univariate Cox regression analysis for two-year all-cause mortality.
| HR | 95 % CI | p value | |
|---|---|---|---|
| Age, an increase of a year | 1.07 | 1.00–1.15 | 0.054 |
| Male | 1.28 | 0.63–2.58 | 0.498 |
| Body mass index, an increase of 1 kg/m2 | 0.85 | 0.77–0.93 | <0.001 |
| Diabetes | 0.64 | 0.26–1.54 | 0.320 |
| Dyslipidemia | 0.59 | 0.29–1.20 | 0.142 |
| Hypertension | 0.65 | 0.32–1.35 | 0.250 |
| Previous myocardial infarction | 1.09 | 0.33–3.57 | 0.887 |
| Congestive heart failure | 1.04 | 0.53–2.05 | 0.900 |
| Peripheral vascular disease | 1.41 | 0.68–2.96 | 0.358 |
| Cerebrovascular disease | 0.72 | 0.22–2.36 | 0.591 |
| Dementia | 1.16 | 0.41–3.28 | 0.787 |
| Chronic obstructive pulmonary disease | 0.49 | 0.20–1.19 | 0.116 |
| CCI score, an increase of 1 point | 1.02 | 0.82–1.27 | 0.851 |
| EuroSCORE II, an increase of 1 point | 1.17 | 1.02–1.33 | 0.021 |
| MELD–XI score, an increase of 1 point | 1.07 | 1.00–1.14 | 0.064 |
| Chronic kidney disease stages 4–5 | 2.69 | 1.10–6.53 | 0.029 |
| Hepatobiliary system impairment | 7.19 | 3.48–14.86 | <0.001 |
| Cachexia | 2.72 | 1.37–5.38 | 0.004 |
| Clinical frailty scale, an increase of 1 point | 1.35 | 0.98–1.85 | 0.065 |
| Renin–angiotensin system inhibitors | 1.38 | 0.70–2.72 | 0.347 |
| Beta blockers | 0.94 | 0.48–1.84 | 0.846 |
| Mineralocorticoid receptor antagonists | 0.45 | 0.23–0.89 | 0.021 |
| Loop diuretics | 0.46 | 0.23–0.93 | 0.031 |
| Statin | 0.63 | 0.32–1.27 | 0.197 |
| Hemoglobin, an increase of 1.0 g/dL | 0.87 | 0.72–1.05 | 0.144 |
| Albumin, an increase of 1.0 g/dL | 0.21 | 0.10–0.43 | <0.001 |
| Creatinine, an increase of 1.0 mg/dL | 1.82 | 1.22–2.74 | 0.004 |
| eGFR, an increase of 10 ml/min/1.73m2 | 0.83 | 0.68–1.02 | 0.071 |
| B–type natriuretic peptide, an increase of 1000 pg/mL | 3.60 | 1.85–7.03 | <0.001 |
| C–reactive protein, an increase of 1.0 mg/dl | 1.13 | 1.00–1.28 | 0.058 |
| Left ventricular end–diastolic volume, an increase of 10 ml | 0.95 | 0.83–1.08 | 0.393 |
| Left ventricular end–systolic volume, an increase of 10 ml | 1.06 | 0.89–1.26 | 0.502 |
| Left ventricular ejection fraction, an increase of 10 % | 0.73 | 0.56–0.95 | 0.020 |
| Left ventricular ejection fraction <40 % | 1.78 | 0.85–3.71 | 0.127 |
| Systolic pulmonary arterial pressure, an increase of 1 mmHg | 1.02 | 0.98–1.05 | 0.333 |
| Systolic pulmonary artery pressure ≥38 mmHg | 2.29 | 1.16–4.53 | 0.017 |
| Mitral valve regurgitation severity ≥2+ | 1.32 | 0.51–3.40 | 0.571 |
| Tricuspid valve regurgitation severity ≥2+ | 1.61 | 0.77–3.36 | 0.207 |
CCI, Charlson Comorbidities Index; CI, confidence interval; eGFR, estimated glomerular filtration rate; MELD–XI, HR, hazard ratio; Model for End–stage Liver Disease eXcluding International normalized ratio.
3.3. Comparison of the 3C score with existing models
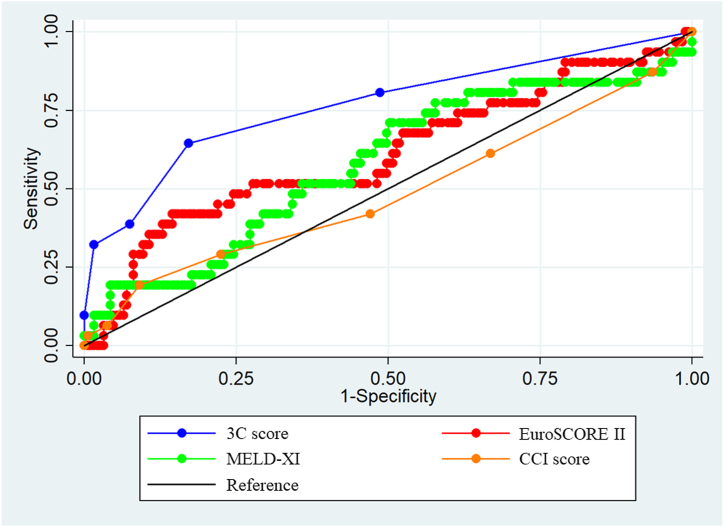
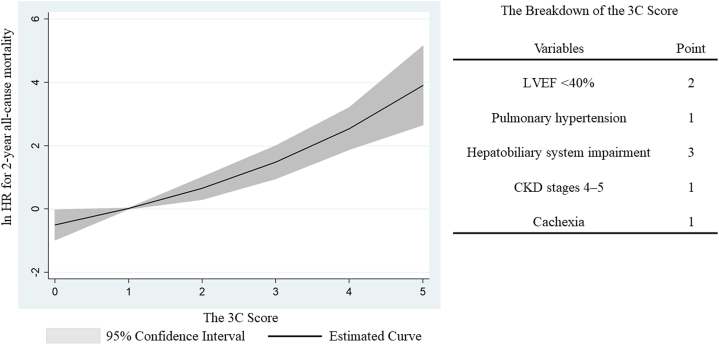
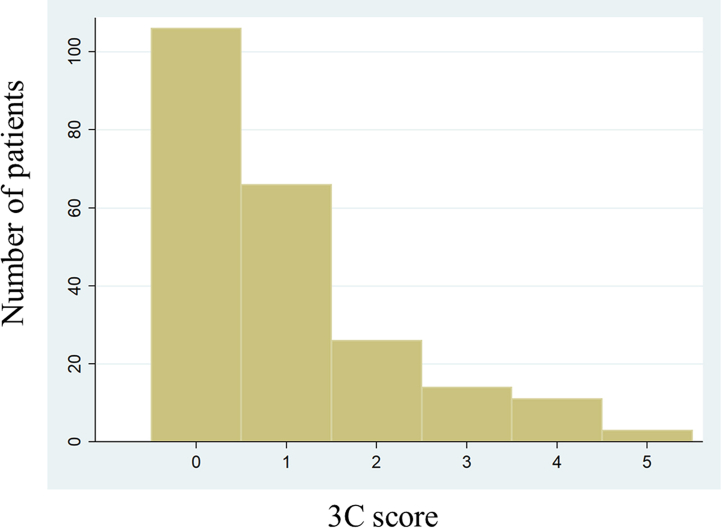
Table 3 indicates the 3C score based on the results of multivariate Cox regression analysis, which identified reduced LVEF, PH, hepatobiliary system impairment, CKD stages 4–5, and cachexia as significant predictors. Each weighted point of the included variables ranged from 1 to 3. Supplemental Fig. 1 shows the distribution of the 3C score. The new score ranged from 0 to 5 points. The C-statics of the 3C score, EuroSCORE II, MELD-XI score, and CCI score were 0.767 (0.666–0.867), 0.610 (0.491–0.729), 0.580 (0.465–0.696), and 0.489 (0.367–0.611), respectively (p < 0.001) (Fig. 1). The cutoff value for the 3C score was 3 points. Compared to patients with a 3C score of <3 points, those with a score of ≥3 points indicated higher 2-year all-cause mortality (HR, 7.08; 95 % CI, 3.55–14.12; p < 0.001). Fractional polynomials demonstrate that the 3C score strongly predicted 2-year all-cause mortality (Fig. 2).
Table 3.
Multiple Cox regression analysis for two–year all–cause mortality.
| Coefficient | HR | 95 % CI | p value | Point | |
|---|---|---|---|---|---|
| LVEF <40 % | 1.58 | 4.84 | 1.71–13.69 | 0.003 | 2 |
| Pulmonary hypertension | 1.12 | 3.05 | 1.49–6.24 | 0.002 | 1 |
| Hepatobiliary system impairment | 2.26 | 9.54 | 4.33–21.00 | <0.001 | 3 |
| CKD stages 4–5 | 1.16 | 3.19 | 1.28–7.97 | 0.013 | 1 |
| Cachexia | 1.04 | 2.84 | 1.42–5.69 | 0.003 | 1 |
CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; LVEF, left ventricular ejection fraction.
Fig. 1.
Comparison of the C-statistics of the 3C score and existing prediction scores
The C-statistic of the 3C score (0.767 [0.666–0.867]) was significantly higher than that of the EuroSCORE (0.610 [0.491–0.729]), the MELD-XI score (0.580 [0.465–0.696]), and the CCI score (0.489 [0.367–0.611]).
3C score, Cardiac and nonCardiac Comorbidities risk score; CCI, Charlson Comorbidities Index; MELD-XI, Model for End-stage Liver Disease eXcluding International normalized ratio.
Fig. 2.
Association of the 3C score with 2-year all-cause mortality
Fractional polynomials show that 2-year all-cause mortality increased with higher 3C scores, including LVEF <40 %, pulmonary hypertension, hepatobiliary system impairment, CKD stages 4–5, and cachexia.
3C score, Cardiac and nonCardiac Comorbidities risk score; CKD, chronic kidney disease; HR, hazard ratio; LVEF, left ventricular ejection fraction.
4. Discussion
The current study provides a new prediction model for assessing cardiac and noncardiac comorbidities in patients following TAVR. The 3C score was constructed based on the weighting of each coefficient. It is noteworthy that the impact of noncardiac comorbidities on two-year all-cause mortality was similar to, or even exceeded that of cardiac comorbidities. Evaluation of hepatobiliary system, renal function, and cachexia should be conducted prior to TAVR for better patient selection.
Even in the setting of cardiac diseases, concomitant noncardiac comorbidities are common and can affect treatment efficacy and prognosis, e.g. the impact of guideline-directed medical therapy has been reported to differ based on noncardiac comorbidities in patients with heart failure [19,20]. According to our 3C score, noncardiac comorbidities were at least as strongly associated with long-term mortality as were cardiac comorbidities. In particular, hepatobiliary system impairment showed the strongest association with two-year all-cause mortality despite the fact that the most patients with such disorders did not progress to liver cirrhosis. Tirado-Conte et al. reported high mortality in patients with Child-Pugh class B or C [21]. Decompensated cirrhosis is generally irreversible, and few options are available to improve its prognosis [22]. To avoid futile treatment, physicians may avoid performing TAVR for patients with advanced stages of liver cirrhosis. Meanwhile, liver impairment without liver cirrhosis may be reversible, and any intervention for such disorders can improve prognosis. Cardiohepatic syndrome could be resolved after TAVR for decompensated heart failure due to severe AS if the duration of congestion is not prolonged. However, congestive hepatopathy is usually subclinical, making it difficult to recognize. Long-term hepatobiliary system impairment would induce irreversible cardiac systolic and diastolic dysfunction, and impaired cardiac beta-adrenergic responsiveness [23] even if patients do not develop liver cirrhosis. Considering the high prevalence of CHF, patients with hepatobiliary system impairment in our cohort may present with advanced stages of the disease. This comorbidity might not be completely cured and remained a poor predictor. Similarly, although hepatitis C virus is highly curable using direct-acting antiviral agents [24] and non-alcoholic steatohepatitis can improve through a combination of a healthy diet and exercise [25], a delay in treatment delivery may impair the efficacy of TAVR. Future studies should investigate whether etiologies of hepatobiliary system impairment or changes in liver parameters following TAVR affect survival prediction. CKD has been reported to be associated with poor prognosis [26] and to attenuate treatment effects [19]. Some patients may present with cardiorenal syndrome due to severe AS leading to decompensated heart failure. Distinguishing between patients with and without reversible renal impairment may contribute to improving predictive ability. Compared to hepatobiliary and renal impairments, there is little room to improve prognosis in patients with cachexia, which may represent chronic inflammation; given that its treatment is difficult, prevention is essential. For selected patients with cachexia, effective strategies may include optimizing their diet, prescribing nutritional supplements, or implementing dietitian-led interventions during hospitalization to achieve daily calorie and protein targets [27]. The adverse clinical impact of reduced LVEF and PH in the current study was consistent with previous studies [28,29]. LVEF and sPAP can be affected by afterload mismatch, removal of which may lead to normalization of such parameters. However, our study demonstrated that both comorbidities were associated with higher long-term mortality. In some patients, such comorbidities persist after TAVR. If reduced LVEF is mainly caused by irreversible myocardial damage, then the procedure is not expected to improve function. Furthermore, some patients might present with pre-capillary PH, which is not affected by TAVR.
Our 3C score is simple and evaluates both cardiac and noncardiac comorbidities. Although the CCI score includes a wide range of comorbidities, the C-statistic was low in the modern era. Either the evolution of medical treatment or lower incidences of certain disorders, such as hematological malignancies, might contribute to the low predictive ability. Furthermore, solid tumors may grow slowly in older patients. Considering the improved current prognoses of AIDS and malignancies, the weight of each disorder should be different between the 1980's and the 2020's. The EuroSCORE II includes some parameters used in our 3C score, such as LVEF, PH, and renal function. However, the C-statistics of the 3C score were higher than those of the EuroSCORE II. This suggests the importance of hepatobiliary system impairment and cachexia in predicting long-term mortality. The MELD-XI score evaluates only a part of comorbidities.
It is noteworthy that the incidence of noncardiac mortality was as high as approximately eighty percent in the present cohort. Similar incidences were observed in previous studies including patients following TAVR [[30], [31], [32]] as well as those with heart failure with preserved LVEF [33]. As a result of successful TAVR, such patients would succumb to noncardiac death or die of natural causes instead of cardiac death. Physicians should not overlook treatable noncardiac events such as infection.
Our new prediction score indicated the importance of both cardiac and noncardiac comorbidities in evaluating long-term mortality in patients following TAVR for severe AS. Some patients may present with irreversible disorders, leading to futile procedures and a poor prognosis. A previous study indicated that the prognosis of a conservative treatment strategy was acceptable among asymptomatic patients with severe AS compared with the prognosis in patients who underwent TAVR or surgical aortic valve replacement [34]. Therefore, it may be reasonable for physicians to consider selecting conservative treatment strategies for asymptomatic patients with severe AS and a high 3C score.
4.1. Limitations
There are some limitations to be addressed. Firstly, the 3C score was not validated in this study because our cohort did not include a sufficient number of patients available for a validation cohort. This score should be assessed in other populations in the future. Secondly, PH was not diagnosed by right heart catheterization but by echocardiography. Nevertheless, the association between invasive and non-invasive assessment has been well investigated [13]. Thirdly, our cohort lacked information on body weight loss over six months. Although our cachexia definition aligned with previous studies [15,20,35], incorporating data on body weight loss might provide a more accurate predictive value. Finally, because of the nature of a retrospective study, detailed etiologies of the hepatobiliary system, renal impairment, and cachexia were not sufficiently investigated. Exclusion of reversible disorders from the 3C score may improve its predictive ability.
5. Conclusions
Cardiac (reduced LVEF and PH) and noncardiac (hepatobiliary system impairment, CKD stages 4–5, and cachexia) comorbidities were associated with a higher incidence of long-term all-cause mortality in older patients following TAVR for severe AS. The 3C score based on these comorbidities may contribute to the risk stratification of such fragile patients and avoid futile procedures.
Funding
None.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
CRediT authorship contribution statement
Satoshi Higuchi: Writing – original draft, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Hidenari Matsumoto: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Methodology, Data curation. Ryota Masaki: Writing – review & editing, Visualization, Resources, Investigation. Seita Kondo: Writing – review & editing, Resources, Investigation. Yasuhide Mochizuki: Writing – review & editing, Visualization, Resources, Investigation. Shiori Fuse: Writing – review & editing, Resources, Investigation. Eiji Toyosaki: Writing – review & editing, Resources, Investigation. Tomoaki Masuda: Writing – review & editing, Visualization, Investigation. Kazuto Maruta: Writing – review & editing, Resources, Investigation. Tadashi Omoto: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation. Atsushi Aoki: Writing – review & editing, Resources, Investigation. Toshiro Shinke: Writing – review & editing, Visualization, Validation, Supervision, Resources, Investigation, Funding acquisition, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36724.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplemental Fig. 1.
Distribution of the 3C score
The comorbidities included in the 3C score were reduced left ventricular ejection fraction, pulmonary hypertension, hepatobiliary system impairment, chronic kidney disease stages 4–5, and cachexia.
3C score, Cardiac and nonCardiac Comorbidities risk score.
References
- 1.Eveborn G.W., Schirmer H., Heggelund G., Lunde P., Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 2.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Leon M.B., Smith C.R., Mack M.J., Makkar R.R., Svensson L.G., Kodali S.K., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5.Levi A., Landes U., Assali A.R., Orvin K., Sharony R., Vaknin-Assa H., et al. Long-term outcomes of 560 consecutive patients treated with transcatheter aortic valve implantation and propensity score-matched analysis of early- versus new-generation valves. Am. J. Cardiol. 2017;119:1821–1831. doi: 10.1016/j.amjcard.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chron. Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Peck K.A., Calvo R.Y., Sise C.B., Johnson J., Yen J.W., Sise M.J., et al. Death after discharge: predictors of mortality in older brain-injured patients. J. Trauma Acute Care Surg. 2014;77:978–983. doi: 10.1097/TA.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 8.Coyan G.N., Chin H., Shah A., Miguelino A.M., Wang Y., Kilic A., et al. Charlson comorbidity index is associated with longer-term mortality and Re-admissions following coronary artery bypass grafting. J. Surg. Res. 2022;275:300–307. doi: 10.1016/j.jss.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Tseng A., Seet J., Phillips E.J. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br. J. Clin. Pharmacol. 2015;79:182–194. doi: 10.1111/bcp.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falzone L., Salomone S., Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai T., Yashima F., Yanagisawa R., Tanaka M., Shimizu H., Fukuda K., et al. Prognostic value of liver dysfunction assessed by MELD-XI scoring system in patients undergoing transcatheter aortic valve implantation. Int. J. Cardiol. 2017;228:648–653. doi: 10.1016/j.ijcard.2016.11.096. [DOI] [PubMed] [Google Scholar]
- 12.Feldman D.R., Romashko M.D., Koethe B., Patel S., Rastegar H., Zhan Y., et al. Comorbidity burden and adverse outcomes after transcatheter aortic valve replacement. J. Am. Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafitte S., Pillois X., Reant P., Picard F., Arsac F., Dijos M., et al. Estimation of pulmonary pressures and diagnosis of pulmonary hypertension by Doppler echocardiography: a retrospective comparison of routine echocardiography and invasive hemodynamics. J. Am. Soc. Echocardiogr. 2013;26:457–463. doi: 10.1016/j.echo.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Stolz L., Orban M., Besler C., Kresoja K.P., Braun D., Doldi P., et al. Cardiohepatic syndrome is associated with poor prognosis in patients undergoing tricuspid transcatheter edge-to-edge valve repair. JACC Cardiovasc. Interv. 2022;15:179–189. doi: 10.1016/j.jcin.2021.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Miura Y., Higuchi S., Kohno T., Shiraishi Y., Kitamura M., Nagatomo Y., et al. Association of potassium level at discharge with long-term mortality in hospitalized patients with heart failure. J. Clin. Med. 2022;11(24):7358. doi: 10.3390/jcm11247358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wernly B., Lichtenauer M., Franz M., Kabisch B., Muessig J., Masyuk M., et al. Model for End-stage Liver Disease excluding INR (MELD-XI) score in critically ill patients: easily available and of prognostic relevance. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mickey R.M., Greenland S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 18.Vittinghoff E., McCulloch C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi S., Kohsaka S., Shiraishi Y., Katsuki T., Nagatomo Y., Mizuno A., et al. Association of renin-angiotensin system inhibitors with long-term outcomes in patients with systolic heart failure and moderate-to-severe kidney function impairment. Eur. J. Intern. Med. 2019;62:58–66. doi: 10.1016/j.ejim.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi S., Kohno T., Kohsaka S., Shiraishi Y., Takei M., Goda A., et al. Different impact of beta-blockers on long-term mortality in heart failure patients with and without chronic obstructive pulmonary disease. J. Clin. Med. 2021;10(19):4378. doi: 10.3390/jcm10194378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirado-Conte G., Rodés-Cabau J., Rodríguez-Olivares R., Barbanti M., Lhermusier T., Amat-Santos I., et al. Clinical outcomes and prognosis markers of patients with liver disease undergoing transcatheter aortic valve replacement: a propensity score-matched analysis. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.117.005727. [DOI] [PubMed] [Google Scholar]
- 22.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J. Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.El Hadi H., Di Vincenzo A., Vettor R., Rossato M. Relationship between heart disease and liver disease: a two-way street. Cells. 2020;9(3):567. doi: 10.3390/cells9030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourlière M., Gordon S.C., Flamm S.L., Cooper C.L., Ramji A., Tong M., et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N. Engl. J. Med. 2017;376:2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 25.Ezpeleta M., Gabel K., Cienfuegos S., Kalam F., Lin S., Pavlou V., et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metabol. 2023;35:56–70.e3. doi: 10.1016/j.cmet.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumonteil N., van der Boon R.M., Tchetche D., Chieffo A., Van Mieghem N.M., Marcheix B., et al. Impact of preoperative chronic kidney disease on short- and long-term outcomes after transcatheter aortic valve implantation: a Pooled-RotterdAm-Milano-Toulouse in Collaboration Plus (PRAGMATIC-Plus) initiative substudy. Am. Heart J. 2013;165:752–760. doi: 10.1016/j.ahj.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Driggin E., Cohen L.P., Gallagher D., Karmally W., Maddox T., Hummel S.L., et al. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 2022;79:1623–1635. doi: 10.1016/j.jacc.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furer A., Chen S., Redfors B., Elmariah S., Pibarot P., Herrmann H.C., et al. Effect of baseline left ventricular ejection fraction on 2-year outcomes after transcatheter aortic valve replacement: analysis of the PARTNER 2 trials. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.118.005809. [DOI] [PubMed] [Google Scholar]
- 29.Parikh R., Varghese B., Khatoon H.N., Kovach J.A., Kavinsky C.J., Tandon R. Increased mortality from complications of pulmonary hypertension in patients undergoing transcatheter aortic valve replacement. Pulm. Circ. 2017;7:391–398. doi: 10.1177/2045893217697709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazui T., Hsu C.H., Hamidi M., Acharya D., Shanmugasundaram M., Lee K., et al. Five-meter walk test before transcatheter aortic valve replacement and 1-year noncardiac mortality. JTCVS Open. 2022;12:103–117. doi: 10.1016/j.xjon.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith C.R., Leon M.B., Mack M.J., Miller D.C., Moses J.W., Svensson L.G., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 32.Senussi M.H., Schindler J., Sultan I., Masri A., Navid F., Kliner D., et al. Long term mortality and readmissions after transcatheter aortic valve replacement. Cardiovasc. Diagn. Ther. 2021;11:1002–1012. doi: 10.21037/cdt-20-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamaru R., Shiraishi Y., Sandhu A.T., Heidenreich P.A., Shoji S., Kohno T., et al. Cardiovascular vs. non-cardiovascular deaths after heart failure hospitalization in young, older, and very old patients. ESC Heart Fail. 2022;10(1):673–684. doi: 10.1002/ehf2.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González-Saldivar H., Rodriguez-Pascual C., de la Morena G., Fernández-Golfín C., Amorós C., Alonso M.B., et al. Comparison of 1-year outcome in patients with severe aorta stenosis treated conservatively or by aortic valve replacement or by percutaneous transcatheter aortic valve implantation (data from a multicenter Spanish registry) Am. J. Cardiol. 2016;118:244–250. doi: 10.1016/j.amjcard.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 35.Higuchi S., Matsumoto H., Masaki R., Hirano T., Fuse S., Tanisawa H., et al. Potential confounders of the obesity paradox in older patients following transcatheter aortic valve replacement. Eur Geriatr Med. 2024;15:179–187. doi: 10.1007/s41999-023-00855-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.