Abstract
Background
Parkinson's disease (PD) is a complex neurodegenerative disorder characterized by dopamine depletion and severe motor impairments. Preladenant, an adenosine A2 receptor antagonist, is an investigational treatment for PD. This systematic review and meta-analysis aimed to critically evaluate the efficacy of Preladenant in improving motor symptoms in patients with PD.
Methods
A comprehensive literature search was conducted in PubMed, Embase, and Cochrane Central Register of Controlled Trials from inception to March 2023, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Randomized controlled trials (RCTs) comparing Preladenant with placebo in PD patients were included. The primary outcome was the change in daily ON time without troublesome dyskinesia. Secondary outcomes included the change in daily OFF time and adverse events. The risk of bias was assessed using the Cochrane Risk of Bias tool.
Results
Four RCTs with a total of 2097 PD patients were included. Pooled analysis showed that Preladenant could generally increase daily ON time (pooled effect 0.15 and 95 % CI: −0.19–0.48) and reduce daily OFF time (pooled effect −0.04 and 95 % CI: −0.43–0.36) compared to placebo, however it was not significant. The included studies had moderate to high heterogeneity. No significant differences in adverse events were observed between Preladenant and placebo.
Conclusion
This meta-analysis suggests that Preladenant may improve motor fluctuations in PD patients by increasing ON time and reducing OFF time. However, the high heterogeneity among studies warrants further large-scale, high-quality RCTs to confirm these findings and establish the long-term safety and efficacy of Preladenant in PD management.
Keywords: Preladenant, Parkinson's disease, Adenosine A2 receptor antagonist, Motor symptoms, Motor fluctuations
1. Introduction
Parkinson's disease (PD) is a prevalent and heterogeneous neurodegenerative disorder affecting millions globally (Bloem et al., 2021). Neurological conditions have emerged as the leading cause of disability worldwide. Notably, PD exhibits the fastest growth among these diseases, as evidenced by age-standardized prevalence, disability, and mortality rates. From 1990–2015, the global PD population increased by 118 %, reaching 6.2 million affected individuals (Global, 2024). This progressive and chronic disorder impacts both patients and their caregivers (Patient, 2024), posing a growing economic and social burden on society (Global, 2016). Epidemiological findings suggest that White men are more susceptible to PD compared to women, particularly those with estrogen deficiency (Zuñiga-Ramírez and Micheli, 2013 Nov 1).
The primary cause of motor deficits in PD is the progressive degeneration of dopamine-producing neurons in the ventral midbrain, leading to reduced dopamine input to the motor structures in the forebrain (striatum). The absence of dopaminergic input to the neostriatum disrupts striatal function, manifesting the characteristic motor symptoms associated with PD, including resting tremor, muscular rigidity, and bradykinesia (Parkinson, 2024a, Fink et al., 1992 Jul, Parkinson, 2024b, Parkinson’s disease, 2024). Restoring dopamine levels is the primary therapeutic strategy, mainly through the use of levodopa, a chemical that can convert to dopamine (Challenges, 2024, Levodopa, 2024). However, prolonged levodopa usage can lose effectiveness and produce undesirable side effects such as dyskinesia and motor fluctuations (Duodenal, 2024, Pharmacokinetic, 2024).
For newly diagnosed and younger PD patients, dopamine agonists are often used as the initial approach to delay levodopa therapy (Time until Need, 2024, An update, 2024). Prolonged dopamine replacement therapy (DRT) has been associated with significant adverse consequences, including heart valve disease, anhedonia, and Dopamine Dysregulation Syndrome (DDS) (Dopamine, 2024). In this stage, adjuvant therapy is needed. One such intervention is the administration of Adenosine 2 A (A2A) receptor antagonists (Bara-Jimenez et al., 2003 Aug 12).
A2A receptors are specifically located on striatopallidal neurons and can form functional heteromeric interactions with dopamine D2 receptors and metabotropic glutamate mGlu5 receptors. Their unique distribution and experimental findings demonstrate improved motor symptoms in animal models of PD, and preliminary clinical trials have positioned A2A receptor antagonists as a promising non-dopaminergic approach for alleviating PD-associated motor deficits. Moreover, evidence suggests that A2A receptor antagonists do not induce neuroplasticity events that may complicate extended dopaminergic treatments (Mori and Shindou, 2003, Pinna et al., 2007, Adenosine, 2024).
Preladenant, an A2A receptor antagonist, offers a promising avenue for enhancing its efficacy. The hypothesis posits that medications like Preladenant may potentially benefit individuals with PD by attenuating A2A receptors (Rose et al., 2022, Factor et al., 2013), thereby amplifying the transmission of D2 receptors, which could be advantageous in managing PD (Zuñiga-Ramírez and Micheli, 2013 Nov 1, Jenner, 2003).
To our knowledge, there is a lack of comprehensive analysis on the effects of preladenant in PD patients. This systematic review aims to address this gap by critically examining the impact of preladenant in individuals diagnosed with PD.
2. Material and method
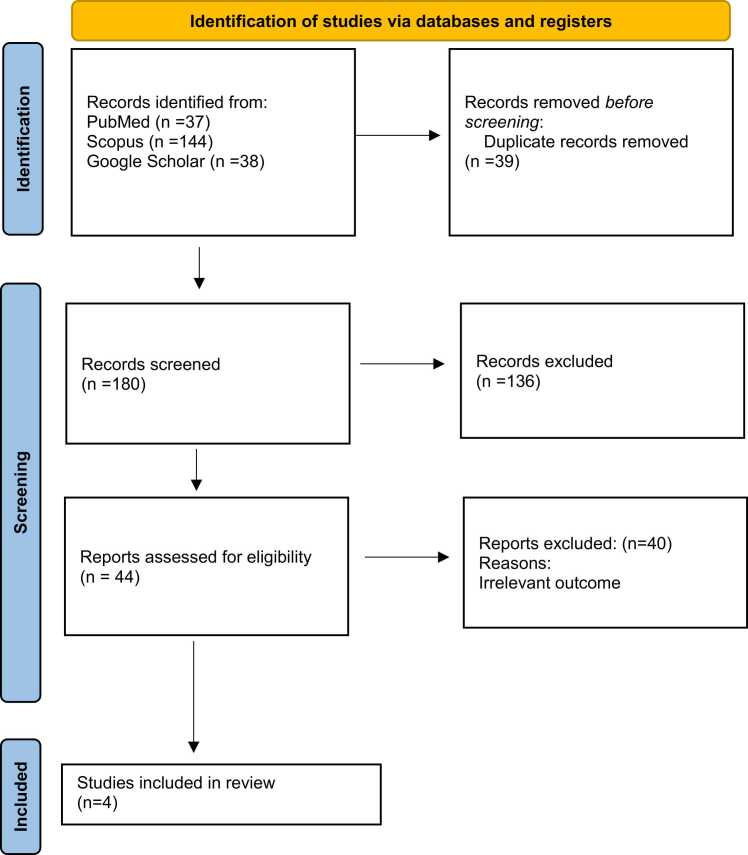
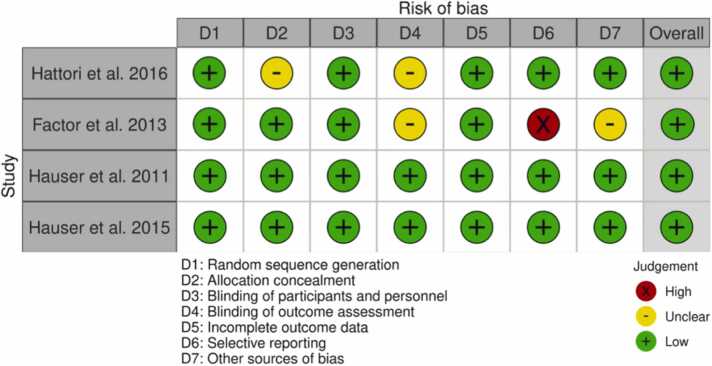
The purpose of this systematic review is to investigate the role of Preladenant in PD patients. The protocol for the study has been filed in the Open Science Framework. In the present study, the search strategy, the screening, and the data selection were all carried out using checklists. It was done in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines Fig. 1, Fig. 2.
Fig. 1.
Flowchart of literature inclusion in accordance with PRISMA guidelines. After a thorough database search (PubMed, Scopus, Google Scholar), 219 articles were found, and 39 duplicates were removed. After screening the title & abstract screening, 44 studies remained, and unrelated articles were deleted. The remaining 4 articles were included.
Fig. 2.
Quality assessment.
2.1. Search strategy
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we conducted a systematic review. We searched the PubMed (Medline), Scopus, and Google Scholar databases from inception to March 2023 using the search terms "(preladenant) AND (Parkinson's) OR (Parkinson)". The detailed search strategy for the MeSH database is provided in Table 1. We screened titles and abstracts of the retrieved studies, and eligible studies were selected for full-text review.
Table 1.
Search strategies for PubMed and Scopus.
| Search engine | Search strategy | Time | Results |
|---|---|---|---|
| Scopus | (TITLE-ABS-KEY (preladenant)) AND ((TITLE-ABS-KEY (parkinson) OR TITLE-ABS-KEY (parkinson's) OR TITLE-ABS-KEY (pd))) | March 13th, 2023 | 144 |
| PubMed | ((parkinson[Title/Abstract]) OR (parkinson's[Title/Abstract]) OR (pd[Title/Abstract])) AND (preladenant[Title/Abstract]) | March 13th, 2023 | 37 |
2.2. Inclusion and exclusion criteria
We included observational studies (cross-sectional, cohort, case-control, case reports, and case series) that evaluated the effect of preladenant on Parkinson's disease. We excluded review articles, editorials, commentaries, in vivo, in vitro, and non-English publications. Additional relevant studies were identified by manually searching the references of the included publications Table 2.
Table 2.
Outcomes of included studies.
| Author | Country | Study design | Participants |
Mean age (years) (SD) |
Sex (female) | Dose of preladenant | Duration of follow up | Adjustment |
Primary Outcome |
Other Outcomes | Combined Treatment | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hauser et al. (2011) (Hauser et al., 2015) | Argentina, Australia, Canada, Chile, Colombia, France, Guatemala, Hong Kong, Italy, New Zealand, Peru, Singapore, South Africa, Spain, and USA | Double-blind, randomized trial | 253 patients who were aged 30 years or older, had been diagnosed with PD at least five years prior, and had been treated with levodopa for two years or more •Group A (n=49): 1 mg preladenant twice daily •Group B (n=49): 2 mg preladenant twice daily •Group C (n=47): 5 mg preladenant twice daily •Group D (n=54): 10 mg preladenant twice daily •Group E (n=47): Placebo |
Group A: 61·3 (8·4) Group B: 63·9 (9·3) Group C: 62·0 (10·3) Group D: 62·4 (10·2) Group E: 62·2 (11·2) |
Group A: 39 % female Group B: 27 %female Group C: 36 %female Group D: 41 %female Group E: 34 % female |
2 mg BID 4 mg BID 10 mg BID 20 mg BID |
3 months | - Significant reduction in mean daily "off" time with 5 mg preladenant twice daily vs. placebo (difference: −1.0 hours; 95 % CI: −2.1–0.0; p=0.0486). - Significant reduction with 10 mg preladenant twice daily vs. placebo (difference: −1.2 hours; 95 % CI: −2.2 to −0.2; p=0.019). - No significant changes were observed with 1 or 2 mg preladenant twice daily versus placebo. |
- Mean Change from Baseline in OFF Time at Each Visit: - Significant reductions in OFF time compared to placebo were observed in the following groups: - 10 mg preladenant twice daily group: Weeks 2, 8, 10, and 12. - 5 mg preladenant twice daily group: Weeks 10 and 12. - Mean Change from Baseline to Week 12 in: - ON time: - Nominally significant increases compared to placebo in the 5 mg and 10 mg preladenant twice daily groups. - ON time without Dyskinesia: - No significant differences compared to placebo in any preladenant group. - ON time with Any Dyskinesia: - Nominally significant increases compared to placebo in the 2 mg and 10 mg preladenant twice daily groups. - ON time with Troublesome Dyskinesia: - No significant differences compared to placebo in any preladenant group. - ON time with Non-Troublesome Dyskinesia: - Nominally significant increases compared to placebo in the 2 mg and 10 mg preladenant twice daily groups. - Change in UPDRS Scores: - Part 1: - Significant reductions compared to placebo in the 5 mg and 10 mg preladenant twice daily groups. - Part 2: - No significant differences compared to placebo in any preladenant group. - Part 3 (Motor): - No significant differences compared to placebo in any preladenant group. - Part 4: - No data reported. |
• Levodopa • Dopamine agonist • Anticholinergic •Amantadine • COMT inhibitor • Monoamine oxidase B inhibitor |
• Group A: - Any treatment-emergent adverse event: 40 (82 %) -Adverse event leading to discontinuation: 12 (24 %) -Severe or life-threatening adverse event: 4 (8 %) -Serious treatment-emergent adverse event: 2 (4 %) - Worsening PD: 5 (10 %) - Somnolence: 5 (10 %) - Dyskinesia: 4 (8 %) - Nausea: 5 (10 %) - Constipation: 6 (12 %) - Insomnia: 3 (6 %) - Dizziness: 1 (2 %) - Fall: 4 (8 %) - Headache: 5 (10 %) - Back pain: 1 (2 %) - Diarrhoea: 2 (4 %) • Group B: -Any treatment-emergent adverse event: 39 (80 %) -Adverse event leading to discontinuation: 2 (4 %) -Severe or life-threatening adverse event: 1 (2 %) -Serious treatment-emergent adverse event: 1 (2 %) - Worsening PD: 4 (8 %) - Somnolence: 4 (8 %) - Dyskinesia: 3 (6 %) - Nausea: 4 (8 %) - Constipation: 1 (2 %) - Insomnia: 3 (6 %) - Dizziness: 4 (8 %) - Fall: 4 (8 %) - Headache: 2 (4 %) - Back pain: 3 (6 %) - Diarrhoea: 3 (6 %) • Group C: -Any treatment-emergent adverse event: 40 (85 %) -Adverse event leading to discontinuation: 5 (11 %) -Severe or life-threatening adverse event: 2 (4 %) -Serious treatment-emergent adverse event: 0 - Worsening PD: 5 (11 %) - Somnolence: 4 (9 %) - Dyskinesia: 4 (9 %) - Nausea: 6 (13 %) - Constipation: 6 (13 %) - Insomnia: 4 (9 %) - Dizziness: 3 (6 %) - Fall: 3 (6 %) - Headache: 4 (9 %) - Back pain: 3 (6 %) - Diarrhoea: 2 (4 %) • Group D: -Any treatment-emergent adverse event: 47 (87 %) -Adverse event leading to discontinuation: 10 (19 %) -Severe or life-threatening adverse event: 9 (17 %) -Serious treatment-emergent adverse event: 2 (4 % - Worsening PD: 8 (15 %) - Somnolence: 7 (13 %) - Dyskinesia: 7 (13 %) - Nausea: 2 (4 %) - Constipation: 2 (4 %) - Insomnia: 5 (9 %) - Dizziness: 6 (11 %) - Fall: 3 (6 %) - Headache: 3 (6 %) - Back pain: 2 (4 %) - Diarrhoea: 2 (4 %) |
|
| Hauser et al. (2015) (Hattori et al., 2016) | Americas, the European Union, Eastern Europe, India, and South Africa | two 12-weeks Phase III, Randomized, placebo-controlled, double-blind clinical Trials | Trial−1= 778 Trial−2= 476 patients with moderate to severe PD experiencing motor fluctuations, a diagnosis based on the UK Parkinson’s Disease Society Brain Bank criteria, a Hoehn-Yahr stage between 2.5 and 4, a stable and optimal treatment regimen including levodopa, and a minimum of 2 hours per day of OFF time as documented in a 3-day PD diary. | Trial−1 = 63.0 [30–86] Trial−2 = 62.9 [35–85] | Trial−1= 36.9 % Trial−2= 35.9 % | 2 mg preladenant BID 5 mg preladenant BID 10 mg preladenant BID |
12 weeks | • Trial−1: - Preladenant 2 mg twice daily: −0.10 hour (95 % CI, −0.69–.46 hour) - Preladenant 5 mg twice daily: −0.20 hour (95 % CI, −0.75–0.41 hour)- Preladenant 10 mg twice daily: −0.00 hour (95 % CI, −0.62–0.53 hour) - Rasagiline mesylate 1 mg/d: −0.30 hour (95 % CI, −0.90–0.26 hour) • Trial−2: - Preladenant 2 mg twice daily: −0.20 hour (95 % CI, −0.72–0.35 hour) - Preladenant 5 mg twice daily: −0.30 hour (95 % CI, −0.86–0.21 hour) - Preladenant was not superior to placebo in reducing OFF time from baseline to week 12. | - Proportion of Responders (≥30 % reduction in mean OFF time from baseline to week 12) - Trial−1: - Percentage of responders: Similar among preladenant, placebo, and rasagiline groups (31.0–36.1 %) - No significant differences between preladenant or rasagiline vs placebo - Trial−2: - Percentage of responders: Approximately 37 % in the preladenant groups - Placebo group: 30.5 % of responders - Change from Baseline in Mean ON Time without Troublesome Dyskinesia - Trial 1: - All preladenant groups and the rasagiline group had numerically larger increases than placebo - None were statistically significant from placebo and no dose response observed - Trial−2: - Mean increases at week 12: Similar across treatment groups (0.6, 0.7, and 0.5 hours) - UPDRS Part 3 Scores - Trial−1: - Changes were generally similar among treatments - No significant differences from placebo except for rasagiline at week 12 - Trial−2: - Changes from baseline were similar among treatments - No significant differences from placebo except for preladenant 5 mg twice daily at week 12. |
• Levodopa • Dopamine agonist • Amantadine • Hydrochloride • COMT inhibitor • Anticholinergic |
• Preladenant Trial−1 (N=460): - Any AE: 250 - Drug-related AE: 140 - Serious AE: 14 - Discontinued due to AE: 33 - Constipation: 26 - Headache: 17 - Dyskinesia: 22 - Dizziness: 15 - Nausea: 14 - Fall: 15 • Preladenant Trial−2 (N=314) - Any AE: 190 - Drug-related AE: 115 - Serious AE: 7 - Discontinued due to AE: 16 - Constipation: 25 - Headache: 21 - Dyskinesia: 14 - Dizziness: 15 - Nausea: 13 - Fall: 15 | |
| Factor et al. (2013) [28] | USA | phase II open-label double-blind trial |
140 patients who were aged 30 years or older, had been diagnosed with PD at least five years prior, and had been treated with levodopa for two years or more on a stable dose for 4 weeks or more • Group A: 1 mg preladenant BID (N=20) • Group B: 2 mg preladenant BID (N=28) • Group C: 5 mg preladenant BID (N=32) • Group D: 10 mg preladenant BID (N=32) • Placebo : (N=28)28) |
62.9±9.8 | 32 % | 1 mg preladenant BID 2 mg preladenant BID 5 mg preladenant BID 10 mg preladenant BID |
36-weeks | - The 5-mg BID preladenant dose appears to balance efficacy and tolerability. - Preladenant 5 mg BID: - Provided OFF time reductions of 1.4–1.9 hours/day throughout the 36-week - Increased - Change from baseline to week 12 in mean OFF time: - None of the preladenant doses demonstrated statistically significant improvement compared to placebo. - Estimated treatment differences for preladenant versus placebo were: - 2 mg BID: 0.7 hours (p=0.0564) - 5 mg BID: −0.5 hours (p=0.1844) - 10 mg BID: 0.3 hours (p=0.3386) - Treatment was well tolerated at doses between 2 and 10 mg BID. - Preladenant did not demonstrate statistically significant efficacy. by 1.2–1.5 hours/day throughout the 36-week treatment period relative to the baseline - Provided sustained OFF time reductions and ON time increases over the 36-week treatment period. |
- Percentage of ON time free of dyskinesia: - Slightly lower at the end of the open-label extension (56.4 %) than the double-blind study baseline (61.6 %). - Averaged 58 %−60 % throughout the open-label extension. - Percentage of ON time with nontroublesome dyskinesia: - Similar at the end of the open-label extension (30.9 %) and the double-blind study baseline (29.3 %). - Averaged 30 % throughout the open-label extension. - Percentage of ON time with troublesome dyskinesia: - Averaged 11 %−12 % throughout the open-label extension. - Slightly greater at the end of the open-label extension (12.7 %) than the double-blind study baseline (9.2 %). -Absolute dyskinesia duration: - Increased incidence of dyskinesia in the open-label extension (33 %) compared to the randomized study (9 %). |
• Levodopa • Other antiparkinsonian drugs |
- Dyskinesia:46(33 %) - Constipation:26 (19 %) - PD deterioration: 19 (14 %) - Fall: 18 (13 %) - Somnolence: 15 (11 %) - Back pain: 14 (10 %) - Arthralgia: 13 (9 %) - Headache: 13 (9 %) - Insomnia: 11 (8 %) - Diarrhea: 11 (8 %) Increased -CPK: 9 (6 %) - Influenza: 9 (6 %) - Nausea: 9 (6 %) - Nasopharyngitis: 9 (6 %) - Pain in extremity: 8 (6 %) - Tremor:7 (5 %) |
|
| Nobutaa Hattori (2016) [29] | Japan | Randomized, placebo-controlled, parallel-group, multisite, double-blind, dose-ranging phase II clinical trial | 450 Japanese Patients between 30 and 85 years of age who had moderate to severe PD, were on a stable regimen of levodopa, and were experiencing motor fluctuations and 2 hours or more /day OFF time Group A: 2 mg preladenant BID (N=111) Group B: 5 mg preladenant BID (N=113) Group C: 10 mg preladenant BID (N=113) Placebo: (N=113) |
Group A: 68.0±7.9 Group B: 67.6±8.3 Group C: 67.8±7.6 Placebo: 65.8±9.1 |
Group A: 49 % Group B: 58 % Group C: 61 % Placebo: 57 % |
2 mg preladenant BID 5 mg preladenant BID 10 mg preladenant BID |
12-weeks | - Change from baseline to week 12 in mean OFF time: - None of the preladenant doses demonstrated statistically significant improvement compared to placebo. - Estimated treatment differences for preladenant versus placebo were: - 2 mg BID: 0.7 hours (p=0.0564) - 5 mg BID: −0.5 hours (p=0.1844) - 10 mg BID: 0.3 hours (p=0.3386) - Treatment was well tolerated at doses between 2 and 10 mg BID. - Preladenant did not demonstrate statistically significant efficacy. |
- Proportion of responders (≥30 % reduction in mean OFF time from baseline to week 12): - Estimated treatment differences vs placebo were not statistically significant: - Preladenant 2 mg BID: 5.7 % (p=0.404) - Preladenant 5 mg BID: 5.7 % (p=0.390) - Preladenant 10 mg BID: 4.9 % (p=0.508) - Change from baseline to week 12 in mean ON time without troublesome dyskinesia: - Estimated treatment differences vs placebo were not statistically significant: - Preladenant 2 mg BID: 0.7 hours (p=0.05) - Preladenant 5 mg BID: 0.5 hours (p=0.18) - Preladenant 10 mg BID: 0.5 hours (p=0.20) |
• Amantadine • Anticholinergics • Dopa decarboxylase inhibitors • Dopamine agonists • Entacapone • Levodopa |
- Discontinued because of AE: Group A: 5 Group B: 5 Group C: 11 Placebo: 4 - Death: Group A: 1 Group B: 0 Group C: 0 Placebo: 0 - Any AE: Group A: 48 Group B: 53 Group C: 61 Placebo: 49 - Drug-related AE: Group A: 17 Group B: 17 Group C: 35 Placebo: 19 - Serious AE: Group A: 8 Group B: 4 Group C: 6 Group Placebo: 3 - Serious drug-related AE: Group A: 1 Group B: 1 Group C: 2 Placebo: 2 - Nasopharyngitis: Group A: 5 Group B: 8 Group C: 7 Group Placebo: 7 - Fall: Group A: 7 Group B: 3 Group C: 6 Placebo: 0 - Dyskinesia: Group A: 3 Group B: 6 Group C: 6 Group Placebo: 2 - Constipation: Group A: 3 Group B: 4 Group C: 7 Placebo: 4 - Worsening PD: Group A: 5 Group B: 4 Group C: 4 Placebo: 4 |
Abbreviations: PD: Parkinson Disease; COMT: catechol-O-methyl transferase; CI: Confidential Interval; UPDRS: Unified Parkinson’s disease rating scale; AE: adverse event; BID: twice daily
2.3. Quality Assessment
Two independent reviewers assessed the included studies' quality and risk of bias using the Joanna Briggs Institute (JBI) Critical Appraisal tools. Any disagreements were resolved through discussion and consensus.
2.4. Data extraction and analysis
Two reviewers independently extracted data using a standardized form. Meta-analyses evaluated the association between preladenant concentrations and PD, considering all the reported variables. The standardized mean difference (SMD) with Cohen's d was used to estimate the effect size, and the I2 statistic and Hedges' H value were used to assess heterogeneity. Subgroup analyses and sensitivity analyses were conducted to explore potential sources of heterogeneity (Salivary, 2024). Publication bias was assessed using funnel plots and Egger's linear regression test. All statistical analyses were performed using the 'meta' and 'metaphor' packages in R software (version 3.4.2), with a significance level of 5 % for two-sided tests.
3. Results
After an extensive search across databases (PubMed, Scopus, Google Scholar), 219 articles were initially identified, with 39 duplicates removed. After screening titles and abstracts, 44 studies remained, and articles with irrelevant data were excluded, leaving four articles eligible for final review. All selected articles were randomized, double-blind, placebo-controlled trials involving a total population of 2097 patients with PD across various countries. The mean age of the patients was 64.15 years, and the follow-up duration ranged from 12 (Hauser et al., 2011, Hauser et al., 2015, Hattori et al., 2016) to 36 weeks (Factor et al., 2013). The studies evaluated the impact of Preladenant on OFF and ON time, with some studies incorporating additional medications alongside Preladenant. Adverse events observed included a range of symptoms.
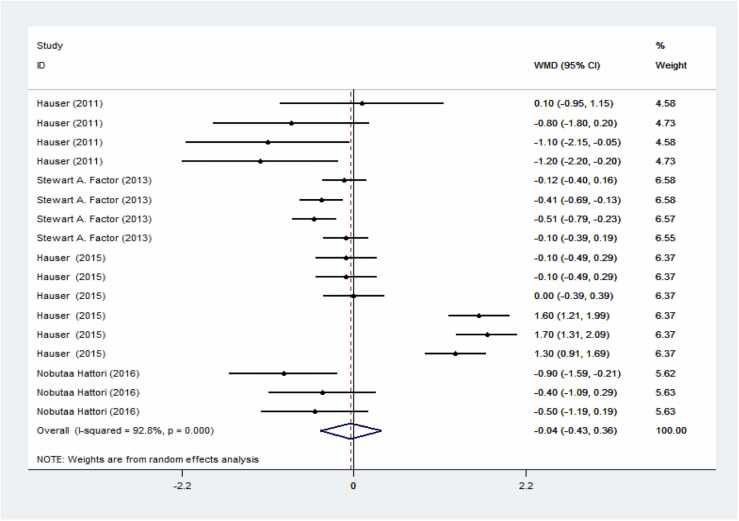
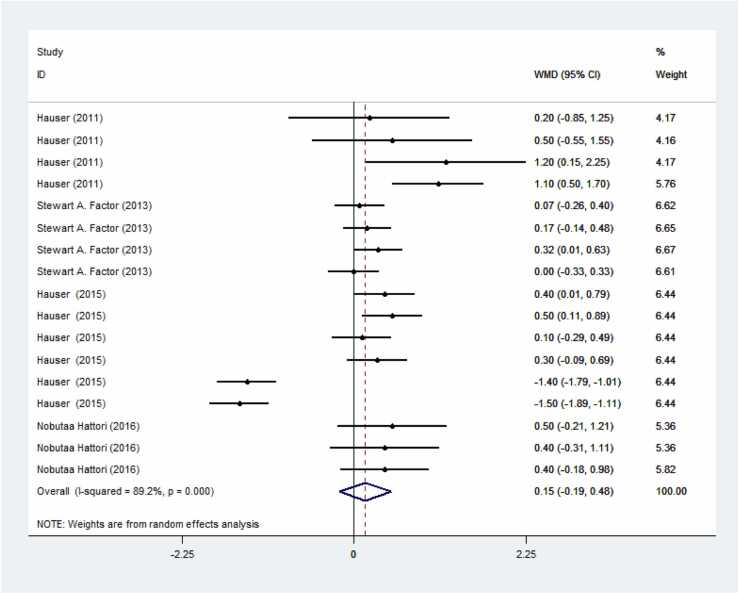
Preladenant demonstrated a reduction in OFF time and an increase in ON time compared to the control group; however, these results were not significant. Specifically, Fig. 3 shows a s decrease in OFF time with a weighted mean difference (WMD) of −0.04 (95 % CI: −0.43–0.36) in patients receiving Preladenant. Fig. 4 indicates an increase in ON time with a WMD of 0.15 (95 % CI: −0.19–0.48).
Fig. 3.
Forest plot of preladenant's impact on PD's OFF time. The result is a significant decrease in OFF time in the group receiving preladenant.
Fig. 4.
Forest plot of the impact of preladenant regarding ON time in PD. The result is a significant increase in ON time in the group receiving preladenant.
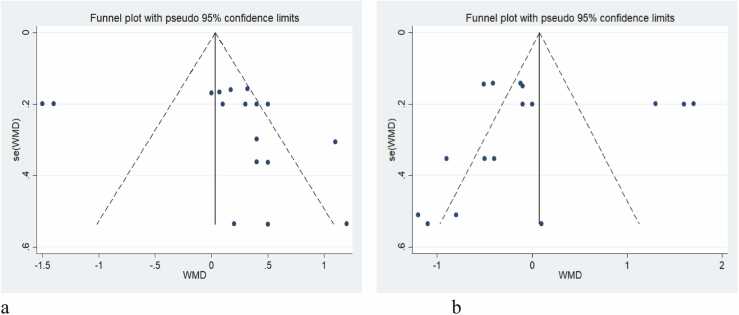
However, the funnel plots in Fig. 5 reveal potential publication bias. The funnel plots were asymmetric for both ON time (Fig. 5a) and OFF time (Fig. 5b). Specifically, the asymmetry in the funnel plot for ON time suggests that smaller studies with less precision tended to report more significant effects, indicating a possible publication bias. The funnel plot for OFF time similarly shows an asymmetry, again implying a relationship between the estimated effect of Preladenant and study precision, suggesting overall publication bias in the included studies.
Fig. 5.
Funnel plot of preladenant’s effect in PD patients, considering OFF time and ON time. The funnel plot was asymmetric, regarding ON time (a) and OFF time (b), showing a relationship between the effect of preladenant estimate and study precision, and overall, publication bias is possible in included studies.
The meta-analysis revealed no linear association between dose and response for both OFF and ON time. Despite the observed publication bias, Preladenant positively affected ON and OFF time in PD patients, with no studies excluded in the sensitivity analysis.
4. Discussion
This systematic review aimed to assess the therapeutic effects of Preladenant in patients diagnosed with PD. The review synthesized data from a total of four studies involving 1379 individuals. Compared to the control and placebo groups, patients who took Preladenant showed an increase in ON time duration and decrease in OFF time periods, although not significant.
Our findings are in line with earlier research on the effects of preladenant. Factor et al (Factor et al., 2013). presented findings from a 36-week open-label extension phase assessing the long-term safety and efficacy of preladenant as an adjunctive therapy for PD with motor fluctuations. A cohort of 140 participants who had previously completed a 12-week double-blind, placebo-controlled trial of preladenant enrolled in the extension study and received a dosage of 5 mg twice daily. Of these, 106 subjects (76 %) completed the extension phase. Efficacy analyses indicated that treatment with preladenant 5 mg twice daily resulted in reductions in OFF time ranging from 1.4 to 1.9 hours per day and increases in ON time of 1.2–1.5 hours per day throughout the 36-week period compared to baseline from the initial double-blind study. There was a slight rise in the percentage of ON time associated with troublesome dyskinesia relative to baseline. These findings suggest that preladenant maintained tolerability and sustained efficacy in ameliorating motor fluctuations in PD over the 36-week open-label treatment duration.
In the same line of thought, Hauser et al (Hauser et al., 2011). investigated the efficacy and safety of preladenant as an adjunctive therapy to levodopa in patients with PD and motor fluctuations through a phase 2, double-blind, randomized trial. The study included 253 patients with PD who were randomized to receive one of four different twice-daily doses of preladenant (1 mg, 2 mg, 5 mg, 10 mg) or placebo. After a 12-week treatment period, 234 patients were included in the efficacy analysis. The findings revealed that patients receiving preladenant at doses of 5 mg and 10 mg twice daily experienced significantly lower daily OFF time compared to those on placebo, with reductions of 1.0 hour and 1.2 hours, respectively, from baseline to week 12. There were also nominal increases in mean daily ON time observed with these doses compared to the placebo. However, neither on time with dyskinesia nor on time with troublesome dyskinesia showed statistically significant increases with preladenant compared to placebo. Overall, adjunctive treatment with preladenant at doses of 5 mg and 10 mg twice daily demonstrated significant efficacy in reducing OFF time without exacerbating dyskinesia.
However, in another study conducted by Hauser et al (Hauser et al., 2015)., they found no appreciable decrease in OFF time when comparing the preladenant with the placebo. The findings from two phase 3 randomized clinical trials (Trial 1 and Trial 2) were presented, investigating the effectiveness and safety of preladenant as an adjunctive treatment to levodopa in patients with PD who experience motor fluctuations. In Trial 1, a total of 778 eligible patients were randomly allocated to receive preladenant (2 mg, 5 mg, or 10 mg twice daily), placebo, or Rasagiline mesylate (1 mg/day). Trial 2 involved 476 eligible patients who were randomly assigned to receive preladenant (2 mg or 5 mg twice daily) or placebo. In both trials, preladenant did not significantly reduce off time compared to placebo at 12 weeks. Similarly, in Trial 1, the active control Rasagiline also failed to demonstrate a significant reduction in off time. Preladenant was generally well-tolerated, with constipation being the most common adverse event. Post hoc analyses of Trial 1 indicated a significant placebo effect in regions such as Turkey, India, and Latin America, with the placebo group showing greater reductions in off time than those receiving preladenant or Rasagiline. Significant reductions in off time for preladenant and Rasagiline were observed in the first 50 % of enrolled patients but not in the latter half. The authors proposed potential explanations for these negative results, including study design or conduct issues. Preladenant's effectiveness in lowering OFF time in PD must be further investigated to make more convincing data.
Hattori et al (Hattori et al., 2016). reported similar findings in their phase 2 study conducted in 2016, where none of the doses of preladenant showed superior efficacy compared to placebo in reducing mean OFF time from baseline to week 12. This study presents findings from a randomized, placebo-controlled, double-blind, 12-week, dose-ranging phase 2 trial evaluating preladenant as adjunctive therapy in Japanese patients diagnosed with moderate to severe PD and experiencing motor fluctuations. Patients were randomly assigned to treatment groups, with a total of 111 patients receiving preladenant at a dose of 2 mg and 113 patients allocated to receive preladenant at doses of 5 mg, 10 mg, or placebo. However, no statistically significant improvements were observed in mean OFF time from baseline to week 12 across any of the preladenant doses compared to placebo. Similarly, key secondary endpoints, including changes in ON time without troublesome dyskinesia and the proportion of treatment responders, did not significantly differ between preladenant groups and placebo, despite numerical trends favoring preladenant. They acknowledged certain limitations of their study. On one hand, the absence of an active comparator made it challenging to assess whether the trial failed or the preladenant was ineffective. On the other hand, using paper diaries to record on and off states may have introduced bias into the data.
The effects of Preladenant on the OFF time and ON time durations in Parkinson's patients have never been thoroughly studied before the publication of this systematic review and meta-analysis. High heterogeneity and the lack of studies in this field are two drawbacks of the study, though. It is advised to carry out more randomized controlled trials (RCTs) and examine Preladenant's impact on a bigger group of Parkinson's patients to solve these shortcomings. These investigations will add to our knowledge of the therapeutic potential of Preladenant in the treatment of PD and offer insightful information.
5. Conclusion
Preladenant, an A2A receptor antagonist, has shown potential benefits in reducing off-time and increasing on-time in patients with PD and motor fluctuations in a phase 2 clinical trial (Hauser et al., 2011). The 5 mg and 10 mg twice daily doses of preladenant reduced off-time by approximately 25–30 % and increased on-time by 10–15 % compared to placebo (Factor et al., 2013). However, the evidence on the efficacy of preladenant is mixed. While the phase 2 trial showed positive results, a subsequent phase 3 trial did not find preladenant to be superior to placebo. Additionally, there are concerns about the potential for preladenant to exacerbate dyskinesia, with up to 33 % of patients experiencing dyskinesia in the open-label extension of the phase 2 trial. The systematic review and meta-analysis cited in the query had limitations, including high heterogeneity among the included studies and a small number of studies evaluating preladenant. Therefore, more high-quality, large-scale, randomized controlled trials are needed to definitively establish the efficacy and safety of preladenant for the treatment of motor symptoms in PD.
In conclusion, while preliminary evidence indicates potential beneficial effects of preladenant on motor symptoms in PD, the current findings are varied, and the safety profile remains incompletely understood. Further research is essential to elucidate the precise role of preladenant in PD management. This meta-analysis suggests that preladenant may enhance motor fluctuations in PD patients by increasing ON time and reducing OFF time. However, due to significant heterogeneity among studies, larger-scale and high-quality RCTs are needed to validate these results and establish preladenant's long-term safety and efficacy in treating PD.
Finding
None
CRediT authorship contribution statement
Ata Akhtari Kohnehshahri: Formal analysis, Supervision. Alireza Ghajari: Methodology, Software, Writing – original draft. Niloofar Deravi: Project administration, Writing – review & editing. Reza Khademi: Writing – original draft. Zahra Jafari Shendi: Investigation, Writing – original draft. Mohammad Amin Karimi: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Hossein Ashkpour Motlagh: Conceptualization, Supervision. Behnaz Mahmoudvand: Investigation, Writing – original draft. Mohaddeseh Belbasi: Investigation, Software, Writing – original draft. Kimia Janeshin: Writing – original draft, Writing – review & editing. Seyed Amirhossein Mazhari: Investigation, Methodology. Mohammad Hossein Etemadi: Writing – original draft, Writing – review & editing. Mahdyieh Naziri: Formal analysis. Narges Safar Firouz: Investigation, Writing – original draft. Sayedeh-Fatemeh Sadat-Madani: Conceptualization, Writing – original draft, Writing – review & editing. Mohammad Abbasalizadeh: Methodology, Writing – original draft. Mahsa Aziz: Writing – review & editing. Afra Darvishi: Validation, Writing – original draft, Writing – review & editing. Matin Bidares: Writing – review & editing. Shafagh Asgarzadeh: Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to thank the researchers whose work was included in this study.
Contributor Information
Mahdyieh Naziri, Email: nazirimahdyieh@yahoo.com.
Hossein Ashkpour Motlagh, Email: Ashkpour.md92@gmail.com.
References
- Adenosine A2A receptor availability in patients with early- and moderate-stage Parkinson’s disease - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/36053386/〉. [DOI] [PMC free article] [PubMed]
- An update on the management of young-onset Parkinson’s disease - PMC [Internet]. [cited 2024 Jun 7]. Available from: 〈https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6065598/〉.
- Bara-Jimenez W., Sherzai A., Dimitrova T., Favit A., Bibbiani F., Gillespie M., et al. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003 Aug 12;61(3):293–296. doi: 10.1212/01.wnl.0000073136.00548.d4. [DOI] [PubMed] [Google Scholar]
- Bloem B.R., Okun M.S., Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–2303. doi: 10.1016/S0140-6736(21)00218-X. Jun 12. [DOI] [PubMed] [Google Scholar]
- Challenges in Parkinson’s disease: restoration of the nigrostriatal dopamine system is not enough - ScienceDirect [Internet]. [cited 2024 Jun 7]. Available from: 〈https://www.sciencedirect.com/science/article/abs/pii/S1474442204007409〉. [DOI] [PubMed]
- Dopamine receptors and Parkinson’s disease - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/25954517/〉.
- Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/15668416/〉. [DOI] [PubMed]
- Factor S.A., Wolski K., Togasaki D.M., Huyck S., Cantillon M., Ho T.W., et al. Long-term safety and efficacy of preladenant in subjects with fluctuating Parkinson’s disease. Mov. Disord. 2013;28(6):817–820. doi: 10.1002/mds.25395. [DOI] [PubMed] [Google Scholar]
- Fink J.S., Weaver D.R., Rivkees S.A., Peterfreund R.A., Pollack A.E., Adler E.M., et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. Mol. Brain Res. 1992 Jul;14(3):186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015 - PubMed [Internet]. [cited 2024 Jun 7]. Available from: https://pubmed.ncbi.nlm.nih.gov/28931491/.
- Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016 - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/30287051/〉.
- Hattori N., Kikuchi M., Adachi N., Hewitt D., Huyck S., Saito T. Adjunctive preladenant: A placebo-controlled, dose-finding study in Japanese patients with Parkinson’s disease. Park. Relat. Disord. 2016;32:73–79. doi: 10.1016/j.parkreldis.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Hauser R.A., Cantillon M., Pourcher E., Micheli F., Mok V., Onofrj M., et al. Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol. 2011;10(3):221–229. doi: 10.1016/S1474-4422(11)70012-6. [DOI] [PubMed] [Google Scholar]
- Hauser R.A., Stocchi F., Rascol O., Huyck S.B., Capece R., Ho T.W., et al. Preladenant as an adjunctive therapy with levodopa in Parkinson disease: two randomized clinical trials and lessons learned. JAMA Neurol. 2015;72(12):1491–1500. doi: 10.1001/jamaneurol.2015.2268. [DOI] [PubMed] [Google Scholar]
- Jenner P. A2A antagonists as novel non-dopaminergic therapy for motor dysfunction in PD. Neurology. 2003;61(11 Suppl 6):S32–S38. doi: 10.1212/01.wnl.0000095209.59347.79. Dec 9. [DOI] [PubMed] [Google Scholar]
- Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/19050033/〉. [DOI] [PubMed]
- Mori A., Shindou T. Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors: a potential mechanism for the antiparkinsonian effects of A2A antagonists. Neurology. 2003;61(11 Suppl 6):S44–S48. doi: 10.1212/01.wnl.0000095211.71092.a0. Dec 9. [DOI] [PubMed] [Google Scholar]
- Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology - PubMed [Internet]. [cited 2024a Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/31733690/〉.
- (15) Parkinson’s disease: A review | Request PDF [Internet]. [cited 2024b Jun 7]. Available from: 〈https://www.researchgate.net/publication/259587074_Parkinson%27s_disease_A_review〉.
- Parkinson’s disease: Autoimmunity and neuroinflammation - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/27497913/〉.
- Patient and caregiver characteristics associated with caregiver burden in Parkinson’s disease: a palliative care approach - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/31735048/〉. [DOI] [PubMed]
- Pharmacokinetic-pharmacodynamic modeling of levodopa in patients with advanced Parkinson disease - PubMed [Internet]. [cited 2024 Jun 7]. Available from: 〈https://pubmed.ncbi.nlm.nih.gov/20216409/〉. [DOI] [PubMed]
- Pinna A., Pontis S., Borsini F., Morelli M. Adenosine A2A receptor antagonists improve deficits in initiation of movement and sensory motor integration in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Synapse. 2007;61(8):606–614. doi: 10.1002/syn.20410. (Aug) [DOI] [PubMed] [Google Scholar]
- Rose R., Mitchell E., Van Der Graaf P., Takaichi D., Hosogi J., Geerts H. A quantitative systems pharmacology model for simulating OFF-Time in augmentation trials for Parkinson’s disease: application to preladenant. J. Pharmacokinet. Pharmacodyn. 2022;49(6):593–606. doi: 10.1007/s10928-022-09825-9. [DOI] [PubMed] [Google Scholar]
- Salivary cortisol in post-traumatic stress disorder: a systematic review and meta-analysis | BMC Psychiatry | Full Text [Internet]. [cited 2024 Jun 7]. Available from: 〈https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-018-1910-9〉. [DOI] [PMC free article] [PubMed]
- Time until Need for Levodopa among New Users of Dopamine Agonists or MAO-B Inhibitors - PMC [Internet]. [cited 2024 Jun 7]. Available from: 〈https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8270692/〉. [DOI] [PMC free article] [PubMed]
- Zuñiga-Ramírez C., Micheli F. Preladenant: An adenosine A2A receptor antagonist for Parkinson"s disease. Future Neurol. 2013 Nov 1;8:639. [Google Scholar]