Abstract
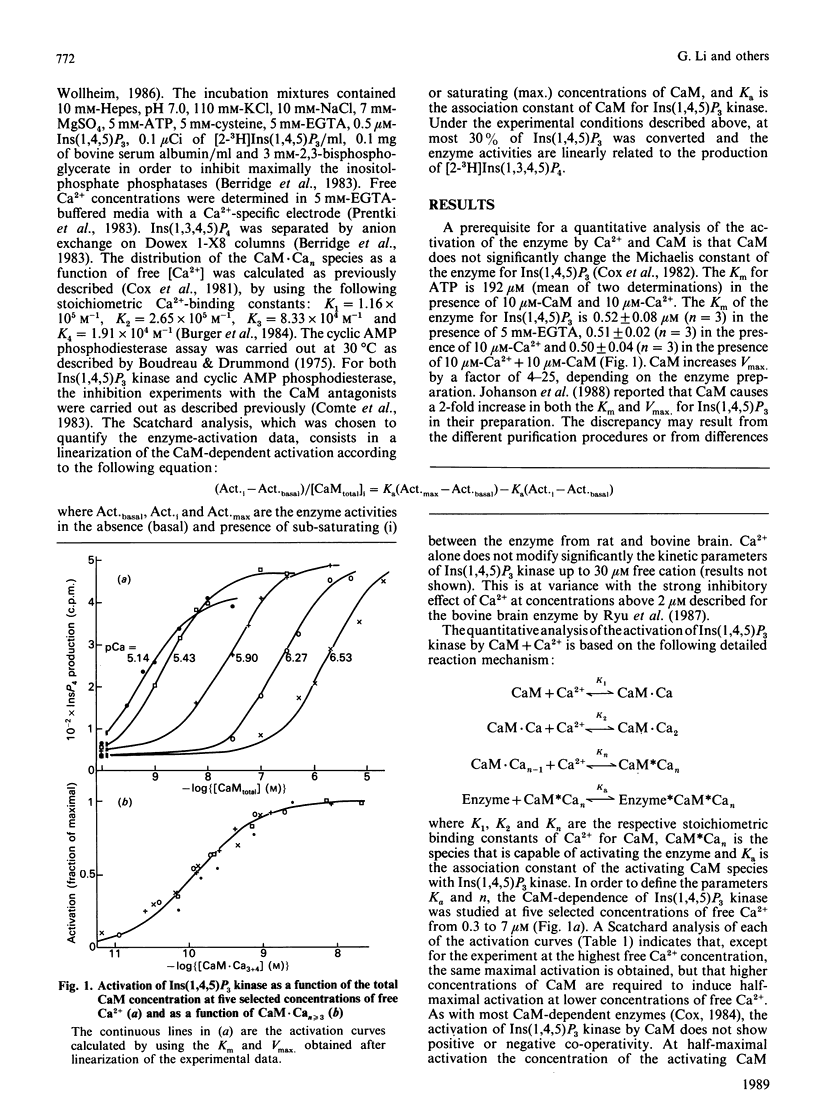
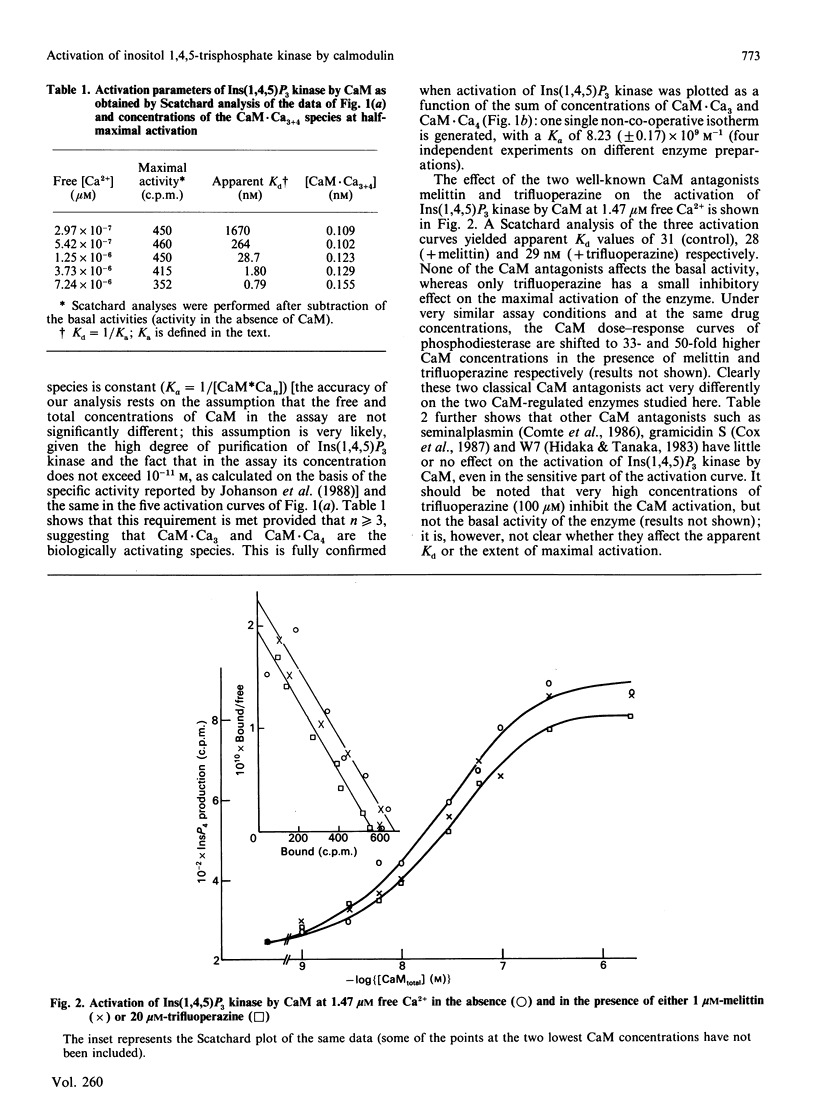
The effect of Ca2+ and calmodulin (CaM) on the activation of purified bovine brain Ins(1,4,5)P3 kinase was quantified and interpreted according to the model of sequential equilibria generally used for other calmodulin-stimulated systems. Two main conclusions can be drawn. (i) CaM.Ca3 and CaM.Ca4 together are the biologically active species in vitro, as is the case for the great majority of other calmodulin targets. (ii) These species bind in a non-co-operative way to the enzyme with an affinity constant of 8.23 x 10(9) M-1, i.e. approx 10-fold higher than for most calmodulin-activated target enzymes. The dose-response curve of the activation of Ins(1,4,5)P3 kinase by calmodulin is not significantly impaired by melittin and trifluoperazine, whereas under very similar assay conditions the half-maximal activation of bovine brain cyclic AMP phosphodiesterase requires over 30-50-fold higher concentrations of CaM when 1 microM melittin or 20 microM-trifluoperazine is present in the assay medium. Similarly, 1 microM of the anti-calmodulin peptides seminalplasmin and gramicidin S, as well as 20 microM of N-(6-aminohexyl)-5-chloro-1-naphthalene-sulphonamide (W7), do not inhibit the activation process. These data suggest that binding and activation of Ins(1,4,5)P3 kinase require surface sites of calmodulin which are different from those involved in the binding of most other target enzymes or of model peptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Comte M., Cox J. A., Wollheim C. B. Calcium-calmodulin stimulates inositol 1,4,5-trisphosphate kinase activity from insulin-secreting RINm5F cells. J Biol Chem. 1987 Jul 15;262(20):9437–9440. [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Boudreau R. J., Drummond G. I. A modified assay of 3':5'-cyclic-AMP phosphodiesterase. Anal Biochem. 1975 Feb;63(2):388–399. doi: 10.1016/0003-2697(75)90361-9. [DOI] [PubMed] [Google Scholar]
- Burger D., Stein E. A., Cox J. A. Free energy coupling in the interactions between Ca2+, calmodulin, and phosphorylase kinase. J Biol Chem. 1983 Dec 10;258(23):14733–14739. [PubMed] [Google Scholar]
- Comte M., Malnoë A., Cox J. A. Affinity purification of seminalplasmin and characterization of its interaction with calmodulin. Biochem J. 1986 Dec 1;240(2):567–573. doi: 10.1042/bj2400567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comte M., Maulet Y., Cox J. A. Ca2+-dependent high-affinity complex formation between calmodulin and melittin. Biochem J. 1983 Jan 1;209(1):269–272. doi: 10.1042/bj2090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Stein E. A. Activation of human erythrocyte Ca2+-dependent Mg2+-activated ATPase by calmodulin and calcium: quantitative analysis. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4265–4269. doi: 10.1073/pnas.79.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Malnoë A., Stein E. A. Regulation of brain cyclic nucleotide phosphodiesterase by calmodulin. A quantitative analysis. J Biol Chem. 1981 Apr 10;256(7):3218–3222. [PubMed] [Google Scholar]
- Cox J. A., Milos M., Comte M. High-affinity formation of a 2:1 complex between gramicidin S and calmodulin. Biochem J. 1987 Sep 1;246(2):495–502. doi: 10.1042/bj2460495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A. Sequential events in calmodulin on binding with calcium and interaction with target enzymes. Fed Proc. 1984 Dec;43(15):3000–3004. [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Hansen R. S., Beavo J. A. Differential recognition of calmodulin-enzyme complexes by a conformation-specific anti-calmodulin monoclonal antibody. J Biol Chem. 1986 Nov 5;261(31):14636–14645. [PubMed] [Google Scholar]
- Hidaka H., Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- Hubbard M. J., Klee C. B. Calmodulin binding by calcineurin. Ligand-induced renaturation of protein immobilized on nitrocellulose. J Biol Chem. 1987 Nov 5;262(31):15062–15070. [PubMed] [Google Scholar]
- Irvine R. F. Inositol phosphates and calcium entry. 1987 Jul 30-Aug 5Nature. 328(6129):386–386. doi: 10.1038/328386b0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Johanson R. A., Hansen C. A., Williamson J. R. Purification of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. J Biol Chem. 1988 Jun 5;263(16):7465–7471. [PubMed] [Google Scholar]
- Kimura Y., Hirata M., Yamaguchi K., Koga T. Activation by calmodulin of inositol-1,4,5-trisphosphate 3-kinase in guinea pig peritoneal macrophages. Arch Biochem Biophys. 1987 Sep;257(2):363–369. doi: 10.1016/0003-9861(87)90578-9. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Specificity of the binding of trifluoperazine to the calcium-dependent activator of phosphodiesterase and to a series of other calcium-binding proteins. Biochim Biophys Acta. 1978 May 3;540(2):197–204. doi: 10.1016/0304-4165(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Malnoë A., Cox J. A., Stein E. A. Ca2+-dependent regulation of calmodulin binding and adenylate cyclase activation in bovine cerebellar membranes. Biochim Biophys Acta. 1982 Jan 12;714(1):84–92. doi: 10.1016/0304-4165(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Mamar-Bachi A., Cox J. A. Quantitative analysis of the free energy coupling in the system calmodulin, calcium, smooth muscle myosin light chain kinase. Cell Calcium. 1987 Dec;8(6):473–482. doi: 10.1016/0143-4160(87)90030-3. [DOI] [PubMed] [Google Scholar]
- Maulet Y., Cox J. A. Structural changes in melittin and calmodulin upon complex formation and their modulation by calcium. Biochemistry. 1983 Nov 22;22(24):5680–5686. doi: 10.1021/bi00293a035. [DOI] [PubMed] [Google Scholar]
- Morris A. J., Downes C. P., Harden T. K., Michell R. H. Turkey erythrocytes possess a membrane-associated inositol 1,4,5-trisphosphate 3-kinase that is activated by Ca2+ in the presence of calmodulin. Biochem J. 1987 Dec 1;248(2):489–493. doi: 10.1042/bj2480489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Janjic D., Wollheim C. B. The regulation of extramitochondrial steady state free Ca2+ concentration by rat insulinoma mitochondria. J Biol Chem. 1983 Jun 25;258(12):7597–7602. [PubMed] [Google Scholar]
- Ryu S. H., Lee S. Y., Lee K. Y., Rhee S. G. Catalytic properties of inositol trisphosphate kinase: activation by Ca2+ and calmodulin. FASEB J. 1987 Nov;1(5):388–393. doi: 10.1096/fasebj.1.5.2824270. [DOI] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Takazawa K., Passareiro H., Dumont J. E., Erneux C. Ca2+/calmodulin-sensitive inositol 1,4,5-trisphosphate 3-kinase in rat and bovine brain tissues. Biochem Biophys Res Commun. 1988 Jun 16;153(2):632–641. doi: 10.1016/s0006-291x(88)81142-2. [DOI] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Hirata M., Kuriyama H. Calmodulin activates inositol 1,4,5-trisphosphate 3-kinase activity in pig aortic smooth muscle. Biochem J. 1987 Jun 15;244(3):787–791. doi: 10.1042/bj2440787. [DOI] [PMC free article] [PubMed] [Google Scholar]


