Abstract
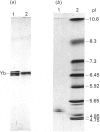
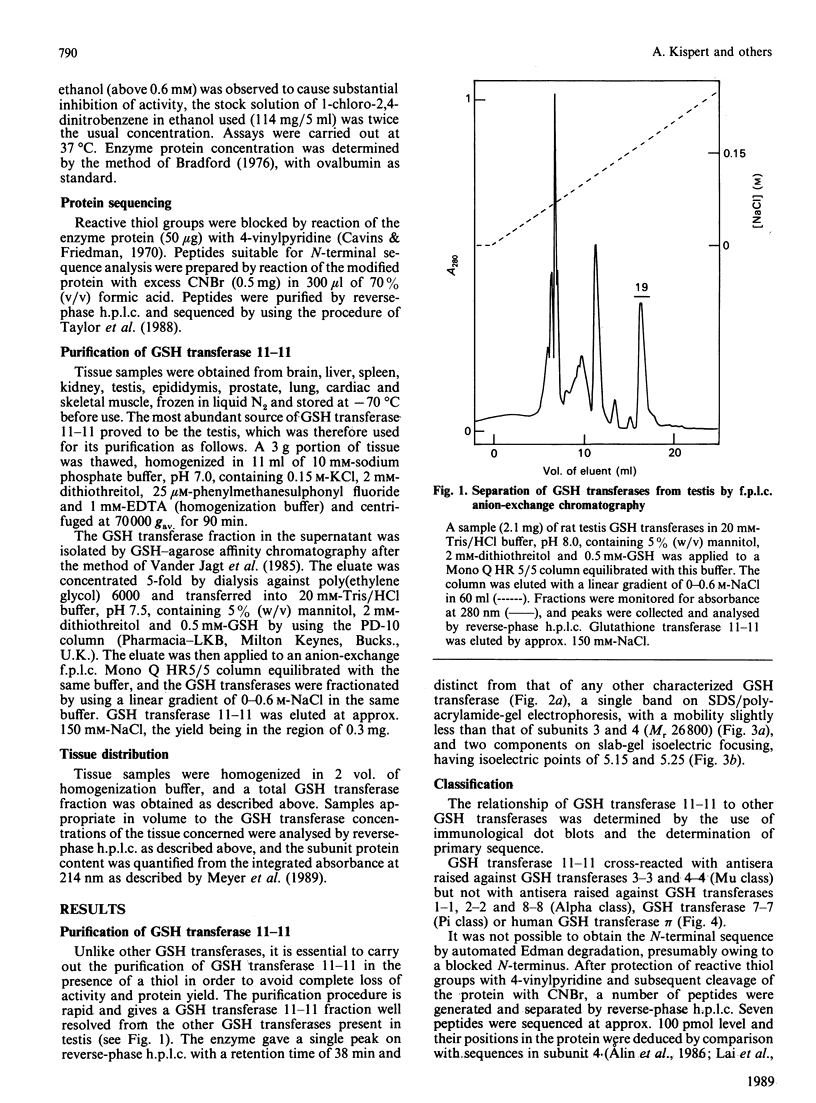
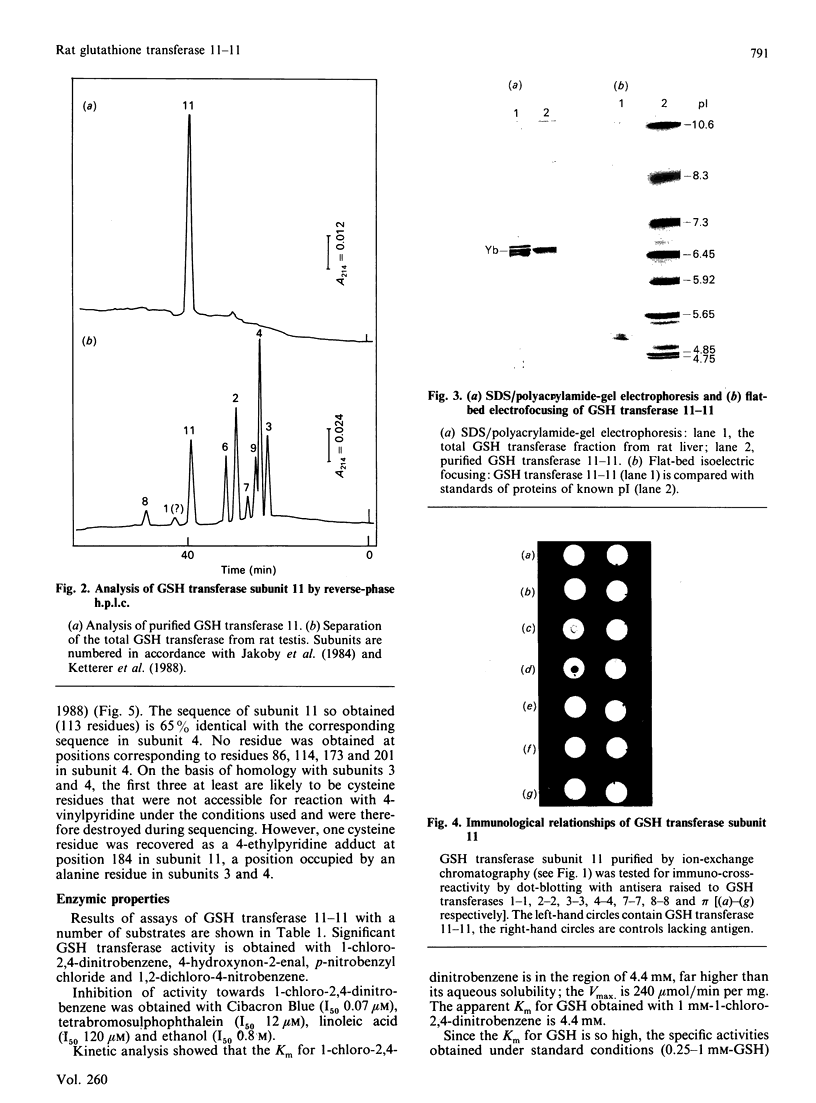
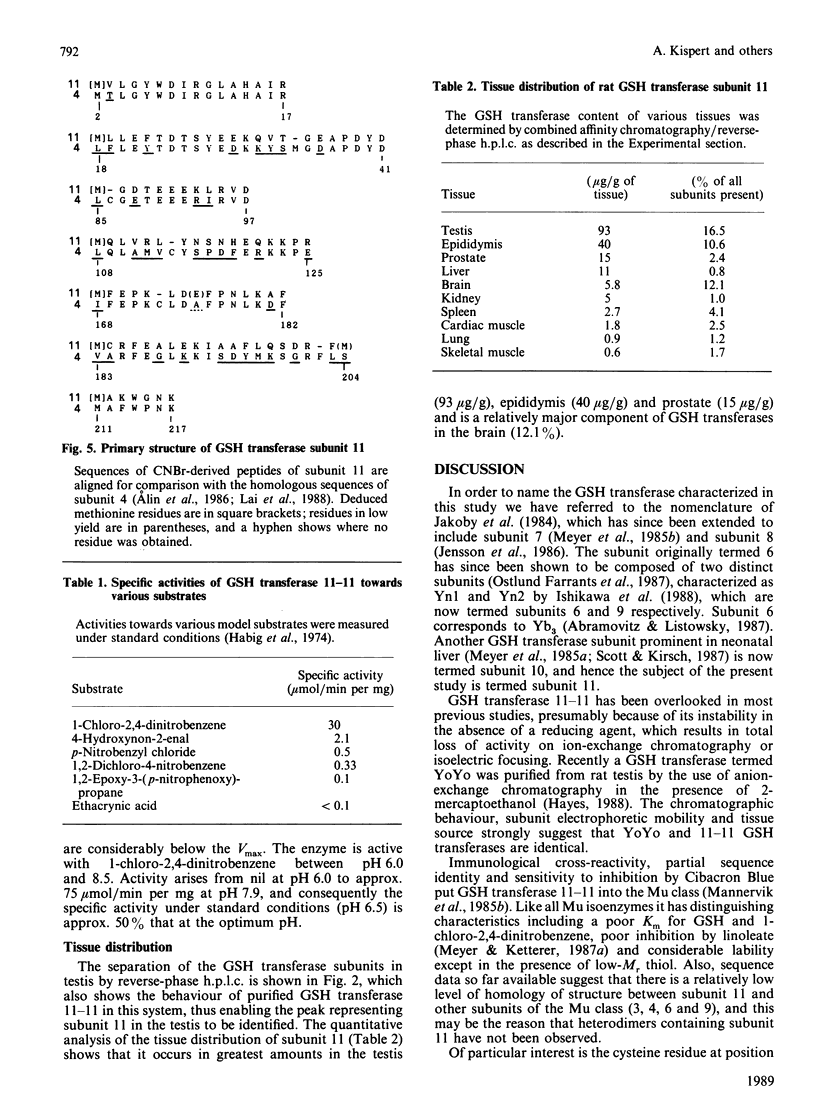
A labile GSH transferase homodimer termed 11-11 was purified from rat testis by GSH-agarose affinity chromatography followed by anion-exchange f.p.l.c. The enzyme is unstable in the absence of thiol(s) and has relatively low affinity for both 1-chloro-2,4-dinitrobenzene (Km 4.4 mM) and GSH (Km(app.) 4.4mM). Its mobility on SDS/polyacrylamide-gel electrophoresis is slightly less than that of subunits 3 and 4 and its pI is 5.2. Subunit 11 has a blocked N-terminal amino acid residue, but after CNBr cleavage fragments accounting for 113 amino acid residues were sequenced and showed 65% homology with corresponding sequences in subunit 4, indicating that it is a member of the Mu family. GSH transferase 11 is a major isoenzyme in testis, epididymis, prostate and brain and present at lower concentrations in other tissues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Listowsky I. Selective expression of a unique glutathione S-transferase Yb3 gene in rat brain. J Biol Chem. 1987 Jun 5;262(16):7770–7773. [PubMed] [Google Scholar]
- Alin P., Danielson U. H., Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985 Jan 7;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Alin P., Mannervik B., Jörnvall H. Cytosolic rat liver glutathione transferase 4-4. Primary structure of the protein reveals extensive differences between homologous glutathione transferases of classes alpha and mu. Eur J Biochem. 1986 Apr 15;156(2):343–350. doi: 10.1111/j.1432-1033.1986.tb09588.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess J. R., Yang H., Chang M., Rao M. K., Tu C. P., Reddy C. C. Enzymatic transformation of PGH2 to PGF2 alpha catalyzed by glutathione S-transferases. Biochem Biophys Res Commun. 1987 Jan 30;142(2):441–447. doi: 10.1016/0006-291x(87)90294-4. [DOI] [PubMed] [Google Scholar]
- Cavins J. F., Friedman M. An internal standard for amino acid analyses: S-beta-(4-pyridylethyl)-L-cysteine. Anal Biochem. 1970 Jun;35(2):489–493. doi: 10.1016/0003-2697(70)90211-3. [DOI] [PubMed] [Google Scholar]
- Coles B., Wilson I., Wardman P., Hinson J. A., Nelson S. D., Ketterer B. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch Biochem Biophys. 1988 Jul;264(1):253–260. doi: 10.1016/0003-9861(88)90592-9. [DOI] [PubMed] [Google Scholar]
- Danielson U. H., Esterbauer H., Mannervik B. Structure-activity relationships of 4-hydroxyalkenals in the conjugation catalysed by mammalian glutathione transferases. Biochem J. 1987 Nov 1;247(3):707–713. doi: 10.1042/bj2470707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D. Selective elution of rodent glutathione S-transferases and glyoxalase I from the S-hexyglutathione-Sepharose affinity matrix. Biochem J. 1988 Nov 1;255(3):913–922. doi: 10.1042/bj2550913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Tsuchida S., Satoh K., Sato K. The subunit structure of a major glutathione S-transferase form, MT, in rat testis. Evidence for a heterodimer consisting of subunits with different isoelectric points. Eur J Biochem. 1988 Oct 1;176(3):551–557. doi: 10.1111/j.1432-1033.1988.tb14313.x. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Jensson H., Guthenberg C., Alin P., Mannervik B. Rat glutathione transferase 8-8, an enzyme efficiently detoxifying 4-hydroxyalk-2-enals. FEBS Lett. 1986 Jul 28;203(2):207–209. doi: 10.1016/0014-5793(86)80743-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Qian B., Grove G., Tu C. P. Gene expression of rat glutathione S-transferases. Evidence for gene conversion in the evolution of the Yb multigene family. J Biol Chem. 1988 Aug 15;263(23):11389–11395. [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Beale D., Tan K. H., Coles B., Ketterer B. Glutathione transferases in primary rat hepatomas: the isolation of a form with GSH peroxidase activity. FEBS Lett. 1985 May 6;184(1):139–143. doi: 10.1016/0014-5793(85)80670-0. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Lalor E., Coles B., Kispert A., Alin P., Mannervik B., Ketterer B. Single-step purification and h.p.l.c. analysis of glutathione transferase 8-8 in rat tissues. Biochem J. 1989 Jun 15;260(3):785–788. doi: 10.1042/bj2600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I. G., Guthenberg C., Mannervik B., Jernström B. Differences in stereoselectivity and catalytic efficiency of three human glutathione transferases in the conjugation of glutathione with 7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. Cancer Res. 1986 May;46(5):2220–2224. [PubMed] [Google Scholar]
- Robertson I. G., Jernström B. The enzymatic conjugation of glutathione with bay-region diol-epoxides of benzo[a]pyrene, benz[a]anthracene and chrysene. Carcinogenesis. 1986 Oct;7(10):1633–1636. doi: 10.1093/carcin/7.10.1633. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Kirsch R. E. The isolation of a fetal rat liver glutathione S-transferase isoenzyme with high glutathione peroxidase activity. Biochim Biophys Acta. 1987 Dec 7;926(3):264–269. doi: 10.1016/0304-4165(87)90212-1. [DOI] [PubMed] [Google Scholar]
- Tan K. H., Meyer D. J., Belin J., Ketterer B. Inhibition of microsomal lipid peroxidation by glutathione and glutathione transferases B and AA. Role of endogenous phospholipase A2. Biochem J. 1984 May 15;220(1):243–252. doi: 10.1042/bj2200243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. H., Meyer D. J., Gillies N., Ketterer B. Detoxification of DNA hydroperoxide by glutathione transferases and the purification and characterization of glutathione transferases of the rat liver nucleus. Biochem J. 1988 Sep 15;254(3):841–845. doi: 10.1042/bj2540841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. B., Vidal A., Torpier G., Meyer D. J., Roitsch C., Balloul J. M., Southan C., Sondermeyer P., Pemble S., Lecocq J. P. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 1988 Feb;7(2):465–472. doi: 10.1002/j.1460-2075.1988.tb02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida S., Izumi T., Shimizu T., Ishikawa T., Hatayama I., Satoh K., Sato K. Purification of a new acidic glutathione S-transferase, GST-Yn1Yn1, with a high leukotriene-C4 synthase activity from rat brain. Eur J Biochem. 1987 Dec 30;170(1-2):159–164. doi: 10.1111/j.1432-1033.1987.tb13681.x. [DOI] [PubMed] [Google Scholar]
- Ujihara M., Tsuchida S., Satoh K., Sato K., Urade Y. Biochemical and immunological demonstration of prostaglandin D2, E2, and F2 alpha formation from prostaglandin H2 by various rat glutathione S-transferase isozymes. Arch Biochem Biophys. 1988 Aug 1;264(2):428–437. doi: 10.1016/0003-9861(88)90308-6. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]