Abstract
Traumatic intracranial cerebral artery dissection (ICAD) in the pediatric population is relatively rare. We report two traumatic ICAD cases in children. Case 1: A 13-year-old boy presented with headache and left hemiparesis after body contact while playing basketball. We found a cerebral infarction in the middle cerebral artery territory and dissection at the bifurcation of the right internal carotid artery and posterior communicating artery. Six days after onset, his right hemiparesis deteriorated, and the infarction progressed. Therefore, bypass surgery was performed. Three months later, he regained the ability to walk without a cane and resumed school. Case 2: A 10-year-old boy fell while skiing and experienced a severe headache several hours later. Neuroradiological examination revealed a subarachnoid hemorrhage in the basal cistern without aneurysm. Six days after admission, magnetic resonance angiography revealed stenotic changes and an irregularly shaped basilar artery (BA). On day 7, an angiogram confirmed BA dissection. The patient's headache gradually improved, and the irregular shape of the BA normalized 3 weeks later. He was discharged without any neurological deficits. Determining whether vascular reconstruction should be performed is challenging. However, we believe that therapeutic intervention should be performed promptly when symptoms or brain images deteriorate.
Keywords: Traumatic, Intracranial cerebral artery dissection, Children, Subarachnoid hemorrhage, Cerebral infarction, Bypass
Introduction
Traumatic intracranial cerebral artery dissection (ICAD) in the pediatric population is relatively rare. However, attention should be paid to this pathology because it may occur owing to hyperextension of the neck in minor sports-related injuries [1]. According to a report by Karibe et al., there were only 10 cases of cerebral ischemia caused by ICAD in 721 children with head injuries, and all patients with ICAD received conservative treatment [2]. The treatment options include surgery, endovascular treatment, and antithrombotic therapy. The natural course of ICAD with ischemia in children remains unclear; nonetheless, the prognosis is mostly good [1,2]. Therefore, it is difficult to determine whether and if vascular reconstruction should be performed for ICAD with ischemia. Conversely, the mortality rate of pediatric patients with subarachnoid hemorrhage (SAH) from traumatic aneurysms is higher in conservatively treated cases than in surgically treated cases; therefore, several investigators advocate for surgical treatment once the diagnosis is made [3,4]. However, most surgically treated cases involved patients with peripheral anterior cerebral artery (ACA) or middle cerebral artery (MCA) aneurysms treated with clipping or trapping, with or without bypass [4].
Herein, we report 2 cases of traumatic ICAD: one case was treated with bypass surgery to prevent progressive cerebral infarction due to intracranial internal carotid artery (ICA) dissection, and the other was treated conservatively for SAH owing to a traumatic dissecting aneurysm in the basilar artery (BA).
Cases presentations
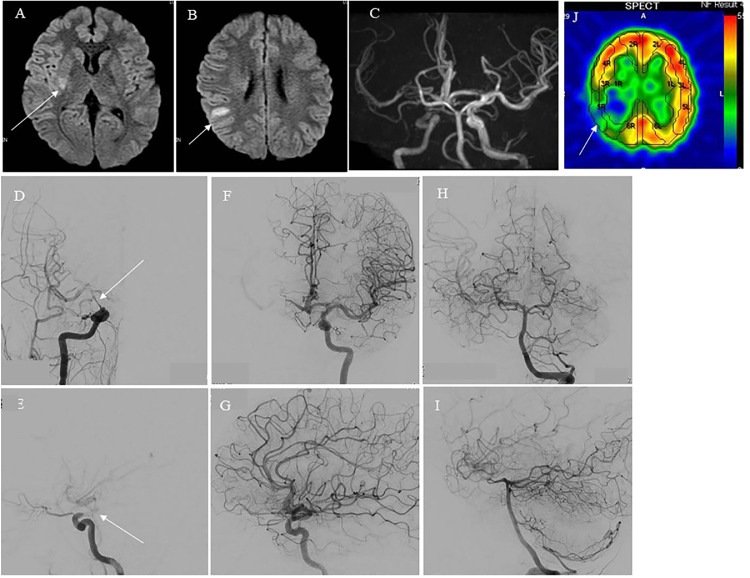
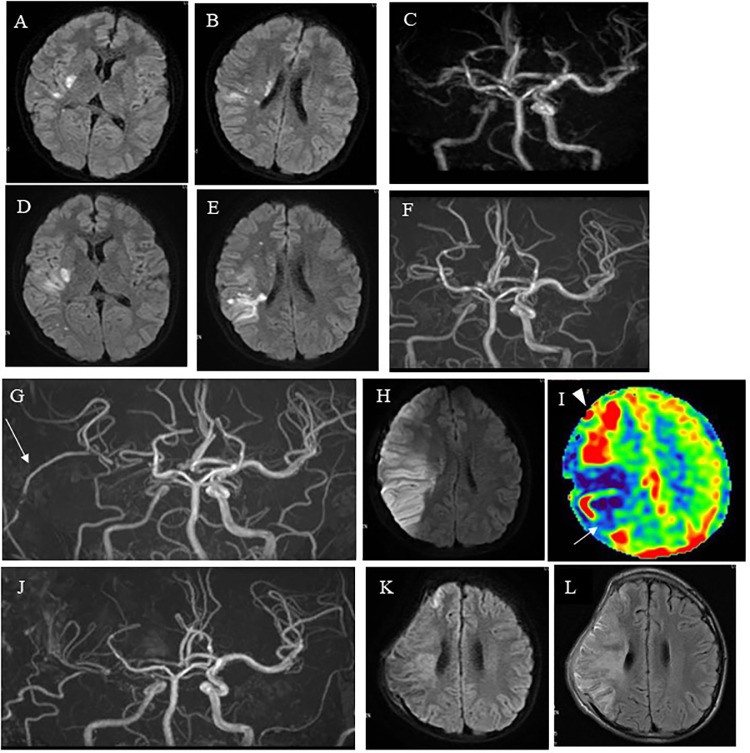
Case 1: A 13-year-old boy presented with sudden onset of severe headache and severe left hemiparesis after mild body contact while playing basketball. His motor weakness on the left side had a manual muscle test (MMT) score of 2 in the ambulance but improved to MMT 4 in the emergency room. Magnetic resonance imaging (MRI) demonstrated right frontal cerebral watershed infarction, and magnetic resonance angiography (MRA) showed stenosis in the right C2 portion of the ICA (Figs. 1A-C). Digital subtraction angiography (DSA) revealed dissection at the bifurcation of the right ICA and posterior communicating artery (P-com), and the main collateral was perfused by reversal of flow from the P-com (Figs. 1D-I). Thus, the true lumen from the cervical ICA was occluded at the ICA and P-com bifurcation, and the C1-2 portion of the ICA was perfused by the reversal of flow from the P-com. Because his neurological status improved and traumatic ICAD was sometimes spontaneously remodeled, careful observation, and heparin administration were initially performed unless neurological deterioration occurred. Single-photon emission tomography (SPECT) performed a day after onset demonstrated low perfusion in the right hemisphere (approximately 70% of the left hemisphere) (Fig. 1J). MRI and MRA 2 days after onset demonstrated slight progression of the right cerebral infarction and no remarkable change in the right MCA flow (Figs. 2A-C). Although the patient's motor weakness did not worsen, his headache gradually worsened. Six days after onset, his left hemiparesis deteriorated (from MMT 4 to MMT 2) and MRI and MRA demonstrated further progression of the right cerebral infarction and deterioration of the right MCA flow (Figs. 2D-F). Therefore, we performed a superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis 7 days after onset. Despite a patent bypass, the cerebral infarction progressed further into the territory of the MCA posterior trunk, resulting in hyperperfusion in the territory of the MCA anterior trunk (Figs. 2G-I). A day after bypass surgery, brain swelling due to infarction and hyperperfusion occurred, and external decompression surgery was performed. Subsequent MRI and MRA revealed no remarkable changes in the neuroradiological findings (Figs. 2J-L), and cranioplasty was performed 1 month later, followed by continued rehabilitation. He was discharged 3 months after onset with mild left hemiparesis but no higher brain dysfunction. He resumed attending junior high school without assistance (modified Rankin Score 2). Two years later, follow-up MRI demonstrated no new infarction, and MRA showed a patent STA-MCA bypass. No cerebrovascular events occurred during the 2-year follow-up period.
Fig. 1.
Neuroradiological findings on admission of Case 1. (A, B) Diffusion-weighted image (DWI) of magnetic resonance imaging (MRI) showing several spotty high signals in the right basal ganglia (white arrow in A) and parietal subcortical area (white arrow in B). (C) Magnetic resonance angiography (MRA) illustrating a faint flow signal in the right internal carotid artery (ICA). (D) Anterior view and (E) lateral view of digital subtraction angiography (DSA) of the right carotid angiogram demonstrating near occlusion of the ICA at the PC2 portion (white arrow in Fig. 1, Fig. 1). (F) Anterior view and (G) lateral view of DSA of the left carotid angiogram demonstrating faint flow of left middle cerebral artery (MCA) via the anterior communicating artery. (H) Anterior view and (I) lateral view of DSA of the left vertebral artery angiogram demonstrating flow of the left MCA via the left posterior communicating artery. (J) 123I-IMP single-photon emission tomography (SPECT) demonstrating reduced cerebral blood flow in the right middle cerebral artery territory, especially in the parietal lobe (white arrow in J).
Fig. 2.
Pre- and post-operative neuroradiological findings of Case 1. (A, B) DWI of the MRI, performed 2 days after onset, showing slight progression of the right cerebral infarction. (C) MRA illustrating a faint flow signal in the right ICA, but no remarkable change compared to MRA on admission. (D, E) The DWI of the MRI, performed 6 days after onset, revealing further progression of the right cerebral infarction. (F) MRA illustrating deterioration of the faint flow signal in the right ICA. (G, H) MRA and DWI, performed 1 day after bypass surgery, revealing a patent bypass (white arrow in G) but further progression of right cerebral infarction in the territory of the MCA posterior trunk (I) 123I-IMP SPECT, performed 1 day after surgery, demonstrating reduction of cerebral blood flow in the territory of the right MCA posterior trunk (white arrow in I) and hyperperfusion in the territory of the MCA anterior trunk (white arrow head in I). (J) MRA, performed 3 weeks after bypass surgery, showing bypass patency. (K, L) DWI and Fluid-attenuated inversion recovery (FLAIR)of MRI, performed 3 weeks after bypass surgery, revealing only natural time-course changes in the right cerebral infarction in the territory of the MCA posterior trunk.
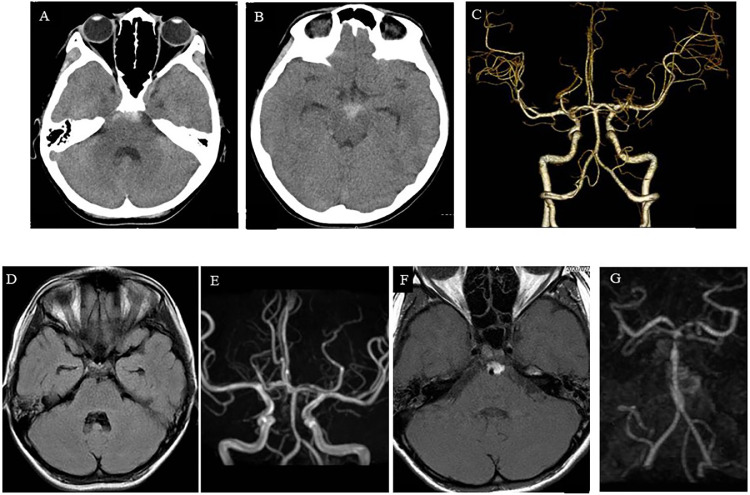
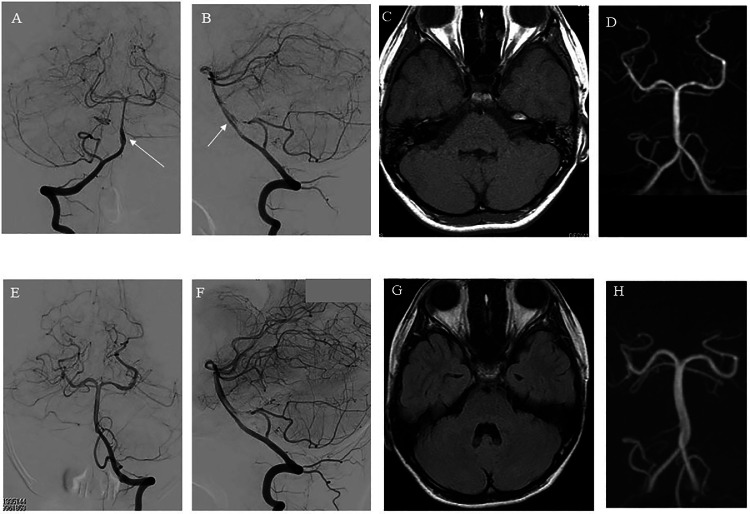
Case 2: A 10-year-old boy fell twice while skiing during a daytime ski lesson. After several hours, the patient presented with severe headache and was admitted to our hospital. Computed tomography demonstrated a basal cistern SAH (Fig. 3A-B), and three-dimensional computed tomographic angiography (3D-CTA) showed no aneurysm (Fig. 3C). Therefore, the patient was diagnosed with traumatic SAH and kept under close observation. Although the SAH on MRI was gradually washed out and MRA demonstrated no obvious abnormalities (Fig. 3D-E), the headache worsened. Six days after admission, follow-up MRI and MRA showed localized SAH around the BA, stenotic change, and irregularly shaped BA (Fig. 3F-G). The patient was suspected with ICAD or early vasospasm after traumatic SAH. Therefore, we carefully observed the patient and planned DSA under general anesthesia. On day 7, we performed DSA, which demonstrated lower BA dissection (Fig. 4A-B). The entry point was located at the distal end, and there was a reversal flow into the pseudolumen. By this time, the headache had almost disappeared. In addition, it was neither easy nor safe to treat ICAD at this point. Therefore, we decided to carefully observe the patient through frequent follow-up brain imaging. However, if the irregular BA shape changed to a protruding shape similar to that of an aneurysm, stent-assisted coil embolization was planned. Fortunately, the headache gradually improved, and the irregular BA shape normalized 3 weeks later (Fig. 4C-F). The patient was discharged from the hospital 3 weeks after onset with no neurological deficits. Two years later, follow-up MRI demonstrated the absence of a new infarction, and MRA showed no abnormality in the BA (Fig. 4G-H). No cerebrovascular events occurred during the 2-year follow-up period.
Fig. 3.
Radiological findings of Case 2. (A, B) Computed tomography performed at admission revealing a subarachnoid hemorrhage (SAH) around the brainstem. (C) Three-dimensional computed tomographic angiography (3D-CTA) anterior view on admission demonstrating no obvious aneurysm. (D) FLAIR on MRI, performed 3 days after onset, demonstrating clearance of the SAH. (E) MRA, conducted 3 days after onset, revealing no obvious aneurysm or vasospasm. (F) FLAIR of MRI, performed 6 days after onset, demonstrating localized SAH around the basilar artery (BA). (G) MRA, conducted 6 days after onset, revealing irregularity of the BA.
Fig. 4.
Radiological findings of Case 2. (A) Anterior view and (B) lateral view of DSA, performed 7 days after onset, demonstrating lower BA dissection (white arrow in Fig. 4, Fig. 4); the entry point was located at the distal end, and reversal flow was observed in the pseudolumen. (C) FLAIR of MRI, conducted 16 days after onset, showing absence of SAH. (D) MRA, performed 16 days after onset, revealing no obvious aneurysm or vasospasm. (E) Anterior view and (F) lateral view of DSA, conducted 21 days after onset, demonstrating no obvious aneurysm or vasospasm (G) FLAIR of MRI, performed 1 year after onset, demonstrating absence of SAH. (H) MRA, performed 1 year after onset, revealing no BA abnormality.
Discussion
Regarding Case 1, we initially chose conservative therapy. One reason was that most children with ICADs underwent conservative treatment, and favorable outcomes were obtained in more than half cases [2]. However, unfavorable outcomes have also been reported in cases of ICAD [2]. The other reason was the natural improvement in left hemiparesis. Therefore, it was reasonable not to perform vascular reconstruction immediately after admission. However, we performed STA-MCA anastomosis after the progression of the cerebral infarction and deterioration of motor weakness. Although the bypass was patent, the brain infarction progressed further, and hyperperfusion occurred. The worsening headache might have been caused by compensatory vasodilation, indicating a limitation of conservative therapy at that point. In hindsight, we should have considered vascular reconstruction a few days earlier, when the patient's headache and left hemiparesis deteriorated and MRI demonstrated that the right watershed infarction had progressed. In other words, we could have performed vascular reconstruction before symptom onset or immediately after symptom deterioration. We chose bypass surgery as a treatment option, which has been reported in only 1 case of ICAD in children. There was a case of a 10-year-old girl who underwent an extracranial-intracranial bypass, and her outcome was moderate disability [1]. Another treatment option is interventional vascular reconstruction; Kikuchi et al. reported successful mechanical thrombectomy with a stent retriever for pediatric ICAD presenting with ischemia [5]. As traumatic ICADs in children requiring vascular reconstruction are very rare, selection of appropriate treatment is challenging. However, considering the need for prompt treatment, interventional radiology (IVR) may be advantageous. Although not limited to traumatic ICADs in children, a latest Japanese nationwide survey suggested patients with intracranial carotid stenotic dissection causing ischemia were at risk of further aggravation, and IVR improved or prevented aggravation [6].
In Case 2, the source of bleeding was initially unknown based on 3D-CTA. Therefore, we first diagnosed the patient with traumatic SAH and carefully monitored him. ICAD was suspected after a few days, and BA dissection was confirmed 7 days after the onset. However, by that time, his headache had almost disappeared. Consequently, we decided that the patient should be carefully observed and undergo frequent brain imaging follow-ups. As mentioned before, the mortality of pediatric patients with SAH from traumatic aneurysms is high in conservatively treated cases; therefore, surgical treatment should be performed once the diagnosis is made [4]. However, most of these surgically treated cases involved patients with peripheral ACA or MCA aneurysms treated with clipping or trapping with or without bypass, [4] and there have been no reports of SAH from a traumatic dissecting aneurysm in the BA. It is not easy or safe to treat ICAD at this point, either by trapping with bypass or IVR. Moreover, there is a possibility of spontaneous remodeling of the dissected BA. Our plan was to carefully observe the patient and perform frequent brain imaging follow-ups. Although not specifically for a traumatic dissecting aneurysm in the BA, there is a report on stent-assisted coiling treatment of a pediatric iatrogenic pseudoaneurysm of the intracranial ICA [7]. Therefore, if the irregular BA shape changed to a protruding shape similar to that of an aneurysm, we planned stent-assisted coil embolization. Fortunately, the patient's natural course was good, eliminating the need for IVR.
Conclusion
Herein, we report two rare cases of ICAD in children. In 1 case, traumatic ICAD with ischemia was treated with bypass surgery. The other case involved conservative treatment of SAH owing to traumatic ICAD, with favorable outcomes. As traumatic ICAD in children is rare, deciding whether to perform vascular reconstruction or careful observation is difficult. If symptoms and imaging changes worsen, it is advisable to immediately consider vascular reconstruction as a potential treatment option.
Patient consent
We obtained the patient's permissions and informed consent to publish their information and images from the guardian.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Karibe H, Hayashi T, Narisawa A, Akamatsu Y, Kameyama M, Nakagawa A, et al. Clinical characteristics of sports-related traumatic cerebrovaxcular occlusive accident: Importance of cervical hyper-extension and/or-rotation as mechanisms of injury. J Japan Soc Neurol Emergenc Crit Care. 2018;30(2):1–8. [Google Scholar]
- 2.Karibe H, Onuma T, Kameyama M, Shida N, Nakagawa A, Shimizu H. Clinical characteristics of traumatic occlusive cerebrovascular disease in children. Nervous Syst Children. 2007;32(1):8–13. [Google Scholar]
- 3.Ventureyra EC, Higgins MJ. Traumatic intracranial aneurysms in childhood and adolescence. Case reports and review of the literature. Childs Nerv Syst. 1994;10(6):361–379. doi: 10.1007/BF00335125. [DOI] [PubMed] [Google Scholar]
- 4.Livshits IM, Berdinov BF, Musa G, Chmutin EG, Levov VA, Chmutin GK, et al. Traumatic intracranial aneurysms (TICA) in children: a description of two clinical cases of successful treatment and review of literature. Childs Nerv Syst. 2022;38(11):2063–2070. doi: 10.1007/s00381-022-05647-9. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi B, Shimbo J, Ando K, Saito S, Watanabe M, Saito A, et al. [Succesful emergency mechanical thrombectomy with stent retriever for pediatric intracranial internal carotid artery dissection presenting with cerebral ischemia: a case report] Curr Pract Neurosurg. 2018;28(1):95–101. [Google Scholar]
- 6.Shimizu H, Ono T, Abe T, Hokari M, Egashira Y, Shimonaga K, et al. Current treatment results of intracranial carotid artery dissection causing cerebral ischemia: a Japanese Nationwide Survey. Neurol Med Chir (Tokyo) 2023;63(2):80–89. doi: 10.2176/jns-nmc.2022-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogilvy CS, Tawk RG, Mokin M, Yang X, Levy EI, Hopkins LN, et al. Stent-assisted coiling treatment of pediatric traumatic pseudoaneurysm resulting from tumor surgery. Pediatr Neurosurg. 2011;47(6):442–448. doi: 10.1159/000339353. [DOI] [PubMed] [Google Scholar]