Abstract
Background
Heparin-induced thrombocytopenia (HIT) is a complication of heparin exposure associated with high risk for morbidity and mortality. Diagnosis and management are complex due to limitations of laboratory testing and the need for nonheparin anticoagulation.
Objectives
To increase the delivery of evidence-based care of patients with suspected and confirmed HIT via electronic consultation (e-consult).
Methods
We describe the creation and implementation of an e-consult service for patients with concern for HIT at a large academic medical center. Hematology physicians with HIT expertise performed real-time chart review of all patients with a positive screening immunoassay result and provided written recommendations in their electronic health record.
Results
Comparison of outcomes for 1 year before and the year after the e-consult service implementation identified improvements in direct thrombin inhibitor stewardship, increased diagnostic accuracy, and decreased length of stay of patients with confirmed HIT.
Conclusion
The e-consult platform is a novel method for rapid, targeted consultative guidance, and this single-institution pilot demonstrates its feasibility and effectiveness to improve the care of patients with suspected and confirmed HIT.
Keywords: heparin, quality improvement, referral and consultation, remote consultation, thrombocytopenia, thrombosis
Essentials
-
•
Heparin-induced thrombocytopenia (HIT) is life-threatening and complex to diagnose and treat.
-
•
We developed an electronic consultation (e-consult) service for patients with positive HIT testing results.
-
•
In patients with suspected HIT, e-consult decreased unnecessary use of bivalirudin.
-
•
In patients with confirmed HIT, e-consult decreased the mean length of hospitalization.
1. Introduction
Heparin-induced thrombocytopenia (HIT) results from an immune reaction to heparin, resulting in thrombocytopenia and a high risk for venous and/or arterial thrombosis. HIT is diagnosed in approximately 20,000 patients per year in the United States alone, and affected patients have an approximately 4-fold higher risk of death compared with those without HIT [1].
Although evidence-based guidelines exist to guide best practices in diagnosis and management [2,3], deviation from guideline-based care is common. Existing literature and evaluation of our internal institutional practice [4] highlight 2 major barriers to optimal care of patients with suspected and confirmed HIT: (1) misinterpretation and/or misuse of diagnostic evaluation and (2) complexities of management of nonheparin anticoagulation.
First, the diagnosis of HIT requires the stepwise use of the 4Ts score to assess clinical probability, a screening immunoassay, and a confirmatory functional test. Each step is associated with high potential for error and/or misinterpretation. Overtesting and misdiagnosis are known to increase patient morbidity and care costs [[5], [6], [7]].
Second, patients with suspected or confirmed HIT require nonheparin anticoagulation. Historically, intravenous direct thrombin inhibitors (DTIs) have been used for acute management, but these agents are associated with high cost and rates of medication error, as well as the potential to increase bleeding risk [[8], [9], [10], [11]]. Use of fondaparinux and oral factor (F)Xa inhibitors provides an opportunity to increase the safety and efficacy of the care of patients with HIT [9,12].
Electronic consultation (e-consult) refers to communication between providers by means of review of the medical record without patient contact, including written recommendations in the electronic health record. e-Consult is being increasingly used to expand access to subspecialty expertise, requiring a lower resource investment compared with traditional face-to-face (FTF) consultation [13,14].
Herein, we describe the implementation of an e-consult service for patients with suspected HIT. The intent of the intervention was to improve evidence-based care of patients with suspected and confirmed HIT, with a hypothesized measurable benefit of (1) an improvement in diagnostic accuracy, (2) a decrease in inappropriate use of DTIs, and (3) a decrease in the length of stay in patients with confirmed HIT.
2. Methods
2.1. Study setting and design
2.1.1. e-Consult service implementation
The HIT e-Consult Service was implemented on February 1, 2021, at a large, academic tertiary care medical center. Any provider at the institution was permitted to obtain an enzyme immunoassay (EIA) to be performed in-house in any patient with clinical concern for HIT. e-Consults were completed by a hematology faculty member (J.E.M. or F.J.O.). EIA assays were run Monday through Friday at approximately 4:00 pm, so the result release time was consistent and during regular business hours. Prior to implementation, the process for notification of a positive EIA result had been that a laboratory staff member would contact the bedside nurse caring for the patient with the positive result, who would then alert the care team. This process was maintained, but an additional step was added to notify the e-consult physician. At the initial stages of the implementation, a laboratory staff member would notify the e-consult physician by email. Later, an automatic consult alert was generated with any positive EIA result and sent directly to the e-consult physician via the electronic health record. In addition, medical providers could place an order for a HIT e-consult prior to receiving the EIA result. All treatment decisions were ultimately at the discretion of the primary service (ie, the patient’s primary providers could decide to follow or not follow e-consult service recommendations).
e-Consult included chart review and documentation with a note in the electronic health record addressing the interpretation of diagnostic testing results and providing treatment recommendations (Figure 1). Complex or urgent concerns (eg, high concern for HIT in a patient with an active heparin order) could also be communicated to the ordering provider by phone at the discretion of the hematologist. The patient was not billed for the e-consult.
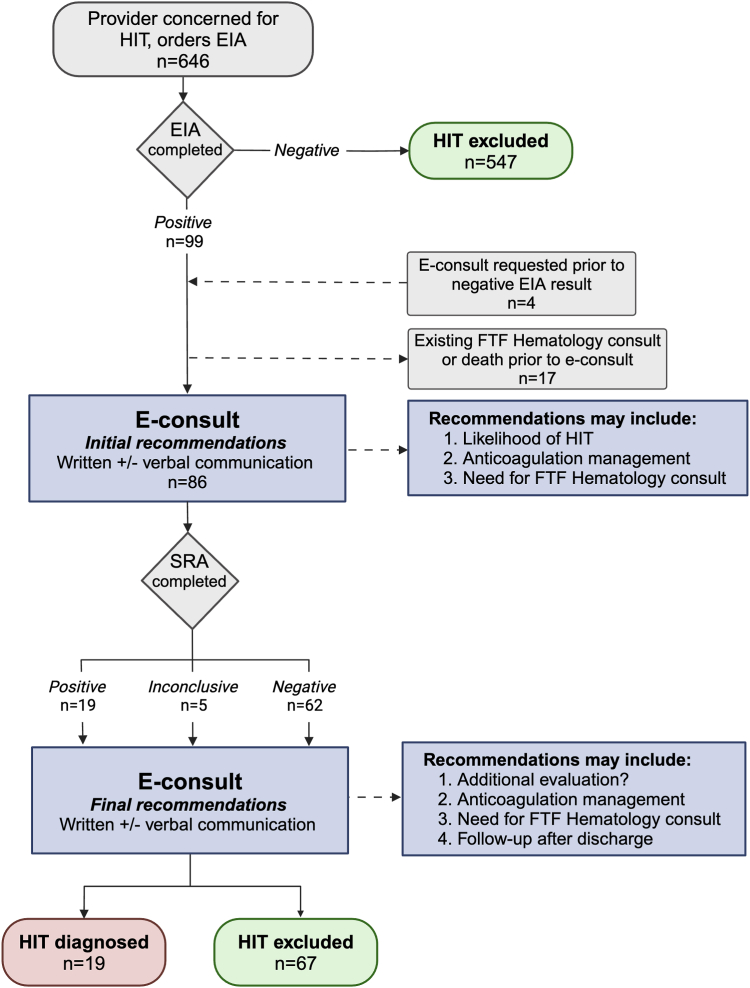
Figure 1.
Process map for the HIT e-Consult Service. Includes patient volumes for year 1 of implementation, along with description of e-consult activities in blue. e-consult, electronic consultation; EIA, enzyme immunoassay; FTF, face-to-face; HIT, heparin-induced thrombocytopenia; SRA, serotonin release assay.
2.1.2. e-Consult service performance evaluation
We compared HIT diagnosis and management for the year preceding the e-consult service (January 1, 2019, to December 31, 2019) to a 1-year period with the e-consult service (February 1, 2021, to January 31, 2022). Cases from 2019 were used for comparison given the significant disruptions in care in 2020 at the onset of the COVID-19 pandemic that may have affected HIT diagnosis and testing. If a patient had multiple e-consults in a single admission, all were included as individual events.
Bivalirudin was the preferred intravenous DTI at the institution throughout the study periods; argatroban was not available. A marked increase in institutional bivalirudin use was observed starting in September 2019, which persisted and was unchanged through the COVID-19 pandemic. The reason for this increase was not known and was a focus of the intervention, so analysis of bivalirudin use was performed continuously before and after implementation.
Data were collected from retrospective chart review and laboratory and pharmacy records. Patients with HIT who died during hospitalization were removed from the analysis of anticoagulation selection after discharge. Major bleeding was defined based on International Society on Thrombosis and Haemostasis criteria [15].
2.1.3. e-Consult service outcomes
Patient-level metrics collected to assess the outcomes of the e-consult service included components of the 4Ts score, EIA result and optical density, functional assay result, time of notification of positive EIA result, time of signed e-consult note, time to perform the e-consult (measured by the clinician in real time and documented in the note), anticoagulation recommendations, bivalirudin start and stop dates, whether an FTF hematology consult was performed before or after e-consult, and discharge disposition. In addition, data on hospital-level bivalirudin use were collected and reported as total days per month and individual patients per quarter. For patients with confirmed HIT, additional data collected included initial nonheparin anticoagulant, nonheparin anticoagulant at discharge, development of thrombosis or bleeding after HIT diagnosis, and length of stay.
2.1.4. HIT testing practices
The initial HIT screening assay was an EIA (Asserachrom HPIA, Diagnostica Stago), which was performed in the hospital core laboratory. If its result was positive, a serotonin release assay (SRA) was reflexively sent to a reference laboratory (Versiti, Milwaukee, Wisconsin), which communicated the result electronically and immediately upon test completion to our institution’s e-consult physicians and laboratory team. A positive SRA result depended on >20% release of serotonin induced by the patient’s serum plus low-dose heparin (0.1 U/mL) and <20% release when high-dose heparin (100 U/mL) was used. An inconclusive result was defined as one where there was release of serotonin not meeting criteria for a positive result (eg, >20% release with both doses of heparin or some release but <20% with low dose along with inhibition of release with high dose). Physicians on the HIT e-Consult Service could request a P-selectin expression assay (PEA, Versiti) if SRA results were inconclusive.
2.2. Statistical analysis
The data were analyzed using Stata version 18 (StataCorp). Descriptive statistics describing the characteristics of the HIT e-Consult Service are displayed in Table 1. Univariate and bivariate results compared outcomes of patients with HIT before and after e-consult service implementation, and tests of significance were conducted as appropriate (chi-square for categorical variables and t-tests for continuous variables).
Table 1.
Features of patients with positive enzyme immunoassay results and/or heparin-induced thrombocytopenia electronic consultation performed.
| Feature | Values |
|---|---|
| Demographic information | |
| Patient age (y), mean (SD) | 57 (14) |
| Patient sex, n (%) | |
| Male | 61 (59) |
| Female | 42 (41) |
| Patient race, n (%) | |
| White | 61 (59) |
| Black | 35 (34) |
| Other | 4 (4) |
| Not documented | 3 (3) |
| Admitting service, n (%) | |
| Cardiovascular surgery | 30 (29) |
| Cardiology (acute care and ICU) | 20 (19) |
| Other ICUs (medical, neurologic, and surgical) | 13 (13) |
| ECMO | 10 (10) |
| Other acute carea | 30 (29) |
| EIA optical density, n (%) | |
| ≤1 | 58 (59) |
| >1-2 | 32 (32) |
| >2 | 9 (9) |
| 4Ts score, n (%) | |
| 0-3 | 30 (30) |
| 4-5 | 51 (52) |
| 6+ | 18 (18) |
| Initial anticoagulation recommendations, n (%) | |
| Start bivalirudin | 29 (34) |
| Start other nonheparin anticoagulant | 12 (14) |
| No need for nonheparin anticoagulant | 45 (52) |
Demographic information and admitting service values include all patients where an electronic consultation (e-consult) was requested (n = 103), including those with a positive EIA result with a subsequent e-consult (n = 99) and patients where an e-consult was requested prior to a negative EIA result (n = 4). EIA optical density, 4Ts score, and initial anticoagulation recommendations include only those patients that had a positive EIA result (n = 99) with a subsequent e-consult performed.
ECMO, extracorporeal membrane oxygenation; EIA, enzyme immunoassay; ICU, intensive care unit.
Including general surgery, internal medicine (hospitalist), liver transplant, medical oncology, neurology, neurosurgery, rehabilitation, trauma, and vascular surgery.
3. Results
During the 1 year after e-consult implementation, 646 EIAs were requested. The HIT e-Consult Service interacted with the medical record of 103 patients with a positive EIA result and 4 who had a negative EIA result, but a consult was requested prior to the test result (Figure 1). In 14 patients with a positive EIA result, an e-consult was not performed because the FTF hematology consult service (“traditional consultation”) was already caring for the patient. Three patients died because of non–HIT-related causes after a positive EIA result and before the e-consult could be performed. Therefore, e-consult was completed for 86 patients, 19 of whom were diagnosed with HIT. Table 1 presents features of the patient population that interacted with the e-consult service.
The mean time for the HIT e-Consult Service to review the medical record and formulate recommendations was 11.2 minutes (SD, 2.88; range, 4-19 minutes). The mean time to communicate recommendations to the consulting provider from reporting of a positive EIA result was 76.2 minutes (SD, 85.5; range, 7-552 minutes).
Features of patients with a positive EIA result and/or a HIT e-consult are presented in Table 1. Almost 60% of patients were admitted through the cardiovascular surgery service and other acute care services (30% each). Thirty percent had a low-risk 4Ts score of between 0 and 3, as calculated by the HIT e-Consult Service.
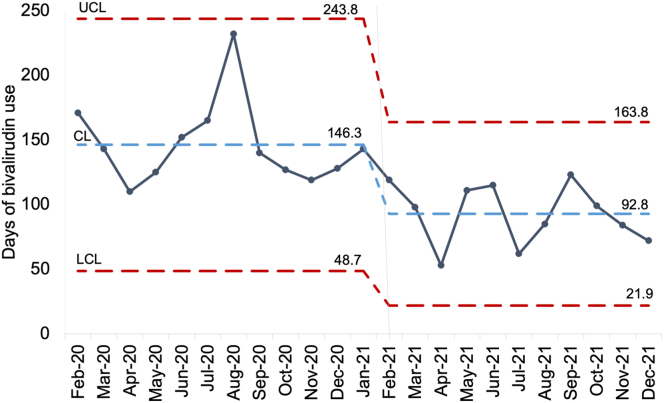
Approximately one third of patients received an initial anticoagulation recommendation to start bivalirudin (34%), 14% to start another nonheparin anticoagulant, and 52% received a recommendation to not prescribe a nonheparin anticoagulant. Figure 2 presents a control chart illustrating the number of days of hospital-wide bivalirudin use before and after intervention. The mean days of bivalirudin use per month decreased from 146.3 in the preintervention (e-consult) period to 92.8 days in the postintervention period. The mean number of unique patients receiving bivalirudin decreased from 56 patients per quarter to 35.3 patients per quarter.
Figure 2.
Days of bivalirudin use per month before and after HIT e-Consult Service implementation. CL, control limit; e-consult, electronic consultation; HIT, heparin-induced thrombocytopenia; LCL, lower control limit; UCL, upper control limit.
In the 1-year period without e-consult, 42% (n = 10) of patients with confirmed HIT were seen by hematology during hospitalization and 16% (n = 3) of discharged patients were seen by hematology for an outpatient follow-up. Inpatient evaluation of patients with confirmed HIT increased to 100% after e-consult implementation, and 80% (n = 12) of discharged patients were seen in the outpatient clinic.
Prior to HIT e-Consult Service implementation, there were 9 inconclusive SRA results, and 5 patients (21% of patients diagnosed with HIT in that period) were treated as if they had HIT. After implementation, there were 5 inconclusive SRA results. In all cases, a PEA was requested by the HIT e-Consult Service and returned negative. Based on these results and our clinical judgment, all 5 patients had the diagnosis of HIT excluded and did not suffer any complications that could have been caused by untreated HIT.
Table 2 presents a bivariate comparison of features of patients with confirmed HIT before and after e-consult implementation. On average, the length of stay decreased from 39 days before to 17 days after (t = 2.92; P < .01). There was a significant increase in the use of oral FXa inhibitors (apixaban and rivaroxaban) as well as fondaparinux both for initial anticoagulation after positive EIA result and at discharge after e-consult implementation. There was no significant difference in the incidence of thrombosis or major bleeding attributed to HIT.
Table 2.
Comparison of features and outcomes of patients with heparin-induced thrombocytopenia before and after HIT e-Consult Service implementation (n = 43).
| Feature | Before, January 1, 2019, to December 31, 2019 (n = 24) | After, February 1, 2021 to January 31, 2022 (n = 19) | t/X2; P value |
|---|---|---|---|
| Demographic information | |||
| Patient age (y), mean (SD) | 57.6 (14.9) | 57.1 (11.7) | 0.11; .91 |
| Patient sex, n (%) | |||
| Male | 11 (46) | 14 (74) | 3.38; .07 |
| Female | 13 (54) | 5 (26) | |
| Patient race, n (%) | |||
| White | 11 (46) | 12 (63) | 1.96; .58 |
| Black | 11 (46) | 6 (32) | |
| Other | 1 (4) | 0 (0) | |
| Unknown | 1 (4) | 1 (5) | |
| Admitting service, n (%) | |||
| Cardiovascular surgery | 9 (37) | 9 (48) | 7.86; <.05 |
| Cardiology (acute care and ICU) | 1 (4) | 5 (26) | |
| Other ICUs (medical, neurologic, and surgical) | 0 (0) | 0 (0) | |
| ECMO | 4 (17) | 0 (0) | |
| Other acute carea | 10 (42) | 5 (26) | |
| Initial anticoagulation, n (%) | |||
| Bivalirudin | 24 (100) | 10 (56) | 13.18; <.001 |
| Oral factor Xa inhibitor | 0 (0) | 6 (33) | |
| Fondaparinux | 0 (0) | 2 (11) | |
| Discharge anticoagulation, n (%) | |||
| Warfarin | 12 (71) | 2 (14) | 10.20; <.01 |
| Oral factor Xa inhibitor | 5 (29) | 11 (79) | |
| Fondaparinux | 0 (0) | 1 (7) | |
| Clinical outcomes | |||
| Incidence of thrombosis, n (%) | 2 (9) | 0 (0) | 1.82; .18 |
| Incidence of major bleeding, n (%) | 3 (14) | 2 (11) | 0.09; .76 |
| Length of stay (d), average (range) | 39 (9-131) | 17 (2-69) | 2.92; <.01 |
Oral factor Xa inhibitors included rivaroxaban and apixaban.
e-consult, electronic consultation; ECMO, extracorporeal membrane oxygenation; HIT, heparin-induced thrombocytopenia; ICU, intensive care unit.
Including general surgery, internal medicine (hospitalist), medical oncology, neurology, neurosurgery, orthopedic surgery, trauma, and vascular surgery.
4. Discussion
Improving the quality of care in HIT diagnosis and management is a well-documented challenge, with a range of attempted interventions reporting varying success [16]. Active interventions that directly involve experts in decision-making have demonstrated improvement in meaningful clinical outcomes, with published examples presented in Table 3 [[17], [18], [19], [20]].
Table 3.
Comparison of literature reports of active intervention with expert guidance to improve heparin-induced thrombocytopenia diagnosis and management.
| Feature | Burnett et al. [17] | Lee et al. [18] | Ritchie et al. [19] | Lim et al. [20] | May et al. (current study) |
|---|---|---|---|---|---|
| Timing of intervention | At the time of screening assay request | At the time of request for i.v. DTI | After screening assay request | After positive screening assay result | After positive screening assay result |
| Team members | Anticoagulation management service: pharmacist(s),a 1 physician, laboratory | Antithrombotic stewardship program: 2 pharmacists, 2 physicians | Hemostatic and antithrombotic stewardship program: 1 pharmacist, 2 physicians | Anticoagulation and Bleeding Management Service: physiciansa | Two physicians, laboratory staff members |
| Health care environment | Large academic medical center | ||||
| Intervention components | Primary intervention: review of all HIT assay orders, with 4Ts score calculation
|
Primary intervention: anticoagulation selection of suspected and confirmed HIT
|
Primary intervention: anticoagulation selection of suspected and confirmed HIT
|
Primary intervention: anticoagulation selection of suspected and confirmed HIT
|
Primary intervention: comprehensive evaluation of suspected and confirmed HIT
|
| Selected outcomes |
|
|
|
|
|
| Estimated cost savings | Assay + drug costs, offset by pharmacist time cost: $75,754 ($58,691-$134,445) over 12 mo | Not estimated | Drug cost: $180,000 over 12 mo Laboratory costs: $68,500 over 12 mo |
Not estimated | Not estimated |
DTI, direct thrombin inhibitor; e-consult, electronic consultation; HIT, heparin-induced thrombocytopenia; i.v., intravenous.
Number of providers not specified.
Herein, we report the use of e-consult to provide real-time guidance after positive in-house screening test result for HIT. With this intervention, we observed improvement in care in 3 domains: (1) interpretation of diagnostic testing, (2) anticoagulation stewardship, and (3) health care utilization.
First, the interpretation of HIT diagnostic testing is inherently challenging and may be less familiar to providers without dedicated training. With e-consult implementation, providers with expertise were able to assess and communicate the likelihood of HIT based on the calculated 4Ts Score and the optical density of the EIA [21], which guided diagnosis and subsequent anticoagulation management. Additionally, prior to the e-consult service, there was variation in the interpretation of inconclusive SRA results, with the majority of patients being diagnosed with HIT without additional testing. With e-consult, we incorporated the PEA in cases of diagnostic uncertainty and were ultimately able to exclude HIT in the majority of patients. We presume that they may have been mistakenly diagnosed as HIT prior to this intervention.
Second, the e-consult service improved efforts in anticoagulation stewardship. There was a significant decrease in the unnecessary use of bivalirudin in patients with suspected and confirmed HIT, decreasing both the number of patients receiving it and the number of days of bivalirudin used hospital-wide (Figure 2). Since available data from the literature suggest that bivalirudin is associated with increased risk of medication errors and bleeding risk, we hypothesize that minimizing its use improved patient safety [8,[10], [11], [12]]. Although we did not directly measure, decreased bivalirudin use also likely contributed to cost savings based on the increased cost of bivalirudin compared with unfractionated heparin and fondaparinux. As a balancing measure for limiting bivalirudin use, we did not see an increase in the incidence of thrombosis in patients with confirmed HIT.
Third, we decreased health care utilization in patients with confirmed HIT by decreasing the average length of stay from 39 days to 17 days. The use of warfarin in HIT requires parenteral anticoagulation with warfarin avoidance until platelet normalization, followed by a parenteral anticoagulant bridge once warfarin is initiated, both of which contribute to a need for extended hospitalization [2]. By increasing prescribing of fondaparinux and oral FXa inhibitors, which can be used during the period of thrombocytopenia and do not require bridging, patients were more promptly discharged for outpatient monitoring. Other potential benefits of warfarin avoidance include a hypothesized lower bleeding risk with oral Xa inhibitors as well as the avoidance of the risk of warfarin-induced skin necrosis and venous gangrene.
The HIT e-Consult Service was created as a nonbillable service. This was due in large part to the complexity of billing for inpatient e-consults in the United States. Although there is a Current Procedural Terminology code for e-consults (99451), there are limitations to this code, including that the consulting service cannot bill for an FTF consultation for the same patient within 14 days [22]. We opted to keep the e-consult nonbillable so that FTF consultation could be billable and completed, if indicated.
Importantly, the analysis of the impact of the HIT e-Consult Service is limited by a small sample size and a single-institution intervention. Furthermore, the optimal time to intervene in HIT diagnosis would be prior to EIA ordering, with many published efforts focused on promoting accurate 4Ts score calculation to avoid unnecessary testing [16]. However, given the high volume of EIA requests at our institution and personnel limitations, including lack of dedicated physician time for this service, the intervention was implemented after the EIA result was obtained. Furthermore, whether the ordering provider had initiated nonheparin anticoagulation at the time of EIA ordering was not assessed. In future efforts, we intend to focus on appropriate EIA ordering and initiation of nonheparin anticoagulation in patients with a 4Ts score of 4 or higher pending the EIA result.
In conclusion, the use of e-consult to provide expert guidance in the diagnosis and management of HIT significantly improved meaningful outcomes, including improving diagnostic accuracy, decreasing unnecessary DTI use, decreasing length of stay, and decreasing care expenditures. Although passive interventions to improve HIT care quality require less resource investment, our intervention and others support investing in increasing availability of HIT care expertise to improve management across health systems. At our institution, this project has served as the initial intervention as part of a broader Anticoagulation Stewardship Program [23], which will guide the care of patients with other complex thrombotic and hemostatic conditions.
Acknowledgments
Funding
No funding was used.
Author contributions
J.E.M. developed the concept, performed the intervention, collected the data, and wrote the manuscript. K.R.H. performed the data analysis, reviewed the manuscript, and gave final approval. F.J.O. performed the intervention, reviewed the manuscript, and gave final approval. G.A.K. developed the electronic tools for the intervention, reviewed the manuscript, and gave final approval. L.J.T. provided the laboratory support for the intervention, assisted in collecting the data, reviewed the manuscript, and gave final approval. M.B.M. assisted in developing the consult service, reviewed the manuscript, and gave final approval.
Relationship disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Bethany Samuelson Bannow
References
- 1.Dhakal B., Kreuziger L.B., Rein L., Kleman A., Fraser R., Aster R.H., et al. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: a population-based study. Lancet Haematol. 2018;5:e220–e231. doi: 10.1016/S2352-3026(18)30046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuker A., Arepally G.M., Chong B.H., Cines D.B., Greinacher A., Gruel Y., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–3392. doi: 10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linkins L.A., Dans A.L., Moores L.K., Bona R., Davidson B.L., Schulman S., et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl):e495S–e530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May J.E., Martin K.D., Taylor L.J., Wallace E.L., Marques M.B. A road paved with good intentions: a platelet count-based alert to facilitate diagnosis of heparin-induced thrombocytopenia. Blood. 2021;138(Suppl 1):756. doi: 10.1182/blood-2021-145891. [DOI] [Google Scholar]
- 5.Marler J., Unzaga J., Stelts S., Oliphant C.S. Consequences of treating false positive heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2015;40:512–514. doi: 10.1007/s11239-015-1236-0. [DOI] [PubMed] [Google Scholar]
- 6.Pishko A.M., Lefler D.S., Gimotty P., Paydary K., Fardin S., Arepally G.M., et al. The risk of major bleeding in patients with suspected heparin-induced thrombocytopenia. J Thromb Haemost. 2019;17:1956–1965. doi: 10.1111/jth.14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi S., Kohli R., McCrae K. Over-testing for heparin induced thrombocytopenia in hospitalized patients. J Thromb Thrombolysis. 2015;40:12–16. doi: 10.1007/s11239-014-1123-0. [DOI] [PubMed] [Google Scholar]
- 8.Joseph L., Casanegra A.I., Dhariwal M., Smith M.A., Raju M.G., Militello M.A., et al. Bivalirudin for the treatment of patients with confirmed or suspected heparin-induced thrombocytopenia. J Thromb Haemost. 2014;12:1044–1053. doi: 10.1111/jth.12592. [DOI] [PubMed] [Google Scholar]
- 9.Aljabri A., Huckleberry Y., Karnes J.H., Gharaibeh M., Kutbi H.I., Raz Y., et al. Cost-effectiveness of anticoagulants for suspected heparin-induced thrombocytopenia in the United States. Blood. 2016;128:3043–3051. doi: 10.1182/blood-2016-07-728030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowther M.A., Warkentin T.E. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood. 2008;111:4871–4879. doi: 10.1182/blood-2007-10-120543. [DOI] [PubMed] [Google Scholar]
- 11.Cooper T., White C.L., Taber D., Uber W.E., Kokko H., Mazur J. Safety and effectiveness outcomes of an inpatient collaborative drug therapy management service for direct thrombin inhibitors. Am J Health Syst Pharm. 2012;69:1993–1998. doi: 10.2146/ajhp120121. [DOI] [PubMed] [Google Scholar]
- 12.Tuleja A., Salvador D., Muka T., Bernhard S., Lenz A., Baumgartner I., et al. Cost-effectiveness analysis of alternative anticoagulation in suspected heparin-induced thrombocytopenia. Blood Adv. 2022;6:3114–3125. doi: 10.1182/bloodadvances.2022007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judson T.J., Mourad M., Wachter R.M. Building a targeted automatic e-consult (TACo) program. Jt Comm J Qual Patient Saf. 2022;48:114–119. doi: 10.1016/j.jcjq.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Pai A., Kotak D., Facher N., Reader K., Kong K., Kolevska T. Development of a virtual benign hematology consultation service: results of a pilot project involving 5 medical centers. Blood. 2019;133:993–995. doi: 10.1182/blood-2018-11-887349. [DOI] [PubMed] [Google Scholar]
- 15.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 16.Cogan J.C., McFarland M.M., May J.E., Lim M.Y. Quality improvement approaches to heparin-induced thrombocytopenia: a scoping review. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnett A.E., Bowles H., Borrego M.E., Montoya T.N., Garcia D.A., Mahan C. Heparin-induced thrombocytopenia: reducing misdiagnosis via collaboration between an inpatient anticoagulation pharmacy service and hospital reference laboratory. J Thromb Thrombolysis. 2016;42:471–478. doi: 10.1007/s11239-016-1381-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee J., Lindsley J., Chasler J., Streiff M.B., Naik R., Shanbhag S., et al. The Impact of an antithrombotic stewardship program on heparin-induced thrombocytopenia management. J Pharm Pract. 2023;36:1343–1349. doi: 10.1177/08971900221116185. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie B.M., Sylvester K.W., Reardon D.P., Churchill W.W., Berliner N., Connors J.M. Treatment of heparin-induced thrombocytopenia before and after the implementation of a hemostatic and antithrombotic stewardship program. J Thromb Thrombolysis. 2016;42:616–622. doi: 10.1007/s11239-016-1408-6. [DOI] [PubMed] [Google Scholar]
- 20.Lim M.Y., Foster J., Rourk A., Greenberg C.S. Initial and long term impact of a multi-disciplinary task force in the diagnosis and management of heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2018;45:130–134. doi: 10.1007/s11239-017-1592-z. [DOI] [PubMed] [Google Scholar]
- 21.Raschke R.A., Gallo T., Curry S.C., Whiting T., Padilla-Jones A., Warkentin T.E., et al. Clinical effectiveness of a Bayesian algorithm for the diagnosis and management of heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15:1640–1645. doi: 10.1111/jth.13758. [DOI] [PubMed] [Google Scholar]
- 22.AAP Division of Health Care Finance 2 new codes developed for interprofessional consultation. AAP news. https://publications.aap.org/aapnews/news/6286/2-new-codes-developed-for-interprofessional ; 2019 [accessed February 8, 2024].
- 23.Burnett A.E., Barnes G.D. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6 doi: 10.1002/rth2.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]