Abstract
In recent times, nanomaterials (NMs) have gained significant attention for their unique properties and wide-ranging applications. This increased interest has driven research aimed at developing more efficient synthetic approaches in the fields of material science. Moreover, today's increasing demand for materials underscores the need for innovative technologies that can effectively scale up production to meet these growing needs. Hence, this review is primarily delve deeply into the template-assisted method i.e., an advance bottom-up approach for NMs synthesis. Furthermore, this review emphasizes to explore the advancements in soft template-based synthetic strategies for nanostructured materials as it provides high control on morphology and size. Therefore, this review specifically organized around on providing an in-depth discussion of the liquid/liquid interface-assisted soft template method, applications, and the factors affecting liquid/liquid interface for NMs synthesis. These key points are instrumental in driving advancements, highlighting the ongoing need for further enhancement and refinement of smart technologies. Finally, we conclude the review by describing the challenges and future perspectives of the liquid/liquid-assisted approach for NMs designing.
Keywords: Hard template, Soft template, Liquid/liquid interface, Nanomaterials designing, Self-assembly
Graphical abstract
Highlights
-
•
Synthesis of nanomaterials (NMs) with controlled morphology.
-
•
Template-assisted synthesis i.e., hard and soft.
-
•
NMs formation at liquid/liquid interface.
-
•
Involvement in self-assembly and interfacial chemistry.
1. Introduction
The growth of urbanization, industrialization, and population directly contributes to heightened pollution levels, significantly impacting both our environment and public health. Concerned about these issues and the applications of nanomaterials (NMs), researchers have frequently focussed on published research reports in the field of material science and nanotechnology as an efficient and sustainable solutions. In the past decade, advancements in nanoscience provided a promising solutions by representing NMs having distinguished quantum size, macroscopic tunnelling, dielectric confinement, surface and volume factors [1]. However, it is difficult to say that the synthesized NMs are ready to be used without processing the materials to make the specific property for applications useful for mankind. It is also well known that the applications of any NMs depend on the close relation between synthesized NMs, their structural features and properties. The synthesis of these cutting-edge materials or NMs have a plethora of applications in various fields (sensor, catalyst, drug delivery, device fabrication, environmental remediation such as wastewater treatment, etc.) [2,3]. NMs are materials that lie in the nanoscale range between 1 and 1000 nm. NMs beautifully show unique physiochemical properties controlled by different parameters like size, morphology, dimensionality, etc [4,5]. These remarkable characteristics of NMs make them unique over bulk materials. Consequently, researchers are not only concentrating on applications but they are also working on modifying the approaches for NMs synthesis to enhance the physiochemical properties for better results. In general, NMs are synthesized by two approaches i.e., top-down and bottom-up. The top-down approaches are focused on the macroscopic or large particles, reduced to nano-sized materials. It comprises physical processes like mechanical grinding, electron beam lithography, ball milling, vapor phase synthesis, spray pyrolysis, inert gas condensation, discharge method, etc [[6], [7], [8]]. The chief downsides of these methods are that they are expensive and comprise substantial setups. The approach is based on the grinding of materials. While bottom-up approaches emphasize the principle of molecular recognition (i.e., self-assembly) that involves the miniaturizing of materials components to the atomic level [9]. The self-assembly is defined by the spontaneous gathering of small particles changes into stable novel NMs with distinct morphology. The forces responsible for self-assembly are hydrogen bonding, hydrophilic/hydrophobic interactions, van der Waals, magnetic, electrostatic, and surface organic molecular forces [[10], [11], [12]]. Bottom-up approach involves chemical processes like a sol-gel, solvothermal, sonochemical, and biological methods (such as plant-based extract, microorganisms, enzymes, biomolecules) etc [7,13]. It is a cost-effective and readily controlled process for the high-yield targeting products as compared to top-down approaches. However, the drawback of this approach is that it needs multifaceted experimental steps that restrict its applications to simple NMs [14]. It further sub-classified into three categories i.e., template-based, solution-based, and vapor-based method [15]. As this review is primarily focused on the sequential key points of the current discussion, our objective is to delve deeply into the template-assisted method i.e., an advance bottom-up approach for NMs synthesis. Nowadays, template-assisted methods for NMs synthesis are an emerging field for researchers. It entails employing scaffolds or templates on the nanoscale level to direct the assembly or growth of NMs. These methods enable precise control over the morphology, dimensions, composition, and enhanced properties of NMs [[16], [17], [18], [19]]. Also, NMs with specific properties can be tailored by manipulating the template structure and deposition step. The tailored NMs are suitable for multipurpose applications in the field of biosensing, catalysis, electronics, and biomedicines. These methods for NMs synthesis are classified into hard and soft template approaches [15,20]. Hard template is a rigid material, whose stable structure directly dictates the size and morphology of sample particle. While, the soft template possess non-rigid structural features. For the fabrication of NMs, different types of templates are used but the removal of the template after the production of NMs is a complex process, and many times the structure of synthesized NMs is disturbed in this process. Dong et al. mentioned that several characteristics of hard template method pose a barrier to its efficient use include their considerably less controllable pore size, the irresistible nucleation that occurs outside the pore leads to a lower yield, and complex processes with high costs [21,22]. For better understanding, it is crucial to grasp the fundamentals of hard and soft template approaches and deeper discussion of them are mentioned in forthcoming sections. Poolakkandy et al. reported that soft-template-assisted synthesis provides high control on morphology and size of the synthesized NMs [15]. Therefore, considering the advantages of the soft template method, our focus is to review and explore its applications in the upcoming discussion. Various interfaces such as solid/liquid (S/L), gas/solid (G/S), liquid/liquid (L/L), and gas/liquid (G/L) are utilized as templates in NMs synthesis. However, it is important to consider the limitations associated with each interface when employing them as templates. Huhn et al. highlighted challenges such as limited control over morphology and difficulties in template removal when employing the solid/liquid interface as a template [23]. Nuruzzaman et al. reported that using the solid/gas interface as a template presents challenges such as limited control over morphology, along with specific conditions required for gas-phase interactions to maintain template stability and achieve desired NMs properties [24]. In 2007, Ariga et al. noted that the gas/liquid interface presents restrictions, as direct interaction between gas-phase species and template materials can hinder precise control over NMs assembly and properties. Additionally, achieving uniform and well-defined NMs structures can be challenging due to the limited templating influence [25]. To overcome these limitations, among different synthesis methods, L/L interface-assisted method as a soft template is the most leading, effective, and efficient in comparison with G/L, G/S, and S/L interfaces [26,27]. This method depends on the interface developed between the two immiscible solvents that's why it is known as the L/L interface-assisted method. The beauty of this method is that it provides the freedom to choose different solvents without any removal of the template with a simple synthesis procedure and is economically favoured. It is exciting to know that synthesized NMs by this method are present in technologies such as electrocatalysis, removal of heavy metal and dye degradation from wastewater, electrochromism, sensing & device fabrication, biological activities supercapacitor, etc., [28,29]. In this review, our objective is to explore the advancements in soft template-based synthetic strategies for nanostructured materials. Therefore, this review specifically organized around on providing an in-depth discussion of the liquid/liquid interface-assisted soft template method, applications, and the factors affecting L/L interface for NMs synthesis. To understand the fundamentals and advancements of the L/L interface-assisted method as a soft template, this review starts with a basic description of the templated-assisted approaches for nanomaterials synthesis (Section 2) which are subdivided into different classes i.e., hard and soft template methods. In the next section, we will understand the details of the L/L interface-assisted method through some reported examples and general mechanisms including basic knowledge of the G/L interface, G/S interface, and S/L interface. Hopefully, this review will help to gain more knowledge about the advanced bottom-up approach i.e., L/L interface-assisted method as a soft template for the fabrication of complicated nanomaterials without any complex process to inspire innovative progress and captivating area of research in nanoscience and nanotechnology for mankind.
2. Template-assisted approaches for nanomaterials synthesis
Over the last two decades, significant strides have been made in developing synthetic strategies for NMs with precise control over size, shape, composition, and spatial arrangement. “Template” is the main component in template-assisted synthesis. It is an entity having nanostructured characteristics and the properties of synthesized NMs depend on the size, morphology, and charge distribution of the template [[30], [31], [32]]. It follows the three steps i.e., template preparation, synthesis of NMs by utilizing a template, and removal of a template (if required), respectively [33]. In the realm of NMs synthesis, a template is fundamentally a material characterized by nanostructured features and hence there exists a wide array of materials that can be considered. These include naturally occurring substances as well as synthetic nanostructures derived from established synthesis methods. The physical and chemical properties of NMs can vary significantly depending on the type of template used in their synthesis [34]. The fabrication of the desired product can be done by chemical (organic reactions i.e., addition, substitution, elimination, etc.) or physical methods like channel replication and surface coating. Likewise, the removal of a template can be done by chemical or physical methods i.e., calcination and dissolution, respectively [35]. By this method, synthesized NMs are achieved with controlled morphology, shape, and dimension. Based on the template types, the template-assisted approaches can be classified as hard and soft template methods.
2.1. Hard template method
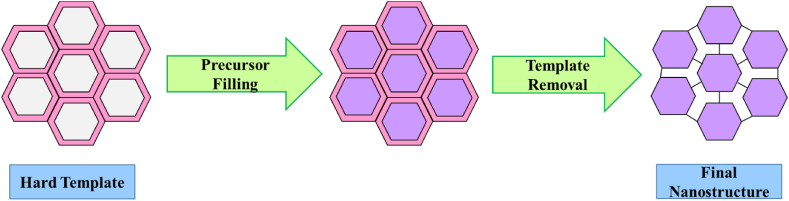
The hard template method is a nanocasting method in which solid porous materials are used as a solid mold. These solid molds have a suitable size, shape, and inside porosity in which liquid materials are allowed to be set for molded structure. These molds are filled with precursor molecules and removed after the formation of the desired nanomaterial without affecting the properties as shown in Fig. 1 [[36], [37], [38]]. It is mostly inorganic and silica-based materials which is widely used as a template. Other than silica, polymer microspheres like polystyrene-acrylate (PSA), etc., porous membranes, mesoporous carbon, porous anodic aluminium oxide (AAO), ion exchange resin, etc., is used as a hard template for NMs synthesis because of their restricted particle size and distinct structures [[39], [40], [41], [42]]. While synthesizing NMs via hard template method it is necessary to consider some important factors i.e., the precursor should be chemically unreactive with the template so judicious selection of precursor and the template is important. The strong interaction leads to the pore blocking of hard template and hence strong interaction between the precursor and hard template should be avoided. For easy diffusion of the precursor into the mesoporous hard template solvent selection is important. To avoid the collapsed structure of desired product after removal of the template a sufficient amount of precursor is necessary [43]. Although the hard template method for NMs synthesis is an outstanding approach for crystalline oxides. However, several characteristics that pose a barrier to its efficient use include their considerably less controllable pore size, the irresistible nucleation that occurs outside the pore and results in a lower yield, and complex processes with high costs [21,22]. The drawback of this method is that at the time of template removal, it may cause damage to NMs structure because of using harsh conditions like etching by HF, etc.
Fig. 1.
Pictorial representation of the synthesis of nanostructure using hard templates.
2.2. Soft template method
In the soft template method, the flexible nanostructures are used as a soft template, formed by intermolecular interaction by weak non-covalent bonds (electrostatic interaction and hydrogen bonding) between the precursors and soft templates as shown in Fig. 2. Unlike the hard template, soft template approaches are generally based on organic materials i.e., soft materials, containing surfactants, flexible organic molecules, block copolymers, interfaces, and so forth are used as soft templates [[42], [43], [44], [45]]. Typically, for the synthesis of NMs co-precipitation, hydrothermal, and sol-gel processes are frequently used as a soft templating methodology but nowadays interface assisted synthesis is also a popular area of research. Similarly, for removing template calcination and solvent extraction are used frequently [2,46,47]. Since the liquid/liquid (L/L) interface-assisted approach as a soft template for the synthesis of NMs is the main theme of the present review. The template preparation is an initial step of NMs synthesis with a soft template which consists of surfactant self-assembly leading to the formation of micelle or liquid crystal. The second step leads to the formation of an inorganic-organic hybrid by adding an inorganic precursor to surfactant self-assembly. At last removal of organic template takes place [48,49].
Fig. 2.
Pictorial representation of the synthesis of nanostructure using Soft templates.
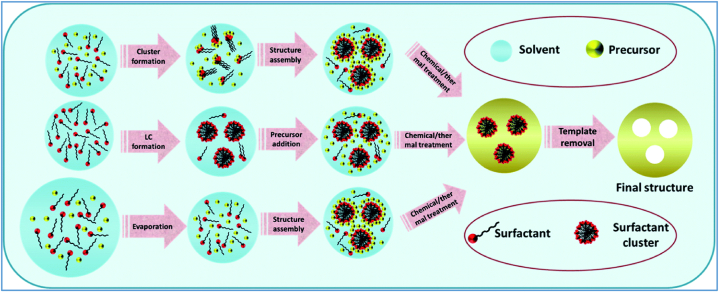
In general soft template-assisted synthesis of nanomaterials follows three mechanisms i.e., cooperative self-assembly (CSA), true crystal liquid templating (TCLT), and evaporation-induced self-assembly (EISA), shown in Fig. 3 [15]. In the CSA mechanism, low concentrations of surfactant are used which leads to the formation of micelle by the assembly of precursors and templates that occur synchronously in various reactions. This step is known as “cooperative assembly”. In the end, desired nanomaterials are obtained by developing a liquid crystal phase that is precipitated by the solution [46]. TLCT mechanism depends on the high concentration of surfactant. In the absence of precursors, the liquid crystal phase is developed and then the precursor is combined with that liquid crystal phase [42,46]. The EISA mechanism follows three steps first is the preparation of a dilute solution of precursors, template, and volatile solvent with appropriate stoichiometry. The second one is a distribution of prepared solution in a huge area to achieve critical micelle concentration (CMC) by solvent evaporation and the last one is a condensation process of precursors near the liquid crystal phase which developed the final product [[50], [51], [52]].
Fig. 3.
General mechanistic steps for soft template-assisted synthesis of nanomaterials via Cooperative self-assembly (CSA), True crystal liquid templating (TCLT), and Evaporation-induced self-assembly (EISA) [15].
After the synthesis of nanomaterials, the soft template removal takes place through different processes like combustion or depolymerization via thermal treatment or calcination, low-temperature chemical treatment, etc. without affecting the morphology and properties of nanomaterials [53]. In contrast to the hard template method, soft templates are gaining more popularity due to their inexpensive cost and ease of synthesis sometimes without the necessity for a time-consuming procedure to remove the template [54]. A comparison between hard and soft template method is shown in Table 1.
Table 1.
Comparative study of hard and soft template method for nanomaterial synthesis.
| Hard Template Method | Soft Template Method |
|---|---|
| Mainly inorganic-based materials like silica, polymer microspheres, porous membranes, mesoporous carbon, AAO, ion exchange resin, etc., are used as hard templates. | Mainly organic-based, surfactant, block copolymers, flexible organic molecules, and interfaces are used as soft templates. |
| By nanocasting process and mesoporous template. | The mechanism follows cooperative self-assembly, True crystal liquid templating, and evaporation-induced self-assembly. |
| Complex synthesis steps. | Synthesis is done in a few steps. |
| Template removal by etching using HF, and high-temperature calcination may cause the damage of nanostructure morphology. | Template removal by low-temperature heat treatment, combustion, or depolymerization. Sometimes no need to remove the template so maintain the morphology of synthesized nanomaterials. |
| Less controllable pore size, and fixed morphology because of the preformed structure of hard template. | Controllable morphology, tunable pore structure, and properties of synthesized nanomaterials. |
| Expensive and time-consuming method. | Less expensive and less time-consuming procedure for template removal. |
The comparative study of hard and soft template approaches shows that the soft template method is a more promising approach for NMs synthesis. It generally avoids complex reaction procedures and is easy to handle. The main aim of this review is to focus on the synthesis of NMs by soft templates i.e., liquid/liquid interface assisted as a soft template. Also, review the fact that the liquid/liquid (L/L) interface-assisted approach for nanomaterials fabrication are more emerging and efficient approach than other interface-assisted synthesis pathways of soft template methods. But to know about the L/L interface-assisted approach it is necessary to gain a little bit of knowledge about other interface-assisted approaches as a soft template for the synthesis of NMs.
3. Synthesis of nanomaterials with interface assisted as soft template
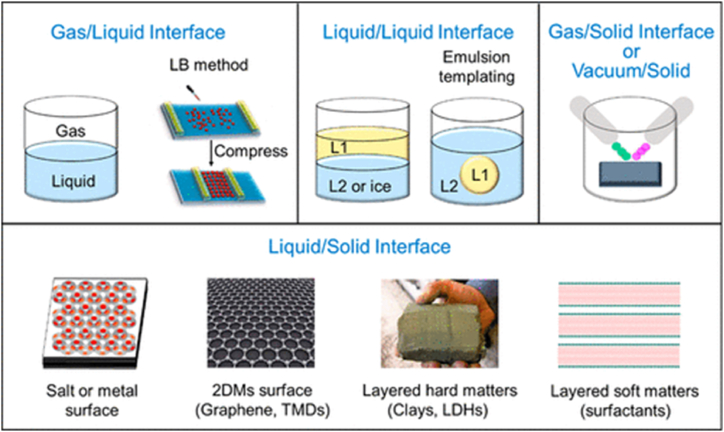
To assemble NMs based on the interfacial assembly is an elegant approach. The interface is something related to the phase boundary between two substances or solvents and the thickness of this interphase ranges from some angstroms to nanometres and even micrometres scale. In material science, the interface is more dynamic at phase boundaries of multi-reactions than the bulk phase [26,55]. The interface forms a unique non-equilibrium atmosphere with improved surface energy for the self-assembly of starting material to start the nucleation, leading to the formation of intermediates at the interface of two phases for the generation of desired nanomaterials [26]. The interfaces as a soft template including in NMs synthesis are gas/liquid, liquid/liquid, gas/solid, and solid/liquid, as shown in Fig. 4 [56,57]. Some reported synthesized NMs by gas/liquid, gas/solid, and solid/liquid interface-assisted approaches are shown in Table 2.
Fig. 4.
Diagrammatic presentation of different interfaces for nanomaterial synthesis, comprising gas/liquid, liquid/liquid, gas/solid, and liquid/solid interfaces [26].
Table 2.
Synthesized nanomaterials via solid/liquid, gas/liquid, and gas/solid interface-assisted approach.
| Interfaces Used | Nanostructures | Morphology | Application | Reference |
|---|---|---|---|---|
| Solid/Liquid | CeO2/Cu2O | Nanocomposite | Catalytic activity for CO oxidation. | [76] |
| Fe3O4/Fe(OH)3 | Flower-like nanocomposite | Nanocatalyst for the cycloaddition reaction of CO2 and epoxides. | [77] | |
| CeO2 | Nanotube | A catalyst for CO oxidation. | [78] | |
| CuO | Nanorod | Photocatalytic degradation of RhB. | [79] | |
| Co3O4 | Mesoporous nanoparticle | Electrochemical capacitive behavior. | [80] | |
| Gas/Liquid | NiO | Flower like-hollow nanosphere | Proposing excellent supercapacitor potentials. | [81] |
| Ag-MnOOH-GO | Nanocomposite | Electrochemical sensing has a good property to catalyze H2O2. | [82] | |
| Pt NWN | Nanowire network sheet | Used in electrocatalytic reactions i.e. ORR and HER, capable of developing next-generation electrocatalysts. | [83] | |
| Cu(OH)2/Au/SiO2 | Ordered nanoarray composite | For detection of trace gaseous VOCs (Volatile organic compounds). | [84] | |
| Janus Nanographene Oxide | 2D lamellar-like nanostructure | Enhancing foam stability in high-temperature reservoirs. | [85] | |
| Quasi 2D Polyaniline (PANI) | Thin Film (2.6–30 nm) | Good chemical vapor sensing ability. | [86] | |
| Ag/WO3/rGO | Nanocomposite | H2S gas sensor | [87] | |
| Fe3O4 | Nanorod | For the photodegradation of phenol as a catalytic carrier. | [88] | |
| SnO2/rGO | Nanocomposite | Sensing of NO2. | [89] | |
| Gas/Solid | Fluorine-doped carbon nanosphere (FPHCs) | Hierarchical porous hollow nanosphere | Suitable for sustainable energy applications. | [90] |
| Pt NWs | Nanowires | Active catalysts toward ORR. | [91] | |
| Cu2S | Tree-like nanostructure | Suggested potential for 1D functional nanosystems. | [92] | |
| AlN | Hollow nanosphere | Suggested applications as potential high-temperature structural materials. | [93] |
3.1. Solid/liquid (S/L) interface assisted synthesis of nanomaterials
The solid/liquid interface assisted synthesis is more suitable for the polymer nanomaterials or functional mesoporous materials with tuned morphology without any destruction in the morphology of the product. It is generally based on the evaporation-induced self-assembly mechanism (EISA). In this method, an ingredient is dissolved in the liquid phase, and the solid phase acts as a template as well as a precursor or reaction field [56,[58], [59], [60]]. In many research articles ice surfaces, solvent crystals, salts, graphene, metal templates, cellulose, paper, and glasses, etc. are reported as solid templates in S/L interface for nanomaterials fabrication (as shown in Table 2) [56,[61], [62], [63], [64], [65], [66], [67], [68]].
3.2. Gas/liquid (G/L) interface synthesis of nanomaterials
The gas/liquid interface synthesis follows the general mechanism of evaporation of the organic solvent in which the hydrophilic substrate is taken in an aqueous surface surrounded with a small amount of organic solution of a hydrophobic substrate. The G/L interface is formed after the evaporation of the organic solvent [69]. This method is broadly used in the synthesis of metal-organic framework (MOF) nanomaterials [70]. It provides room for the synthesis of thin nanostructures up to 0.6 nm because the precursor cannot be delivered from the gas side [71]. Although this method is suitable for nanomaterials consisting of hydrophilic and hydrophobic substrates the major drawback of this method is for large-yield production it needs a large G/L interface (as shown in Table 2) [27].
3.3. Gas/solid (G/S) interface synthesis of nanomaterials
The main methodology used in solid/gas-assisted method is a chemical vapor deposition (CVD), vapor phase transport (VPT), and physical vapor deposition (PVD) for the synthesis of nanomaterials [27,72,73]. The CVD is a very common method utilized, in which the gaseous component is diffused at the surface of the substrate by adsorption i.e. solid/gas interface. This interface results in the formation of nucleation, growth, and combination, and the desired nanoproduct is formed with the bi-product. Later, gaseous products and unreactive components are desorbed from the surface and removed (as shown in Table 2) [27,74,75].
Among all mentioned interface synthesis approaches the liquid/liquid interface as a soft template method for the miniaturization of materials is a piqued and emerging field for researchers nowadays. It is a flexible method of synthesis for tuned nanomaterials with modified morphologies. The detailed views about this method are discussed in section 4.
4. Synthesis of nanomaterials with liquid/liquid (L/L) interface as a soft template
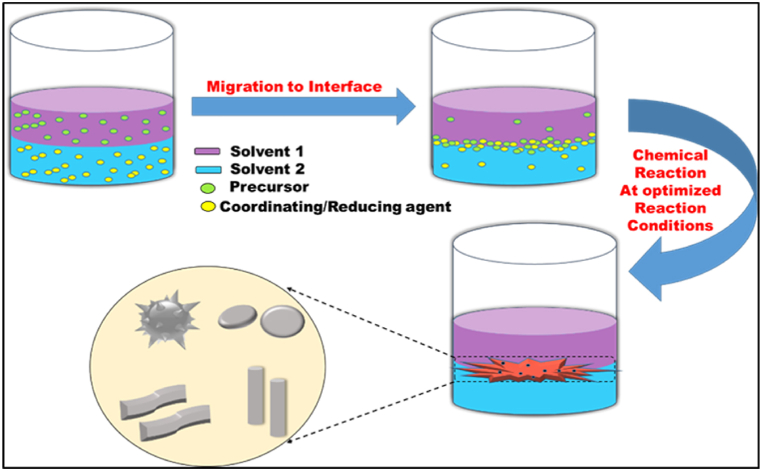
The fundamentals of the L/L interface-assisted method are based on the self-assembly of solid NMs at the interface of the two immiscible liquids. Examples of two immiscible solvents used for chemical reactions include chloroform/water, DCE (dichloroethane)/water, DCM (dichloromethane)/water, toluene/water, n-hexane/water, etc., are more pronounced [29,94]. In this process, the precursor should be dissolved in one of the two solvents, coordinating and reducing agent in another solvent. It is a widely used method for the fabrication of metal-organic frameworks and polymer NMs [26,95]. The general mechanism for liquid/liquid interface-assisted synthesis is shown in Fig. 5. The liquid-liquid interface-assisted method offers opportunities for synthesizing NMs with controlled thickness, morphology, uniformity, and transparency under appropriate reaction conditions, using a straightforward and practical approaches [29].
Fig. 5.
Schematic diagram of the methodology for L/L interface-assisted synthesis of NMs.
Before delving into specific examples, it's crucial to understand the fundamental principles of this approach. The question arises: why do solid particles assemble at the interface of solvents? This phenomenon is driven by interfacial energies and the minimization of interfacial area. Immiscible solvents exhibit distinct intermolecular forces and do not blend at a molecular level. At their interface, there exists notable interfacial tension owing to the disparity in cohesive energies between the two solvents. Solid particles can lower this tension by adsorbing at the interface, where they can partially shield the solvent-solvent interactions. Also, the accumulation of solid particles at the interface reduces the free energy of the system. This happens because the particles move to locations where they minimize the exposed surface area between the two immiscible phases, thereby lowering the associated free energy of the system. Hence, these solid particles tends to minimize the high energy of the L/L interface and decrease the interfacial area, thereby immigrate at the interface which is thermodynamically favoured [96,97]. Due to numerous parameters such as particle size, roughness factor, chemical and surface composition, shape, etc., which influence this process, achieving optimized reaction conditions is crucial in experiments [29,98,99]. The effective interaction between solid particles is dependent on factors such as the nature of the interface, size, shape, and chemical composition of the particles. Under favourable conditions, the accumulation and interaction of solid particles are stable. Moshrefi et al. described a copper nanoparticle-based flexible electrocatalytic composite electrode material at the micro interface between two immiscible electrolyte solutions (ITIES). This setup was used for the simultaneous electropolymerization of 2,2′:5′,2″-terthiophene (TT) and reduction of copper(II) ions, demonstrating a promising approach for generating electrocatalytic materials for carbon capture [100]. A facile method of using green solvents, low catalyst loading, high yields, short reaction times, and the ability to reuse the catalyst was reported by Hoseini in 2014. This study demonstrated the synthesis of palladium nanoparticles by reduction of an organopalladium(II) complex at the oil–water interface and it has been found that the product have high potential for catalysing the reaction of aryl chloride derivatives [101]. Yao reported a method for single-crystal silver nanosheets at room temperature via a chloroform-water interfacial reaction route with o-ethoxyaniline (OEA) in chloroform as reductant. A remarkable SERS (surface-enhanced Raman scattering) response and high reproducibility was observed [102]. A novel approach for molybdenum disulphide synthesis at the liquid–liquid interface was demonstrated by Higgins in 2019 for scaleup the 2D materials [103]. A gold-silver alloy preparation method with poly(3,4-ethylenedioxythiophene) in hexane/water interface was introduced by Puthiyottil in 2023 and this study proved a non-enzymatic electrochemical detection of histamine [104]. Liu prepared a cobalt based metal organic framework nanosheets using liquid/liquid interface as an electrode for high-performance asymmetric supercapacitor [105]. Table 3 provides detailed data on the synthesis at the liquid-liquid interface and their applications. This study demonstrates that nanomaterials produced by this method are sufficiently stable and potentially effective for diverse applications in the current scenario. These investigations also revealed good control on morphology and size of the NMs by adjusting the conditions at the interface. However, under unfavourable conditions, the particles may precipitate at the liquid-liquid interface or agglomerate [29,106]. There is no need for struggling with the removal of templates after synthesizing NMs using this method. It can produce nanomaterials in large quantities due to efficient nucleation and growth at the interface. Therefore, it is typically scalable and can be adapted for large-scale synthesis without major modifications, provided there is stability in the liquid-liquid interface. For bulky production, parallel laminar flow in a microflow reactor may be suitable due to their sufficiently small channels to ensure the solution is stable flow [107,108]. This method required environmentally friendly solvents and reduces the production of hazardous waste compared to other methods, thereby enhancing its sustainability for future applications. Also, the liquid/liquid interface provides a straightforward method for functionalizing and modifying nanomaterial surfaces, thereby improving their stability and functionality. From Table 3, it is clear that this method supports the synthesis of a wide range of NMs, including metals, metal oxides, and composites, which are suitable for multipurpose applications.
Table 3.
List of recently synthesized nanomaterials via liquid/liquid interface method with their applications.
| Nanostructures | Interfaces used as soft-template | Morphology | Application | Reference |
|---|---|---|---|---|
| PSS-free PEDOT | Chloroform/Water | Nanocomposite | Electrochromism Applications. |
[109] |
| PPy/MnO2 | Chloroform/Water | Nanosheet | Electrochemical sensor for nicotine. | [110] |
| PEDOT/graphene | Chloroform/Water | Nanocomposite | Electrochromism Applications. |
[109] |
| ZnO | Water/Air | Nanosheet | Sensing and device fabrication. | [111] |
| Nickle Hydroxide | n-Butanol/Water | Nanoflakes | Non-enzymatic electrochemical sensor to detect glutamic acid. | [112] |
| CuO | n-Octanol/Water | Hollow Nanosphere | Surface photovoltage properties and photocatalytic activity. | [113] |
| Fe2O3 | Benzene/Water | Durian-like hollow sphere | Removal of heavy metal ion Cr(VI). | [114] |
| NiCo2O4 | n-Butanol/Water | Nanoflake | Electrochemical detection of dopamine. | [115] |
| MnO/MnO2 | DMF/Water | Nanoparticle, Microbar, Nanoflake | Applications in lithium-ion batteries. | [116] |
| PPy/Ag | Chloroform/Water | Nanosheet | H2O2 sensing and antibacterial activity. | [117] |
| Fe2O3 | Toluene/Water | Cauliflower-like | Organic dye degradation and heavy metal ions removal in water treatment, photocatalytic degradation in the presence of H2O2 for rhodamine B. | [118] |
| MnO/MnO2 | DCM/Water | Urchin-like | Catalytic degradation of methylene blue. | [119] |
| Au nanoring arrays | Oil/Water | Nanoring | – | [120] |
| PT/Au | n-Hexane/Water | Nanocomposite thin film | Electrochromic applications. | [121] |
| Sulfonated Graphene/Polyaniline | Chloroform/Water | Nanocomposite | Electrochemical measurements for use in energy storage and conversion devices. | [122] |
| Pure FeNPs | Toluene/Water | Spherical nanoparticle | Show superparamagnetic properties for biomedical and industrial applications. | [123] |
| AgNBs | RAIL(redox-active ionic liquid) ([FcMDDA+] [TFPB−])/Water |
Chain like-Nanobelt | Substrate for plasmonic applications. | [124] |
| PdNFAs | RAIL/Water | Vertically aligned nanofibre arrays | Electrocatalytic activity towards ethanol oxidation reaction. | [125] |
| Bis(dipyrrinato) zinc(II) complex | dichloromethane/pyridine (100:1) solution/Water | Nanosheet | – | [126] |
| MoO3 MoS2 MoSe2 |
Toluene/Water | Nanobelt | Illustrated for electrochemical applications. | [127] |
| Nanosheets | ||||
| PT/Au-NPs/CNT | n-Hexane/Water | Nanocomposite | Electrochemical sensor for dopamine. | [128] |
4.1. Reaction occurred at liquid/liquid interface assisted method
The reaction occurring at the interface of two immiscible liquids have been extensively studied. These reactions exhibit behaviour distinct from those in the bulk phase, largely driven by the unique characteristics of this region. Reduction, precipitation, polymerization, and complexation reactions were typically observed to occur at the interface of two immiscible liquids. These can be easily understood by examining examples of nanomaterials fabrication in recent years. Johans et al. synthesized the polyphenylpyrrole-coated silver nanospheroids of 3–20 nm size via electrochemical reduction at the liquid-liquid interface. In this process, silver metal ions were transferred from the aqueous phase to the organic phase. Subsequently, a slow homogeneous electron transfer reaction occurred from N-phenylpyrrole to the silver ion, followed by polymerization and growth of the metal clusters [129]. A novel method involves using a liquid-liquid interfacial reaction to produce noble metal palladium nanoparticles at the interface between 1,2-dichloroethane and water was also introduced by Lee in 2009 for studying the growth kinetics of the formation. The study concluded that the reaction was initiated by the reduction of ammonium tetrachloropalladate salt, resulting in the formation of palladium nanoparticles with sizes ranging from 50 to 100 nm [130]. Likewise, oxidation at the liquid-liquid interface are employed for NMs fabrication. In this, oxidation of precursor molecules or ions present in one phase by oxidizing agents present in the other phase occurred. Kelgenbaeva et al. proposed a method for synthesizing superparamagnetic hematite nanoparticles of approx. 10 nm size using liquid/liquid interface method in which water and toluene were taken as a solvents [123]. Likewise, complexation at the liquid-liquid interface is also employed for the formation of metal organic frameworks (MOF) NMs. In this, a metal ion in an aqueous phase can complex with organic ligands dissolved in an organic phase at the liquid-liquid interface leads to the nucleation and growth of MOF NMs. For example, a bluish film with a dark color at the interphase was observed on spreading hexahydroxytriphenylene (HHTP) solution made in ethyl acetate onto an aqueous cuprous acetate solution, within a few minutes at room temperature [131]. Likewise, Yang et al. proposed a novel method for rapid synthesis of well-crystalline 2D MOF for nitro reduction via liquid/liquid interfacial approach. In this, Cu-CoPc (2D MOFs with Cu2+ coordinated to CoPc), where Pc is phthalocyanine, based structures prepared for the catalytic reduction of nitroaromatics [132].
4.2. Factors affecting the liquid/liquid interface assisted method for nanomaterials synthesis
Factors influencing the liquid/liquid interface assisted NMs synthesis include various parameters that affect the reaction dynamics and NMs characteristics. One of such factor is the choice of liquids. For example, Cheng et al. considered toluene water interface for iron oxide NMs preparation and a cauliflower like morphology was observed [118]. While, Cheng et al. prepared iron oxide durian-like hollow spherical NMs using benzene water as an interface [114]. Hence, the selection of immiscible liquid have tendency to affect the stability of interface as well as solubility of precursors, which in turn affect the growth kinetics and physicochemical properties of NMs. Likewise, one such factor is the use of surfactants. Polymeric micelles typically exhibit greater stability compared to low molecular weight surfactant micelles due to the increased interfacial free energy [133]. The surface chemistry of the liquid/liquid interface may also affect the physiochemical properties of NMs. The distribution of NMs depends on interfacial tension of the liquid/liquid interface. Likewise, pH is another factor plays an important role as it change the surface charge. Fernando et al. reported that the pH increased may leads to decreased particle size within a specific time frame. As a consequence, pH conditions preferred to reduced aggregation and enhanced particle stability [134]. Similarly, light may also affect the liquid/liquid interface which results in photoisomerization of the synthesized NMs [135]. Similarly, temperature is a factor that influences the liquid-liquid interface, thereby affecting the physicochemical properties of nanomaterials. Therefore, many approaches are devised to optimise the factors which led to the creation of smart liquid/liquid systems with vast potential for intricate and practical applications. However, despite considerable advancements, there is still a need for further enhancement and refinement of these technologies.
5. Conclusion and future perspectives
In recent times, nanomaterials have garnered increased attention due to their distinct properties and diverse applications. This heightened interest has spurred research into developing more effective synthetic approaches within the fields of nanoscience and nanotechnology. In today's era, there is a rising demand for materials that necessitates the development of new and intelligent technologies to scale up production. The soft template assisted liquid-liquid interface method as mentioned in this review can potentially serve as a solution to meet this demand. The L/L-assisted approach has several advantages compared with other interface-assisted synthesis pathways. In the L/L-assisted approach, to perform the reaction no sophisticated instruments are required, and can be easily done in any environmental conditions. The reaction doesn't follow complex synthesis steps and nanomaterials are synthesized with controlled morphology without any removal of the template. It is extremely reproducible. This review summarized several reported examples of the broadness of the liquid/liquid assisted approach for the fabrication of nanomaterials, covering organic, inorganic, and hybrid nanomaterials prepared by the different immiscible solvents. That means it provides room to play with solvents beautifully. The potentiality of novel liquid-liquid interfaces is another experimental frontier that is yet to be explored and a variety of fundamental questions are open to answer. Consequently, a lot of experimental investigations as well as theoretical efforts are necessary to explore all these objectives and enhance the applicability of the liquid/liquid interface-assisted method for nanomaterials designing. Additionally, the liquid-liquid interface method has high potential for producing two-dimensional metal-organic frameworks (MOFs), which have garnered significant attention due to their exceptional properties in diverse research fields such as catalysis, biomedicine, energy storage, and sensing applications etc. In the future, there is optimism about fabricating nanomaterials using the liquid-liquid interface method, where miscible green solvents could potentially replace the selection of immiscible liquids. This approach holds promise for advancing environmentally friendly and sustainable practices in nanomaterial synthesis. Therefore, this review benefits future studies of template-assisted approaches and nanomaterials at liquid/liquid interface. We believe these findings could provide novel synthesis pathways with controlled morphology and their multidisciplinary applications.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Nisha Gangwar: Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Chinky Gangwar: Writing – review & editing. Joy Sarkar: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to the Head, Department of Chemistry, University of Lucknow, Lucknow (U.P.) India, for providing basic infrastructure. Mr. Ajeet Singh and Mr. Bharat Kaushik (Senior Research Fellow) BITS PILANI, Pilani main campus, Pilani, Rajasthan India for their support and help in accessing the recent articles.
References
- 1.Xie Y., Kocaefe D., Chen C., Kocaefe Y. Review of research on template methods in preparation of nanomaterials. J. Nanomater. 2016;2016(1) doi: 10.1155/2016/2302595. [DOI] [Google Scholar]
- 2.Oni O.P., Hu Y., Tang S., Yan H., Zeng H., Wang H., Ma L., Yang C., Ran J. Syntheses and applications of mesoporous hydroxyapatite: a review. Mater. Chem. Front. 2023;7:9. [Google Scholar]
- 3.Wang F., Seo J.-H., Luo G., Starr M.B., Li Z., Geng D., Yin X., Wang S., Fraser D.G., Morgan D., Ma Z., Wang X. Nanometre-thick single-crystalline nanosheets grown at the water–air interface. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dardun V., Pinto T., Benaillon L., Veyre L., Galipaud J., Camp C., Meille V., Thieuleux C. Easy preparation of small crystalline Pd2Sn nanoparticles in solution at room temperature. Dalton Trans. 2023;52(7):2157. doi: 10.1039/D2DT03476J. [DOI] [PubMed] [Google Scholar]
- 5.El-Khawaga A.M., Zidan A., El-Mageed A.I.A.A. Preparation methods of different nanomaterials for various potential applications: a review. J. Mol. Struct. 2023;1281 doi: 10.1016/j.molstruc.2023.135148. [DOI] [Google Scholar]
- 6.Zhao C., Zhang H., Si W., Wu H. Mass production of two-dimensional oxides by rapid heating of hydrous chlorides. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil N., Bhaskar R., Vyavhare V., Dhadge R., Khaire V., Patil Y. Overview on methods of synthesis of nanoparticles. Int. J. Curr. Pharmaceut. Res. 2021;13(2):11. [Google Scholar]
- 8.Mailly D. Nanofabrication techniques. EPJ ST. 2009;172(1):333. doi: 10.1140/epjst/e2009-01058-x. [DOI] [Google Scholar]
- 9.Gangwar C., Yaseen B., Kumar I., Singh N.K., Naik R.M. Growth kinetic study of tannic acid mediated monodispersed silver nanoparticles synthesized by chemical reduction method and its characterization. ACS Omega. 2021;6(34) doi: 10.1021/acsomega.1c03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q., Dong D., Si K.J., Sikdar D., Yap L.W., Premaratne M., Cheng W. Shape transformation of constituent building blocks within self-assembled nanosheets and nano-origami. ACS Nano. 2018;12(2):1014. doi: 10.1021/acsnano.7b08334. [DOI] [PubMed] [Google Scholar]
- 11.Niehues M., Engel S., Ravoo B.J. Photo-responsive self-assembly of plasmonic magnetic janus nanoparticles. Langmuir. 2021;37(37) doi: 10.1021/acs.langmuir.1c01979. [DOI] [PubMed] [Google Scholar]
- 12.Li X., Chen C., Niu Q., Li N.-W., Yu L., Wang B. Self-assembly of nanoparticles at solid–liquid interface for electrochemical capacitors. Rare Met. 2022;41(11):3591. [Google Scholar]
- 13.Rangarajan M., Vasanthakumari R., Vikram S. Superparamagnetic iron oxide nanoparticles from coprecipitation: composition, size, and magnetization. Nanosci. Nanotechnol. 2014;14:1. [Google Scholar]
- 14.Mei J., Liao T., Kou L., Sun Z. Two‐dimensional metal oxide nanomaterials for next‐generation rechargeable batteries. Adv. Mater. 2017;29(48) doi: 10.1002/adma.201700176. [DOI] [PubMed] [Google Scholar]
- 15.Poolakkandy R.R., Menamparambath M.M. Soft-template-assisted synthesis: a promising approach for the fabrication of transition metal oxides. Nanoscale Adv. 2020;2(11):5015. doi: 10.1039/d0na00599a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghimbeu C.M., Le Meins J.-M., Zlotea C., Vidal L., Schrodj G., Latroche M., Vix-Guterl C. Controlled synthesis of NiCo nanoalloys embedded in ordered porous carbon by a novel soft-template strategy. Carbon. 2014;67:260. [Google Scholar]
- 17.Rodríguez-San-Miguel D., Montoro C., Zamora F. Covalent organic framework nanosheets: preparation, properties and applications. Chem. Soc. Rev. 2020;49(8):2291. doi: 10.1039/c9cs00890j. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z., Nie K., Qu X., Li X., Li B., Yuan Y., Chong S., Liu P., Li Y., Yin Z., Huang W. General bottom-up colloidal synthesis of nano-monolayer transition-metal dichalcogenides with high 1t′-phase purity. JACS. 2022;144(11):4863. doi: 10.1021/jacs.1c12379. [DOI] [PubMed] [Google Scholar]
- 19.Fujii Y., Zhou S., Shimada M., Kubo M. Synthesis of monodispersed hollow mesoporous organosilica and silica nanoparticles with controllable shell thickness using soft and hard templates. Langmuir. 2023;39(13):4571. doi: 10.1021/acs.langmuir.2c03121. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W., Dai L. Mesoporous metal nanomaterials: developments and electrocatalytic applications. Chem.--Eur. J. 2024;30(25) doi: 10.1002/chem.202400402. [DOI] [PubMed] [Google Scholar]
- 21.Lu A.-H., Schüth F. Nanocasting pathways to create ordered mesoporous solids. C R Chim. 2005;8(3–4):609. [Google Scholar]
- 22.Lu A.H., Schüth F. Nanocasting: a versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006;18(14):1793. [Google Scholar]
- 23.Hühn D., Kantner K., Geidel C., Brandholt S., De I., Cock, Soenen S.J., Rivera Gil P., Montenegro J.M., Braeckmans K., Müllen K., Nienhaus G.U., Klapper M., Parak W.J. Polymer-coated nanoparticles interacting with proteins and cells: focusing on the sign of the net charge. ACS Nano. 2013;7(4):3253. doi: 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- 24.Nuruzzaman M., Rahman M.M., Liu Y., Naidu R. Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J. Agric. Food Chem. 2016;64(7):1447. doi: 10.1021/acs.jafc.5b05214. [DOI] [PubMed] [Google Scholar]
- 25.Ariga K., Hill J.P., Ji Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007;9(19):2319. doi: 10.1039/B700410A. [DOI] [PubMed] [Google Scholar]
- 26.Dong R., Zhang T., Feng X. Interface-assisted synthesis of 2D materials: trend and challenges. Chem. Rev. 2018;118(13):6189. doi: 10.1021/acs.chemrev.8b00056. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki K., Uchida Y., Nishiyama N. Bottom‐up synthesis of nanosheets at various interfaces. ChemPlusChem. 2023 doi: 10.1002/cplu.202300255. [DOI] [PubMed] [Google Scholar]
- 28.Divya V., Sangaranarayanan M.V. Nanomaterials at liquid/liquid interfaces-A review. J. Nanosci. Nanotechnol. 2015;15(9):6863. doi: 10.1166/jnn.2015.10727. [DOI] [PubMed] [Google Scholar]
- 29.Zarbin A.J.G. Liquid–liquid interfaces: a unique and advantageous environment to prepare and process thin films of complex materials. Mater. Horiz. 2021;8(5):1409. doi: 10.1039/d0mh01676d. [DOI] [PubMed] [Google Scholar]
- 30.Vázquez M. Woodhead Publishing; 2015. Magnetic Nano-And Microwires: Design, Synthesis, Properties and Applications. [Google Scholar]
- 31.Li H., Wang L., Wei Y., Yan W., Feng J. Preparation of templated materials and their application to typical pollutants in wastewater: a review. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.882876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrakchi F., Ahmed M.J., Khanday W.A., Asif M., Hameed B.H. Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. Int. J. Biol. Macromol. 2017;98:233. doi: 10.1016/j.ijbiomac.2017.01.119. [DOI] [PubMed] [Google Scholar]
- 33.Scarpelli F., Mastropietro T.F., Poerio T., Godbert N. Mesoporous TiO2 thin films: state of the art, Mater. for Sus. Environ. Times. 2018;508(1):135. [Google Scholar]
- 34.Liu Y., Goebl J., Yin Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013;42(7):2610. doi: 10.1039/C2CS35369E. [DOI] [PubMed] [Google Scholar]
- 35.Petkovich N.D., Stein A. Controlling material macrostructures and mesostructures through combined hard and soft templating. Chem. Soc. Rev. 2013;42:3721. doi: 10.1039/c2cs35308c. [DOI] [PubMed] [Google Scholar]
- 36.Bhosale S.R., Bhosale R.R., Jagadhane K.S., Gore A.H., Kolekar G.B., Kolekar S.S., Anbhule P.V. Recent trends in synthetic Top-down approach for Mesoporous Carbon: a seminal review. J. mater. nanosci. 2023;10(1):601. [Google Scholar]
- 37.Liu X.-Y., Wang K.-X., Chen J.-S. Template-directed metal oxides for electrochemical energy storage. Energy Stor. Mater. 2016;3:1. [Google Scholar]
- 38.Yang H., Zhao D. Synthesis of replica mesostructures by the nanocasting strategy. J. Mater. Chem. 2005;15(12):1217. [Google Scholar]
- 39.Xie Y., Kocaefe D., Chen C., Kocaefe Y. Review of research on template methods in preparation of nanomaterials. J. Nanomater. 2016;2016:1. [Google Scholar]
- 40.Platschek B., Keilbach A., Bein T. Mesoporous structures confined in anodic alumina membranes. Adv. Mater. 2011;23(21):2395. doi: 10.1002/adma.201002828. [DOI] [PubMed] [Google Scholar]
- 41.Kawamura G., Muto H., Matsuda A. Hard template synthesis of metal nanowires. Front. Chem. 2014;2:104. doi: 10.3389/fchem.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nugraha R.E., Fauziyah N.A., Wira G.R. Templated growth formation of mesoporous silica materials: a soft-hard template approach. Nusant. Sci. Technol. Proc. 2022:318. [Google Scholar]
- 43.Savic S., Vojisavljevic K., uč a-Nešić M.P., Zivojevic K., Mladenovic M., Knezevic N. Hard template synthesis of nanomaterials based on mesoporous silica. Metall. & Mater. Eng. 2018;24(4):225. [Google Scholar]
- 44.Meng Y., Gu D., Zhang F., Shi Y., Yang H., Li Z., Yu C., Tu B., Zhao D. Ordered mesoporous polymers and homologous carbon frameworks: amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 2005;44(43):7053. doi: 10.1002/anie.200501561. [DOI] [PubMed] [Google Scholar]
- 45.Li W., Wu Z., Wang J., Elzatahry A.A., Zhao D. A perspective on mesoporous TiO2 materials. Chem. Mater. 2014;26(1):287. [Google Scholar]
- 46.Soler-Illia G.J.A.A., Azzaroni O. Multifunctional hybrids by combining ordered mesoporous materials and macromolecular building blocks. Chem. Soc. Rev. 2011;40(2):1107. doi: 10.1039/c0cs00208a. [DOI] [PubMed] [Google Scholar]
- 47.Fan J., Boettcher S.W., Tsung C.-K., Shi Q., Schierhorn M., Stucky G.D. Field-Directed and confined molecular assembly of mesostructured materials: basic principles and new opportunities. Chem. Mater. 2008;20(3):909. doi: 10.1021/cm702328k. [DOI] [Google Scholar]
- 48.Pal N., Bhaumik A. Soft templating strategies for the synthesis of mesoporous materials: inorganic, organic–inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013;189:21. doi: 10.1016/j.cis.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Ajami D., Liu L., Rebek J., Jr. Soft templates in encapsulation complexes. Chem. Soc. Rev. 2015;44(2):490. doi: 10.1039/c4cs00065j. [DOI] [PubMed] [Google Scholar]
- 50.Wang W., Faulkner D., Moir J., Ozin G.A. The effect of solvent in evaporation-induced self-assembly: a case study of benzene periodic mesoporous organosilica. Sci. China Chem. 2011;54:1920. [Google Scholar]
- 51.Grosso D., Cagnol F., Aa Soler-Illia GJ.d., Crepaldi E.L., Amenitsch H., Brunet-Bruneau A., Bourgeois A., Sanchez C. Adv. Funct. Mater. 2004;14:309. [Google Scholar]
- 52.Hu D., Wang E., Wang A., Li H., Duan A. Synthesis of wrinkle silica nanospheres by solvent evaporation-induced self-assembly method for hydrogenation reaction. Mater. Today Chem. 2023;27 doi: 10.1016/j.mtchem.2022.101280. [DOI] [Google Scholar]
- 53.Petkovich N.D., Stein A. Controlling macro-and mesostructures with hierarchical porosity through combined hard and soft templating. Chem. Soc. Rev. 2013;42(9):3721. doi: 10.1039/c2cs35308c. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., Li L., Su H., Huang W., Dong X. Binary metal oxide: advanced energy storage materials in supercapacitors. J. Mater. Chem. A. 2015;3(1):43. [Google Scholar]
- 55.Dallas P., Georgakilas V. Interfacial polymerization of conductive polymers: generation of polymeric nanostructures in a 2-D space. Adv. Colloid Interface Sci. 2015;224:46. doi: 10.1016/j.cis.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Chondath S.K., Menamparambath M.M. Interface-assisted synthesis: a gateway to effective nanostructure tuning of conducting polymers. Nanoscale Adv. 2021;3(4):918. doi: 10.1039/d0na00940g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L., Zhang S., Liu X., Ge X. Recent advances in water-mediated multiphase catalysis. Curr. Opin. Colloid Interface Sci. 2023;65 doi: 10.1016/j.cocis.2023.101691. [DOI] [Google Scholar]
- 58.Oaki Y., Sato K. Crystal-controlled polymerization: recent advances in morphology design and control of organic polymer materials. J. Mater. Chem. A. 2018;6(46) [Google Scholar]
- 59.Huang X., Li S., Huang Y., Wu S., Zhou X., Li S., Gan C.L., Boey F., Mirkin C.A., Zhang H. Synthesis of hexagonal close-packed gold nanostructures. Nat. Commun. 2011;2(1):292. doi: 10.1038/ncomms1291. [DOI] [PubMed] [Google Scholar]
- 60.Duan L., Wang C., Zhang W., Ma B., Deng Y., Li W., Zhao D. Interfacial assembly and applications of functional mesoporous materials. Chem. Rev. 2021;121(23) doi: 10.1021/acs.chemrev.1c00236. [DOI] [PubMed] [Google Scholar]
- 61.Zhao G., Li J., Ren X., Hu J., Hu W., Wang X. Highly active MnO2 nanosheet synthesis from graphene oxide templates and their application in efficient oxidative degradation of methylene blue. RSC Adv. 2013;3(31) doi: 10.1039/C3RA40942B. [DOI] [Google Scholar]
- 62.Zhou J., Gao X., Liu R., Xie Z., Yang J., Zhang S., Zhang G., Liu H., Li Y., Zhang J. Synthesis of graphdiyne nanowalls using acetylenic coupling reaction. JACS. 2015;137(24):7596. doi: 10.1021/jacs.5b04057. [DOI] [PubMed] [Google Scholar]
- 63.Choi I.Y., Lee J., Ahn H., Lee J., Choi H.C., Park M.J. High‐conductivity two‐dimensional polyaniline nanosheets developed on ice surfaces. Angew. Chem. Int. Ed. 2015;54(36) doi: 10.1002/anie.201503332. [DOI] [PubMed] [Google Scholar]
- 64.Patil K.R., Hwang Y.K., Kim M.-J., Chang J.-S., Park S.-E. Preparation of thin films comprising palladium nanoparticles by a solid–liquid interface reaction technique. J. Colloid Interface Sci. 2004;276(2):333. doi: 10.1016/j.jcis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Wang X., Liu Q., Wu S., Xu B., Xu H. Multilayer polypyrrole nanosheets with self‐organized surface structures for flexible and efficient solar–thermal energy conversion. Adv. Mater. 2019;31(19) doi: 10.1002/adma.201807716. [DOI] [PubMed] [Google Scholar]
- 66.Guo X., Bai N., Tian Y., Gai L. Free-standing reduced graphene oxide/polypyrrole films with enhanced electrochemical performance for flexible supercapacitors. J. Power Sources. 2018;408:51. [Google Scholar]
- 67.Barpuzary D., Kim K., Park M.J. Two-dimensional growth of large-area conjugated polymers on ice surfaces: high conductivity and photoelectrochemical applications. ACS Nano. 2019;13(4):3953. doi: 10.1021/acsnano.8b07294. [DOI] [PubMed] [Google Scholar]
- 68.Dreyer, S D.R., Park C.W., R Bielawski, Ruoff R.S. “The chemistry of graphene oxide. Chem. Soc. Rev. 2010;39(1):228. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 69.Matsuoka R., Sakamoto R., Hoshiko K., Sasaki S., Masunaga H., Nagashio K., Nishihara H. Crystalline graphdiyne nanosheets produced at a gas/liquid or liquid/liquid interface. JACS. 2017;139(8):3145. doi: 10.1021/jacs.6b12776. [DOI] [PubMed] [Google Scholar]
- 70.Kambe T., Sakamoto R., Hoshiko K., Takada K., Miyachi M., Ryu J.-H., Sasaki S., Kim J., Nakazato K., Takata M., Nishihara H. π-Conjugated nickel bis(dithiolene) complex nanosheet. JACS. 2013;135(7):2462. doi: 10.1021/ja312380b. [DOI] [PubMed] [Google Scholar]
- 71.Sun X., Wu K.-H., Sakamoto R., Kusamoto T., Maeda H., Ni X., Jiang W., Liu F., Sasaki S., Masunaga H. Bis (aminothiolato) nickel nanosheet as a redox switch for conductivity and an electrocatalyst for the hydrogen evolution reaction. Chem. Sci. 2017;8(12):8078. doi: 10.1039/c7sc02688a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan H., Wang J., Li X., You H., Li X., Pei C., Huang X., Li H. Direct CVD growth of MoS2 on chemically and thermally reduced graphene oxide nanosheets for improved photoresponse. Apl. Mater. 2021;9(5) doi: 10.1063/5.0047015. [DOI] [Google Scholar]
- 73.Soni A., Kushavah D., Lu L.-S., Chang W.-H., Pal S.K. Ultrafast exciton trapping and exciton–exciton annihilation in large-area CVD-grown monolayer WS2. J. Phys. Chem. C. 2021;125(43) doi: 10.1021/acs.jpcc.1c06267. [DOI] [Google Scholar]
- 74.Chondath S.K., Menamparambath M.M. Interface-assisted synthesis: a gateway to effective nanostructure tuning of conducting polymers. Nanoscale Adv. 2021;3(4):918. doi: 10.1039/d0na00940g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shang N.G., Au F.C.K., Meng X.M., Lee C.S., Bello I., Lee S.T. Uniform carbon nanoflake films and their field emissions. Chem. Phys. Lett. 2002;358(3):187. doi: 10.1016/S0009-2614(02)00430-X. [DOI] [Google Scholar]
- 76.Bao H., Zhang Z., Hua Q., Huang W. Compositions, structures, and catalytic activities of CeO2@Cu2O nanocomposites prepared by the template-assisted method. Langmuir. 2014;30(22):6427. doi: 10.1021/la501406w. [DOI] [PubMed] [Google Scholar]
- 77.Qu J., Cao C.-Y., Dou Z.-F., Liu H., Yu Y., Li P., Song W.-G. Synthesis of cyclic carbonates: catalysis by an iron-based composite and the role of hydrogen bonding at the solid/liquid interface. ChemSusChem. 2012;5(4):652. doi: 10.1002/cssc.201100839. [DOI] [PubMed] [Google Scholar]
- 78.Chen G., Xu C., Song X., Zhao W., Ding Y., Sun S. Interface reaction route to two different kinds of CeO2 nanotubes. Inorg. Chem. 2008;47(2):723. doi: 10.1021/ic701867f. [DOI] [PubMed] [Google Scholar]
- 79.Lee K.-T., Chen Y.-A., Lu S.-Y. Solid-liquid interface based biphasic reaction for nanomaterial preparation: bundled CuO nanorods as an example and their outstanding photocatalytic efficiencies. ChemistrySelect. 2017;2(11):3276. doi: 10.1002/slct.201700568. [DOI] [Google Scholar]
- 80.Zheng M.-b., Cao J., Liao S.-t., Liu J.-s., Chen H.-q., Zhao Y., Dai W.-j., Ji G.-b., Cao J.-m., Tao J. Preparation of mesoporous Co3O4 nanoparticles via Solid−Liquid route and effects of calcination temperature and textural parameters on their electrochemical capacitive behaviors. J. Phys. Chem. C. 2009;113(9):3887. doi: 10.1021/jp810230d. [DOI] [Google Scholar]
- 81.Cao C.-Y., Guo W., Cui Z.-M., Song W.-G., Cai W. Microwave-assisted gas/liquid interfacial synthesis of flowerlike NiO hollow nanosphere precursors and their application as supercapacitor electrodes. J. Mater. Chem. 2011;21(9):3204. doi: 10.1039/C0JM03749D. [DOI] [Google Scholar]
- 82.Bai W., Nie F., Zheng J., Sheng Q. Novel silver nanoparticle–manganese oxyhydroxide–graphene oxide nanocomposite prepared by modified silver mirror reaction and its application for electrochemical sensing. ACS Appl. Mater. Interfaces. 2014;6(8):5439. doi: 10.1021/am500641d. [DOI] [PubMed] [Google Scholar]
- 83.Zhu E., Liu Y., Huang J., Zhang A., Peng B., Liu Z., Liu H., Yu J., Li Y.-R., Yang L., Duan X., Huang Y. Bubble-Mediated large-scale hierarchical assembly of ultrathin Pt nanowire network monolayer at gas/liquid interfaces. ACS Nano. 2023;17(14) doi: 10.1021/acsnano.3c04771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu Y., Yuan X., Jia C., Lei B., Zhang H., Zhao Z., Zhu S., Zhao Q., Cai W. Self-Assembled bifunctional copper hydroxide/gold-ordered nanoarray composites for fast, sensitive, and recyclable SERS detection of hazardous benzene vapors. Nanomaterials. 2023;13(13):2016. doi: 10.3390/nano13132016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun N., Yao X., Xu Z., Li J., Yang N., Lyu D., Zhao G., Dai C. Janus nanographene oxide with aerophilic/hydrophilic characteristics for enhancing foam stability in high-temperature reservoirs. J. Mol. Liq. 2023;371 doi: 10.1016/j.molliq.2022.121087. [DOI] [Google Scholar]
- 86.Zhang T., Qi H., Liao Z., Horev Y.D., Panes-Ruiz L.A., Petkov P.S., Zhang Z., Shivhare R., Zhang P., Liu K., Bezugly V., Liu S., Zheng Z., Mannsfeld S., Heine T., Cuniberti G., Haick H., Zschech E., Kaiser U., Dong R., Feng X. Engineering crystalline quasi-two-dimensional polyaniline thin film with enhanced electrical and chemiresistive sensing performances. Nat. Commun. 2019;10(1):4225. doi: 10.1038/s41467-019-11921-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gui Y., Wu J., Tian K., Guo H., Qin X., Qin X., Guo X., Fang C., Liu P. Ultraviolet-induced gas sensing performance of Ag/WO3/rGO nanocomposites for H2S gas sensors. ACS Appl. Electron. Mater. 2023;5(7):3625. doi: 10.1021/acsaelm.3c00349. [DOI] [Google Scholar]
- 88.Zhang C., Mo Z., Guo R., Teng G., Zhao G. Magnetic-field-induced synthesis of Fe3O4 nanorods by a gas–liquid interfacial process: microstructure control, magnetic and photocatalytic properties. Mater. Res. Bull. 2014;53:116. doi: 10.1016/j.materresbull.2014.02.014. [DOI] [Google Scholar]
- 89.Gui Y.-h., Wang H.-y., Tian K., Yang L.-l., Guo H.-s., Zhang H.-z., Fang S.-m., Wang Y. Enhanced gas sensing properties to NO2 of SnO2/rGO nanocomposites synthesized by microwave-assisted gas-liquid interfacial method. Ceram. Int. 2018;44(5):4900. doi: 10.1016/j.ceramint.2017.12.080. [DOI] [Google Scholar]
- 90.Hu J., Zhang L. Achieving F-doped porous hollow carbon nanospheres with ultrahigh pore volume via a gas–solid interface reaction. J. Mater. Chem. A. 2021;9(48) doi: 10.1039/D1TA07749J. [DOI] [Google Scholar]
- 91.Ma Y., Gao W., Shan H., Chen W., Shang W., Tao P., Song C., Addiego C., Deng T., Pan X., Wu J. Platinum-based nanowires as active catalysts toward oxygen reduction reaction: in situ observation of surface-diffusion-assisted, solid-state oriented attachment. Adv. Mater. 2017;29(46) doi: 10.1002/adma.201703460. [DOI] [PubMed] [Google Scholar]
- 92.Lai C., Wu Q., Chen J., Wen L., Ren S. Large-area aligned branched Cu2S nanostructure arrays: room-temperature synthesis and growth mechanism. Nanotechnology. 2010;21(21) doi: 10.1088/0957-4484/21/21/215602. [DOI] [PubMed] [Google Scholar]
- 93.Zheng J., Song X., Zhang Y., Li Y., Li X., Pu Y. Nanosized aluminum nitride hollow spheres formed through a self-templating solid–gas interface reaction. J. Solid State Chem. 2007;180(1):276. doi: 10.1016/j.jssc.2006.10.011. [DOI] [Google Scholar]
- 94.Catalán-Toledo J., Djafari J., Mas-Torrent M., Crivillers N. Redox-Active Au nanoparticles self-assembled at liquid–liquid interface via C–Au functionalization for dye degradation electrocatalysis. ACS Appl. Nano Mater. 2024;7(5):5242. [Google Scholar]
- 95.Zhao M., Lu Q., Ma Q., Zhang H. Two‐dimensional metal–organic framework nanosheets. Small Methods. 2017;1(1–2) [Google Scholar]
- 96.Hua X., Bevan M.A., Frechette J. Reversible partitioning of nanoparticles at an oil–water interface. Langmuir. 2016;32(44) doi: 10.1021/acs.langmuir.6b02255. [DOI] [PubMed] [Google Scholar]
- 97.Biswas S., Drzal L.T. A novel approach to create a highly ordered monolayer film of graphene nanosheets at the Liquid−Liquid interface. Nano Lett. 2009;9(1):167. doi: 10.1021/nl802724f. [DOI] [PubMed] [Google Scholar]
- 98.Davidovits P., Kolb C.E., Williams L.R., Jayne J.T., Worsnop D.R. Mass accommodation and chemical reactions at gas− liquid interfaces. Chem. Rev. 2006;106(4):1323. doi: 10.1021/cr040366k. [DOI] [PubMed] [Google Scholar]
- 99.Ariga K. Liquid interfacial nanoarchitectonics: molecular machines, organic semiconductors, nanocarbons, stem cells, and others. Curr. Opin. Colloid Interface Sci. 2023;63 doi: 10.1016/j.cocis.2022.101656. [DOI] [Google Scholar]
- 100.Moshrefi R., Przybyła H., Stockmann T.J. Simultaneous electro-generation/polymerization of Cu nanocluster embedded conductive poly (2, 2′: 5′, 2′′-terthiophene) films at micro and macro liquid/liquid interfaces. Sci. Rep. 2023;13(1):1201. doi: 10.1038/s41598-023-28391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoseini S.J., Bahrami M., Dehghani M. Formation of snowman-like Pt/Pd thin film and Pt/Pd/reduced-graphene oxide thin film at liquid–liquid interface by use of organometallic complexes, suitable for methanol fuel cells. RSC Adv. 2014;4(27) doi: 10.1039/C4RA01625D. [DOI] [Google Scholar]
- 102.Yao K.-S., Zhao H.-L., Wang N., Li T.-J., Zhao S., Lu W.-W. Facile synthesis of ultra-large, single-crystal Ag nanosheet-assembled films at chloroform-water interface. J. Solid State Chem. 2019;278 doi: 10.1016/j.jssc.2019.120912. [DOI] [Google Scholar]
- 103.Higgins E.P.C., McAdams S.G., Hopkinson D.G., Byrne C., Walton A.S., Lewis D.J., Dryfe R.A.W. Room-temperature production of nanocrystalline molybdenum disulfide (MoS2) at the Liquid−Liquid interface. Chem. Mater. 2019;31(15):5384. doi: 10.1021/acs.chemmater.8b05232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puthiyottil N., Kanakkayil S., Pillai N.P., Rajan A., Parambath S.K., Krishnamurthy R.G., Chatanathodi R., Menamparambath M.M. In situ engineering of Au–Ag alloy embedded PEDOT nanohybrids at a solvent/non-solvent interface for the electrochemical enzyme-free detection of histamine. J. Mater. Chem. B. 2023;11(5):1144. doi: 10.1039/d2tb02637f. [DOI] [PubMed] [Google Scholar]
- 105.Liu Q., Guo Z., Wang C., Guo S., Xu Z., Hu C., Liu Y., Wang Y., He J., Wong W.Y. A cobalt‐based metal‐organic framework nanosheet as the electrode for high‐performance asymmetric supercapacitor. Adv. Sci. 2023 doi: 10.1002/advs.202207545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bowden N., Terfort A., Carbeck J., Whitesides G.M. Self-assembly of mesoscale objects into ordered two-dimensional arrays. Science. 1997;276(5310):233. doi: 10.1126/science.276.5310.233. [DOI] [PubMed] [Google Scholar]
- 107.He Y., Kim K.-J., Chang C.-h. Segmented microfluidic flow reactors for nanomaterial synthesis. Nanomaterials. 2020;10(7):1421. doi: 10.3390/nano10071421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neyt N.C., Riley D.L. Application of reactor engineering concepts in continuous flow chemistry: a review. React. Chem. Eng. 2021;6(8):1295. [Google Scholar]
- 109.Pinto C.S., Souza V.H.R., Schmidt A., Zarbin A.J.G. PSS-free PEDOT and PEDOT/graphene transparent films: synthesis, characterization and electrochromism. Synth. Met. 2023;293 [Google Scholar]
- 110.Chondath S.K., Sreekala A.P.K., Farzeena C., Varanakkottu S.N., Menamparambath M.M. Interfacial tension driven adsorption of MnO 2 nanoparticles at the liquid/liquid interface to tailor ultra-thin polypyrrole sheets. Nanoscale. 2022;14(31) doi: 10.1039/d2nr02130g. [DOI] [PubMed] [Google Scholar]
- 111.Wang F., Seo J.-H., Luo G., Starr M.B., Li Z., Geng D., Yin X., Wang S., Fraser D.G., Morgan D. Nanometre-thick single-crystalline nanosheets grown at the water–air interface. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poolakkandy R.R., Ar N., Padmalayam K.A., Gk R., Menamparambath M.M. Nickel hydroxide nanoflake/carbon nanotube composites for the electrochemical detection of glutamic acid using in vitro stroke model. ACS Appl. Nano Mater. 2023;6(2):1347. [Google Scholar]
- 113.Zhu J., Qian X. From 2-D CuO nanosheets to 3-D hollow nanospheres: interface-assisted synthesis, surface photovoltage properties and photocatalytic activity. J. Solid State Chem. 2010;183(7):1632. [Google Scholar]
- 114.Cheng X.-L., Jiang J.-S., Hu M., Mao G.-Y., Liu Z.-W., Zeng Y., Zhang Q.-H. Liquid–liquid interface-assisted solvothermal synthesis of durian-like α-Fe2O3 hollow spheres constructed by nano-polyhedrons. CrystEngComm. 2012;14(9):3056. [Google Scholar]
- 115.Poolakkandy R.R., Neelakandan A.R., Puthiyaparambath M.F., Krishnamurthy R.G., Chatanathodi R., Menamparambath M.M. Nickel cobaltite/multi-walled carbon nanotube flexible sensor for the electrochemical detection of dopamine released by human neural cells. J. Mater. Chem. C. 2022;10(8):3048. [Google Scholar]
- 116.Chen Z., Wang C., Chen M., Ye C., Lin Z., Xing L., Liao Y., Xu M., Cao G., Li W. Highly effective fabrication of two dimensional metal oxides as high performance lithium storage anodes. J. Mater. Chem. A. 2019;7(8):3924. [Google Scholar]
- 117.Chondath S.K., Poolakkandy R.R., Kottayintavida R., Thekkangil A., Gopalan N.K., Vasu S.T., Athiyanathil S., Menamparambath M.M. Water–chloroform interface assisted microstructure tuning of polypyrrole–silver sheets. ACS Appl. Mater. Interfaces. 2019;11(1):1723. doi: 10.1021/acsami.8b18943. [DOI] [PubMed] [Google Scholar]
- 118.Cheng X.-L., Jiang J.-S., Jin C.-Y., Lin C.-C., Zeng Y., Zhang Q.-H. Cauliflower-like α-Fe2O3 microstructures: toluene–water interface-assisted synthesis, characterization, and applications in wastewater treatment and visible-light photocatalysis. Chem. Eng. J. 2014;236:139. [Google Scholar]
- 119.Liu Y., Chen Z., Shek C.-H., Wu C.M.L., Lai J.K.L. Hierarchical mesoporous MnO2 superstructures synthesized by soft-interface method and their catalytic performances. ACS Appl. Mater. Interfaces. 2014;6(12):9776. doi: 10.1021/am502191k. [DOI] [PubMed] [Google Scholar]
- 120.Clarke T.B., Colón G.S., Dick J.E. Tunable gold nanoring arrays by electrodeposition. Adv. Mater. Technol. 2023;8(13) doi: 10.1002/admt.202201946. [DOI] [Google Scholar]
- 121.Inagaki C.S., Oliveira M.M., Zarbin A.J.G. Direct and one-step synthesis of polythiophene/gold nanoparticles thin films through liquid/liquid interfacial polymerization. J. Colloid Interface Sci. 2018;516:498. doi: 10.1016/j.jcis.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 122.Hao Q., Wang H., Yang X., Lu L., Wang X. Morphology-controlled fabrication of sulfonated graphene/polyaniline nanocomposites by liquid/liquid interfacial polymerization and investigation of their electrochemical properties. Nano Res. 2011;4(4):323. doi: 10.1007/s12274-010-0087-4. [DOI] [Google Scholar]
- 123.Kelgenbaeva Z., Omurzak E., Takebe S., Sulaimankulova S., Abdullaeva Z., Iwamoto C., Mashimo T. Synthesis of pure iron nanoparticles at liquid–liquid interface using pulsed plasma. J Nanopart Res. 2014;16(9):2603. doi: 10.1007/s11051-014-2603-z. [DOI] [Google Scholar]
- 124.Zhang Y., Nishi N., Sakka T. Interface-templated synthesis of single-crystalline silver chain-like nanobelts at the liquid-liquid interface between water and redox-active ionic liquid. Colloids Surf. A Physicochem. Eng. Asp. 2020;597 doi: 10.1016/j.colsurfa.2020.124747. [DOI] [Google Scholar]
- 125.Zhang Y., Nishi N., Sakka T. Template-free and spontaneous formation of vertically aligned Pd nanofiber arrays at the liquid–liquid interface between redox-active ionic liquid and water. ACS Appl. Mater. Interfaces. 2019;11(26) doi: 10.1021/acsami.9b05255. [DOI] [PubMed] [Google Scholar]
- 126.Sakamoto R., Yagi T., Hoshiko K., Kusaka S., Matsuoka R., Maeda H., Liu Z., Liu Q., Wong W.Y., Nishihara H. Photofunctionality in porphyrin‐hybridized bis (dipyrrinato) zinc (II) complex micro‐and nanosheets. Angew. Chem. 2017;129(13):3580. doi: 10.1002/anie.201611785. [DOI] [PubMed] [Google Scholar]
- 127.Prabhu R.B., Singh K.K., Alex C., John N.S. A general route to free-standing films of nanocrystalline molybdenum chalcogenides at a liquid/liquid interface under hydrothermal conditions. Appl. Surf. Sci. 2020;511 doi: 10.1016/j.apsusc.2020.145579. [DOI] [Google Scholar]
- 128.Inagaki C.S., Oliveira M.M., Bergamini M.F., Marcolino-Junior L.H., Zarbin A.J.G. Facile synthesis and dopamine sensing application of three component nanocomposite thin films based on polythiophene, gold nanoparticles and carbon nanotubes. J. Electroanal. Chem. 2019;840:208. doi: 10.1016/j.jelechem.2019.03.066. [DOI] [Google Scholar]
- 129.Johans C., Clohessy J., Fantini S., Kontturi K., Cunnane V.J. Electrosynthesis of polyphenylpyrrole coated silver particles at a liquid–liquid interface. Electrochem. Commun. 2002;4(3):227. doi: 10.1016/S1388-2481(02)00256-4. [DOI] [Google Scholar]
- 130.Lee W.P., Chen H., Dryfe R., Ding Y. Kinetics of nanoparticle synthesis by liquid–liquid interfacial reaction. Colloids Surf. A Physicochem. Eng. Asp. 2009;343(1):3. doi: 10.1016/j.colsurfa.2009.01.040. [DOI] [Google Scholar]
- 131.Rubio‐Giménez V., Galbiati M., Castells‐Gil J., Almora‐Barrios N., Navarro‐Sánchez J., Escorcia‐Ariza G., Mattera M., Arnold T., Rawle J., Tatay S. Bottom‐up fabrication of semiconductive metal–organic framework ultrathin films. Adv. Mater. 2018;30(10) doi: 10.1002/adma.201704291. [DOI] [PubMed] [Google Scholar]
- 132.Yang J., Kong L., Huang C., Wang C., Wei S., Zhou L. Liquid-Liquid interfacial approach for rapid synthesis of Well-Crystalline Two-Dimensional Metal-Organic frameworks for nitro reduction. Chem. Eng. J. 2024;485 doi: 10.1016/j.cej.2024.149969. [DOI] [Google Scholar]
- 133.Cabral H., Miyata K., Osada K., Kataoka K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018;118(14):6844. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 134.Fernando I., Zhou Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere. 2019;216:297. doi: 10.1016/j.chemosphere.2018.10.122. [DOI] [PubMed] [Google Scholar]
- 135.Fu Y., Zhao S., Chen W., Zhang Q., Chai Y. Self-assembly of nanoparticles with stimulated responses at liquid interfaces. Nano Today. 2024;54 doi: 10.1016/j.nantod.2023.102073. [DOI] [Google Scholar]