Abstract
Acute myeloid leukemia (AML), a highly heterogeneous myeloid malignancy, remains a challenge in terms of proper risk stratification. In this study, we developed a novel pyroptosis prognostic model based on pyroptosis-related gene pairs, which exhibited excellent prognostic performance across multiple cohorts (N = 1506) and accurately predicted both adult and pediatric AML prognosis. Additionally, we integrated the pyroptosis risk model with other clinical risk factors to construct a highly operational nomogram. Moreover, our findings indicate a significant correlation between elevated pyroptosis risk scores and increased stemness of AML. Using CIBERSORT immune analysis, we found a decreased proportion of resting NK cells and activated mast cells in the high-risk group. Through analyzing the correlation between chemotherapy drug response sensitivity and risk scores, we found that AZD1332 and BPD-0008900 were extremely sensitive in the high-risk group.
Keywords: Pyroptosis, Gene pairs, Prognostic model, Acute myeloid leukemia, Bioinformatics
1. Introduction
AML is a malignant hematological neoplasm characterized by clonal expansion and blockage of myeloid precursors differentiation. It has a poor prognosis with a five-year survival rate of only 24 % [1]. Despite significant improvements in the prognosis of pediatric patients with AML over the past 40 years, the disease remains heavily reliant on comprehensive diagnosis, intensive treatment, and effective care, resulting in wide variations in survival rates across different regions [2]. Therefore, deciphering the general prognostic biomarkers and relevant genetic molecular levels has become urgent for treatment guidance.
Pyroptosis is a programmed cell death process mediated by gasdermin proteins, which are involved in inflammation. Under the influence of various inducing factors, the cleavage of gasdermin proteins by CASP proteins triggers cell membrane perforation, leading to the release of inflammatory mediators inside pyroptotic cells following a series of inflammatory reactions [3]. Numerous studies have shown that pyroptosis is strongly associated with the occurrence, proliferation, invasion, recurrence, and response to chemotherapy in various cancers. In melanoma, GSDME inhibits tumor growth, and NLRP3 can prevent melanoma metastasis [4,5]. In breast cancer, high expression of GSDMB indicates tumor progression and poor response to HER-2 targeted therapy [6], whereas downregulation of GSDME indicates lower survival rates [7]. The inflammasome and IL-1β are strongly associated with tumor recurrence, proliferation, angiogenesis, and invasion [8]. The downregulation of GSDMC in colon cancer suggests a decrease in the number of cancer cells, while its overexpression leads to cell proliferation and tumor formation [9]. In gastric cancer, GSDMA and GSDMB act as tumor suppressor and oncogenes, respectively [10,11]. High expression of GSDME following chemotherapy indicates pyroptosis mode [12], and the NLRP3 pathway plays an important role in the development of gastric cancer [13]. DFNA5 upregulation in liver cancer inhibits cancer cell proliferation [14]. In lung cancer, high expression of GSDMD indicates tumor metastasis and poor prognosis. Silencing GSDMD weakens the EGFR/AKT pathway, thereby inhibiting the proliferation of non-small cell lung cancer cells [15]. The inflammasome is also closely related to lung cancer subtypes, tumor stages, infiltration, and chemotherapy sensitivity [16]. In cervical cancer, there is positive feedback between pyroptosis and immune cells [17]. In leukemia, studies on pyroptosis mainly focus on the treatment of AML. Tp92 [18], DPP8/9 inhibitors [19], and pyridoxine [20] have been shown to induce pyroptosis in leukemia cell lines and mouse models. In addition, CAR-T cell therapy for B-cell leukemia induces pyroptosis, leading to cytokine release syndrome [21]. Reducing pyroptosis may reduce the occurrence of cytokine release syndrome in CAR-T cell therapy.
Although several studies have attempted to construct prognostic models for AML based on pyroptosis-associated genes, the reproducibility of these models has been limited by the inability to precisely quantify gene expression [[22], [23], [24], [25], [26], [27]]. To address this challenge, Kong et al. developed an immunity and pyroptosis-related prognostic (IPRP) signature using pairwise gene comparisons, which improved the generalization performance of the model across multiple platforms [28]. In this study, we used the same approach to construct a pyroptosis model that demonstrated robust cross-platform generalization and excellent performance in predicting the prognosis of both pediatric and adult patients with AML. In addition, we compared immune infiltration and sensitivity to chemotherapy between AML subgroups based on the prognostic model. By analyzing the differences in these factors, we aimed to gain a more comprehensive understanding of the underlying biological mechanisms that contribute to AML prognosis.
2. Method
2.1. Data collection and preprocessing
We obtained gene expression, clinical, and survival information from five cohorts (GSE37642, GSE12417, the Cancer Genome Atlas (TCGA) [29], Beat AML [30], and Therapeutically Applicable Research to Generate Effective Treatments (TARGET)) comprising 1506 samples. The GSE37642 and GSE12417 datasets were obtained from the Gene Expression Omnibus (GEO) public database. The specific cohort information can be found in Supplementary Table 1. Microarray gene expression profiling was rigorously performed on a cohort of 138 leukemia stem cells (LSCs)-positive and 89 LSC-negative cell fractions, as cataloged in the dataset GSE76008. Additionally, we chose 40 pyroptosis-related genes from the Gene Set Enrichment Analysis (GSEA) website (https://www.gsea-msigdb.org/gsea/msigdb/index.jsp) [31]. Transcriptome values were made log2 transform in all cohorts.
2.2. Prognostic model construction and evaluation
In the GSE37642 dataset, a random sample of 2/3 (n = 368) was selected as the training set, and the remaining 1/3 (n = 185) was used as the internal validation set. Four additional arrays, GSE12417, TCGA, Beat AML, and TARGET, were used for external validation. First, KM survival analysis was performed on 40 pyroptosis-related genes in the GSE37642 array, and 12 pyroptosis-related genes with FDR less than 0.05 were selected to generate gene pairs. The intersection of available genes in the 5 arrays resulted in 11,431 genes that were used to generate (12 × 11,431) gene pairs. Binary values were generated for each gene pair, such as CASP3|TP53 = 1 indicating that CASP3 expression was higher than TP53 expression in a particular sample, and CASP3|TP53 = 0 indicating that CASP3 expression was lower than or equal to TP53 expression. Gene pairs with frequencies greater than 0.8 or less than 0.2 between samples were removed, and Cox univariate analysis was performed on the remaining gene pairs (with FDR<0.05) resulting in 1636 prognosis-related gene pairs. Lasso Cox regression analysis was then performed on the 1636 gene pairs, and the best penalty coefficient was used to identify the 87 gene pairs that contributed highly to prognosis. Finally, a prognostic model was constructed through iterative multivariate Cox regression analysis (with p < 0.01 and using the bidirectional stepwise method to remove variables with collinearity), resulting in 16 pyroptosis-related gene pairs in the signature. The risk scores were calculated using the following formula:
RiskScore = CASP3|ATF6*-0.5+CASP3|CD96*0.47 + BAK1|FKBP5*-0.52 + BAK1|PRF1*0.72 + IL18|MCM10*0.42+CASP5|CGREF1*-0.57+CASP5|SPP1*-0.44+CYCS|DDIT4*-0.48+CASP4|SOCS2*-0.63+NLRP1|RSU1*-0.88+NLRP1|VNN1*-0.52+CHMP4A|EIF2S3*0.67+CHMP4A|REC8*-0.85+CHMP6|ARHGEF3*-0.59+CHMP6|DHCR24*-0.59+CHMP6|WDR3*-0.54.
The samples were divided into high-risk and low-risk groups based on the median risk score. Time ROC was used to evaluate the prognostic performance of the risk model using ‘timeROC’ package. Fig. 1A–B illustrates the flow of this analysis.
Fig. 1.
Flowchart of our study. (A) The overall flowchart. (B) The flowchart of constructing pyroptosis-related gene-pairs signature.
2.3. Independence testing and nomogram construction
To validate the independent predictive ability of the pyroptosis-related gene-pairs prognostic model, we conducted multiple factor Cox regression analysis in the Beat AML cohort to verify its condition as an independent prognostic factor. The reason for choosing the Beat AML cohort was based on comprehensive clinical information. The nomogram plot is highly clinically applicable, and we constructed nomograms for the pyroptosis-related prognostic model and several other independent risk factors using the “rms” package. We then used time ROC curves to validate the prognostic prediction accuracy of the combined model.
2.4. Immune microenvironment analysis
We performed immune microenvironment analysis using CIBERSORT [32] to investigate the proportions of various immune cells in AML samples. We also analyzed the differences in infiltrating immune cells between the high-risk and low-risk groups. The immune and stromal scores, along with the comprehensive ESTIMATE score, were derived utilizing the 'estimate' package [33].
2.5. Chemotherapy drug sensitivities analysis
We utilized the “oncopredict” package to perform ridge regression-based estimation of the IC50 values for multiple chemotherapy drugs in each AML sample [34]. Our training data consisted of the drug sensitivity and transcriptome information of cell lines from the Genomics of Drug Sensitivity in Cancer (GDSC) database [35]. We further explored the relation between drug sensitivity and risk scores of AML samples through correlation analysis, which helped to identify chemotherapy drugs having significant associations with AML risk scores.
2.6. Statistical analysis
All analyses in this study were performed using R language (version 4.0.5). Kruskal-Wallis or rank sum tests were used for group comparisons. The “survival” and “survminer” packages were utilized for generating Kaplan-Meier survival plots, while the log-rank test was employed to compare the survival curves of two or more groups. The LASSO regression analysis was conducted using the ‘glmnet’ package [36]. Pearson's correlation analysis was used to assess correlations between variables. To estimate the population Area Under the Curve (AUC), we applied the bootstrap resampling method, performing 10,000 iterations with replacements, each comprising several samples commensurate with the original dataset size. This approach ensured a statistically robust approximation of the AUC value. The concordance index (C-index) served as a comparative metric for assessing the prognostic prediction performance across diverse prognostic models. The “meta” package was utilized for the amalgamation of C-indices from multiple datasets, employing a random-effects model to synthesize these metrics. Data visualization was achieved using the “ggplot2” package. The FDR method was used to correct for multiple comparisons, and statistical significance was defined as p < 0.05.
3. Results
3.1. Prognostic model construction and evaluation
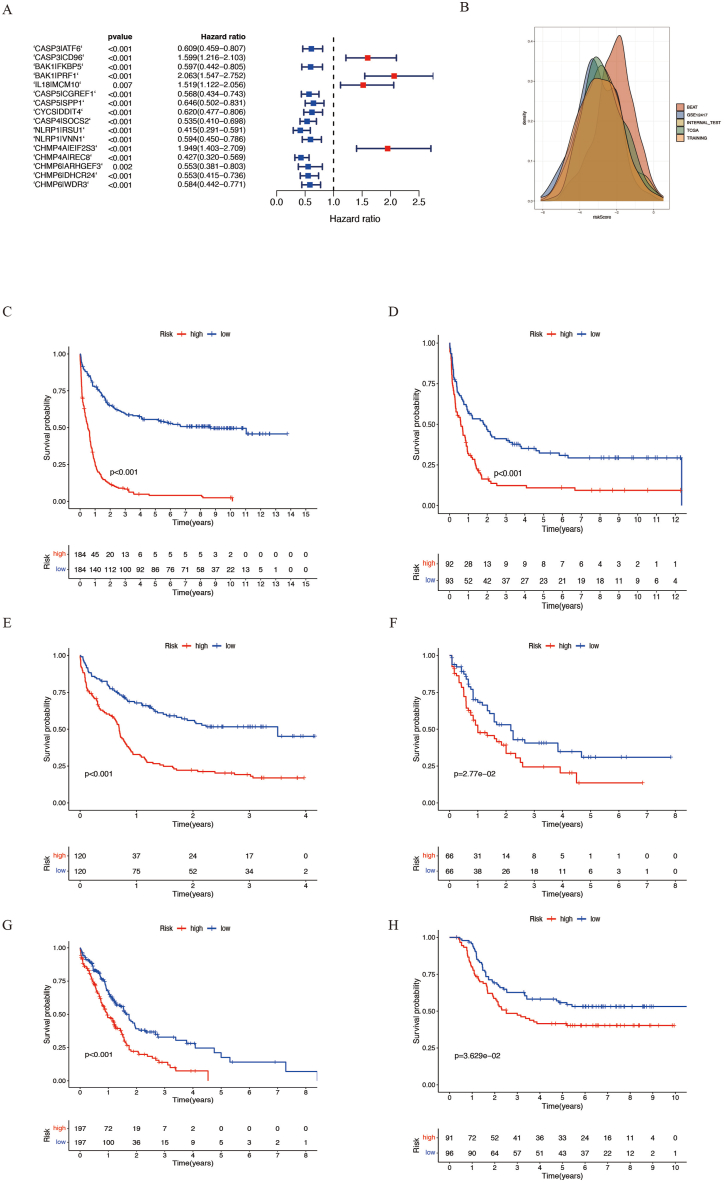
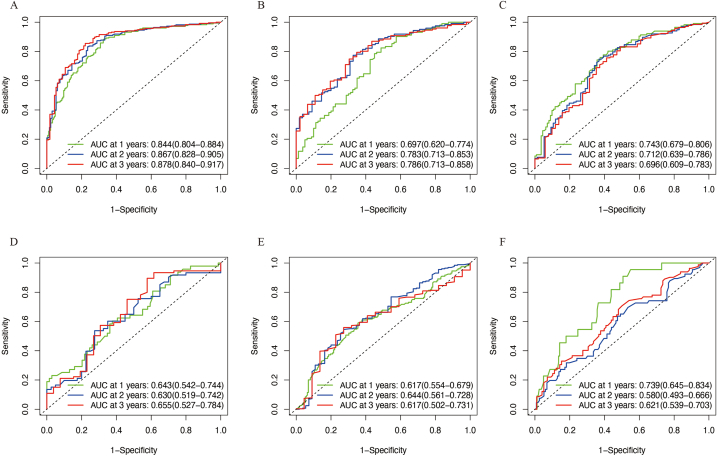
To construct a gene-pairing prognosis model, we first performed survival analysis on 40 pyroptosis-related genes in the GSE37642 array. Based on a threshold FDR of <0.05, we identified 12 pyroptosis-related genes associated with prognosis, including IL18, ELANE, CASP5, NLRP1, BAX, GSDMB, CHMP4A, CHMP6, CASP3, BAK1, CYCS, and CASP4 (Supplementary Table 2). Next, we searched for gene pairs for each of these 12 genes, as described in the Methods section and Supplementary Table 3 and Fig. 1B. After LASSO screening and multiple-factor Cox regression analysis, we constructed a pyroptosis-related gene-pairs signature consisting of 16 gene pairs, including CASP3|ATF6, CASP3|CD96, BAK1|FKBP5, BAK1|PRF1, IL18|MCM10, CASP5|CGREF1, CASP5|SPP1, CYCS|DDIT4, CASP4|SOCS2, NLRP1|RSU1, NLRP1|VNN1, CHMP4A|EIF2S3, CHMP4A|REC8, CHMP6|ARHGEF3, CHMP6|DHCR24, and CHMP6|WDR3 (Fig. 2A and Supplementary Fig. 1). Using the risk score calculation formula (see Methods and Supplementary Tables 4 and 5), we calculated the risk scores for each sample and divided them into high- and low-risk groups using the median value. In the GSE37642 cohort, we randomly selected two-third of the samples as the training group and the remaining one-third as the internal validation group. Four other cohorts, TCGA, Beat AML, GSE12417, and TARGET were used as external validation groups. We visualized the risk value distribution in all groups using density plots and found that the risk values were consistent across all cohorts (Fig. 2B). By comparing the survival rates between the high- and low-risk groups, we found that the prognosis of the high-risk group was significantly worse than that of the low-risk group in all cohorts (Fig. 2C–H). In addition, we evaluated the prognostic predictive performance of the risk score using time-dependent ROC curves (Fig. 3A–F). The findings demonstrated excellent predictive performance for 1-, 2-, and 3-year survival rates across all cohorts, indicating a high degree of generalizability of the model.
Fig. 2.
Construction of pyroptosis-related gene-pairs signature. (A) Forest plot showing the hazard ratios of 16 pyroptosis-related gene pairs. (B) Distribution of risk scores in different cohorts. (C–H) Kaplan-Meier curves showing the overall survival differences between high and low-risk groups in different cohorts including GSE37642 training set (C), GSE37642 internal validation set (D), GSE12417 (E), TCGA (F), Beat AML (G), and TARGET (H).
Fig. 3.
Evaluation of pyroptosis-related gene-pairs signature. Time ROC curves were used to assess the predictive performance of the model for 1-, 2-, and 3-year overall survival in AML patients. The cohorts represented in (A–F) are GSE37642 training set, GSE37642 internal validation set, GSE12417, TCGA, Beat AML, and TARGET datasets, respectively.
Through differential analysis, we examined the distribution of risk scores among different age, sex, racial, and cytogenetic risk groups in TCGA and Beat AML cohorts (Fig. 4A–B). Our results showed that in the Beat AML array, the risk scores of AML patients over 60 years old were significantly higher than those of patients under 60 years old, whereas no significant difference was observed in TCGA. There were no significant differences in the risk scores between female and male groups or between the white and non-white racial groups. However, in different cytogenetic risk groups (favorable vs intermediate vs poor), the risk scores increased in the poor group compared with those in the intermediate group, and in the intermediate group compared with those in the favorable group, and this finding was consistent across both cohorts. Furthermore, we sought to explore the potential association between pyroptosis risk scores and the stemness of AML. Upon comparing the distribution of pyroptosis risk scores between the LSC-positive and LSC-negative groups, we observed a significant increase in risk scores within the LSC-positive cohort. Additionally, we assessed the discriminative ability of the pyroptosis risk scores to identify LSC-positivity, achieving an average AUC value of 0.689 across 10,000 bootstrap iterations, indicating a robust capacity to differentiate LSC-positive samples. Moreover, utilizing the LSC17 model, we calculated the leukemia stemness risk scores for AML samples within the BEAT AML and TCGA cohorts, noting a significant upregulation of LSC17 risk scores in the high-risk group (Fig. 4C). These findings underscore the close link between pyroptosis and the stemness properties of AML leukemic stem cells.
Fig. 4.
Distribution of risk scores in different clinical subgroups. Subgroups included different age groups (<60 vs. ≥60 years old), different gender groups (female vs. male), different race groups (Caucasian vs. non-Caucasian), and different cytogenetic risk groups (favorable vs. intermediate vs. poor). Cohorts represented in (A) and (B) are Beat AML and TCGA datasets, respectively. (C) The violin plot on the far left delineates the distribution of pyroptosis risk scores within the LSC-positive and LSC-negative cohorts. The adjacent plot details the distribution of AUC values for distinguishing the LSC-positive group, obtained through 10,000 bootstrap replications, with a mean value of 0.689. The third and fourth plots from the left show the stratification of LSC17 risk scores between high and low pyroptosis risk categories within the TCGA and BEAT AML datasets, respectively.
3.2. Prognostic independence test and nomogram construction
To validate that whether pyroptosis-related gene-pairs signature is an independent predictor of prognosis, we conducted univariate Cox regression analysis on several clinical variables and model risk groups in the Beat AML cohort. Then, we performed multivariate Cox regression analysis on variables that were significantly associated with prognosis (Fig. 5A–B). Our findings revealed that previous non-myeloid neoplasms, age, previous cumulative chemotherapy, previous MDS/MPN, and the risk score of pyroptosis-related prognostic model were all independent prognostic factors.
Fig. 5.
Construction of Nomogram. The forest plots in (A) and (B) represent the results of univariate Cox regression and multivariate Cox regression analysis, respectively. The purpose was to demonstrate that the pyroptosis model is an independent prognostic factor. (C) Nomogram combining the pyroptosis risk model and four other independent risk factors. (D) The upper left segment presents the nomogram's calibration curve, while the remaining sections display Time-Dependent ROC curves that illustrate the predictive accuracy of the composite model for 1-year, 2-year, and 3-year overall survival among patients with AML.
To enhance the utility of the model in clinical practice, we integrated five independent prognostic indicators to develop a comprehensive nomogram (Fig. 5C). This nomogram facilitates the prediction of survival probabilities for patients with AML by correlating their cumulative scores with potential outcomes. According to the calibration curve depicted in Fig. 5D, there was a substantial concordance between the survival probabilities projected by the nomogram and the observed survival rates, underscoring the nomogram's robust predictive capability. Through time-dependent ROC curves, we found that the combined model had higher accuracy in predicting 1-, 2-, and 3-year survival rates than the individual prognostic factors (Fig. 5D).
3.3. Immunological microenvironment analysis
Pyroptosis has been found to be strongly associated with the immune microenvironment. In our study, we employed the CIBERSORT method to analyze the proportions of 22 immune cell types in AML samples across five cohorts and compared the differences in immune cell composition between the high- and low-risk groups (Supplementary Fig. 2). To verify the reliability of the data, we selected immune cells that exhibited significant differences in at least three cohorts, including NK resting cells and activated mast cells. Our analysis revealed a significant decrease in the proportion of NK resting cells in the high-risk group across three cohorts (GSE37642, GSE12417, and Beat AML) (Fig. 6A). Furthermore, the proportion of activated mast cells significantly decreased in the high-risk group across the TCGA, Beat AML, and TARGET cohorts (Fig. 6B). We also investigated the distribution differences in immune score, stromal score, and ESTIMATE score between the high-risk and low-risk groups. Notably, in the TCGA, BEAT AML, and GSE71014 cohorts, the low-risk groups exhibited a significant elevation in ESTIMATE scores. Furthermore, in the BEAT AML and GSE71014 datasets, the immune scores were significantly higher in the low-risk group, whereas in the TCGA and GSE71014 datasets, the low-risk group demonstrated higher stromal scores. These findings suggest that the low-risk groups may be more inclined towards an immunologically "hot" tumor phenotype, characterized by a more active immune response (Supplementary Fig. 3).
Fig. 6.
Comparison of the infiltration of resting NK cells and activated mast cells between high and low-risk groups. The five boxplots represent the results of different cohorts, from left to right: GSE37642, GSE12417, TCGA, Beat AML, and TARGET datasets. (A) and (B) represent the results of resting NK cells and activated mast cells, respectively.
3.4. Chemotherapy response sensitivities analysis
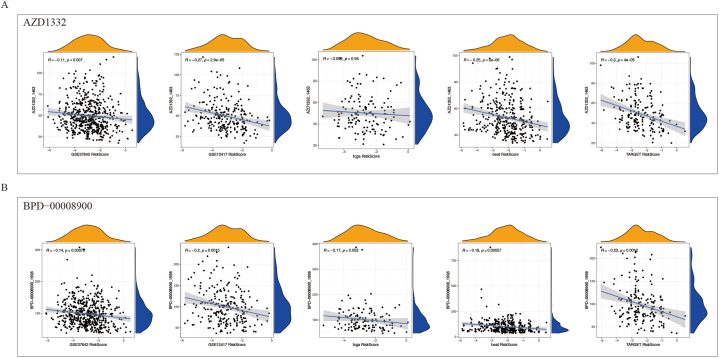
Chemotherapy remains to serve as the primary treatment option for AML. We determined the sensitivity of AML samples to chemotherapy drugs based on their gene expression levels and identified drugs that were significantly correlated with the risk score through correlation analysis. To validate the robustness of our findings, we selected drugs that were significantly correlated across at least four cohorts and ultimately identified two drugs, namely AZD1332 and BPD-0008900. Except for the TCGA array, both drugs exhibited significant negative correlation with the risk score in the other cohorts (Fig. 7A–B). Therefore, these two drugs may offer more effective therapeutic strategies for high-risk AML patients.
Fig. 7.
Correlation between sensitivity to two chemotherapy drugs (AZD1332 and BPD-0008900) and risk scores in AML samples. The five columns represent the results of different cohorts, from left to right: GSE37642, GSE12417, TCGA, Beat AML, and TARGET datasets. (A) and (B) represent the results of AZD1332 and BPD-0008900, respectively. The x-axis represents the risk score based on the pyroptosis model, and the y-axis represents the estimated IC50 value of the drugs.
3.5. Model comparison
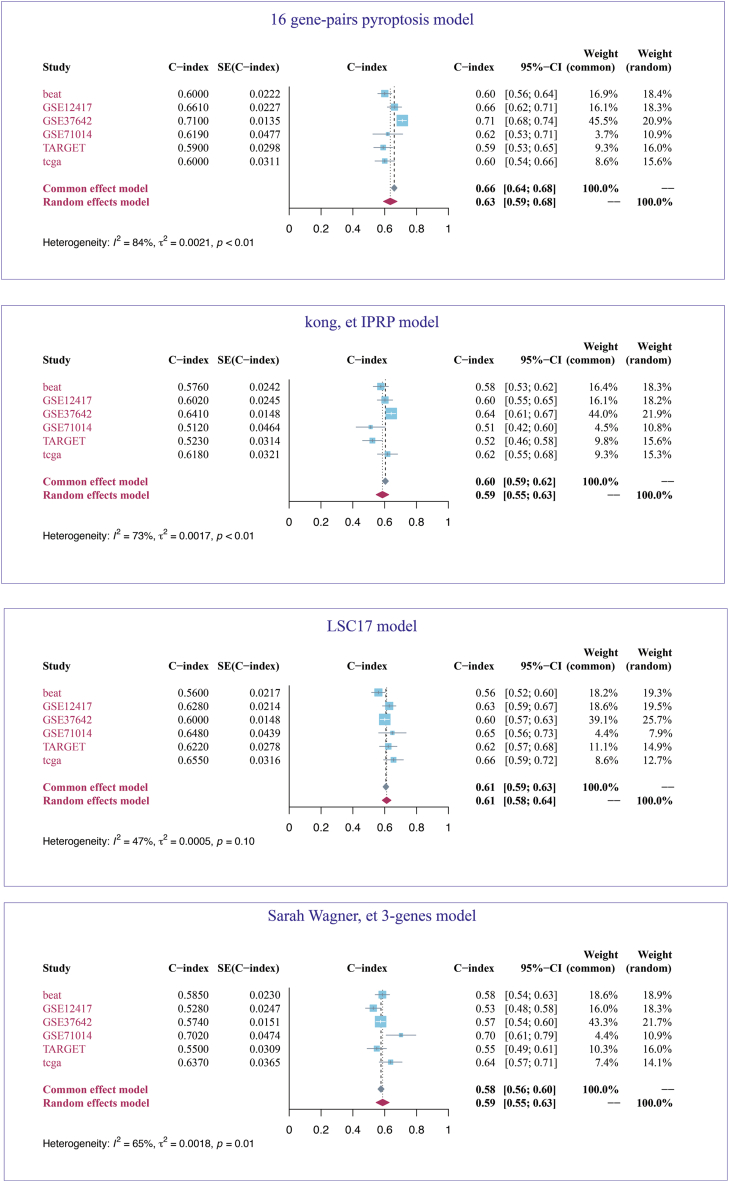
We compared our pyroptosis prognostic model with several published models. The C-index was selected as the metric to evaluate model performance. To explore the stability of the models, we assessed them across six AML cohorts (TCGA, BEAT AML, GSE71014, GSE12417, GSE37642 and TARGET). Given the significant heterogeneity among the cohorts, particularly considering the pediatric patients with AML in the TARGET dataset, we computed an aggregate C-index using a random-effects model. Our findings revealed that our pyroptosis model exhibited the highest pooled C-index at 0.63, surpassing the LSC17, three genes, and Kong et al. IPRP model, which had aggregate C-index of 0.61, 0.59, and 0.59, respectively (Fig. 8).
Fig. 8.
The prognostic predictive performance of four models is presented here. Featured at the top is the 16 gene-pairs pyroptosis model, which is the prognostic model developed in this study. The forest plot in the center of the figure illustrates the 95 % confidence intervals for the C-index across six distinct cohorts. A random-effects model has been employed to integrate the C-index from all cohorts.
4. Discussion
In the present study, we aimed to develop a prognostic model for AML based on pyroptosis-related genes. After analyzing 40 pyroptosis-related genes, we constructed a 16-gene-pair signature that exhibited high accuracy in predicting AML prognosis across different cohorts and platforms. To make the model more user-friendly, a nomogram was created by incorporating additional clinical variables. In addition, we analyzed the relation between the stemness of AML and risk score. To better understand the molecular immune mechanisms related to the pyroptosis prognostic model, we conducted an immune microenvironment analysis and immune score. Moreover, we explored the role of risk scores in guiding chemotherapy by analyzing the treatment responses.
During the screening of pyroptosis-related gene pairs in AML, we found that 12 pyroptosis-related genes were significantly correlated with the prognosis through univariate Cox regression analysis of 40 pyroptosis-related genes, including IL18, ELANE, CASP5, NLRP1, BAX, GSDMB, CHMP4A, CHMP6, CASP3, BAK1, CYCS, and CASP4. High expression of CHMP4A and CYCS was associated with a poor prognosis, whereas high expression of the other 10 genes predicted a better prognosis. It was demonstrated that CHMP4A was a high risk factor for liver cancer [37]. High CYCS expression also predicted a poor prognosis in multiple myeloma and neck squamous cell carcinoma [38,39]. Whereas high expression of CASP3/4/5 and GSDMB predicts a good prognosis, suggesting that pyroptosis in AML is mainly mediated by the non-canonical pathway or Caspase-3/8-mediated pathways [3]. In addition, through LASSO regression and multivariate Cox regression analysis of pyroptosis-related gene pairs, we obtained 16 pyroptosis-related gene pairs, including CASP3|ATF6, CASP3|CD96, BAK1|FKBP5, BAK1|PRF1, IL18|MCM10, CASP5|CGREF1, CASP5|SPP1, CYCS|DDIT4, CASP4|SOCS2, NLRP1 | RSU1, NLRP1 | VNN1, CHMP4A | EIF2S3, CHMP4A | REC8, CHMP6 | ARHGEF3, CHMP6 | DHCR24, and CHMP6 | WDR3.
Most of these gene pairs were strongly associated with immune processes and tumor development. ATF6 is a transcription factor that promotes the expression of important genes involved in the unfolded protein response under endoplasmic reticulum (ER) stress. It was demonstrated that pyroptosis-related gene was involved in the renal toxicity caused by cadmium, and cadmium activates the ER stress pathway mediated by ATF6 [40]. In addition, tanshinone IIA reduced pyroptosis in Mtb-infected macrophages and inhibited ER stress [41]. This suggests that ER stress mediated by ATF6 may be related to pyroptosis in AML. CD96 has been proved to be the specific marker and the therapeutic target of AML-LSCs. The expression of FKBP5 is positively correlated with sensitivity to cytarabine in the treatment of AML and can elevate the apoptotic effect of cytarabine by promoting the dephosphorylation of AKT-Ser473 [42]. PRF1 encodes perforin protein, which is involved in recognizing exogenous components by the immune system [43]. In cervical cancer, immune cells promote pyroptosis by releasing perforin via a positive feedback mechanism. Therefore, the positive feedback of immune cell may also exist in AML. SOCS2 controls hematopoietic stem cell proliferation and stemness by inhibiting JAK-STAT pathways through feedback effects and is highly expressed in multiple AML sub-populations with poor prognosis (such as MLL and BCR/ABL abnormalities) [44]. CGREF1 is significantly correlated with cisplatin resistance in osteosarcomas [45]. Compared with observations in normal bone marrow stromal cells, SPP1 is significantly elevated in stromal cells during the diagnosis, recurrence, and remission stages of AML [46]. In AML, EIF2S3 inhibits tumor cell proliferation and participates in cell cycle regulation via the MAPK/ERK signaling pathway [47]. REC8 promotes cancer angiogenesis, cancer cell stemness, metastasis and invasion in multiple digestive tract tumors (such as gastric, colon, and liver cancers) [[48], [49], [50]]. In IGH-BCL2+ diffuse large B-cell lymphomas, the SOX9-DHCR24-cholesterol biosynthesis axis exhibits carcinogenic effect [51]. ARHGEF3 regulates HDAC inhibitor-induced AML differentiation via RhoA-dependent pathways [52]. In pancreatic cancer, WDR3 overexpression activates the Hippo pathway [53]. Simultaneously, WDR3 promotes the stemness of cancer cells by inhibiting the expression of RASSF1A in prostate cancer [54]. Survival analysis showed that high DDIT4 expression may be a poor prognostic factor in AML [55].
The gene transcription level obtained from second-generation sequencing and microarray analysis is only a relative quantification. Therefore, the signature constructed based on gene expression is influenced by platform and batch effects. We used binary values based on the comparison of gene pairs to solve this problem and improved the generalization ability of the model. The density plot in Fig. 2B shows that the risk values remain in a consistent range across all the arrays. Our pyroptosis-related prognostic model showed excellent performance in predicting the prognosis of adult and pediatric patients with AML. This suggests that pyroptosis may affect the prognosis of AML throughout all the age.
There is growing evidence that LSCs play key roles in AML development and maintenance [56]. LSCs can self-renewal and resist drug, which are the main reasons for the persistence and recurrence of leukemia [57]. In this study, we found that the LSC-positive group had a higher pyroptosis risk score than that of the LSC-negative group, and the high-risk group showed a significant increase in leukemia stemness score compared with that of the low-risk group. In addition, Li et al. demonstrated that triggering pyroptosis increases the sensitivity of cancer stem cells to chemotherapy [58]. Therefore, targeting pyroptosis in LSCs may be a new strategy for treating AML.
We found that immune cells infiltration and immune scores were significantly reduced in the high-risk group. Zhang et al. divided renal clear cell carcinoma samples into two groups according to pyroptosis-related genes, and found that the infiltration of resting NK cells in one group was significantly increased [59]. Tan et al. constructed a prognostic model for gliomas based on pyroptosis-related lncRNAs, and activated mast cells in the high-risk group also showed low infiltration [60]. In addition, the combination of venetoclax and hypomethylating agents can induce pyroptosis of AML cells. Therefore, we infer that high levels of immune cells infiltration in the low-risk group may inhibit AML progression of by promoting pyroptosis [61].
Finally, we analyzed the role of the pyroptosis-related prognostic model in directing AML treatment. Based on susceptibility and correlation analysis, the high-risk group was highly sensitive to AZD1332, a neurotrophic tyrosine kinase receptor (TRK) inhibitor, and BPD-00008900. Approximately 44 % of AML patients express TRKA mRNA, and up to 30 % encode activated TRKA mutations. It was demonstrated that Nerve Growth Factor (NGF) can binds specifically to TRKA and promote AML cell proliferation by phosphorylation of ERK and AKT. In addition, selective TRK inhibitors can effectively reduce the burden of AML and improve survival. Tang et al. demonstrated that the expression level of NGF was correlated with pyroptosis [62]. Therefore, the TRK inhibitor AZD1332 may be the potential therapy strategy for the high-risk patients.
This study had some limitations. First, all data were obtained from the public databases. Although the quality of the public database is good, its content is determined by the research purpose of the uploader, and the integrity of the clinical information is also different. For example, it is not completely ideal when we used the existing clinical information in the database to prove that our model is an independent prognostic factor. Second, both immune-infiltration analysis and drug sensitivity analysis were based on algorithm analysis. Subsequently, a large number of clinical samples and experiments are required to confirm our conclusions.
In summary, we constructed a prognostic model for pyroptosis based on pyroptosis-related gene pairs, that showed good prognostic effects in adult and pediatric AML arrays. Simultaneously, the clinical information was combined to construct a nomogram. In addition, pyroptosis risk scores were found to be related with the leukemia stemness in AML. Immune infiltration was analyzed in the two risk groups. Finally, drug sensitivity analysis showed that the pyroptosis-related prognostic model plays a potential role in directing the treatment of AML.
Funding
This work was supported by the National Natural Science Foundation of China (82070175, 81400093 and 32270791), the Scientific Research Project of Hunan Provincial Health Commission (C202303046332), Huxiang Youth Talent Support Program (2022RC1205) and International Centre for Genetic Engineering and Biotechnology – ICGEB (CRP/CHN22-03_EC).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
CRediT authorship contribution statement
Huifang Zhang: Methodology, Data curation, Conceptualization. Hongkai Zhu: Data curation, Conceptualization. Yue Sheng: Methodology, Funding acquisition. Zhao Cheng: Supervision, Funding acquisition. Hongling Peng: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT in order to edit language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the High Performance Computing Center at Central South University for providing the computing resources.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36624.
Contributor Information
Yue Sheng, Email: shengyue@csu.edu.cn.
Zhao Cheng, Email: echocz@126.com.
Hongling Peng, Email: penghongling@csu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- 1.Shallis R.M., Wang R., Davidoff A., Ma X., Zeidan A.M. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi: 10.1016/j.blre.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Reinhardt D., Antoniou E., Waack K. Pediatric acute myeloid leukemia-past, present, and future. J. Clin. Med. 2022;11 doi: 10.3390/jcm11030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct. Targeted Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers C., Erkes D.A., Nardone A., Aplin A.E., Fernandes-Alnemri T., Alnemri E.S. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019;10:1689. doi: 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow M.T., Sceneay J., Paget C., Wong C.S., Duret H., Tschopp J., et al. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72:5721–5732. doi: 10.1158/0008-5472.Can-12-0509. [DOI] [PubMed] [Google Scholar]
- 6.Hergueta-Redondo M., Sarrió D., Molina-Crespo Á., Megias D., Mota A., Rojo-Sebastian A., et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Beeck K.O., Van Laer L., Van Camp G. DFNA5, a gene involved in hearing loss and cancer: a review. Ann. Otol. Rhinol. Laryngol. 2012;121:197–207. doi: 10.1177/000348941212100310. [DOI] [PubMed] [Google Scholar]
- 8.Voronov E., Shouval D.S., Krelin Y., Cagnano E., Benharroch D., Iwakura Y., et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miguchi M., Hinoi T., Shimomura M., Adachi T., Saito Y., Niitsu H., et al. Gasdermin C is upregulated by inactivation of transforming growth factor β receptor type II in the presence of mutated apc, promoting colorectal cancer proliferation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeki N., Usui T., Aoyagi K., Kim D.H., Sato M., Mabuchi T., et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium, Genes. Chromosomes Cancer. 2009;48:261–271. doi: 10.1002/gcc.20636. [DOI] [PubMed] [Google Scholar]
- 11.Komiyama H., Aoki A., Tanaka S., Maekawa H., Kato Y., Wada R., et al. Alu-derived cis-element regulates tumorigenesis-dependent gastric expression of GASDERMIN B (GSDMB) Genes Genet. Syst. 2010;85:75–83. doi: 10.1266/ggs.85.75. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Yin B., Li D., Wang G., Han X., Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem. Biophys. Res. Commun. 2018;495:1418–1425. doi: 10.1016/j.bbrc.2017.11.156. [DOI] [PubMed] [Google Scholar]
- 13.Li S., Liang X., Ma L., Shen L., Li T., Zheng L., et al. MiR-22 sustains NLRP3 expression and attenuates H. pylori-induced gastric carcinogenesis. Oncogene. 2018;37:884–896. doi: 10.1038/onc.2017.381. [DOI] [PubMed] [Google Scholar]
- 14.Wang C.J., Tang L., Shen D.W., Wang C., Yuan Q.Y., Gao W., et al. The expression and regulation of DFNA5 in human hepatocellular carcinoma DFNA5 in hepatocellular carcinoma. Mol. Biol. Rep. 2013;40:6525–6531. doi: 10.1007/s11033-013-2581-8. [DOI] [PubMed] [Google Scholar]
- 15.J. Gao, X. Qiu, G. Xi, H. Liu, F. Zhang, T. Lv, et al., Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer, Oncol. Rep. 40 (2018) 1971-1984. 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed]
- 16.Kong H., Wang Y., Zeng X., Wang Z., Wang H., Xie W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015;36:7501–7513. doi: 10.1007/s13277-015-3473-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z., Zhang Y., Xia S., Kong Q., Li S., Liu X., et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo X., Zhang X., Gan L., Zhou C., Zhao T., Zeng T., et al. The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL-8 secretion. J. Cell Mol. Med. 2018;22:6039–6054. doi: 10.1111/jcmm.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okondo M.C., Johnson D.C., Sridharan R., Go E.B., Chui A.J., Wang M.S., et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 2017;13:46–53. doi: 10.1038/nchembio.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W., Liu S., Li Y., Wang Y., Deng Y., Sun W., et al. Pyridoxine induces monocyte-macrophages death as specific treatment of acute myeloid leukemia. Cancer Lett. 2020;492:96–105. doi: 10.1016/j.canlet.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Fang Y., Chen X., Wang Z., Liang X., Zhang T., et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- 22.He X., Jiang Y., Yu X., He F., Gao H. A gene signature comprising seven pyroptosis-related genes predicts prognosis in pediatric patients with acute myeloid leukemia. Acta Haematol. 2022;145:627–641. doi: 10.1159/000526346. [DOI] [PubMed] [Google Scholar]
- 23.Huang K., Xie L., Wang F. A novel defined pyroptosis-related gene signature for the prognosis of acute myeloid leukemia. Genes. 2022;13 doi: 10.3390/genes13122281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S., Luo D., Luo J., Liang H., Zhi Y., Wang D., et al. Construction of a pyroptosis-related signature for prognostic prediction and characterization of immune microenvironment in acute myelogenous leukemia. Int. J. Gen. Med. 2022;15:2913–2927. doi: 10.2147/ijgm.S352062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao R., Wang H., Wang J., Lu S., He R., Lu Y. Comprehensive analysis of a pyroptosis-related gene signature of clinical and biological value in acute myeloid leukaemia. Int. Immunopharmacol. 2022;108 doi: 10.1016/j.intimp.2022.108802. [DOI] [PubMed] [Google Scholar]
- 26.Zhong G., Guo C., Shang Y., Cui Z., Zhou M., Sun M., et al. Development of a novel pyroptosis-related LncRNA signature with multiple significance in acute myeloid leukemia. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.1029717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou T., Qian K., Li Y.Y., Cai W.K., Yin S.J., Wang P., et al. The pyroptosis-related gene signature predicts prognosis and reveals characterization of the tumor immune microenvironment in acute myeloid leukemia. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.951480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong W., He L., Zhu J., Brück O., Porkka K., Heckman C.A., et al. An immunity and pyroptosis gene-pair signature predicts overall survival in acute myeloid leukemia. Leukemia. 2022;36:2384–2395. doi: 10.1038/s41375-022-01662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein J.N., Collisson E.A., Mills G.B., Shaw K.R., Ozenberger B.A., Ellrott K., et al. The cancer genome Atlas pan-cancer analysis project. Nat. Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyner J.W., Tognon C.E., Bottomly D., Wilmot B., Kurtz S.E., Savage S.L., et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562:526–531. doi: 10.1038/s41586-018-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshihara K., Shahmoradgoli M., Martínez E., Vegesna R., Kim H., Torres-Garcia W., et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeser D., Gruener R.F., Huang R.S. oncoPredict: an R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief. Bioinform. 2021;22 doi: 10.1093/bib/bbab260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W., Soares J., Greninger P., Edelman E.J., Lightfoot H., Forbes S., et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41:D955–D961. doi: 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Li T., Zhang X. Identification of a pyroptosis-related prognostic signature combined with experiments in hepatocellular carcinoma. Front. Mol. Biosci. 2022;9 doi: 10.3389/fmolb.2022.822503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C., Liang H., Bian S., Hou X., Ma Y. Construction of a prognosis model of the pyroptosis-related gene in multiple myeloma and screening of core genes. ACS Omega. 2022;7:34608–34620. doi: 10.1021/acsomega.2c04212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen B., Luo Y., Kang X., Sun Y., Jiang C., Yi B., et al. Development of a prognostic prediction model based on a combined multi-omics analysis of head and neck squamous cell carcinoma cell pyroptosis-related genes. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.981222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou X., Ding F., Zhang X., Ding X., Gao H., Wu Q. Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum stress and pyroptosis through XBP-1s deacetylation in human renal tubular epithelial cells. Arch. Toxicol. 2019;93:965–986. doi: 10.1007/s00204-019-02415-8. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Fu Y., Sun J., Shen J., Liu F., Ning B., et al. Tanshinone IIA alleviates NLRP3 inflammasome-mediated pyroptosis in Mycobacterium tuberculosis-(H37Ra-) infected macrophages by inhibiting endoplasmic reticulum stress. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114595. [DOI] [PubMed] [Google Scholar]
- 42.Mitra A.K., Crews K., Pounds S., Cao X., Downing J.R., Raimondi S., et al. Impact of genetic variation in FKBP5 on clinical response in pediatric acute myeloid leukemia patients: a pilot study. Leukemia. 2011;25:1354–1356. doi: 10.1038/leu.2011.74. https://www.ncbi.nlm.nih.gov/pubmed/21483441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergelli M., Hemmer B., Muraro P.A., Tranquill L., Biddison W.E., Sarin A., et al. Human autoreactive CD4+ T cell clones use perforin- or Fas/Fas ligand-mediated pathways for target cell lysis. J. Immunol. Baltim. Md 1950. 1997;158:2756–2761. [PubMed] [Google Scholar]
- 44.Vitali C., Bassani C., Chiodoni C., Fellini E., Guarnotta C., Miotti S., et al. SOCS2 controls proliferation and stemness of hematopoietic cells under stress conditions and its deregulation marks unfavorable acute leukemias. Cancer Res. 2015;75:2387–2399. doi: 10.1158/0008-5472.Can-14-3625. [DOI] [PubMed] [Google Scholar]
- 45.Xie M., Dai H., Gu Q., Xiao C., Wang H., Lei Y., et al. Identification of genes contributing to cisplatin resistance in osteosarcoma cells. FEBS Open Bio. 2023;13:164–173. doi: 10.1002/2211-5463.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yehudai-Resheff S., Attias-Turgeman S., Sabbah R., Gabay T., Musallam R., Fridman-Dror A., et al. Abnormal morphological and functional nature of bone marrow stromal cells provides preferential support for survival of acute myeloid leukemia cells. Int. J. Cancer. 2019;144:2279–2289. doi: 10.1002/ijc.32063. [DOI] [PubMed] [Google Scholar]
- 47.Lu J., Chen S., Tan H., Huang Z., Li B., Liu L., et al. Eukaryotic initiation factor-2, gamma subunit, suppresses proliferation and regulates the cell cycle via the MAPK/ERK signaling pathway in acute myeloid leukemia. J. Cancer Res. Clin. Oncol. 2021;147:3157–3168. doi: 10.1007/s00432-021-03712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han J., Bai Y., Wang J., Xie X.L., Li A.D., Ding Q., et al. REC8 promotes tumor migration, invasion and angiogenesis by targeting the PKA pathway in hepatocellular carcinoma. Clin. Exp. Med. 2021;21:479–492. doi: 10.1007/s10238-021-00698-9. [DOI] [PubMed] [Google Scholar]
- 49.Liu M., Xu W., Su M., Fan P. REC8 suppresses tumor angiogenesis by inhibition of NF-κB-mediated vascular endothelial growth factor expression in gastric cancer cells. Biol. Res. 2020;53:41. doi: 10.1186/s40659-020-00307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X., Xie X., Liu T., Chen S., Wang Y., Zhang J., et al. REC8 enhances stemness and promotes metastasis of colorectal cancer through BTK/Akt/β-catenin signaling pathway. Transl. Oncol. 2022;15 doi: 10.1016/j.tranon.2021.101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen Y., Zhou J., Nie K., Cheng S., Chen Z., Wang W., et al. Oncogenic role of the SOX9-DHCR24-cholesterol biosynthesis axis in IGH-BCL2+ diffuse large B-cell lymphomas. Blood. 2022;139:73–86. doi: 10.1182/blood.2021012327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Amato L., Dell'Aversana C., Conte M., Ciotta A., Scisciola L., Carissimo A., et al. ARHGEF3 controls HDACi-induced differentiation via RhoA-dependent pathways in acute myeloid leukemias. Epigenetics. 2015;10:6–18. doi: 10.4161/15592294.2014.988035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su W., Zhu S., Chen K., Yang H., Tian M., Fu Q., et al. Overexpressed WDR3 induces the activation of Hippo pathway by interacting with GATA4 in pancreatic cancer. J. Exp. Clin. Cancer Res. 2021;40:88. doi: 10.1186/s13046-021-01879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W., Xie A., Xiong J., Li S., Yang L., Liu W. WDR3 promotes stem cell-like properties in prostate cancer by inhibiting USF2-mediated transcription of RASSF1A. J. Gene Med. 2023:e3498. doi: 10.1002/jgm.3498. [DOI] [PubMed] [Google Scholar]
- 55.Cheng Z., Dai Y., Pang Y., Jiao Y., Liu Y., Cui L., et al. Up-regulation of DDIT4 predicts poor prognosis in acute myeloid leukaemia. J. Cell Mol. Med. 2020;24:1067–1075. doi: 10.1111/jcmm.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheng Y., Yu C., Liu Y., Hu C., Ma R., Lu X., et al. FOXM1 regulates leukemia stem cell quiescence and survival in MLL-rearranged AML. Nat. Commun. 2020;11:928. doi: 10.1038/s41467-020-14590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han L., Dong L., Leung K., Zhao Z., Li Y., Gao L., et al. METTL16 drives leukemogenesis and leukemia stem cell self-renewal by reprogramming BCAA metabolism. Cell Stem Cell. 2023;30:52–68.e13. doi: 10.1016/j.stem.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y.T., Tan X.Y., Ma L.X., Li H.H., Zhang S.H., Zeng C.M., et al. Targeting LGSN restores sensitivity to chemotherapy in gastric cancer stem cells by triggering pyroptosis. Cell Death Dis. 2023;14:545. doi: 10.1038/s41419-023-06081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Chen X., Fu Q., Wang F., Zhou X., Xiang J., et al. Comprehensive analysis of pyroptosis regulators and tumor immune microenvironment in clear cell renal cell carcinoma. Cancer Cell Int. 2021;21:667. doi: 10.1186/s12935-021-02384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanzhu G., Li N., Li Z., Zhou R., Shen L. Molecular subtypes and prognostic signature of pyroptosis-related lncRNAs in glioma patients. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.779168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye F., Zhang W., Fan C., Dong J., Peng M., Deng W., et al. Antileukemic effect of venetoclax and hypomethylating agents via caspase-3/GSDME-mediated pyroptosis. J. Transl. Med. 2023;21:606. doi: 10.1186/s12967-023-04481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghisoli M.L., Fang W., Graham T.C., Levy A.G., Zeng L., Kannan S., et al. Understanding the role of TRKA receptor in acute myeloid leukemias: from proliferation and pro-survival signals to a novel therapeutic approach. Blood. 2008;112:3789. doi: 10.1182/blood.V112.11.3789.3789. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.