Summary
Cordycepin, a natural derivative of adenosine from Cordyceps militaris, can inhibit the replication of the dengue virus (DENV). Here, we investigated its antiviral and anti-inflammatory effects in DENV infected cells. Cordycepin significantly inhibited DENV-2 infection, virion production, and viral protein synthesis. It also reduced DENV-induced cytokine/chemokine production, including RANTES, IP-10, IL-6, and TNF-α. Mechanistically, cordycepin targeted the DENV NS5 protein, suppressing RANTES expression and hindering viral replication. Additionally, it inhibited the NF-κB pathway, leading to reduced nuclear translocation and signaling deactivation. PCR array analysis revealed cordycepin’s suppression of 46 genes associated with DENV-induced inflammation. These findings highlight cordycepin’s dual potential as an antiviral and anti-inflammatory agent against DENV, making it as a promising candidate for dengue treatment, targeting both viral and host factors.
Subject areas: Pharmacology, Natural sciences, Biological sciences, Microbiology
Graphical abstract

Highlights
-
•
Cordycepin inhibits DENV-2 infection by over 1000-fold at 800 μM
-
•
Cordycepin suppresses DENV-induced cytokine/chemokine production
-
•
Cordycepin targets DENV NS5 protein, suppressing RANTES and hindering replication
-
•
NF-κB pathway involvement in cordycepin’s anti-inflammatory action revealed
Pharmacology; Natural sciences; Biological sciences; Microbiology
Introduction
Dengue virus (DENV) poses a significant global public health challenge, with reported cases spanning over 100 countries and an annual infection rate of 96 million individuals, displaying varying degrees of severity and a 2.5% fatality rate.1,2 Transmission occurs through Aedes aegypti and Aedes albopictus mosquitoes, involving four genetically distinct serotypes, namely DENV-1, DENV-2, DENV-3, and DENV-4. The viral genome is translated into a single polyprotein, cleaved by both host and viral protease to yield three structural proteins (capsid, C; pre-membrane, prM; envelope, E), and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5), crucial for viral replication and particle maturation.3,4,5,6,7 Reinfection with a different DENV serotype can lead to severe clinical manifestations.8 Severe dengue is characterized by a disruption in blood vessel integrity, resulting in vascular leakage, primarily due to an aberrant and dysregulated immune response. DENV infection triggers the production of various cytokines/chemokines, including interleukin-1 (IL-1), IL-6, IL-8, IL-10, chemokine (C-X-C motif) ligand 10 (CXCL-10 or IP-10), chemokine ligand 2 (CCL-2), vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), interferon-α (IFN-α), interferon-γ (IFN-γ), regulated upon activation, normal T cell expressed and secreted (RANTES), and macrophage inflammatory protein (MIP-1β), detected in the plasma of dengue patients.9,10,11,12 Autopsies of fatal dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) cases caused by DENV-2 revealed apoptotic cells in the liver, brain, intestinal, and lung tissues. The detection of apoptotic microvascular endothelial cells (ECs) in the intestinal and lung tissues explains the patient’s plasma leakage.13 The up-regulation of IL-8 and RANTES in DENV-2-infected ECs leads to the accumulation of these chemokines at serosal sites and causes local vascular leakage, which has been observed to accompany apoptosis.12
While two licensed dengue vaccines (Dengvaxia and TAK-003) have demonstrated efficacy in reducing dengue severity, they come with limitations. Dengvaxia is less effective in children under 9 years old and those without prior DENV infection,14 while TAK-003 exhibits high efficacy for DENV-2 but lower effectiveness against other serotypes.15,16,17 These limitations impede widespread vaccine use, and currently, there are no licensed drugs available for dengue treatment. Clinical management relies on supportive care and symptomatic treatment. Therefore, there is an urgent need to identify an ideal drug that can effectively reduce viral replication and suppress the inflammatory response for the treatment of dengue. Considering the stage of infection in patients, it is important to note that by the time of diagnosis, many cell types have already been infected, even though viremia levels in DENV-infected patients peak only within the first two days after the onset of symptoms and subsequently decline over time.18 The pathogenesis and disease progression occur after the virus enters the cells and is amplified by the overreaction of the immune response. Accordingly, rather than blocking viral entry by targeting the viral envelope protein function, inhibitors that can reduce virus synthesis and lower the risk of cytokine production—particularly the risk of a cytokine storm—have received significant attention. Nonstructural proteins such as NS1 and NS5 are highlighted as primary targets because they are responsible for viral RNA synthesis and are involved in the inflammatory response.19
Various medicinal plants and bioactive compounds show promise in controlling DENV infection. Notable examples include alpha-mangostin (α-MG) sourced from the pericarp of mangosteen fruit (Garcinia mangostana Linn),19,20,21 andrographolide extracted from Andrographis paniculate,22,23,24 castanospermine obtained from the Castanospermum australe tree (black bean or Moreton Bay chestnut),25,26,27 and (2R,4R)-1,2,4-trihydroxyheptadec-16-yne (THHY) isolated from avocado (Persea americana Mill).28 These bioactive compounds have demonstrated the ability to inhibit the replication cycle of DENV across all four serotypes. Notably, α-MG stands out as a particularly promising candidate for both antiviral and anti-inflammatory effects against DENV infection. However, a limitation arises from its low selectivity index (SI).19,29
Cordycepin (3′-deoxyadenosine) is a major bioactive compound isolated from the caterpillar fungus, Cordyceps militaris. Its discovery in the early 1950s marked its prominence in traditional Chinese medicine.30,31 Extensive research has highlighted the diverse pharmacological effects of cordycepin, encompassing its roles as an antitumor agent,32,33,34 anti-inflammatory compound,35,36,37 antioxidant,38,39 and contributor to anti-aging, antimicrobial, and antiviral properties.40,41,42 Importantly, studies have consistently reported negligible or very low cytotoxic effects of cordycepin on various cultured cells.41
In our prior study, we demonstrated the inhibitory potential of cordycepin against DENV infection and replication by its binding ability to the methyltransferase (MTase) and RNA-dependent RNA polymerase (RdRp) domains of DENV NS5, as predicted through molecular docking.43 Cordycepin also hindered infection by all four DENV serotypes in Vero cells. In this investigation, we transitioned to the A549 human lung epithelial cell line, a commonly used model in DENV research. A previous report identified viral antigens in alveolar macrophages, pulmonary vascular endothelium, and monocytes within lung blood vessels using immunohistochemistry.44 Lung epithelial cells, susceptible to DENV infection, exhibited a significant increase in IL-6 and RANTES expression, mirroring levels observed in severe dengue patients, suggesting them as potential targets for DENV with critical roles in lung-related immunopathogenesis.45
Furthermore, we highlight the involvement of the NF-κB pathway, a pivotal transcription factor for regulating cytokine/chemokine expression.46 Our exploration delved into the interaction between cordycepin and DENV protein, as well as the NF-κB complex. Additionally, we scrutinized cytokine/chemokine expression post-cordycepin treatment and investigated the gene expression profile using real-time PCR array for the NF-κB signaling pathway. Our finding revealed that cordycepin has the capacity to impede this pathway. Consequently, the outcomes of this study strongly suggest the potential of cordycepin as a dual-action agent, serving both as an antiviral and anti-inflammation drug against DENV infection.
Results
Cordycepin inhibited DENV-2 infection and reduced infectious progeny production
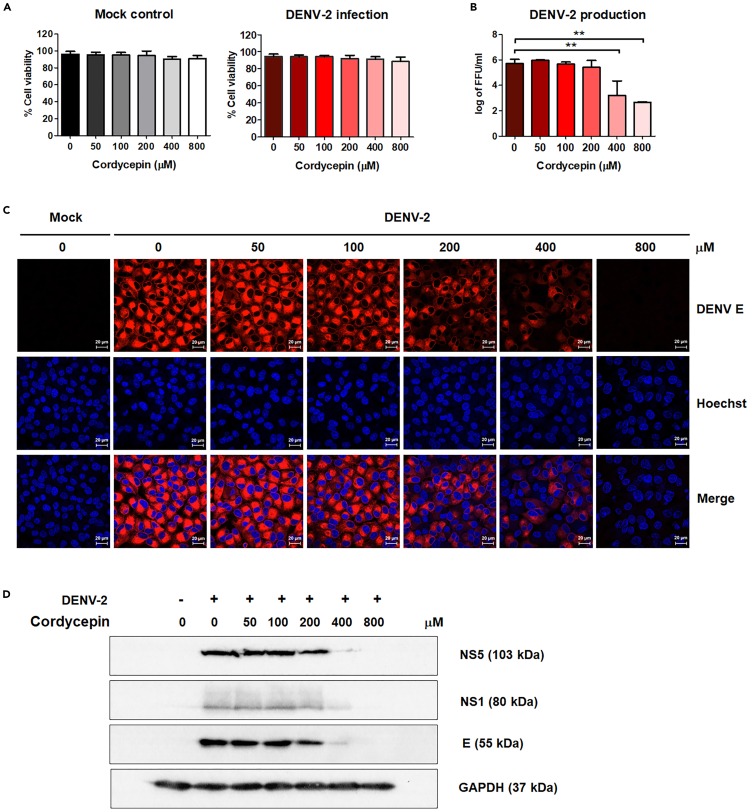
To assess the inhibitory effects of cordycepin on DENV in A549 cells, as previously reported in Vero cells, A549 cells were either infected with DENV-2 at MOI 5 or left uninfected as a mock control. Subsequently, the cells were treated with cordycepin at concentrations of 0, 50, 100, 200, 400, and 800 μM. After 24 h post-infection (hpi), cells and supernatants were collected to evaluate cell viability and viral production, respectively. The results indicated that cordycepin exhibited no toxicity to the cells, with both mock and DENV-infected cells displaying comparable viability exceeding 85% (Figure 1A). Actually, we increased the concentration of cordycepin to 1,000 μM, as shown in the Figures S1A and S1E, but we found that the virus reduction effect was not significantly different from that observed with 800 μM. Additionally, cell viability at 1,000 μM cordycepin was reduced to 80% and unhealthy cell morphology appeared. This reduction in cell viability at the higher concentration adversely affected virus production. Therefore, we selected 800 μM cordycepin as the maximum dose for our experiments. Cordycepin effectively reduced virus production in a dose-dependent manner, with concentrations of 400 and 800 μM resulting in a substantial 200- to 1,000-fold reduction (Figure 1B). This reduction correlated with a decrease in the number of virus-infected cells, as evidenced by immunofluorescence staining of DENV E protein (Figure 1C), and a decrease in the intracellular expression of viral proteins (NS5, NS1, and E), as demonstrated by immunoblot analysis (Figure 1D).
Figure 1.
Anti-dengue virus (DENV) activity of cordycepin in DENV-2-infected A549 cells
A549 cells were infected with DENV-2 for 2 h and subsequently treated with cordycepin concentrations ranging from 0 to 800 μM. Cells and supernatants were collected at 24 h post-infection.
(A) Cell viability percentages were assessed using propidium iodide (PI) staining and flow cytometry.
(B) Virus production in culture supernatants was determined by focus-forming unit (FFU)assay.
(C) Intracellular DENV E protein was visualized through immunofluorescence staining and confocal microscopy (63× magnification; scan zoom 0.6; 20-μM scale bar; DENV E in red and nucleus in blue).
(D) The expression levels of DENV NS5, NS1, and E proteins were examined via immunoblot analysis. Results represent three independent experiments (∗∗p < 0.01).
Furthermore, hepatic cell lines (HepG2 and Huh7) and a kidney cell line (HEK293T) were infected with DENV-2 and treated with cordycepin at various concentrations. The results demonstrated that cordycepin effectively inhibit virus production in all tested target cells (Figure S1). These findings collectively suggest that cordycepin can inhibit DENV infection in various cell types, consistent with a previous study conducted on Vero cells.
Cordycepin directly bound to DENV NS5 protein
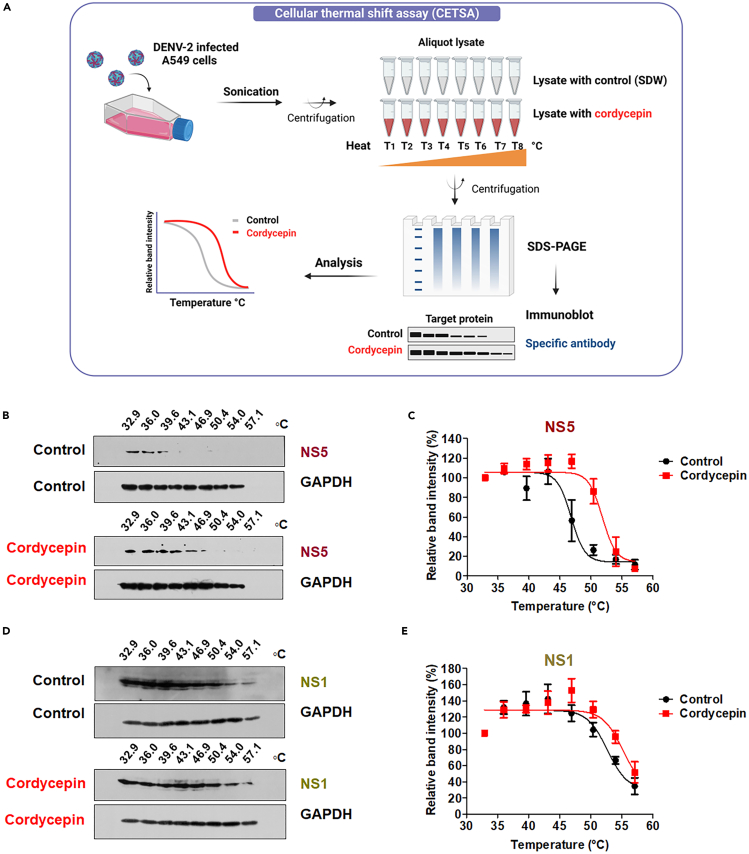
Upon observing the inhibitory effect of cordycepin on DENV infection, we conducted further investigations to validate its direct interaction with dengue viral proteins. We employed Cellular Thermal Shift Assay (CETSA) to probe the potential physical binding between cordycepin and dengue non-structural (NS) proteins, given that cordycepin impedes viral RNA replication.43 CETSA operates on the principle that direct drug binding alters the conformation and thermal stability of the target protein, rendering the protein complex resistant to thermal denaturation.47 DENV-infected A549 cell lysates were incubated with or without 800 μM of cordycepin (the maximum concentration affecting DENV infection) and subjected to heating at eight different temperatures (Figure 2A). Immunoblot revealed a thermal shift profile for DENV NS5, indicating that cordycepin can shield NS5 during heating to temperatures ranging from 30°C to 60°C. The protein bands displayed enhanced stability compared to the control (Figure 2B and 2C and see also Figure S2A). We further conducted CETSA on DENV NS1 by treating cells with or without cordycepin, revealing that the thermal shift profile of DENV NS1 exhibited a similar pattern at different temperatures compared to the control (Figure 2D and 2E and see also Figure S2B). GAPDH served as an internal loading control to ensure equal sample loading (Figure 2B and 2D). Consequently, the CETSA results demonstrated that cordycepin directly binds to the NS5 protein, with a higher binding affinity than to the NS1 protein of DENV, thereby influencing the reduction of virus production.
Figure 2.
Investigation of the direct interaction between cordycepin and DENV NS5 protein using Cellular Thermal Shift Assay (CETSA)
(A) Schematic representation of CETSA, generated using the BioRender web server.
(B and D) Immunoblot analysis of DENV NS5 and NS1 in the CETSA binding assay performed in the presence of cordycepin or SDW (control) at the specified temperatures. GAPDH served as an internal loading control to ensure equal protein loading.
(C and E) Quantification of band intensities of NS5 and NS1 proteins at each temperature, normalized to the band intensity of samples at 32.9°C (the lowest temperature). Results are representative of three independent experiments.
NS5 is a bifunctional protein consisting of MTase and RdRp domains, responsible for initiating viral RNA capping and viral replication, respectively. Previous studies have shown that the binding of certain small compounds to these proteins can inhibit DENV infection by blocking RNA replication steps.48,49,50 To evaluate the ability of cordycepin to bind to the S-adenosyl methionine/S-adenosyl homocysteine (SAM/SAH) binding site of MTase and the N pocket of RdRp—sites where small compound binding has been shown to affect protein function and inhibit DENV infection,48,49,50 we conducted detailed molecular docking studies. The results showed that cordycepin effectively occupied both the SAM/SAH binding site of MTase and the N pocket of RdRp (Figure 3). Ligand-receptor interaction analysis highlighted the significant role of hydrogen bonding in these bindings. Specifically, the C2 hydroxyl group of cordycepin formed a hydrogen bond with the nitrogen atom in the imidazole side chain of His110 in MTase (Figures 3B and S3A). For the RdRp interaction, the C2 hydroxyl group of cordycepin formed a hydrogen bond with the nitrogen atom in the amino group of Arg737, while the C5 hydroxyl group formed hydrogen bonds with the nitrogen atoms in the amino groups of Thr794 and Trp795, and the hydrogen atom in the adenine of cordycepin bound to the carboxylic group of Ser796 (Figures 3D and S3B). In addition to hydrogen bonding, cordycepin formed pi-alkyl interactions with Lys105 and Ile147 of MTase, contributing to its stabilization in SAM/SAH binding site (Figures 3B and S3A). Furthermore, amino acid residues in the RdRp N pocket, including His711, Arg729, and Met761, engaged in non-covalent pi-alkyl interactions with cordycepin (Figures 3D and S3B).
Figure 3.
Docking of cordycepin molecule onto DENV NS5 proteins
The interaction between cordycepin and DENV NS5 was investigated using molecular docking technique. Three-dimension structures of NS5 MTase (PDB: 1R6A) and RdRp (PDB: 5JJR) were utilized as the receptor, with cordycepin designated as the ligand. Panels A-D present docking poses obtained from the SwizzDock program, illustrating the interaction of cordycepin with both NS5 MTase and RdRp.
(A) Cordycepin (depicted in red CPK feature) occupies the protein pocket of NS5 MTase (multicolor surface feature).
(B) The intermolecular interaction between cordycepin (red stick) and MTase (green ribbon) is shown (upper left), with a zoom-in on the interaction with NS5 residues (His110, Lys105, and Ile147) (upper right).
(C) Cordycepin (red CPK feature) occupies the protein pocket of NS5 RdRp (multicolor surface feature).
(D) The intermolecular interaction between cordycepin (red stick) and RdRp (green ribbon) is depicted (lower left), with a zoom-in on the interaction with NS5 residues (Arg737, Thr794, Trp795, and Ser796) (lower right).
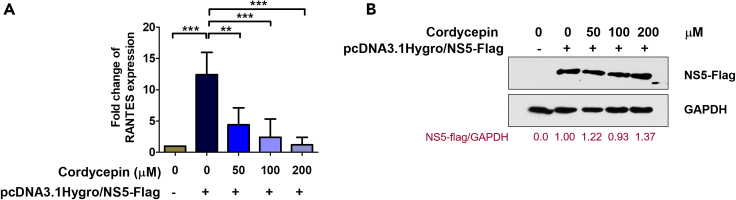
Cordycepin suppressed DENV NS5-induced RANTES expression
In our previous study conducted in HEK293T cells through transient transfection with plasmid DNA containing the full-length DENV NS5 fused with FLAG tag, we identified RANTES as the predominant cytokine/chemokine mRNA expression.51 Furthermore, our investigations revealed that DENV NS5 enhances RANTES production by interaction with a death-domain-associated protein (Daxx), known for its typical interaction with NF-κB.52 To elucidate the inhibition of cordycepin to DENV NS5 affects RANTES expression. HEK293T cells were transfected with NS5 plasmid or empty vector control. Twenty-four hours post-transfection, cells were treated with 0, 50, 100, and 200 μM of cordycepin, which were determined to be non-toxic doses in HEK293T cells (Figure S4), and incubated for an additional 24 h. Cell samples were collected and subjected to RANTES expression analysis using real-time RT-PCR. The results demonstrated a significant increase in RANTES expression levels following NS5 induction compared to the control. However, this elevated RANTES expression was effectively reduced by cordycepin treatment at concentrations ranging from 50 μM to 200 μM compared to the untreated control (Figure 4A). To evaluate NS5 expression in all conditions, equal amounts of protein from each condition underwent immunoblot analysis with an anti-FLAG antibody. The results indicated comparable levels of NS5 protein expression across all conditions (Figure 4B). These findings suggest that cordycepin can inhibit virus infection through its interaction with the NS5 protein, consequently leading to the inhibition of RANTES expression.
Figure 4.
Cordycepin inhibits the expression of RANTES in HEK293T cells transfected with NS5
(A) HEK293T cells were transfected with DENV NS5 plasmid or vector control, followed by treatment with 0–200 μM cordycepin. Cells were harvested 24 h post-treatment for the assessment of RANTES mRNA levels using real-time RT-PCR.
(B) The expression of NS5 protein was determined by immunoblot analysis using an anti-FLAG antibody. The relative levels of total FLAG-tagged NS5 protein were normalized by GAPDH protein and presented as fold-change values compared to untreated cells. Data are represented as means ± SD; n = 3 (∗∗p < 0.01, ∗∗∗p < 0.001).
Cordycepin reduced DENV-2-induced cytokine and chemokine secretion
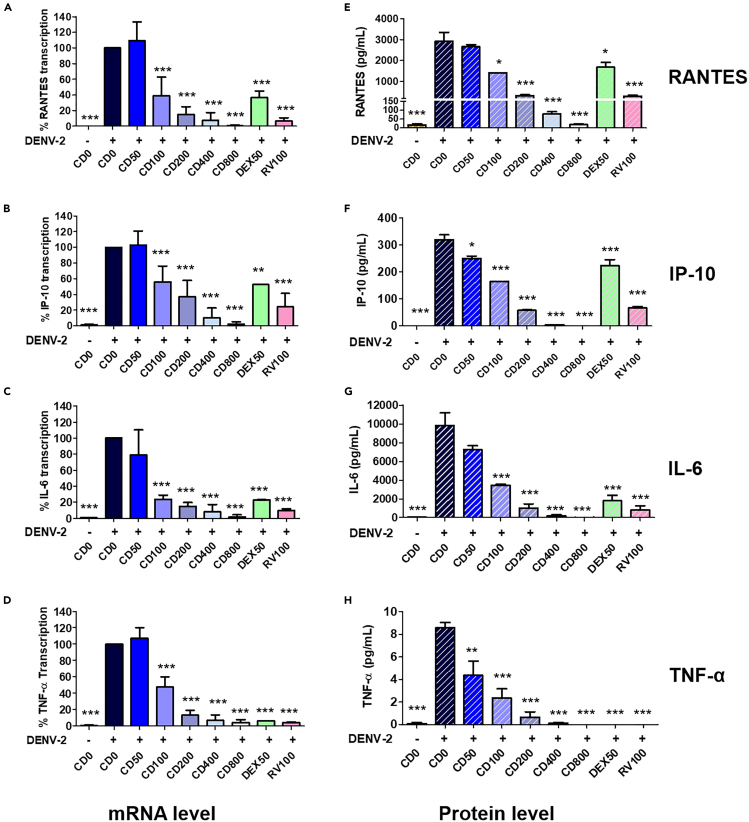
Considering the established correlation between proinflammatory cytokine production during DENV infection and the severity of the associated diseases in patients, we conducted further investigations to explore the potential of cordycepin in modulating cytokine and chemokine expression. DENV-2-infected A549 cells, treated or untreated with cordycepin for 24 h post-treatment, were collected for RNA extraction, and the mRNA levels of RANTES, IP-10, IL-6, and TNF-α were analyzed using real-time RT-PCR. Additionally, the protein levels of these cytokines/chemokines in the supernatant were quantified through a multiplex bead-based immunoassay. The results demonstrated a significant increase in the levels of cytokines/chemokines in A549 cells infected with DENV compared to the mock control (Figures 5A–5H). Notably, treatment with 100–800 μM of cordycepin significantly reduced the mRNA levels of all tested cytokines/chemokines. Treatment with 50 μM of dexamethasone and 100 μM of ribavirin also exhibited an impact on the expression of those cytokines/chemokines (Figures 5A–5D). Consistent with the mRNA levels, cordycepin significantly reduced the levels of secreted cytokines/chemokines in the supernatants in a dose-dependent manner (Figures 5E–5H).
Figure 5.
Cordycepin inhibits the expression of cytokines and chemokines induced by DENV
A549 cells were infected with DENV-2 and treated with 0–800 μM cordycepin (CD), 50 μM DEX, or 100 μM RV for 24 h.
(A–D) Real-time RT-PCR was used to determine the mRNA levels of RANTES, IP-10, IL-6, and TNF-α in virus-infected cells.
(E–H) Multiplex bead-based immunoassay was employed to measure the protein levels of RANTES, IP-10, IL-6, and TNF-α in the supernatants. Data are represented as means ± SD; n = 3 (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Cordycepin suppressed DENV-2-induced NF-κB activation
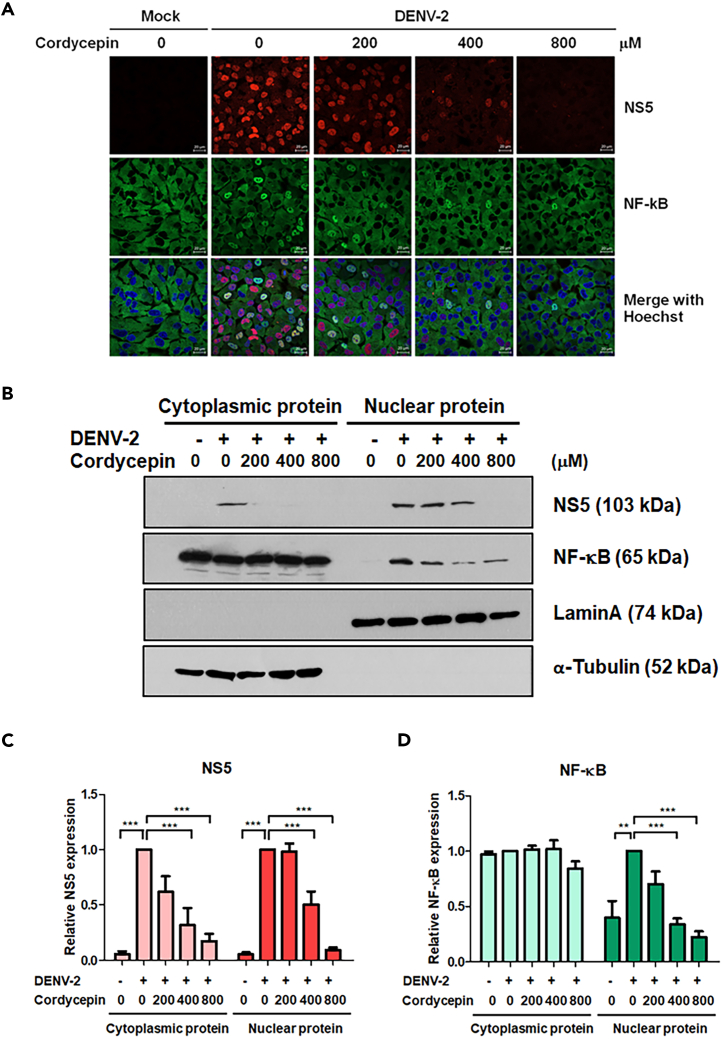
The NF-κB signaling pathway plays a crucial role in regulating cytokine/chemokine expression.46 To explore the impact of cordycepin on cytokine/chemokine production through the NF-κB pathway, we investigated the translocation of NF-κB (p65) using immunofluorescence assay and subcellular fractionation followed by immunoblot analysis. A549 cells were infected with DENV-2 for 2 h and treated with cordycepin at concentrations of 0, 200, 400, and 800 μM. At 24 hpi, cells were fixed, permeabilized, and stained with anti-NF-κB antibody. DENV-infected A549 cells exhibited NF-κB translocation into the nucleus, in contrast to the mock control, whereas the treatment with cordycepin reduced the nuclear translocation of NF-κB (p65) (Figure 6A). This reduction in nuclear translocation was evident from the noticeable decrease in the band intensity of NF-κB (p65) in the nuclear fraction, as observed in the immunoblot results shown in Figure 6B. The relative band intensities of DENV NS5 and NF-κB were determined by normalization to the band intensities of Lamin A (nuclear fraction) and α-tubulin protein (cytoplasmic fraction) at each cordycepin concentration (Figure 6C and 6D). Our findings demonstrated that treatment with cordycepin significantly reduced the levels of NS5 in both the cytoplasm and nucleus in a dose-dependent manner, aligning with cordycepin’s reported effect on DENV infection. Specifically, treatment with 400 and 800 μM of cordycepin resulted in a significant reduction of NF-κB in the nuclear fraction by 52.4% and 77.2%, respectively, correlating with the IFA results. These results suggest that cordycepin inhibits cytokine/chemokine expression by suppressing the NF-κB signaling pathway.
Figure 6.
Cordycepin inhibits nuclear translocation of NF-κB in DENV-infected A549 cells
A549 cells were infected with DENV-2 at MOI 5 and treated with 0, 200, 400, and 800 μM of cordycepin for 24 h.
(A) Immunofluorescence staining and confocal microscopy (63× magnification; scan zoom 0.6; 20-μM scale bar) were used to examine the cellular localization of DENV NS5 and NF-κB (DENV NS5 in red, NF-κB in green, and nucleus in blue).
(B) Immunoblot analysis determined DENV NS5 and NF-κB levels in both cytoplasmic and nuclear fractions, with Lamin A and α-tubulin serving as internal controls for the nuclear and cytoplasmic fractions, respectively.
(C and D) Densitometry scanning with ImageJ software quantified the relative band intensities of NS5 and NF-κB expression. Data are represented as means ± SD; n = 3. (∗p < 0.05, ∗∗∗p < 0.001).
Cordycepin directly interacted with NF-κB and its protein complex
Given that IκB undergoes phosphorylation in response to virus infection, subsequently releasing the NF-κB protein into the nucleus and promoting proinflammatory cytokine production,46 we sought to investigate whether cordycepin directly interacts with NF-κB and/or IκB using CETSA. DENV-infected A549 cell lysates were incubated with or without 800 μM of cordycepin and subjected to heating at temperatures ranging from 37°C to 70°C. The CETSA results indicated that cordycepin stabilized both NF-κB (Figures 7A, 7B, and S5A) and IκB (Figures 7C, 7D, and S5B) against thermal denaturation. This was evident from the higher intensity of the protein bands following exposure to increasing temperatures ranging from 50°C to 70°C, compared to the control. In contrast, the GAPDH control exhibited a constant band intensity, unaffected by the increasing temperature. These CETSA results support the notion of cordycepin directly interacting with NF-κB and IκB, suggesting its potential to disrupt the NF-κB signaling pathway.
Figure 7.
Assessment of the interaction between cordycepin and NF-κB and IκB using Cellular Thermal Shift Assay (CETSA)
(A and C) Immunoblot analysis of NF-κB and IκB in the CETSA binding assay conducted in the presence of cordycepin or SDW (control) at the indicated temperatures. GAPDH served as an internal loading control to ensure equal protein loading.
(B and D) Quantification of band intensities for NF-κB and IκB at each temperature, normalized to the band intensity of the samples at 37.9°C (the lowest temperature). Results are representative of three independent experiments.
Subsequently, we assessed the impact of cordycepin on the expression levels of IκB and IKKβ following a 2-h incubation with DENV-2. Cordycepin treatment at concentrations ranging from 0 to 800 μM for 2 h showed a reduction in the level of IκB protein. However, there was no significant effect observed on NF-κB or IKKβ at the 2 h post-treatment, as demonstrated by immunoblot results in Figures 8A–8D. Additionally, an immunofluorescence assay was performed to observe IκB expression in DENV-infected cells in response to cordycepin treatment. The results confirmed that cordycepin effectively reduces IκB expression, with a significant reduction in fluorescence intensity when DENV-2-infected cells were exposed to 200–800 μM cordycepin (Figure 8E and 8F). These findings suggest that cordycepin may inhibit NF-κB activation by interfering with IκB expression at an early stage, thereby modulating its downstream targets and resulting in the reduction of nuclear NF-κB and the suppression of the NF-κB signaling pathway.
Figure 8.
Cordycepin modulates IκB at the early phase of NF-κB signaling
(A) A549 cells were infected with DENV-2 and treated with various concentrations of cordycepin. At 2 h post-treatment, immunoblot analysis was conducted to detect the expression of NF-κB, IκB, and IKKβ in cell lysates.
(B–D) Quantification analysis of NF-κB, IκB, and IKKβ proteins using densitometry scanning with ImageJ software, normalized to untreated DENV-infected cells.
(E) The cellular location of IκB was assessed using immunofluorescence staining, with IκB shown in green and the nucleus in blue.
(F) Mean fluorescence intensity of three to four focal areas on each slide was calculated in triplicate using Zen 2.3 Lite software (Zeiss). Data are represented as means ± SD; n = 3 (∗∗p < 0.01, ∗∗∗p < 0.001).
Molecular profile of NF-κB signaling in cordycepin-treated DENV-2-infected cells
To delve deeper into the mechanisms underlying the antiviral and anti-inflammatory properties of cordycepin, we conducted a molecular profiling of the NF-κB signaling pathway using PCR array. DENV-infected A549 cells were treated with or without 800 μM cordycepin with uninfected cells serving as a parallel control. Total RNA was extracted from the cells, converted to cDNA, and subjected to amplification using a real-time PCR array. A comparative analysis of the expression levels of 84 candidate genes was performed between uninfected (mock) cells and untreated DENV-2-infected cells (Table S2). The results revealed that 46 genes were upregulated more than 2-fold in untreated DENV-2-infected cells, and all of these 46 genes were downregulated upon treatment with cordycepin (Figure 9). Differential expression data from the NF-κB pathway array are presented as fold changes and detailed in Table S2. Among these upregulated genes, CCL5 (RANTES) exhibited the highest expression in response to DENV-2 infection, with a 3,415.78-fold increase compared to mock cells (mock control with no treatment, set as 1). Subsequent treatment of DENV-2-infected cells with 800 μM of cordycepin significantly reduced the expression of CCL5 (RANTES) to 7.71-fold compared to the mock. Additionally, cordycepin significantly downregulated well-characterized inflammatory markers during DENV infection (e.g., ICAM1, CXCL8, TNF, CCL2, CSF1, CSF2, and CSF3), as well as key genes of the NF-κB pathway in both the canonical pathway (NFKB1, NFKBIE, NFKBIA, REL, RELA, TRAF2, RIP1) and the non-canonical pathway (NFKB2, RELB). In conclusion, our findings demonstrate that cordycepin inhibits the production of cytokines and chemokines by targeting the NF-κB signaling pathway.
Figure 9.
PCR array profiling of NF-κB signaling pathway in DENV-infected A549 cells with or without cordycepin treatment
(A) Schematic representation of the real-time PCR setup and array analysis, generated using the BioRender web server.
(B) The data illustrate fold changes in gene expression relative to the uninfected DENV group (mock control with no treatment, set as 1). Dark gray bars depict DENV-infected A549 cells without cordycepin treatment, while red bars represent DENV-infected A549 cells treated with cordycepin.
Discussion
The precise origins of severe dengue remain elusive; nevertheless, compelling evidence supports that an aberrant immune response during the early stages of infection initiates severe disease. In individuals with severe dengue, a notable increase in various inflammatory cytokines and chemokines is observed compared to those experiencing mild infection.9,11,53 This dysregulated and aberrant immune response leads to diminished viral clearance and excessive inflammation, contributing to a cytokine storm. Therefore, an ideal drug for DENV infection treatment should not only effectively inhibit virus replication but also simultaneously suppress cytokine production. Our research group has identified that Cordyceps militaris (CM) extract and its primary bioactive compound, cordycepin, exhibit potent anti-DENV activity in Vero cells.43 However, the anti-inflammatory effects of cordycepin against DENV infection remain unclear. Cordycepin not only demonstrates antiviral activity against DENV but also hinders significant pathogenic RNA viruses, including hepatitis C virus (HCV)42 and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).54 Cordycepin inhibits HCV RNA replication, showcasing its anti-HCV activity.42 In the first in vitro study, cordycepin was found to inhibit SARS-CoV-2 (VOC-202012/01) in Vero E6 cells, targeting all three primary stages of the SARS-CoV-2 infection in humans: virus entry, replication, and the pathogenic stage.54 Based on a computational approach, cordycepin exhibits the highest strong binding affinity with spike protein (S), main protease (Mpro) enzyme,55 and polymerase enzyme (RdRp)56 of SARS-CoV-2. These findings suggest that cordycepin possesses intriguing qualities, notably a broad-spectrum antiviral activity.
In this investigation, we explored the dual role of cordycepin as an antiviral and anti-inflammatory agent in combating DENV infection, while delving into its underlying mechanism. Our findings reveal that cordycepin demonstrates minimal cytotoxicity on A549 cells, with the half-maximal cytotoxicity concentration (CC50) of 1,588.04 ± 115.73 μM (Figures 1 and S4). Notably, cordycepin not only exhibited significant reduction in DENV infection in Vero cells, as observed in a previous study, but also demonstrated efficacy in A549, HepG2, Huh7, and HEK293T cells in the current investigation (Figure S1). This highlights the enduring anti-DENV effect of cordycepin across diverse cellular contexts and suggests a potential target for cordycepin within viral factors.
Our investigation then delved into the mechanism, employing molecular docking and CETSA to substantiate the direct interaction of cordycepin with either DENV or host cellular proteins. CETSA, a label-free method widely employed for drug targeting analysis within intact cells,47,57 revealed that cordycepin enhances the thermal stability of DENV NS5. This indicates its potential to shield the protein from thermally induced aggregation and degradation. However, DENV NS1, with or without cordycepin, displayed no significant change in thermal stability. This observation aligns with evidence showcasing the potent reduction of DENV RNA synthesis by cordycepin. In silico molecular docking further illustrated cordycepin’s binding to DENV NS5 at both the MTase and RdRp domains, crucial for RNA synthesis.43 In line with previous reports, our study demonstrated the binding of cordycepin to His110, Lys105, and Ile147 of MTase, and Arg737, Thr794, Trp795, and Ser796 of RdRp. Interestingly, sequence alignment showed that these binding residues are conserved among all DENV serotypes and other flaviviruses (Figure S6), suggesting the possibility of cross-protective activity of cordycepin. Since all serotypes of DENV naturally co-circulate, the broad activity of cordycepin could improve the clinical benefit of DENV treatment; however, further experiments are needed to confirm this activity. Like other small compounds, the main limitation of cordycepin application is the potential for mutation escape caused by point mutations of the target residues. Alanine substitution of the binding residues of MTase and RdRp affected the orientation of cordycepin at the binding site, resulting in a reduction of binding affinity (Figure S7). Therefore, acquired mutations should be considered, even though the binding residues are highly conserved.
As cordycepin is recognized as a natural analogue of adenosine, concerns arise regarding its potential to inhibit the transcription process and its mutagenic potential during DNA replication in host cells. Our PCR array results showed that cordycepin does not inhibit the transcription process in host cells. This is indicated by the stable expression of at least five housekeeping genes—actin, beta (ACTB), beta-2-microglobulin (B2M), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), hypoxanthine phosphoribosyltransferase 1 (HPRT1), and ribosomal protein, large, P0 (RPLP0)—under both cordycepin treatment and untreated conditions in mock-infected and DENV-2-infected cells. Moreover, cordycepin itself did not show any mutagenic potential in the Ames test.58
Collectively, our results establish that cordycepin hinders DENV replication through a direct interaction with the NS5 protein. Furthermore, cordycepin, recognized as a distinct natural analogue of adenosine, exhibits the potential to inhibit DENV replication by disrupting RNA synthesis, akin to its observed effect in the SARS-CoV-2 model.54 Cordycepin efficiently inhibits SARS-CoV-2 replication by incorporating itself into the newly synthesized RNA strands instead of using actual ATP. This incorporation leads to inducing lethal mutagenesis or reducing the severity of the infection.54
Moreover, it has been established that DENV NS5 induces RANTES expression by modulating the NF-κB signaling pathway.51 The nuclear translocation of NS5 enhances RANTES expression by competitively binding with the host protein death-domain-associated protein (Daxx), thereby releasing NF-κB and augmenting its binding to the RANTES promoter.51,52 In this context, we investigated the impact of cordycepin on RANTES expression in HEK293T cells transiently transfected with FLAG-tagged NS5. Notably, we observed a dose-dependent reduction in RANTES expression with cordycepin treatment, while NS5 expression remained consistent across all conditions (Figure 4). This suggests that cordycepin influences the functionality of NS5. The interaction between cordycepin and NS5 may disrupt the interactions between NS5 and its associated proteins, leading to the inhibition of NF-κB binding to the RANTES promoter and, consequently, a decrease in RANTES expression.
From another perspective, cordycepin may directly interact with host proteins in the NF-κB signaling pathway, thereby ultimately inhibiting NS5-induced RANTES expression. DENV infection, particularly through the DENV NS5 protein, is known to activate NF-κB and release inflammatory molecules.19,51,59 In our study, cordycepin treatment significantly reduced DENV-induced cytokine/chemokine production, including RANTES, IP-10, IL-6, and TNF-α in A549 cells (Figure 10). These results are likely attributed to reduced viral factors, such as NS5, leading to decreased NF-κB activation after exposure to cordycepin (Figure 1). Additionally, the direct impact of cordycepin on NF-κB has been considered, given its inhibitory role in the NF-κB signaling pathway, as proposed in various inflammatory models.46 For instance, Jeong et al. (2010) demonstrated that cordycepin reduces IL-1β and TNF-α by suppressing NF-κB activation in LPS-induced BV2 microglial cells.60 Similar observations were reported in LPS-stimulated RAW 264.7 macrophages35 and in a mouse model of LPS-induced acute lung injury.61 Based on evidence from both in vitro and in vivo studies, we conclude that cordycepin has the potential to reduce cytokine production by suppressing NF-κB activation.
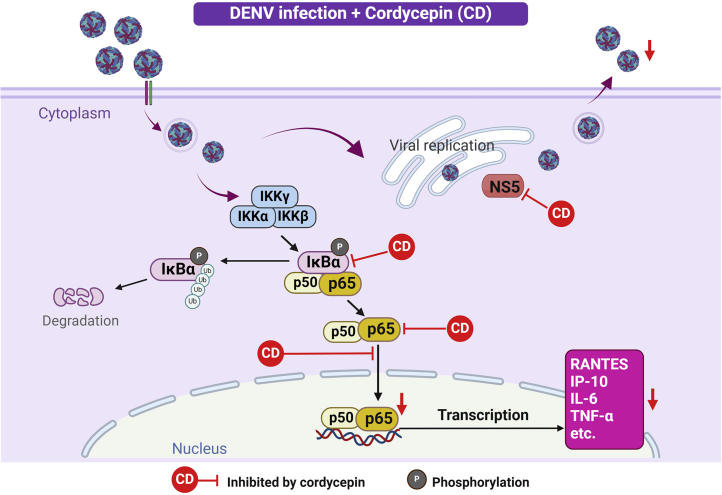
Figure 10.
Schematic diagram illustrating the probable mechanism of cordycepin in DENV-infected A549 cells
Cordycepin inhibits DENV infection, replication and production by directly interacting with the NS5 protein. Additionally, cordycepin influences cytokine/chemokine production through the NF-κB signaling pathway, as indicated by the ones marked with a red line.
Noteworthy is that treating DENV-infected cells with 100 μM of cordycepin did not impact DENV protein and virion production (Figure 1), but it significantly influenced the expression levels of DENV-induced cytokines and chemokines (Figure 5). This suggests that cordycepin can potentially suppress cytokine and chemokine expression through a pathway independent of DENV induction. We conducted additional tests to explore the binding of cordycepin to NF-κB and IκB using CETSA. The results indicated an increase in the thermal stability of NF-κB and IκB compared to the control, suggesting that cordycepin also interacts with NF-κB and IκB (Figure 7). Furthermore, we have unequivocally demonstrated that cordycepin reduces DENV NS5, inhibits NF-κB nuclear translocation, and this association is linked to the inhibition of cytokine/chemokine production (Figure 6).
In our final assessment, we examined the expression profiles of 84 genes associated with the NF-κB signaling pathway in DENV-infected A549 cells treated with cordycepin, comparing them to both uninfected and untreated control cells. Notably, our findings revealed a significant upregulation of the CCL5 gene (RANTES) in untreated DENV-2-infected cells, aligning closely with the clinical characteristics of dengue patients.9,11,62 Surprisingly, among the 46 genes that were upregulated in DENV-2-infected A549 cells, all exhibited downregulation in response to cordycepin treatment. These genes are integral components of both NF-κB signaling pathway and NF-κB responsive pathways (Figures 9 and 10 and Table S2). Collectively, our results strongly indicate that cordycepin exerts potent anti-inflammatory effects by selectively targeting specific proteins, particularly within the canonical pathway. This effect is notably manifested through the inhibition of interleukin-1 receptor-associated kinase 2 (IRAK2) and TNF receptor-associated factor 2 (TRAF2) expression. Consequently, cordycepin interacts with both IκB and NF-κB (p65), hindering the nuclear translocation of NF-κB (p65) and ultimately resulting in the downregulation of inflammatory gene expression (Figures 5, 6, 7, 8, 9, and 10 and Table S2).
In conclusion, cordycepin presents itself as a promising anti-DENV agent with a dual mechanism of action. Firstly, it directly targets the virus by interacting with DENV NS5, disrupting viral replication, and consequently reducing DENV infection and virion production. Simultaneously, cordycepin addresses the inflammatory response triggered by DENV infection. Beyond its impact on viral factors, cordycepin interacts with host elements, specifically NF-κB and IκB, preventing the nuclear translocation of NF-κB and deactivating NF-κB signaling. This dual approach effectively suppresses DENV-induced cytokine and chemokine production. Consequently, cordycepin emerges as a potent anti-DENV agent, offering a comprehensive strategy to alleviate the severity of dengue symptoms.
Limitations of the study
Limitations of this study include the evaluation of cordycepin solely in an in vivo model, which may not fully replicate human responses. The study lacks comprehensive evidence regarding cordycepin’s efficacy in treating dengue-infected animals. Future research should focus on assessing animal survival rates post-infection and cordycepin treatment, measuring viral load, evaluating inflammatory responses, and elucidating cordycepin’s role in viral pathogenesis. These additional investigations are crucial for establishing a more robust understanding of cordycepin’s therapeutic potential in dengue infection.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Pa-thai Yenchitsomanus (ptyench@gmail.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All original data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
The authors express gratitude to Miss. Nunghathai Sawasdee from Siriraj Center of Research Excellence for Cancer Immunotherapy (SiCORE-CIT) and the Division of Molecular Medicine, Research Department, Faculty of Medicine Siriraj Hospital, Mahidol University, for valuable technical advice and the provision of reagents. Additionally, our thanks go to Dr. Sasiprapa Khunchai from the Department of Anatomy, Faculty of Medical Science, Naresuan University, for providing the DENV NS5 plasmid used in this study. This study received support from multiple sources, including the Research Fund of Chulabhorn International College of Medicine under Contract No: G 5/2565 and the Division of Molecular Medicine in the Research Department at the Faculty of Medicine Siriraj Hospital, Mahidol University. Furthermore, financial assistance was provided by the Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma at Thammasat University and partially supported by Chiang Mai University.
Author contributions
Conceptualization: P.Y.; Methodology: P.S., P.Y., and M.T.; Validation: P.S. and M.T.; Formal analysis: P.S., A.P., K.C., and M.T.; Investigation: P.S., A.P., and K.C.; Resources: M.T. and P.Y.; Data curation: P.S.; Writing - Original Draft: P.S., A.P., and M.T.; Writing –Review and Editing: A.P., T.L., S.N., M.T., and P.Y.; Supervision: T.L., S.N., M.T., and P.Y.; Funding Acquisition: M.T. and P.Y.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| mouse anti-dengue E protein clone 4G2 (D1-4G2-4-15) | ATCC | Cat# HB-112; RRID: AB_2940755 |
| mouse anti-dengue virus NS1 glycoprotein (clone DN3) | Abcam | Cat# ab41616; RRID: AB_869448 |
| mouse anti-dengue NS5 (clone GT353) | Invitrogen | Cat# MA5-17295; RRID: AB_2538761 |
| mouse anti-GAPDH (0411) | Santa Cruz | Cat# SC-47724; RRID: AB_627678 |
| mouse anti-FLAG M2 | Sigma-Aldrich | Cat# F1804; RRID: AB_262044 |
| mouse anti-Lamin A (133A2) | Cell Signaling | Cat# 86846; RRID: AB_2800093 |
| rabbit anti-α-tubulin | Cell Signaling | Cat# 2144; RRID: AB_2210548 |
| rabbit anti-NF-κB p65 (D14E12) | Cell Signaling | Cat# 8242; RRID: AB_10859369 |
| rabbit anti-IκB-alpha | Cell Signaling | Cat# 9242; RRID: AB_331623 |
| rabbit anti-IKKβ (D30C6) | Cell Signaling | Cat# 8943; RRID: AB_11024092 |
| HRP-conjugated rabbit anti-mouse | Dako | Cat# P0260; RRID: AB_2636929 |
| HRP-conjugated swine anti-rabbit | Dako | Cat# P0217; RRID: AB_2728719 |
| Cy3-conjugated goat anti-mouse | Invitrogen | Cat# A10521; RRID: AB_10373848 |
| Alexa Fluor 488-conjugated donkey anti-rabbit | Invitrogen | Cat# A21206; RRID: AB_2535792 |
| Bacterial and virus strains | ||
| DENV-2 strain 16681 | provided by the Armed Forces Research Institute of Medical Sciences, Thailand | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM | Gibco | Cat# 12100046 |
| MEM | Gibco | Cat# 61100-053 |
| FBS | Gibco | Cat# 10270098 |
| L-glutamine | Sigma-Aldrich | Cat# G8540 |
| Penicillin | Sigma-Aldrich | Cat# P3032 |
| streptomycin | Sigma-Aldrich | Cat# S9137 |
| Cordycepin | Sigma-Aldrich | Cat# C3394 |
| dexamethasone | Sigma-Aldrich | Cat# D4902 |
| ribavirin | Sigma-Aldrich | Cat# R9644 |
| Popidium iodide | ImmunoTools | Cat# 3149001PI |
| Lipofectamine 2000 | Invitrogen | Cat# 11668-027 |
| Tragacanth gum | Sigma-Aldrich | Cat# G1128 |
| DAB | Sigma-Aldrich | Cat# D5637 |
| paraformaldehyde | Sigma-Aldrich | Cat# P6148 |
| Hoechst 33342 | Invitrogen | Cat# H3570 |
| Trizol reagent | Invitrogen | Cat# 15596026 |
| Western lightning ECL-PRO | PerkinElmer | Cat# NEL121001EA |
| Critical commercial assays | ||
| SuperScriptIII reverse transcriptase | Invitrogen | Cat# 18080-051 |
| SYBR Green master mixes | Bio-Rad laboratories | Cat# 1725124 |
| subcellular protein fractionation kit | Thermo Scientific | Cat# 78840 |
| CBA Human Soluble Protein Flex Set System | BD Biosciences | Cat# 558264 |
| Qubit RNA IQ assay kit | Invitrogen | Cat# Q33221 |
| RNeasy kit | Qiagen | Cat# 74104 |
| RT2 First Strand Kit | Qiagen | Cat# 330404 |
| RT2 SYBR Green qPCR Mastermixes | Qiagen | Cat# 330500 |
| RT2 profiler PCR array for the human NF-κB signaling pathway plus | Qiagen | Cat# 330231/PAHS-025Y |
| Experimental models: Cell lines | ||
| A549 | ATCC | CCL-185 |
| HEK293T | ATCC | CRL-3216 |
| Vero | ATCC | CCL-81 |
| C6/36 | Dr. Sansanee Noisakran | N/A |
| Oligonucleotides | ||
| Primers for RANTES, see Table S1 | Tarasuk et al.19 | N/A |
| Primers for IP-10, see Table S1 | Tarasuk et al.19 | N/A |
| Primers for IL-6, see Table S1 | Tarasuk et al.19 | N/A |
| Primers for TNF-α, see Table S1 | Tarasuk et al.19 | N/A |
| Primers for β-actin, see Table S1 | Tarasuk et al.19 | N/A |
| Recombinant DNA | ||
| Plasmid: pcDNA3.1Hygro | Invitrogen | Cat# V87020 |
| Plasmid: pcDNA3.1Hygro/DENV NS5-Flag | Khunchai S. et al.51 | N/A |
| Software and algorithms | ||
| FCAP Array™ software | BD Biosciences | https://www.bdbiosciences.com/en-us/products/instruments/software-informatics/instrument-software/ |
| CCDC software | Cambridge Crystallographic Data Center, Inc. | https://www.ccdc.cam.ac.uk/structures/ |
| Discovery Studio Visualizer 2020 | Dassault Systèmes SE | https://discover.3ds.com/discovery-studio-visualizer-download-thank-you |
| ImageJ | ImageJ | https://imagej.nih.gov/ij |
| GraphPad Prism 5.0 | GraphPad Software | https://www.graphpad.com/ |
| Zen 2.3 Lite software | Zeiss | https://www.zeiss.com/microscopy/en/products/software/zeiss-zen-lite.html |
| BioEdit software | Informer Technologies, Inc | https://bioedit.software.informer.com/ |
| BioRender | BioRender.com | https://app.biorender.com/ |
Experimental model and study participant details
Cell lines
Human lung epithelial cells (A549; ATCC CCL-185) and human epithelial kidney cells (HEK293T; ATCC CRL-3216) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. African green monkey kidney cells (Vero; ATCC CCL-81) were cultured in Minimum Essential Medium (MEM) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cell lines were maintained at 37°C in a humidified incubator containing 5% CO2.
Virus
Dengue virus serotype 2 (DENV-2) strain 16681 was produced in the C6/36 cell line. Culture supernatants were collected and centrifuged to remove cells debris, then aliquoted and stored at −80°C until use.
Method details
Chemical compounds
Cordycepin (CD) from Cordyceps militaris, dexamethasone (DEX), and ribavirin (RV) were obtained from Sigma-Aldrich MO, USA. Stock of all chemical compounds were aliquoted and stored at −20°C.
Antibodies
The following primary and secondary antibodies were used: mouse anti-dengue E protein clone 4G2 produced from hybridoma cell D1-4G2-4-15 (ATCC), mouse anti-dengue virus NS1 glycoprotein clone DN3 (Abcam, Cambridge, UK), mouse anti-dengue NS5 clone GT353 (Invitrogen Corporation, Carlsbad, CA, USA), mouse anti-GAPDH (Santa Cruz Biotechnology, Dallas, TX, USA), mouse anti-FLAG M2 (Sigma), mouse anti-Lamin A (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-α-tubulin (Cell Signaling Technology), rabbit anti-NF-κB p65 (Cell Signaling Technology), rabbit anti-IκB (Cell Signaling Technology), rabbit anti-IKKβ (Cell Signaling Technology), horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG antibody (Dako, Glostrup, Denmark), HRP-conjugated swine anti-rabbit IgG antibody (Dako), Cy3-conjugated goat anti-mouse IgG antibody (Invitrogen), and Alexa Fluor 488-conjugated donkey anti-rabbit IgG antibody (Invitrogen).
Dengue virus infection, DENV NS5 plasmid transfection and compound treatment
A549 cells were infected with DENV-2 at multiplicities of infection (MOI) at 5 and incubated at 37°C for 2 h (h). Uninfected cells served as a negative control (mock), processed concurrently. Post-infection, cells were washed with PBS and treated with varying concentrations of cordycepin. Harvesting of cells and supernatants occurred at 24 h post-infection (hpi). Cell viability was evaluated through propidium iodide (PI) staining and flow cytometry. Viral RNA levels in infected cells were quantified using real-time RT-PCR. The infectious virus amount and cytokine/chemokine levels in culture supernatants were determined by focus-forming unit (FFU) assay and multiplex bead-based immunoassay, respectively.
HEK293T cells were transfected with pcDNA3.1 Hygro (vector control) or a plasmid containing DENV NS5 with an FLAG tag, using lipofectamine 2000 (Invitrogen), following the manufacturer’s instructions and a previous study.51 Following transfection for 24 h, cells were exposed to various cordycepin concentrations and incubated at 37°C for an additional 24 h. Transfected cells were then collected for the determination of RANTES mRNA expression through real-time RT-PCR.
Focus-forming unit assay
Virus titers were determined through Focus-Forming Unit (FFU) assays in Vero cells, following established protocols.19 In brief, 2.5 × 104 cells per well were seeded in a 96-well plate, and 10-fold serial dilutions of viruses were added. The mixture was then incubated at 37°C for 2 h. Infected Vero cells were overlaid with 1.5% tragacanth gum (Sigma-Aldrich) and further incubated for 3 days. The fixed and permeabilized cells were treated with the 4G2 monoclonal antibody at room temperature (RT) for 1 h. After washing with PBS, the cells were incubated with the HRP-conjugated rabbit anti-mouse IgG antibody (dilution 1:1,000) at RT for 1 h. Foci were visualized by incubation with the 3,3′-diaminobenzidine (DAB) substrate (Sigma-Aldrich) for 5 min (min) at RT. Virus-infected foci were counted, and the virus titers were reported as foci forming units (FFU)/mL.
Immunofluorescence assay
For the immunofluorescence assay (IFA), A549 cells were seeded on coverslips in a 24-well plate for 24 h. Subsequent to infection and compound treatment, cells were fixed with 4% paraformaldehyde (PFA) in PBS for 20 min and permeabilized with 0.2% Triton X-100 in PBS for 10 min. At 24 h post-infection (hpi), cells were rinsed with PBS and then incubated with the primary antibody for 1 h at room temperature (RT). Following PBS washing, cells were treated with the fluorescent dye-conjugated secondary antibody and Hoechst 33342 (Invitrogen), both diluted in 2% FBS in PBS, for 1 h at RT. Fluorescence signals were observed using confocal laser scanning microscopy (LSM 800, Zeiss, Jena, Germany).
Quantification of RNA levels
Total RNA was isolated from the cells using TRIzol reagent (Invitrogen) following the manufacturer’s protocols. Subsequently, the RNA was transcribed into cDNA using SuperScriptIII reverse transcriptase (Invitrogen). Real-time RT-PCR was set up using SYBR Green master mixes (Bio-Rad laboratories, Hercules, CA, USA) with specific primers for human RANTES, IP-10, IL-6, TNF-α, and β-actin (Table S1) and cDNA templates. The real-time RT-PCR was conducted utilizing the LightCycler 480 instrument (Roche Diagnostics, Basel, Switzerland). The cycle threshold (Ct) values of each gene were normalized with β-actin and analyzed by comparison with DENV-infected cells without treatment.
Cellular thermal shift assay (CETSA)
This assay followed established methods as described previously.47,57,63 In brief, DENV-2-infected A549 cells were trypsinized, washed with PBS, and subsequently resuspended in PBS supplemented with protease inhibitors. The cells were lysed on ice through sonication and then subjected to centrifugation at 20,000 × g at 4°C for 20 min. The cell lysate was divided equally into two aliquots and incubated with 800 μM cordycepin or its diluent control at room temperature for 30 min. The resulting mixture was dispensed into eight PCR tubes, which were heated at different temperatures for 3 min (Biometra Tprofessional Basic Gradient 96 Thermal Cycler, Analytik Jena AG), followed by cooling at room temperature for 3 min. The heated samples underwent centrifugation at 20,000 × g at 4°C for 20 min, and the supernatants were transferred to new tubes for subsequent analysis by immunoblotting method.
Molecular docking
The MOL2 format of cordycepin (CCDC number 129360, CSD Entry: CORDCP01) was acquired from CCDC software.64 The structure underwent preparation and conversion to pdbqt format using AutoDock Tools version 1.5.6.65 The crystal structure of DENV-2 MTase (PDB entry 1R6A) and DENV-3 RdRp (PDB entry 5I3P) were obtained from the Protein Data Bank.66 After eliminating water and inhibitors, the proteins were readied as targets with grid box sizes of 30 × 40×40 and 20 × 20 × 24 Å, respectively, to encompass the designated ligand binding sites. Molecular docking was executed to illustrate cordycepin’s interaction with DENV NS5 MTase and RdRp proteins, utilizing the rigid docking of Autodock Vina. The intermolecular interactions were analyzed, and the docking poses were prepared using Discovery Studio Visualizer 2020 (Dassault Systèmes SE, Vélizy-Villacoublay, France).
Subcellular fractionation
The isolation of cytosol and nuclear fractions from A549 cells infected with DENV-2 and treated with cordycepin was carried out using the Thermo Scientific subcellular protein fractionation kit (Waltham, MA, USA). A549 cells were initially plated in a 6-well plate with approximately 1 × 106 cells per well. Subsequently, the treated cells were pelleted, and a cold cytoplasmic extraction buffer (CEB) totaling 200 μL was used to resuspend the cells. The homogenate was gently mixed at 4°C for 10 min and then subjected to centrifugation at 500 × g for 5 min at 4°C. The resulting supernatant constituted the cytosolic fraction. The pellet containing the nuclei underwent incubation at 4°C for 10 min in a cold membrane extraction buffer (MEB) and was then centrifuged at 3,000 × g for 5 min at 4°C. Subsequently, the pellet was subjected to a further 30-min incubation at 4°C in a cold nuclear extraction buffer (NEB). The samples were then centrifuged at 5,000 × g for 5 min at 4°C, resulting in the isolation of the nuclear fraction. Finally, both cytoplasmic and nuclear fractions were analyzed using immunoblotting method.
Multiplex bead-based immunoassay
To assess cytokine/chemokine secretion in DENV-infected A549 cells with or without cordycepin treatment, the levels of RANTES, IP-10, IL-6, and TNF-α were measured in culture supernatants at 24 hpi using the BD CBA Human Soluble Protein Flex Set System (BD Biosciences, San Diego, CA, USA). Analysis was performed using the BD Accuri C6 Flow Cytometer in accordance with the manufacturer’s instructions. Flow cytometry standard data files were processed using FCAP Array software (BD Biosciences) to generate standard curves and calculate sample concentrations.
Immunoblot analysis
Cell lysis was achieved by incubating cells in RIPA buffer (1% Triton X-100, 20 mM Tris-HCl pH 7.5, 5 mM EDTA, 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate) on ice for 30 min, followed by mixing with loading buffer (50 mM Tris-HCl pH 6.8, 2% SDS, 0.1% bromophenol blue and 10% glycerol in the presence or absence of 5% β-mercaptoethanol). The resulting protein samples were separated via 10% SDS-PAGE and transferred onto nitrocellulose membranes. After blocking with 5% skimmed milk in PBS, the membranes were subjected to overnight incubation at 4°C with primary antibodies. Subsequently, the membranes underwent PBS washes and were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature, followed by visualization using chemiluminescence substrate (Western lightning ECL-PRO, PerkinElmer, Waltham, MA, USA).
Real-time PCR array
Mock and DENV-2-infected A549 cells, either treated or untreated with 800 μM cordycepin, were prepared following the previously outlined procedure. Total RNA was extracted using the RNeasy Kit (Qiagen, Hilden Germany). The Qubit RNA IQ assay kit with the Qubit 4 Fluorometer (Invitrogen) was utilized to assess RNA quality and concentration. Initially, 1 μg of RNA sample underwent transcription into cDNA using the RT2 First Stand Kit (Qiagen). Subsequently, the cDNA was combined with RT2 SYBR Green qPCR Mastermix (Qiagen). Each PCR component mixture was then added to the RT2 profiler PCR array for the human NF-κB signaling pathway plus (PAHS-025Y, Qiagen), encompassing 84 genes associated with the NF-κB signaling pathway, along with a panel of 5 housekeeping genes (ACTB, B2M, GAPDH, HPRT1 and RPLP0) for data normalization. The real-time PCR cycling program was executed using a Roche LightCycler 480 (Roche Diagnostics). Ct values were exported to an Excel file and uploaded onto the web portal http://www.qiagen.com/geneglobe. The results are presented as fold increase or decrease relative to the mock with no treatment (control group set as 1).
Quantification and statistical analysis
All data were analyzed based on a minimum of three independent experiments utilizing GraphPad Prism version 5.0 (GraphPad Software, CA, USA). The results are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was conducted, followed by Tukey’s multiple comparison test to evaluate differences between groups. p-values less than 0.05 were deemed statistically significant. Significant differences from the relevant control are indicated by asterisks (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001).
Published: August 13, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110711.
Contributor Information
Mayuri Tarasuk, Email: mayuri.tarasuk@gmail.com.
Pa-thai Yenchitsomanus, Email: ptyench@gmail.com.
Supplemental information
References
- 1.Bhatt P., Sabeena S.P., Varma M., Arunkumar G. Current Understanding of the Pathogenesis of Dengue Virus Infection. Curr. Microbiol. 2021;78:17–32. doi: 10.1007/s00284-020-02284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paranjape S.M., Harris E. Control of dengue virus translation and replication. Curr. Top. Microbiol. Immunol. 2010;338:15–34. doi: 10.1007/978-3-642-02215-9_2. [DOI] [PubMed] [Google Scholar]
- 4.Barrows N.J., Campos R.K., Liao K.C., Prasanth K.R., Soto-Acosta R., Yeh S.C., Schott-Lerner G., Pompon J., Sessions O.M., Bradrick S.S., Garcia-Blanco M.A. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018;118:4448–4482. doi: 10.1021/acs.chemrev.7b00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perera R., Kuhn R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008;11:369–377. doi: 10.1016/j.mib.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller D.A., Young P.R. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013;98:192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., Jones C.T., Mukhopadhyay S., Chipman P.R., Strauss E.G., et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman M.G., Alvarez M., Halstead S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 9.Srikiatkhachorn A., Mathew A., Rothman A.L. Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol. 2017;39:563–574. doi: 10.1007/s00281-017-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozza F.A., Cruz O.G., Zagne S.M.O., Azeredo E.L., Nogueira R.M.R., Assis E.F., Bozza P.T., Kubelka C.F. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect. Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John D.V., Lin Y.-S., Perng G.C. Biomarkers of severe dengue disease – a review. J. Biomed. Sci. 2015;22:83. doi: 10.1186/s12929-015-0191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avirutnan P., Malasit P., Seliger B., Bhakdi S., Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
- 13.Limonta D., Capó V., Torres G., Pérez A.B., Guzmán M.G. Apoptosis in tissues from fatal dengue shock syndrome. J. Clin. Virol. 2007;40:50–54. doi: 10.1016/j.jcv.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Hadinegoro S.R., Arredondo-García J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R., Muhammad Ismail H.I.H., Reynales H., Limkittikul K., Rivera-Medina D.M., et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 15.Rivera L., Biswal S., Sáez-Llorens X., Reynales H., López-Medina E., Borja-Tabora C., Bravo L., Sirivichayakul C., Kosalaraksa P., Martinez Vargas L., et al. Three-year Efficacy and Safety of Takeda’s Dengue Vaccine Candidate (TAK-003) Clin. Infect. Dis. 2022;75:107–117. doi: 10.1093/cid/ciab864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres-Flores J.M., Reyes-Sandoval A., Salazar M.I. Dengue Vaccines: An Update. BioDrugs. 2022;36:325–336. doi: 10.1007/s40259-022-00531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salje H., Alera M.T., Chua M.N., Hunsawong T., Ellison D., Srikiatkhachorn A., Jarman R.G., Gromowski G.D., Rodriguez-Barraquer I., Cauchemez S., et al. Evaluation of the extended efficacy of the Dengvaxia vaccine against symptomatic and subclinical dengue infection. Nat. Med. 2021;27:1395–1400. doi: 10.1038/s41591-021-01392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Arruda T.B., Bavia L., Mosimann A.L.P., Aoki M.N., Sarzi M.L., Conchon-Costa I., Wowk P.F., Duarte Dos Santos C.N., Pavanelli W.R., Silveira G.F., Bordignon J. Viremia and Inflammatory Cytokines in Dengue: Interleukin-2 as a Biomarker of Infection, and Interferon-α and -γ as Markers of Primary versus Secondary Infection. Pathogens. 2023;12 doi: 10.3390/pathogens12111362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarasuk M., Songprakhon P., Chieochansin T., Choomee K., Na-Bangchang K., Yenchitsomanus P.T. Alpha-mangostin inhibits viral replication and suppresses nuclear factor kappa B (NF-κB)-mediated inflammation in dengue virus infection. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-20284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarasuk M., Songprakhon P., Chimma P., Sratongno P., Na-Bangchang K., Yenchitsomanus P.T. Alpha-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 2017;240:180–189. doi: 10.1016/j.virusres.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Panda K., Alagarasu K., Patil P., Agrawal M., More A., Kumar N.V., Mainkar P.S., Parashar D., Cherian S. In Vitro Antiviral Activity of α-Mangostin against Dengue Virus Serotype-2 (DENV-2) Molecules. 2021;26 doi: 10.3390/molecules26103016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panraksa P., Ramphan S., Khongwichit S., Smith D.R. Activity of andrographolide against dengue virus. Antiviral Res. 2017;139:69–78. doi: 10.1016/j.antiviral.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Paemanee A., Hitakarun A., Wintachai P., Roytrakul S., Smith D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed. Pharmacother. 2019;109:322–332. doi: 10.1016/j.biopha.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 24.Li F., Khanom W., Sun X., Paemanee A., Roytrakul S., Wang D., Smith D.R., Zhou G.C. Andrographolide and Its 14-Aryloxy Analogues Inhibit Zika and Dengue Virus Infection. Molecules. 2020;25 doi: 10.3390/molecules25215037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altamish M., Khan M., Baig M.S., Pathak B., Rani V., Akhtar J., Khan A.A., Ahmad S., Krishnan A. Therapeutic Potential of Medicinal Plants against Dengue Infection: A Mechanistic Viewpoint. ACS Omega. 2022;7:24048–24065. doi: 10.1021/acsomega.2c00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitby K., Pierson T.C., Geiss B., Lane K., Engle M., Zhou Y., Doms R.W., Diamond M.S. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J. Virol. 2005;79:8698–8706. doi: 10.1128/jvi.79.14.8698-8706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe S., Rathore A.P.S., Sung C., Lu F., Khoo Y.M., Connolly J., Low J., Ooi E.E., Lee H.S., Vasudevan S.G. Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antiviral Res. 2012;96:32–35. doi: 10.1016/j.antiviral.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y.H., Tseng C.K., Wu H.C., Wei C.K., Lin C.K., Chen I.S., Chang H.S., Lee J.C. Avocado (Persea americana) fruit extract (2R,4R)-1,2,4-trihydroxyheptadec-16-yne inhibits dengue virus replication via upregulation of NF-κB-dependent induction of antiviral interferon responses. Sci. Rep. 2019;9:423. doi: 10.1038/s41598-018-36714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yongpitakwattana P., Morchang A., Panya A., Sawasdee N., Yenchitsomanus P.T. Alpha-mangostin inhibits dengue virus production and pro-inflammatory cytokine/chemokine expression in dendritic cells. Arch. Virol. 2021;166:1623–1632. doi: 10.1007/s00705-021-05017-x. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham K.G., Manson W., Spring F.S., Hutchinson S.A. Cordycepin, a Metabolic Product isolated from Cultures of Cordyceps militaris (Linn.) Link. Nature. 1950;166:949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- 31.Qin P., Li X., Yang H., Wang Z.-Y., Lu D. Therapeutic Potential and Biological Applications of Cordycepin and Metabolic Mechanisms in Cordycepin-Producing Fungi. Molecules. 2019;24:2231. doi: 10.3390/molecules24122231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H., Naura A.S., Errami Y., Ju J., Boulares A.H. Cordycepin blocks lung injury-associated inflammation and promotes BRCA1-deficient breast cancer cell killing by effectively inhibiting PARP. Mol. Med. 2011;17:893–900. doi: 10.2119/molmed.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui Z.Y., Park S.J., Jo E., Hwang I.H., Lee K.B., Kim S.W., Kim D.J., Joo J.C., Hong S.H., Lee M.G., Jang I.S. Cordycepin induces apoptosis of human ovarian cancer cells by inhibiting CCL5-mediated Akt/NF-κB signaling pathway. Cell Death Discov. 2018;4:62. doi: 10.1038/s41420-018-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan M.A., Tania M. Cordycepin and kinase inhibition in cancer. Drug Discov. Today. 2023;28 doi: 10.1016/j.drudis.2022.103481. [DOI] [PubMed] [Google Scholar]
- 35.Choi Y.H., Kim G.Y., Lee H.H. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des. Devel. Ther. 2014;8:1941–1953. doi: 10.2147/dddt.S71957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin S., Moon S., Park Y., Kwon J., Lee S., Lee C.K., Cho K., Ha N.J., Kim K. Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines. Immune Netw. 2009;9:255–264. doi: 10.4110/in.2009.9.6.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W., Zhang L., Sun S., Tang L.-s., He S.-m., Chen A.-q., Yao L.-n., Ren D.-L. Cordycepin inhibits inflammatory responses through suppression of ERK activation in zebrafish. Dev. Comp. Immunol. 2021;124 doi: 10.1016/j.dci.2021.104178. [DOI] [PubMed] [Google Scholar]
- 38.Olatunji O.J., Feng Y., Olatunji O.O., Tang J., Ouyang Z., Su Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed. Pharmacother. 2016;81:7–14. doi: 10.1016/j.biopha.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Ramesh T., Yoo S.-K., Kim S.-W., Hwang S.-Y., Sohn S.-H., Kim I.-W., Kim S.-K. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp. Gerontol. 2012;47:979–987. doi: 10.1016/j.exger.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Lee C.-T., Huang K.-S., Shaw J.-F., Chen J.-R., Kuo W.-S., Shen G., Grumezescu A.M., Holban A.M., Wang Y.-T., Wang J.-S., et al. Trends in the Immunomodulatory Effects of Cordyceps militaris: Total Extracts, Polysaccharides and Cordycepin. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.575704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radhi M., Ashraf S., Lawrence S., Tranholm A.A., Wellham P.A.D., Hafeez A., Khamis A.S., Thomas R., McWilliams D., de Moor C.H. A Systematic Review of the Biological Effects of Cordycepin. Molecules. 2021;26:5886. doi: 10.3390/molecules26195886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueda Y., Mori K., Satoh S., Dansako H., Ikeda M., Kato N. Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 2014;447:341–345. doi: 10.1016/j.bbrc.2014.03.150. [DOI] [PubMed] [Google Scholar]
- 43.Panya A., Songprakhon P., Panwong S., Jantakee K., Kaewkod T., Tragoolpua Y., Sawasdee N., Lee V.S., Nimmanpipug P., Yenchitsomanus P.T. Cordycepin Inhibits Virus Replication in Dengue Virus-Infected Vero Cells. Molecules. 2021;26 doi: 10.3390/molecules26113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jessie K., Fong M.Y., Devi S., Lam S.K., Wong K.T. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 45.Lee Y.-R., Su C.-Y., Chow N.-H., Lai W.-W., Lei H.-Y., Chang C.-L., Chang T.-Y., Chen S.-H., Lin Y.-S., Yeh T.-M., Liu H.-S. Dengue viruses can infect human primary lung epithelia as well as lung carcinoma cells, and can also induce the secretion of IL-6 and RANTES. Virus Res. 2007;126:216–225. doi: 10.1016/j.virusres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez Molina D., Jafari R., Ignatushchenko M., Seki T., Larsson E.A., Dan C., Sreekumar L., Cao Y., Nordlund P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341:84–87. doi: 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 48.Lim S.P., Noble C.G., Seh C.C., Soh T.S., El Sahili A., Chan G.K.Y., Lescar J., Arora R., Benson T., Nilar S., et al. Potent Allosteric Dengue Virus NS5 Polymerase Inhibitors: Mechanism of Action and Resistance Profiling. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tambunan U.S.F., Nasution M.A.F., Azhima F., Parikesit A.A., Toepak E.P., Idrus S., Kerami D. Modification of S-Adenosyl-l-Homocysteine as Inhibitor of Nonstructural Protein 5 Methyltransferase Dengue Virus Through Molecular Docking and Molecular Dynamics Simulation. Drug Target Insights. 2017;11 doi: 10.1177/1177392817701726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benarroch D., Egloff M.P., Mulard L., Guerreiro C., Romette J.L., Canard B. A structural basis for the inhibition of the NS5 dengue virus mRNA 2'-O-methyltransferase domain by ribavirin 5'-triphosphate. J. Biol. Chem. 2004;279:35638–35643. doi: 10.1074/jbc.M400460200. [DOI] [PubMed] [Google Scholar]
- 51.Khunchai S., Junking M., Suttitheptumrong A., Kooptiwut S., Haegeman G., Limjindaporn T., Yenchitsomanus P.T. NF-κB is required for dengue virus NS5-induced RANTES expression. Virus Res. 2015;197:92–100. doi: 10.1016/j.virusres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Khunchai S., Junking M., Suttitheptumrong A., Yasamut U., Sawasdee N., Netsawang J., Morchang A., Chaowalit P., Noisakran S., Yenchitsomanus P.T., Limjindaporn T. Interaction of dengue virus nonstructural protein 5 with Daxx modulates RANTES production. Biochem. Biophys. Res. Commun. 2012;423:398–403. doi: 10.1016/j.bbrc.2012.05.137. [DOI] [PubMed] [Google Scholar]
- 53.McBride W.J., Bielefeldt-Ohmann H. Dengue viral infections; pathogenesis and epidemiology. Microbes Infect. 2000;2:1041–1050. doi: 10.1016/s1286-4579(00)01258-2. [DOI] [PubMed] [Google Scholar]
- 54.Rabie A.M. Potent Inhibitory Activities of the Adenosine Analogue Cordycepin on SARS-CoV-2 Replication. ACS Omega. 2022;7:2960–2969. doi: 10.1021/acsomega.1c05998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma A.K., Aggarwal R. Repurposing potential of FDA-approved and investigational drugs for COVID-19 targeting SARS-CoV-2 spike and main protease and validation by machine learning algorithm. Chem. Biol. Drug Des. 2021;97:836–853. doi: 10.1111/cbdd.13812. [DOI] [PubMed] [Google Scholar]
- 56.Bibi S., Hasan M.M., Wang Y.B., Papadakos S.P., Yu H. Cordycepin as a Promising Inhibitor of SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp) Curr. Med. Chem. 2022;29:152–162. doi: 10.2174/0929867328666210820114025. [DOI] [PubMed] [Google Scholar]
- 57.Jafari R., Almqvist H., Axelsson H., Ignatushchenko M., Lundbäck T., Nordlund P., Martinez Molina D. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 2014;9:2100–2122. doi: 10.1038/nprot.2014.138. [DOI] [PubMed] [Google Scholar]
- 58.Cho M.A., Lee D.S., Kim M.J., Sung J.M., Ham S.S. Antimutagenicity and cytotoxicity of cordycepin isolated from Cordyceps militaris. Food Sci. Biotechnol. 2003;12:472–475. [Google Scholar]
- 59.Medin C.L., Fitzgerald K.A., Rothman A.L. Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J. Virol. 2005;79:11053–11061. doi: 10.1128/jvi.79.17.11053-11061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong J.W., Jin C.Y., Kim G.Y., Lee J.D., Park C., Kim G.D., Kim W.J., Jung W.K., Seo S.K., Choi I.W., Choi Y.H. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2010;10:1580–1586. doi: 10.1016/j.intimp.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Lei J., Wei Y., Song P., Li Y., Zhang T., Feng Q., Xu G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018;818:110–114. doi: 10.1016/j.ejphar.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 62.Islam M., Kalita T., Saikia A.K., Begum A., Baruah V., Singh N., Borkotoky R., Bose S. Significance of RANTES-CCR5 axis and linked downstream immunomodulation in Dengue pathogenesis: A study from Guwahati, India. J. Med. Virol. 2019;91:2066–2073. doi: 10.1002/jmv.25561. [DOI] [PubMed] [Google Scholar]
- 63.Jensen A.J., Martinez Molina D., Lundbäck T. CETSA: a target engagement assay with potential to transform drug discovery. Future Med. Chem. 2015;7:975–978. doi: 10.4155/fmc.15.50. [DOI] [PubMed] [Google Scholar]
- 64.Centre, T.C.C.D. CCDC software. https://www.ccdc.cam.ac.uk.
- 65.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Information N.C.f.B. PubChem Compound Summary for CID 6303, Cordycepin. 2004. https://pubchem.ncbi.nlm.nih.gov/compound/Cordycepin
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All original data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.