Abstract
The effect of cooking aerosol on the human heart was investigated in this study. The heart rate and blood pressure of 33 healthy adults were monitored before, exactly after, and two hours post-exposure (30 minutes, 60 minutes, 90 minutes, and 120 minutes after cooking). One hundred twenty grams of ground beef was fried in sunflower oil for twenty minutes using a gas stove without ventilation. Ultrafine particles, indoor temperature, relative humidity, carbon dioxide, oil, and meat temperatures were monitored during the experiment. The average particle emission rate (S) and average decay rate (a+k) for meat frying were found to be 2.09×1013 (SD=3.94 ×1013, R2=0.98, P <0.0001) particles/min, and 0.055 (SD=0.019, R2=0.91, P <0.0001) particles/min, respectively. No statistically significant changes in diastolic blood pressure (DBP) and heart rate (HR) were observed. The average systolic blood pressure (SBP) statistically significantly increased from 98 mmHg (before the exposure) to 106 mmHg 60 minutes after the exposure. The results suggested that frying emission statistically significantly impacted blood pressure.
Keywords: Frying aerosol, Ultrafine particles, Systolic blood pressure, Diastolic blood pressure, Heart rate
Graphical Abstract
Highlights
-
•
Systolic blood pressure increased 60 minutes after exposure to frying aerosol.
-
•
Observed no significant changes in diastolic blood pressure.
-
•
Observed no significant changes in heart rate.
1. Introduction
Investigating indoor air quality is important as people spend most of their time indoors [1]. Emissions from a variety of indoor particle sources were reported in the literature. Studies identified tobacco smoking, cooking, kerosene heating, and wood burning as the most significant particular matter (PM) sources [2]. Among all sources of indoor PM, cooking has been recognized to be among the highest emitting sources of airborne particles [4], [5], [6], [7], [3]. Cooking can produce particles with concentrations 10 times higher than the background levels [8], [9]. The number of particles produced during cooking can be affected by cooking type, fuel, and ingredients [10], [12], [13], [14], [11], [15]. Studies showed that the concentrations of ultrafine particles (UFPs) and PM2.5 during cooking with oil, such as frying, are higher than cooking with water [16], [17]. It has been demonstrated in the literature that cooking with a gas stove results in higher concentrations of UFPs and PM than cooking using an electric stove [19], [18], [20], [21], [22], [23], [17].
Recent studies discovered particles emitted during different cooking activities could be smaller than 10 nm. For example, particles emitted from stir-frying using a gas stove and electric hot plate were reported to be as small as 1 nm[24]. Also, the average mode diameter values that have been observed for gas stove particles ranged from 4.4 to 7 nm and for the electric stove, from 3.2 to 22 nm [23]. Amouei Torkmahalleh et al. [10] characterized particle size distribution as small as 10 nm for seven different cooking oils and showed the heated oil is a particle source [18]. Saito et al. [25] showed that fat in the meat was the main component that generated Polycyclic Aromatic Hydrocarbons (PAHs) during thermal cooking. The percentages of total PAHs in all particles with diameters <0.43 µm were found to be 61 % in trout, 77 % in beef, and 66 % in pork. Frying beefsteak in a room equipped with a gas stove hood using margarine produced significantly higher levels of aldehydes than virgin olive oil, soybean oil, and rapeseed oil. The quantified mean concentration of trans, trans-2, 4-decadienal, 2, 4-decenal, trans-2-decenal, 2-undecenal, alkanals, and alkenals were 10.33 µg/m3, 25.33 µg/m3, 25.33 µg/m3, 20.67 µg/m3, 426 µg/m3 and 55.7 µg/m3, respectively, during frying with margarine [26]. Particles can enter the blood circulation and translocate to other organs, such as the heart [27], [28], [29]. Kreyling et al. [28] reported that insoluble iridium UFPs were transported to the extrapulmonary organs, including the heart and brain. Manganese was demonstrated to be translocated to the brain through blood circulation. The translocation rate of particles into the blood circulation could vary with particle composition, size, and morphology [27].
Several studies showed cooking as a source of fine and ultrafine particles that can affect brain activity and respiratory and cardiovascular systems [31], [32], [33], [34], [30]. Several clinical studies investigated the cardiovascular impact of cooking fumes through inhalation. These studies monitored heart rate [35] and blood pressure [36], [32], [37] before, during, and several hours after exposure. Some findings reported statistically significant increases in systolic blood pressure (SBP) during the post-exposure period, but no changes in diastolic blood pressure (DBP) were reported. Cosselman et al. [38] investigated the effect of diesel exhaust particles on humans. They showed an increase in systolic blood pressure (SBP) 30–60 minutes post-exposure, but no statistically significant changes in diastolic blood pressure (DBP) were observed. Fedak et al. [39] compared the SBP and DBP changes after exposure to different cook stove fumes and concluded an increase in SBP and no changes in DBP after 24 hours post-exposure. Soppa et al. [37] investigated the blood pressure changes of 54 healthy volunteers with exposure to PM from candle burning, toasting bread, and frying sausages. One hour after the exposure to particles from toasting bread, SBP increased by 1.5 and 2.2 mmHg per 10 µg/m3 increase in PM10 and PM2.5, respectively. Particles emitted during candle burning and frying sausages did not affect the blood pressure. Gabdrashova et al. [32] reported increased SBP 90–120 minutes after exposure to UFPs from frying beef on an electric stove. The authors reported there were no changes in DBP.
Hypertension, commonly known as high blood pressure, is a prevalent medical condition affecting millions worldwide. It is a chronic disorder characterized by elevated blood pressure levels, and if left untreated, it can lead to severe health complications. According to the World Health Organization (WHO), hypertension is a leading risk factor for cardiovascular diseases including coronary artery disease, heart failure, and stroke [40], and renal impairment. As one of the leading causes of preventable morbidity and mortality worldwide, hypertension demands careful attention and comprehensive research to understand its underlying mechanisms and clinical implications [41]. Hypertension significantly impacts various organ systems, posing serious health risks to affected individuals. Sustained elevated blood pressure levels can lead to a myriad of clinical complications, making it a critical public health concern. Furthermore, it plays a pivotal role in developing and progressing chronic kidney disease, leading to renal impairment and ultimately requiring dialysis or transplantation in severe cases [42]. Additionally, hypertension contributes to vascular damage, leading to retinopathy, a condition affecting the eyes' blood vessels and potentially causing visual impairment [43]. Moreover, its adverse effects extend to the central nervous system, with evidence linking hypertension to cognitive decline and an increased risk of developing dementia [44].
One important potential confounding bias due to study protocol in some studies was that study participants were outside the study facility during the post-exposure time, which might have exposed them to other cooking sources, especially during their stay at home and traffic emissions during commuting. Thus, a study protocol that avoids outside uncontrolled exposures during the study period can produce more reliable and valid results. The objective of the present study was to investigate the cardiovascular impact of exposure to UFPs from gas stove cooking up to 2 hours post-exposure through a controlled clinical study. This investigation employed a protocol that prevents study participants from being exposed to PM from outside sources other than cooking during the study period. This paper aims to delve into the clinical impacts of hypertension on these vital organ systems, highlighting the urgency for early detection, effective management, and timely intervention to mitigate the detrimental consequences associated with this prevalent medical condition.
2. Materials and methods
Study Participants: Thirty-three physically and mentally healthy adults (between 18 and 65 years old) were recruited for this study In the first step, potential study volunteers were asked to complete an interview form, being informed of this process in the study recruitment advertisements. Then the research team reviewed the interview forms, and unqualified participants were excluded according to previously established health criteria. The interview form (FS1) described the detailed criteria for inclusion and exclusion. Non-healthy people (suffer from respiratory, cardiovascular, nervous disease or mental disease), smokers (cigarette, Shisha, etc.), alcohol users, pregnant women, drug addicts, and stressed people were excluded. Data on potential study volunteers' lifestyles, such as physical activities, cooking habits, and general health conditions, were collected in the interview form (FS1).
Eligible study volunteers were provided with a consent form that informed them of the aim and procedures of the study, including schedule, research facility address, and cooking protocols; and information concerning the right to withdraw their participation and information, including their health condition and test results, from the database at any time during the study. Those who signed the consent form (FS2) were invited to participate in the study. The study participants' characteristics listed in Table 1 represent the entire group eligible for the following analyses. The ethics committee of Nazarbayev University has approved the procedure of this study under approval code 115/12022019.
Table 1.
Personal and exposure session characteristics of 33 study participants.
| Personal characteristic | Measure |
|---|---|
| Age [years], mean (SD) | 38 (14.8) |
| Female, n (%) | 66 % |
| Weight [kg], mean (SD) | 68.9 (13.1) |
| Height [cm], mean (SD) | 165.3 (8.0) |
| BMI, mean (SD) | 25.4 (4.5) |
| Baseline blood pressure | |
| Systolic, mean (SD) | 97.9 (13.5) |
| Diastolic, mean (SD) | 70.8 (9.5) |
| Heart rate, mean (SD) | 81 (6.5) |
| Participant exposure characteristic | |
| Indoor temperature,°C, mean (SD) | 26.7 (2.1) |
| Indoor humidity, %, mean (SD) | 46.0 (8.5) |
Environmental Measurements: Indoor temperature, CO2 concentration, and relative humidity were monitored using a Smart Meter model AZ-7755, with 1 min manual logging intervals. A NanoTracer (Philips Aerasense, Netherlands) was utilized to measure particle number concentrations in the 10–300 nm range up to 106 particles/cm3 at 10-second intervals. During the measurements, NanoTracer was placed next to the study participants. A digital thermometer (Model 54IIB, Fluke, Everett, USA) equipped with two K-type thermocouple probes (Model THS-103–020, ThermoWorks, USA) was used to monitor the oil and chicken temperatures with 1-minute logging intervals. At the end of the experiment day, the doors and windows were opened such that the concentration of particles declined to the background level, and the room was ready for the next study participants.
Effect assessments: Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were measured with an Omron10 Model BP786N. This Omron was previously compared with a reference blood pressure clinical cuff and good agreement was obtained [32]. Blood pressure and heart rate were measured in the sitting positions six times, including 30 minutes after the study participants arrived at the experiment house (step 1), at the end of cooking (step 2), 30 minutes after the cooking (step 3), one hour after the cooking (step 4), 90 minutes after the cooking (step 5), and two hours after the cooking (step 6). The cooking experiments commenced immediately after step 1. The rationale for this measurement schedule was to ensure that any significant changes in blood pressure during post-exposure were not overlooked. We’ve classified the systolic blood pressure as normal (SBP < 120), elevated (120 < SBP <130), stage 1 high blood pressure (130 < SBP < 140), and stage 2 high blood pressure (SBP > 140) and diastolic blood pressure to normal (DBP < 80), stage 1 high blood pressure (80 < DBP < 89), and stage 2 high blood pressure (DBP > 90) [40].
Experimental Protocol: The experiment was conducted in a kitchen in a two-bedroom apartment equipped with a gas stove. The kitchen volume was 18.75 m3. The doors and windows were closed during the experiment to limit the penetration of outdoor particles into the experimental house. Lack of thermal comfort could be an important element for residents, contributing to cardiovascular and respiratory problems [45]. However, the average ambient temperature and humidity were 26.7 °C and 46 % during the experiments, respectively, in the recommended range according to the ASHRAE 55–2013 standard [46] for indoor air temperature and humidity level. One to three participants stayed at the apartment daily to participate in the experiments. Study participants sat on a sofa in the living room during the experiments and were allowed to talk or read to avoid monotony. A researcher performed cooking, while study participants were asked to sit next to the stove only during the cooking. Other indoor sources and volatile organic compounds (VOCs), such as detergents, food additives, perfumes, and cosmetic materials, were prohibited during the experiment to exclude the other sources of particles. Particle number concentrations were measured during the experiments. The NanoTracer was next to the study participants before, during, and after the exposure to assess their exposure to UFPs.
On each experiment day, two or more volunteers participated in the experiments simultaneously. A hundred grams of low-fat ground beef (according to the seller) which is widely used during cooking at homes and restaurants, 25 g shredded onion, 1 g pepper, 1 g turmeric, and 1 g salt were mixed. Three pan kebabs (each 40 g) were made from this mixture, weighing approximately 40 g each. Clean gloves were used to prepare pan kebabs. A PTFE-coated aluminum pan (25 cm diameter) was heated on a gas stove for 2 minutes, and then 21 ml sunflower oil was added to the pan. Heating the empty pan did not produce significant emissions of particles larger than 10 nm; thus, PNC did not increase. The background concentration was on average 11,300 particles/cm3 and reached 11,400 particles/cm3 during the heating of the empty pan (P value=0.582, CL-95 %). After six minutes, three pieces of pan kebabs were placed into the pan. Pan kebabs were turned over three times at the 11th, 14th, and 17th-minute marks from the start of cooking. After 20 minutes, the stove was switched off. No range hood was applied in this study.
Control Experiments: Ten participants were asked to attend the control study. During the experiments, they sat on a sofa in the living room and were allowed to talk or read to avoid monotony. They only had to sit next to the stovewhile the stove was off, as no actual cooking was performed in the control experiments.
Data Analyses: The non-parametric Friedman test was employed since the normality test failed to assess the effects of cooking versus non-cooking on the human heart [47]. Considering a null hypothesis (H0) that the populations represented by the multiple conditions possess the identical distribution of scores, the Friedman test uncovers any overall discrepancies across related means. Accordingly, the alternative hypothesis asserts that the distribution of scores in at least one related population differs from the others. If the Friedman test revealed a statistically significant difference between the populations, the Wilcoxon test [48] was used as a post hoc test to determine the source of the variations. The null hypotheses are as follows:
H0: the means of the measurements of a condition at all time points remain unchanged and are equal. H1: At least the one-time point mean is statistically significantly different. The associated groups are the subjects before, during, and after cooking, and µs are the population means.
Emission rates: The mass balance approach (Eq. 1) was used to estimate the particle number emission rate [11], assuming the well-mixed environment and constant emission rate.
| (1) |
Cin: indoor number or mass concentration
Cout: outdoor number or mass concentration to be negligible compared to Cin during cooking
P: penetration coefficient
a: air exchange rate
K: deposition rate
S: emission rate
V: volume of the kitchen
The concentration variations with time during the decay period (S=0) were plugged into Eq. 1 to estimate the decay rate (a+k). Assuming the Cout to be constant, Eq. 1 leads to:
| (2) |
The decay rate is equivalent to the negative slope of the graph versus time. Where Cin,t is indoor number concentration (particles/cm3) at any time, Cin,b is background concentration (particles/cm3), and is the maximum concentration (particles/cm3).
| (3) |
| (4) |
The estimated decay rate was plugged into (3), (4) to estimate the S [49]. is the difference between the measured indoor and background concentrations. The y-intercept of the graph versus is. Given (a+k) and the apartment's volume, S was calculated.
3. Results
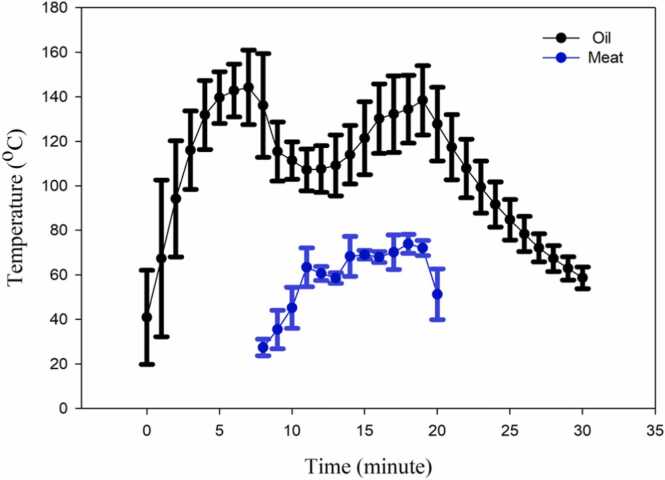
Exposure assessment: Fig. 1 presents the average oil and meat temperature versus time during cooking. Time zero shows the moment that the pan was put on the stove and the gas stove was switched on. After two minutes, the oil was added to the pan. It was observed that the oil temperature increased from 40℃ to 150 ℃ during the first eight minutes of cooking. When the meat was placed into the pan (8 minutes after the cooking), the oil temperature dropped and reached 110 ℃ at minute 14. This drop in temperature was due to the lower temperature of the meat compared to the heated oil. A second peak (140 ℃) was observed at minute 20 due to the continuous heating, and then the stove was switched off with a subsequent drop in the oil temperature. The meat temperature increased to 65℃ after 3 minutes of heating. Turning over the meat (after 11, 14, and 17 minutes of cooking) resulted in temperature drops, as shown in Fig. 1.
Fig. 1.
Average oil and meat temperature changes with time during the frying of beef (The plots represent the average value and the SD. Each data point on the graph is the mean and the error bar is SD).
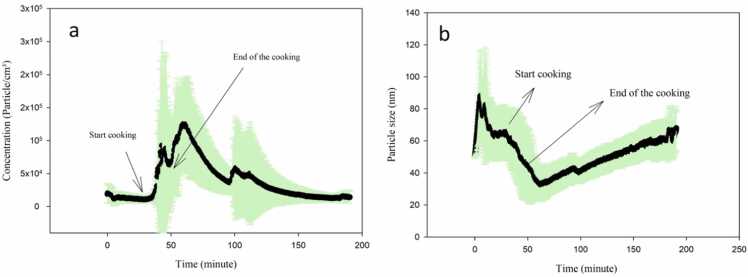
Fig. 2 presents the average particle size and number concentration with time. It is demonstrated that cooking beef kebab produced ultrafine particles. The minimum average diameter at minute 60 in particle size figure is compatible with average particle number concentration figure, suggesting that the highest particle number concentration was associated with the smallest diameter particles. Fig. 2 shows that the diameter of the particles during the experiments was under 100 nm, and the mode diameter was 49.5 nm which shows the presence of UFPs. During the grilling on the stove, 98 % of the emitted particles were in the ultrafine range, and a mode diameter of approximately 20 nm [19] was observed. Wallace et al. [23] reported the mode diameter while cooking rice with water on an electric stove to be approximately 15 nm. The mode diameter during frying bacon using the gas stove was reported to be 84.1 nm [20].
Fig. 2.
Average particle number concentration (a) and particle size (b) with time during the experiments.
The initial background concentration was approximately 1.1×104 particle/cm3. The first peak concentration due to cooking was found at minute 45, which resulted in a particle concentration of 8.6×104 particle/cm3. The second and third concentration peaks were observed at minutes 60 and 100 during the decay period, which could be attributed to the coagulation of particles smaller than 10 nm (below the instrument's detection limit) to larger than 10 nm to be detectable by the instrument. A study by Zhang et al. [17] reported an average particle concentration of 6.04×105 particle/cm3 at high temperatures, and the mode diameter ranged from 60 to 90 nm while frying chicken with a gas stove. However, cooking with the medium temperature resulted in a mode diameter between 30 and 50 nm and an average particle concentration of 2.65×105 particle/cm3. The maximum particle concentration produced during frying pork meat and frying chips was 2.7×105 particle/cm3 and 2.3×105 particle/cm3, respectively [50], which is consistent with the concentration of this study.
The average particle number emission rate (S) and the average particle number decay rate (a+k) for meat frying in our experiments were found to be 2.09×1013 (SD=3.94×1013, R2=0.98, P <0.0001) particles/min, and 0.055 (SD=0.019, R2=0.91, P <0.0001) min−1, respectively. Giorgio [50] reported an average particle number emission factor of 7.7×1010 and 8.8×1010 particles/min for frying chips and pork meat, respectively, which is lower than the concentration of this study.
Heart responses:Fig. 3 shows the SBP, DBP, and HR average values for the six steps of the experiments for the control group. A statistically significant increase in SBP was observed between steps 1 and 4 (p= 0.001) and a significant decrease between steps 4 and 5 (p= 0.049). No significant changes were observed for HR and DBP for the control group. This observation suggest that SBP could change during the day, and thus, diurnal effect could be important in the current study.
Fig. 3.
SBP, DBP, and HR average values for the six steps of the experiments for the control group, including step1 (before the cooking), step 2 (end of the cooking), step 3 (30 minutes after cooking), step 4 (60 minutes after cooking), step 5 (90 minutes after cooking), step 6 (120 minutes after cooking) – Plots represent the average value and the SD. Each data point on the graph is the mean, and the error bar is SD).
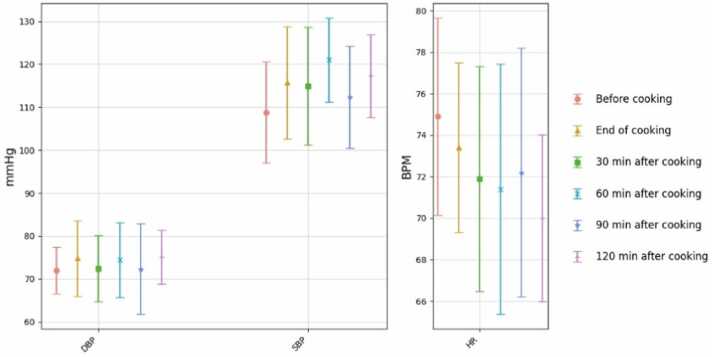
Statistically significant increases in SBP were observed between steps 1 and 4 (p= 0.009), 1 and 6 (p= 0.012), 2 and 4 (p= 0.018), and 2 and 6 (p= 0.018). Fig. 4 presents the average SBP, DBP, and HR values for the six steps of the cooking experiments. The average SBP increased from 98 mm Hg (step 1) to 106.4 and 106.5 mm Hg (steps 4 and 6, respectively). The HR decreased during the post-exposure (after cooking) period compared to the before exposure (before cooking). Our recent study made similar observations [32]. However, the Friedman test showed no statistically significant differences in diastolic blood pressure and heart rates up to two hours post-exposure (the six measurement steps) (Table S1). Results showed that 15 % of study participants entered the elevated stage of SBP, and 30 % entered the stage 1 high DBP after exposure.
Fig. 4.
SBP, DBP, and HR average values for the six steps of the experiments for 33 study participants, including step1 (before the cooking), step 2 (end of the cooking), step 3 (30 minutes after cooking), step 4 (60 minutes after cooking), step 5 (90 minutes after cooking), step 6 (120 minutes after cooking) – Plots represent the average value and the SD. Each data point on the graph is the mean, and the error bar is SD).
These observations agree with similar studies in the literature. For example, exposure to diesel exhaust particles SBP increased after two hours while no effects on DBP up to 24 hours post-exposure were observed [38].
4. Discussion
The observed increases in SBP are likely due to exposure to the cooking emission, but the mechanism is unclear. Several studies showed the translocation of nanoparticles to the blood. Husain et al. [51] observed the translocation of nanoparticles into the blood that changed the blood biomarkers. Black carbon particles were observed on the fetal side of the human placenta [52] and in healthy children's urine [53] due to chronic exposure to ambient PM. On the other hand, other studies [54], [55], [56] didn’t report any evidence of particle translocation into the human blood during short-term exposure. There is currently no evidence that cooking particles can enter into blood circulation, given their aggregated branched chain-like structure or rectangular morphology [19].
Systemic inflammation and autonomic nervous system activation [57], [58] could be why cardiovascular changes are observed. Several clinical studies have investigated the effect of exposure to cooking fumes [59], [60] and diesel engine particles [61] on blood. These studies reported no systemic inflammation in study participants' blood samples. SBP and DBP have different regulatory pathways, showing different responses to external stimuli [62]. Some studies revealed that SBP underwent greater changes than DBP due to increased arterial stiffness after exposure to PM [59], [39]. The SBP is developed when the heart contracts, thus pumping blood through the arteries; which is influenced by the sympathetic nervous system and stress stimuli [36]. The DBP is the pressure in the vessels) the heart is at rest [36]. Inhaled particles can translocate to the brain through olfactory pathways and stimulate the nervous system [63], [64]. One plausible reason for observed changes in systolic blood pressure could be sympathetic activities originated from nervous system stimulation [58].
In our previous study [32] we employed the same experimental protocol and cooking recipe as the current study but used an electric stove, finding increased SBP 90 minutes after exposure to UFPs. However, the present study showed a faster (after 60 minutes) blood pressure response to the inhaled UFPs. The observed faster response could be attributed to the higher particle concentrations reported during frying using a gas stove (the present study) compared to an electric stove which is consistent with the literature [65]. Diesel exhaust inhalation increases SBP but does not significantly affect heart rate or diastolic pressure [38]. An increase in SBP and no changes in DBP also were observed 24 h post-exposure to different cook stoves [39].
The observed effect could potentially be attributed to particles and gases, particularly the influence of smell, as previous research suggests a connection between taste, smell perception, and blood pressure. This observation raises the possibility that changes in SBP may be influenced by olfactory stimuli, such as the smell of cooking meat. Research has indicated that the presence of both self-reported altered taste and smell perception was associated with more significant increases in SBP over a two-year follow-up period, with an adjusted mean difference of 5.1 mmHg (95 % CI: 0.1–10.0) and a p-value of 0.04, compared to those without altered perception. However, when analyzing altered taste or smell perception individually, no significant association was found [66]. Thus, the observed effects could be due to both gases and particles. However, a study showed that the neurological impact of diesel emissions comes from UFPs rather than gas emissions [67].
In our study, we observed similar increases in SBP in both the control group and during cooking experiments. Blood pressure fluctuates due to numerous internal and external factors, with behavioral influences playing a significant role in daily variations. We rigorously controlled for various factors such as food consumption, sodium intake, drinking, smoking, coffee and tea consumption, alcohol consumption, and bathing during the experiments. The observed changes in SBP during both control and cooking scenarios could be attributed to both cooking aerosol and diurnal effects, where blood pressure typically rises upon waking in the morning and decreases during sleep at night, although it varies throughout the day and night [68]. An analysis using time of day in a comparable model accounted for 33 % of the observed variation in blood pressure [69].
5. Limitations of this study
The Omron 10 blood pressure monitor used in this study was not a reference instrument, and thus, uncertainties are higher in the measurements than would be obtained in reference instruments. In our recent study [32], the blood pressure data for 10 study participants were measured using an Omron BP monitor and a clinical blood pressure cuff. The reported 10 % bias in measured SBP using Omron 10 does not impact this study's measurement analyses. While in this study, we compared the changes in BP and HR during and after cooking; for exact measurements, the reference instruments such as a Holter monitor and a clinical BP cuff would be required. The study participants could talk during the experiments; thus, blood pressure measurements could have been biased [61]. This study did not exclude the potential effects of cooking emitted gases and participants’ activities, including reading, watching movies, etc., on blood pressure. Thus, these tors factors could be confounders in our study. One potential limitation of this study is that the sample size calculation (power analysis) was not performed. Thus, the sample size used in the study may not be representative of the population being studied, and the results may not be generalizable to the larger population.
6. Conclusion
Frying emission statistically significantly increased SBP during the post-exposure time, while it had no statistically significant effects on DBP and HR. The impact of the inhaled aersol from frying beef on SBP was faster when a gas stove was used than an electric stove. Results of this study were obtained by comparing exposure data with exposure references; for more representative data, a future study extending the control measurement day to a full 24 hours would be preferred.
7. Future work
We acknowledge the importance of controlling for potential confounding variables, such as the contributions of gases (smell) and particles, as well as the diurnal effects on blood pressure. To better understand the specific impact of particles on blood pressure, future research should focus on developing methodologies to differentiate exposure effects from diurnal variations. Additionally, addressing the potential confounding influence of smell would benefit from implementing measures to control olfactory stimuli. Exposure level could be another potential factor. Future research should investigate how changes in SBP are dose-dependent on the amount of ultrafine particles.
CRediT authorship contribution statement
Saule Zhumambayeva: Writing – review & editing, Methodology. Fatma Ozturk: Writing – review & editing. Motahareh Naseri: Writing – original draft, Investigation, Formal analysis. Mehrdad Jafari Tadi: Writing – review & editing, Methodology, Data curation. Abilova Aigerim Sultanbekovna: Writing – original draft, Investigation. Mehdi Amouei Torkmahalleh: Supervision, Funding acquisition, Conceptualization. Milad Malekipirbazari: Software, Formal analysis. Dhawal Shah: Writing – review & editing, Resources, Project administration, Supervision. Elzira Kenzhegaliyeva: Formal analysis. Giorgio Buonanno: Writing – review & editing. Luca Stabile: Writing – review & editing. Philip K. Hopke: Writing – review & editing. Sergei Sabanov: Writing – review & editing, Funding acquisition. Flemming Casse: Writing – review & editing. Byron Crape: Writing – review & editing.
Acknowledgements
This research has been funded by Nazarbayev University under the Collaborative Research Grants (grant number: 11022021CRP1503, D.S., M.A.T., and B.C., and grant number: 091019CRP2104 S.S. and M.A.T.).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2024.101716.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Data Availability
Data will be made available on request.
References
- 1.Aquilina, N.J. and Camilleri, S.F. 2022. Impact of daily household activities on indoor PM2. 5 and Black Carbon concentrations in Malta. Building and Environment 207, 108422.
- 2.Long C.M., Suh H.H., Koutrakis P. Characterization of indoor particle sources using continuous mass and size monitors. J. Air Waste Manag. Assoc. 2000;50:1236–1250. doi: 10.1080/10473289.2000.10464154. [DOI] [PubMed] [Google Scholar]
- 3.Baeza_Romero M.T., Dudzinska M.R., Amouei Torkmahalleh M., Barros N., Coggins A.M., Ruzgar D.G., Kildsgaard I., Naseri M., Rong L., Saffell J. A review of critical residential buildings parameters and activities when investigating indoor air quality and pollutants. Indoor Air. 2022;32 doi: 10.1111/ina.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhangar S., Mullen N., Hering S., Kreisberg N., Nazaroff W. Ultrafine particle concentrations and exposures in seven residences in northern California. Indoor Air. 2011;21:132–144. doi: 10.1111/j.1600-0668.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- 5.MacNeill M., Kearney J., Wallace L., Gibson M., Héroux M.E., Kuchta J., Guernsey J.R., Wheeler A.J. Quantifying the contribution of ambient and indoor-generated fine particles to indoor air in residential environments. Indoor Air. 2014;24:362–375. doi: 10.1111/ina.12084. [DOI] [PubMed] [Google Scholar]
- 6.Nasir Z.A., Colbeck I. Particulate pollution in different housing types in a UK suburban location. Sci. Total Environ. 2013;445:165–176. doi: 10.1016/j.scitotenv.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Wallace L. Indoor sources of ultrafine and accumulation mode particles: size distributions, size-resolved concentrations, and source strengths. Aerosol Sci. Technol. 2006;40:348–360. [Google Scholar]
- 8.Caracci E., Canale L., Buonanno G., Stabile L. Effectiveness of eco-feedback in improving the indoor air quality in residential buildings: mitigation of the exposure to airborne particles. Build. Environ. 2022;226 [Google Scholar]
- 9.Wan M.-P., Wu C.-L., To G.-N.S., Chan T.-C., Chao C.Y. Ultrafine particles, and PM2. 5 generated from cooking in homes. Atmos. Environ. 2011;45:6141–6148. [Google Scholar]
- 10.Amouei Torkmahalleh M., Goldasteh I., Zhao Y., Udochu N.M., Rossner A., Hopke P., Ferro A. PM2. 5 and ultrafine particles emitted during heating of commercial cooking oils. Indoor Air. 2012;22:483–491. doi: 10.1111/j.1600-0668.2012.00783.x. [DOI] [PubMed] [Google Scholar]
- 11.Amouei Torkmahalleh M., Ospanova S., Baibatyrova A., Nurbay S., Zhanakhmet G., Shah D. Contributions of burner, pan, meat and salt to PM emission during grilling. Environ. Res. 2018;164:11–17. doi: 10.1016/j.envres.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Olson D.A., Burke J.M. Distributions of PM2. 5 source strengths for cooking from the Research Triangle Park particulate matter panel study. Environ. Sci. Technol. 2006;40:163–169. doi: 10.1021/es050359t. [DOI] [PubMed] [Google Scholar]
- 13.See S.W., Balasubramanian R. Chemical characteristics of fine particles emitted from different gas cooking methods. Atmos. Environ. 2008;42:8852–8862. [Google Scholar]
- 14.Taner S., Pekey B., Pekey H. Fine particulate matter in the indoor air of barbeque restaurants: Elemental compositions, sources and health risks. Sci. Total Environ. 454. 2013:79–87. doi: 10.1016/j.scitotenv.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Wallace L.A., Ott W.R., Weschler C.J. Ultrafine particles from electric appliances and cooking pans: experiments suggesting desorption/nucleation of sorbed organics as the primary source. Indoor Air. 2015;25:536–546. doi: 10.1111/ina.12163. [DOI] [PubMed] [Google Scholar]
- 16.See S., Balasubramanian R. Risk assessment of exposure to indoor aerosols associated with Chinese cooking. Environ. Res. 2006;102:197–204. doi: 10.1016/j.envres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q., Gangupomu R.H., Ramirez D., Zhu Y. Measurement of ultrafine particles and other air pollutants emitted by cooking activities. Int. J. Environ. Res. Public Health. 2010;7:1744–1759. doi: 10.3390/ijerph7041744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amouei Torkmahalleh M. Springer; 2022. Cooking Aerosol. Handbook of Indoor Air Quality; pp. 1–40. [Google Scholar]
- 19.Buonanno G., Morawska L., Stabile L. Particle emission factors during cooking activities. Atmos. Environ. 2009;43:3235–3242. [Google Scholar]
- 20.Jørgensen R.B., Strandberg B., Sjaastad A.K., Johansen A., Svendsen K. Simulated restaurant cook exposure to emissions of PAHs, mutagenic aldehydes, and particles from frying bacon. J. Occup. Environ. Hyg. 2013;10:122–131. doi: 10.1080/15459624.2012.755864. [DOI] [PubMed] [Google Scholar]
- 21.Rim D., Wallace L.A., Persily A.K. Indoor ultrafine particles of outdoor origin: importance of window opening area and fan operation condition. Environ. Sci. Technol. 2013;47:1922–1929. doi: 10.1021/es303613e. [DOI] [PubMed] [Google Scholar]
- 22.To W., Yeung L.L. Effect of fuels on cooking fume emissions. Indoor Built Environ. 2011;20:555–563. [Google Scholar]
- 23.Wallace L., Wang F., Howard-Reed C., Persily A. Contribution of gas and electric stoves to residential ultrafine particle concentrations between 2 and 64 nm: size distributions and emission and coagulation rates. Environ. Sci. Technol. 2008;42:8641–8647. doi: 10.1021/es801402v. [DOI] [PubMed] [Google Scholar]
- 24.Patel S., Sankhyan S., Boedicker E.K., DeCarlo P.F., Farmer D.K., Goldstein A.H., Katz E.F., Nazaroff W.W., Tian Y., Vanhanen J. Indoor particulate matter during HOMEChem: Concentrations, size distributions, and exposures. Environ. Sci. Technol. 2020;54:7107–7116. doi: 10.1021/acs.est.0c00740. [DOI] [PubMed] [Google Scholar]
- 25.Saito E., Tanaka N., Miyazaki A., Tsuzaki M. Concentration and particle size distribution of polycyclic aromatic hydrocarbons formed by thermal cooking. Food Chem. 2014:285–291. doi: 10.1016/j.foodchem.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 26.Sjaastad A.K., Svendsen K. Exposure to mutagenic aldehydes and particulate matter during panfrying of beefsteak with margarine, rapeseed oil, olive oil or soybean oil. Ann. Occup. Hyg. 2008;52:739–745. doi: 10.1093/annhyg/men060. [DOI] [PubMed] [Google Scholar]
- 27.Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., Potter R., Maynard A., Ito Y., Finkelstein J. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreyling W., Semmler M., Erbe F., Mayer P., Takenaka S., Schulz H., Oberdörster G., Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health, Part A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 29.Oberdörster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 30.Amouei Torkmahalleh M., Naseri M., Nurzhan S., Gabdrashova R., Bekezhankyzy Z., Gimnkhan A., Malekipirbazari M., Jouzizadeh M., Tabesh M., Farrokhi H. Human exposure to aerosol from indoor gas stove cooking and the resulting nervous system responses. Indoor Air. 2022;32 doi: 10.1111/ina.12983. [DOI] [PubMed] [Google Scholar]
- 31.Buonanno G., Marks G.B., Morawska L. Health effects of daily airborne particle dose in children: direct association between personal dose and respiratory health effects. Environ. Pollut. 2013;180:246–250. doi: 10.1016/j.envpol.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 32.Gabdrashova R., Nurzhan S., Naseri M., Bekezhankyzy Z., Gimnkhan A., Malekipirbazari M., Tabesh M., Khanbabaie R., Crape B., Buonanno G. The impact on heart rate and blood pressure following exposure to ultrafine particles from cooking using an electric stove. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141334. [DOI] [PubMed] [Google Scholar]
- 33.Naseri M., Jouzizadeh M., Tabesh M., Malekipirbazari M., Gabdrashova R., Nurzhan S., Farrokhi H., Khanbabaie R., Mehri-Dehnavi H., Bekezhankyzy Z. The impact of frying aerosol on human brain activity. NeuroToxicology. 2019;74:149–161. doi: 10.1016/j.neuro.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Pacitto A., Stabile L., Viana M., Scungio M., Reche C., Querol X., Alastuey A., Rivas I., Álvarez-Pedrerol M., Sunyer J. Particle-related exposure, dose and lung cancer risk of primary school children in two European countries. Sci. Total Environ. 2018;616:720–729. doi: 10.1016/j.scitotenv.2017.10.256. [DOI] [PubMed] [Google Scholar]
- 35.Rizza V., Stabile L., Vistocco D., Russi A., Pardi S., Buonanno G. Effects of the exposure to ultrafine particles on heart rate in a healthy population. Sci. Total Environ. 2019;650:2403–2410. doi: 10.1016/j.scitotenv.2018.09.385. [DOI] [PubMed] [Google Scholar]
- 36.Du B., Gao J., Chen J., Stevanovic S., Ristovski Z., Wang L., Wang L. Particle exposure level and potential health risks of domestic Chinese cooking. Build. Environ. 2017;123:564–574. [Google Scholar]
- 37.Soppa V.J., Schins R.P., Hennig F., Nieuwenhuijsen M.J., Hellack B., Quass U., Kaminski H., Sasse B., Shinnawi S., Kuhlbusch T.A. Arterial blood pressure responses to short-term exposure to fine and ultrafine particles from indoor sources–A randomized sham-controlled exposure study of healthy volunteers. Environ. Res. 2017;158:225–232. doi: 10.1016/j.envres.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Cosselman K.E., Krishnan M., Oron R., Jansen A.P., Peretz K., Sullivan A., Larson, T.V J.H., Kaufman J.D. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension. 2012;59:943–948. doi: 10.1161/HYPERTENSIONAHA.111.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedak K.M., Good N., Walker E.S., Balmes J., Brook R.D., Clark M.L., Cole-Hunter T., Devlin R., L'Orange C., Luckasen G. Acute effects on blood pressure following controlled exposure to cookstove air pollution in the STOVES study. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whelton, W.J.J.A.C.C. 2017. 2017 Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults.
- 41.American Heart Association [AHA], 2021. https://www.heart.org/en/health-topics/high-blood-pressure/the-facts-about-high-blood-pressure.
- 42.Lewington S.J.L. Prospective studies collaboration. Age-Specif. Relev. usual blood Press. Vasc. Mortal.: a meta-Anal. Individ. data One Million adults 61 Prospect. Stud. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 43.Wong T.Y., Klein R., Sharrett A.R., Duncan B.B., Couper D.J., Tielsch J.M., Klein B.E., Hubbard L.D.J.J. Retinal arteriolar narrowing and risk of coronary heart disease in men and women: the Atherosclerosis Risk in Communities Study. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 44.Iadecola C., Yaffe K., Biller J., Bratzke L.C., Faraci F.M., Gorelick P.B., Gulati M., Kamel H., Knopman D.S., Launer L.J.J.H. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. 2016;68:e67–e94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumareswaran K., Rajapaksha I., Jayasinghe G.J.E., Buildings Energy poverty, occupant comfort. Wellbeing Intern. Displac. People’S. Resid. Sri Lanka. 2021;236 [Google Scholar]
- 46.Arens, E. 2016. ANSI/ASHRAE Addendum g to ANSI/ASHRAE Standard 55-2013 Thermal Environmental Conditions for Human Occupancy. vol.
- 47.Friedman M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J. Am. Stat. Assoc. 1937;32:675–701. [Google Scholar]
- 48.Wilcoxon F. Individual comparisons by ranking methods. Biom. Bull. 1945;1:80–83. [Google Scholar]
- 49.Wallace L.A., Emmerich S.J., Howard-Reed C. Source strengths of ultrafine and fine particles due to cooking with a gas stove. Environ. Sci. Technol. 2004;38:2304–2311. doi: 10.1021/es0306260. [DOI] [PubMed] [Google Scholar]
- 50.Buonanno G., Johnson G., Morawska L., Stabile L. Volatility characterization of cooking-generated aerosol particles. Aerosol Sci. Technol. 2011;45:1069–1077. [Google Scholar]
- 51.Husain M., Wu D., Saber A.T., Decan N., Jacobsen N.R., Williams A., Yauk C.L., Wallin H., Vogel U., Halappanavar S. Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice. Nanotoxicology. 2015;9:1013–1022. doi: 10.3109/17435390.2014.996192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bové H., Bongaerts E., Slenders E., Bijnens E.M., Saenen N.D., Gyselaers W., Van Eyken P., Plusquin M., Roeffaers M.B., Ameloot M. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019;10:1–7. doi: 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saenen N.D., Bové H., Steuwe C., Roeffaers M.B., Provost E.B., Lefebvre W., Vanpoucke C., Ameloot M., Nawrot T.S. Children’s urinary environmental carbon load. A novel marker reflecting residential ambient air pollution exposure? Am. J. Respir. Crit. Care Med. 2017;196:873–881. doi: 10.1164/rccm.201704-0797OC. [DOI] [PubMed] [Google Scholar]
- 54.Brown J.S., Zeman K.L., Bennett W.D. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am. J. Respir. Crit. Care Med. 2002;166:1240–1247. doi: 10.1164/rccm.200205-399OC. [DOI] [PubMed] [Google Scholar]
- 55.Mills N.L., Amin N., Robinson S.D., Anand A., Davies J., Patel D., de la Fuente J.M., Cassee F.R., Boon N.A., MacNee W. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am. J. Respir. Crit. Care Med. 2006;173:426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- 56.Wiebert P., Sanchez-Crespo A., Falk R., Philipson K., Lundin A., Larsson S., Möller W., Kreyling W.G., Svartengren M. No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhal. Toxicol. 2006;18:741–747. doi: 10.1080/08958370600748455. [DOI] [PubMed] [Google Scholar]
- 57.Brook R.D., Rajagopalan S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Langrish J.P., Mills N.L., Chan J.K., Leseman D.L., Aitken R.J., Fokkens P.H., Cassee F.R., Li J., Donaldson K., Newby D.E. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part. Fibre Toxicol. 2009;6:1–9. doi: 10.1186/1743-8977-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumgartner J., Carter E., Schauer J.J., Ezzati M., Daskalopoulou S.S., Valois M.-F., Shan M., Yang X. Household air pollution and measures of blood pressure, arterial stiffness and central haemodynamics. Heart. 2018;104:1515–1521. doi: 10.1136/heartjnl-2017-312595. [DOI] [PubMed] [Google Scholar]
- 60.Widdicombe J., Lee L.-Y. Airway reflexes, autonomic function, and cardiovascular responses. Environ. Health Perspect. 2001;109:579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng D., Giovannini R., Murray A. Effect of respiration, talking and small body movements on blood pressure measurement. J. Hum. Hypertens. 2012;26:458–462. doi: 10.1038/jhh.2011.53. [DOI] [PubMed] [Google Scholar]
- 62.Pieters N., Koppen G., Van Poppel M., De Prins S., Cox B., Dons E., Nelen V., Panis L.I., Plusquin M., Schoeters G. Blood pressure and same-day exposure to air pollution at school: associations with nano-sized to coarse PM in children. Environ. Health Perspect. 2015;123:737–742. doi: 10.1289/ehp.1408121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crüts B., van Etten L., Törnqvist H., Blomberg A., Sandström T., Mills N.L., Borm P.J. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part. Fibre Toxicol. 2008;5:1–6. doi: 10.1186/1743-8977-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorman D.C., McManus B.E., Parkinson C.U., Manuel C.A., McElveen A.M., Everitt J.I. Nasal toxicity of manganese sulfate and manganese phosphate in young male rats following subchronic (13-week) inhalation exposure. Inhal. Toxicol. 2004;16:481–488. doi: 10.1080/08958370490439687. [DOI] [PubMed] [Google Scholar]
- 65.Amouei Torkmahalleh M., Gorjinezhad S., Unluevcek H.S., Hopke P.K. Review of factors impacting emission/concentration of cooking generated particulate matter. Sci. Total Environ. 2017;586:1046–1056. doi: 10.1016/j.scitotenv.2017.02.088. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y.H., Huang Z., Vaidya A., Li J., Curhan G.C., Wu S., Gao X. A longitudinal study of altered taste and smell perception and change in blood pressure. Nutr., Metab., Cardiovasc. Dis.: NMCD. 2018;28:877–883. doi: 10.1016/j.numecd.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Driessen A., Crüts B., van Etten L., Crüts A., Fokkens P.H.B., Cassee F.R., Borm P.J.A. In: NanoFormulation. Tiddy G., Tan R., editors. The Royal Society of Chemistry; 2012. Short-term Exposure to Nanoparticle-rich Diesel Engine Exhaust Causes Changes in Brain Activity but not in Cognitive Performance in Human Volunteers; p. 0. [Google Scholar]
- 68.Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens. Res. 2011;34:281–285. doi: 10.1038/hr.2010.241. [DOI] [PubMed] [Google Scholar]
- 69.Clark L.A., Denby L., Pregibon D., Harshfield G.A., Pickering T.G., Blank S., Laragh J.H. A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J. Chronic Dis. 1987;40:671–681. doi: 10.1016/0021-9681(87)90103-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Data Availability Statement
Data will be made available on request.