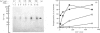Abstract
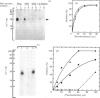
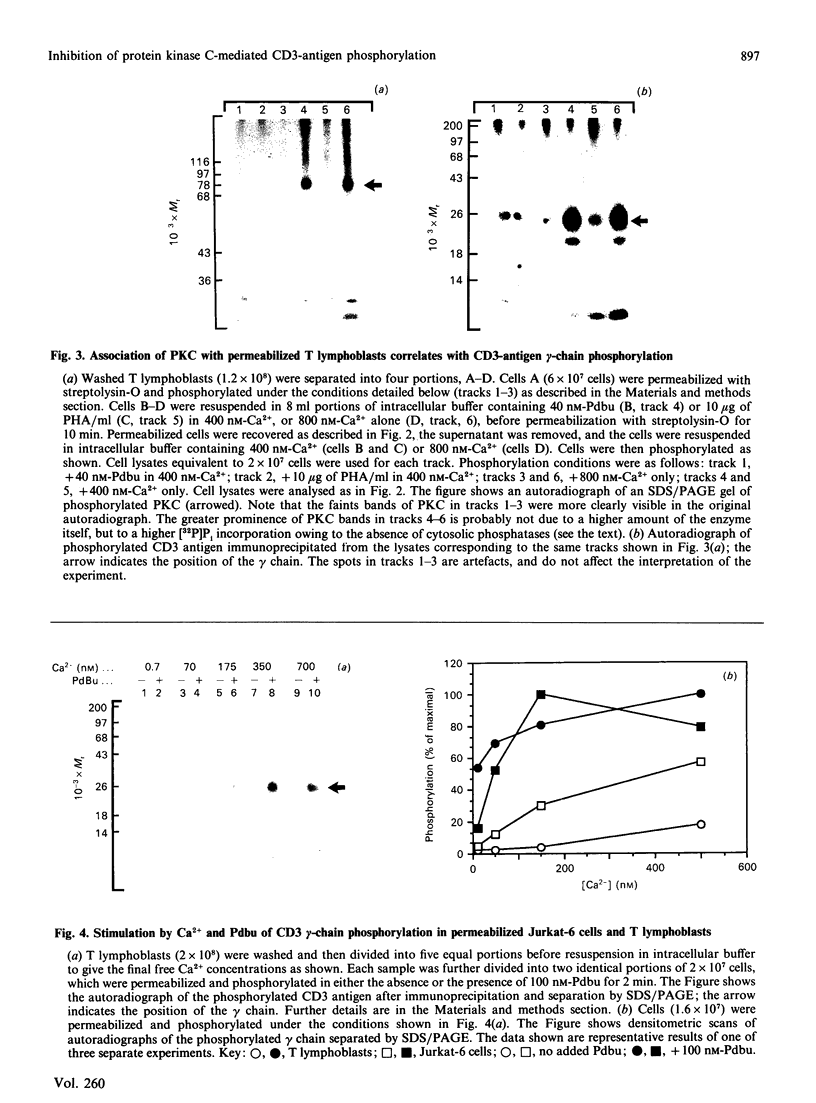
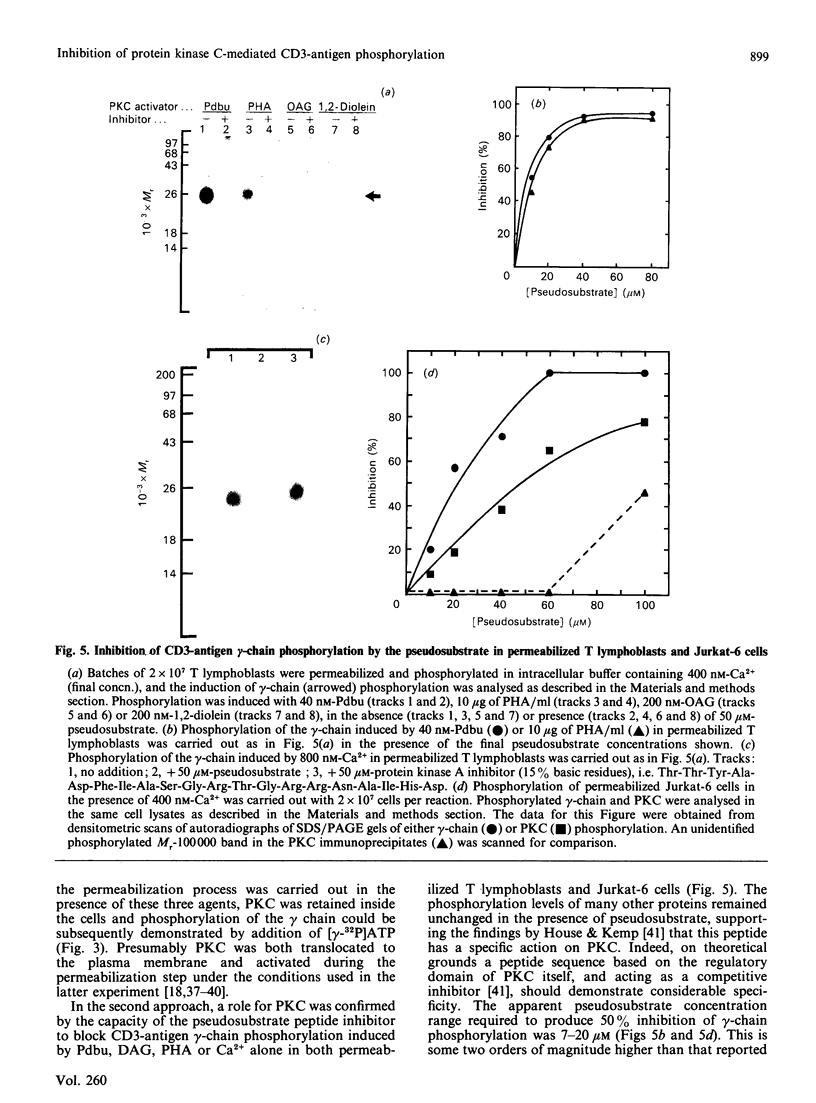
Activation of human T lymphocytes leads to the phosphorylation of the CD3-antigen gamma polypeptide. We have investigated a possible role for protein kinase C (PKC) in mediating this phosphorylation event by using T cells permeabilized with streptolysin-O in the presence of 120 mM-K+ buffers containing Ca2+-EGTA. The gamma-chain was phosphorylated by [gamma-32P]ATP in permeabilized T lymphoblasts in the presence of phorbol 12,13-dibutyrate (Pdbu) or phytohaemagglutinin (PHA). Ca2+ alone in the range 0.5-1.0 microM also induced gamma-chain phosphorylation in some T-lymphoblast preparations; that in Jurkat-6 cells occurred at lower concentrations (50-500 nM). Two experimental approaches were used to investigate the possible involvement of PKC. Firstly, when permeabilization was carried out in buffer lacking free Ca2+, PKC was lost from the cells, and gamma-chain phosphorylation could then no longer be induced on subsequent addition of Pdbu or PHA in 400 nM-Ca2+, or 800 nM-Ca2+ alone, to permeabilized cells. However, when permeabilization was carried out in the presence of these three agents, PKC was translocated to intracellular membranes, and subsequent addition of [gamma-32P]ATP to these cells then resulted in gamma-chain phosphorylation. In the second approach, induction of gamma-chain phosphorylation by Pdbu, 1-oleoyl-2-acetylglycerol, 1,2-diolein, PHA or Ca2+ alone was effectively blocked by permeabilizing T cells in the presence of a PKC pseudosubstrate peptide (50 microM). Pseudosubstrate concentrations in the range 7-20 microM inhibited gamma-chain phosphorylation by 50%. In contrast, addition of four other 'irrelevant' basic peptides (50 microM) did not result in detectable inhibition, and 50 microM-pseudosubstrate did not inhibit the phosphorylation of 17 other polypeptides isolated from permeabilized T cells. These data suggest that Pdbu-, 1,2-diacylglycerol-, PHA- and Ca2+-induced phosphorylation of the CD3-antigen gamma chain in permeabilized T cells is mediated by PKC.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Hexham J. M., Crumpton M. J. The association of type 1, type 2A and type 2B phosphatases with the human T lymphocyte plasma membrane. Biochem J. 1988 Dec 15;256(3):885–892. doi: 10.1042/bj2560885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Bonifacino J. S., Samelson L. E., Klausner R. D. Disulfide linkage of the zeta and eta chains of the T cell receptor. Possible identification of two structural classes of receptors. J Biol Chem. 1988 Jul 15;263(20):9874–9878. [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Properties of membrane-inserted protein kinase C. Biochemistry. 1988 Oct 4;27(20):7589–7593. doi: 10.1021/bi00420a003. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Breitmeyer J. B., Daley J. F., Levine H. B., Schlossman S. F. The T11 (CD2) molecule is functionally linked to the T3/Ti T cell receptor in the majority of T cells. J Immunol. 1987 Nov 1;139(9):2899–2905. [PubMed] [Google Scholar]
- Cantrell D. A., Verbi W., Davies A., Parker P., Crumpton M. J. Evidence that protein kinase C differentially regulates the human T lymphocyte CD2 and CD3 surface antigens. Eur J Immunol. 1988 Sep;18(9):1391–1396. doi: 10.1002/eji.1830180914. [DOI] [PubMed] [Google Scholar]
- Cantrell D., Davies A. A., Londei M., Feldman M., Crumpton M. J. Association of phosphorylation of the T3 antigen with immune activation of T lymphocytes. Nature. 1987 Feb 5;325(6104):540–542. doi: 10.1038/325540a0. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Davies A. A., Cantrell D. A., Hexham J. M., Parker P. J., Rothbard J., Crumpton M. J. The human T3 gamma chain is phosphorylated at serine 126 in response to T lymphocyte activation. J Biol Chem. 1987 Aug 15;262(23):10918–10921. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Friedrich B., Gullberg M. The role of protein kinase C in early activation vs. growth of T lymphocytes. Eur J Immunol. 1988 Mar;18(3):489–492. doi: 10.1002/eji.1830180327. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Noreus K., Cantrell D. A., Gullberg M. Activation-dependent phosphorylation of endogenous protein kinase C substrates in quiescent human T lymphocytes. Immunobiology. 1988 Mar;176(4-5):465–478. doi: 10.1016/s0171-2985(88)80027-5. [DOI] [PubMed] [Google Scholar]
- Herrero C., Cornet M. E., Lopez C., Barreno P. G., Municio A. M., Moscat J. Ca2+-induced changes in the secondary structure of a 60 kDa phosphoinositide-specific phospholipase C from bovine brain cytosol. Biochem J. 1988 Nov 1;255(3):807–812. doi: 10.1042/bj2550807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L., Nakabayashi H., Yoshida Y. Biochemical characterization of rat brain protein kinase C isozymes. J Biol Chem. 1988 Oct 15;263(29):14839–14845. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J., Weyand C., Goronzy J. Antigen recognition by a human T cell clone leads to increases in inositol trisphosphate. J Immunol. 1987 Mar 1;138(5):1322–1324. [PubMed] [Google Scholar]
- Isakov N., Altman A. Human T lymphocyte activation by tumor promoters: role of protein kinase C. J Immunol. 1987 May 15;138(10):3100–3107. [PubMed] [Google Scholar]
- Ito T., Tanaka T., Yoshida T., Onoda K., Ohta H., Hagiwara M., Itoh Y., Ogura M., Saito H., Hidaka H. Immunocytochemical evidence for translocation of protein kinase C in human megakaryoblastic leukemic cells: synergistic effects of Ca2+ and activators of protein kinase C on the plasma membrane association. J Cell Biol. 1988 Sep;107(3):929–937. doi: 10.1083/jcb.107.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Gross R. W. Identification and purification of sheep platelet phospholipase A2 isoforms. Activation by physiologic concentrations of calcium ion. J Biol Chem. 1986 Aug 15;261(23):10467–10470. [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Merćep M., Bonifacino J. S., Garcia-Morales P., Samelson L. E., Klausner R. D., Ashwell J. D. T cell CD3-zeta eta heterodimer expression and coupling to phosphoinositide hydrolysis. Science. 1988 Oct 28;242(4878):571–574. doi: 10.1126/science.2845582. [DOI] [PubMed] [Google Scholar]
- Moscat J., Moreno F., Herrero C., López C., García-Barreno P. Endothelial cell growth factor and ionophore A23187 stimulation of production of inositol phosphates in porcine aorta endothelial cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):659–663. doi: 10.1073/pnas.85.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. E., Bouic P., Lattanze G. R., Stevenson H. C., Miller P., Dirienzo W., Stefanini G. F., Galbraith R. M. Reaction of T lymphocytes with anti-T3 induces translocation of C-kinase activity to the membrane and specific substrate phosphorylation. J Immunol. 1987 May 15;138(10):3519–3524. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Konno Y., Imajoh S., Suzuki K. A novel phorbol ester receptor/protein kinase, nPKC, distantly related to the protein kinase C family. Cell. 1988 Jun 3;53(5):731–741. doi: 10.1016/0092-8674(88)90091-8. [DOI] [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Poggi A., Kozumbo W. J., Moretta L., Moretta A. Transmembrane signalling via the T11-dependent pathway of human T cell activation. Evidence for the involvement of 1,2-diacylglycerol and inositol phosphates. Eur J Immunol. 1987 Jan;17(1):55–60. doi: 10.1002/eji.1830170110. [DOI] [PubMed] [Google Scholar]
- Patel M. D., Samelson L. E., Klausner R. D. Multiple kinases and signal transduction. Phosphorylation of the T cell antigen receptor complex. J Biol Chem. 1987 Apr 25;262(12):5831–5838. [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Berry N., Oda T., Ase K., Kikkawa U., Nishizuka Y. Isolation of protein kinase C subspecies from a preparation of human T lymphocytes. FEBS Lett. 1988 Jul 18;234(2):387–391. doi: 10.1016/0014-5793(88)80122-4. [DOI] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Ca2+ mobilization primes protein kinase C in human platelets. Ca2+ and phorbol esters stimulate platelet aggregation and secretion synergistically through protein kinase C. Biochem J. 1988 Oct 1;255(1):309–318. [PMC free article] [PubMed] [Google Scholar]
- Sitkovsky M. V., Pasternack M. S., Lugo J. P., Klein J. R., Eisen H. N. Isolation and partial characterization of concanavalin A receptors on cloned cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1519–1523. doi: 10.1073/pnas.81.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D. R., Holz R. W. Effects of phorbol esters, diglyceride, and cholinergic agonists on the subcellular distribution of protein kinase C in intact or digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Dec 25;261(36):17099–17106. [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Hou D., Orloff D. G., Modi W. S., Seuanez H., O'Brien S. J., Klausner R. D. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]
- Young S., Rothbard J., Parker P. J. A monoclonal antibody recognising the site of limited proteolysis of protein kinase C. Inhibition of down-regulation in vivo. Eur J Biochem. 1988 Apr 5;173(1):247–252. doi: 10.1111/j.1432-1033.1988.tb13991.x. [DOI] [PubMed] [Google Scholar]