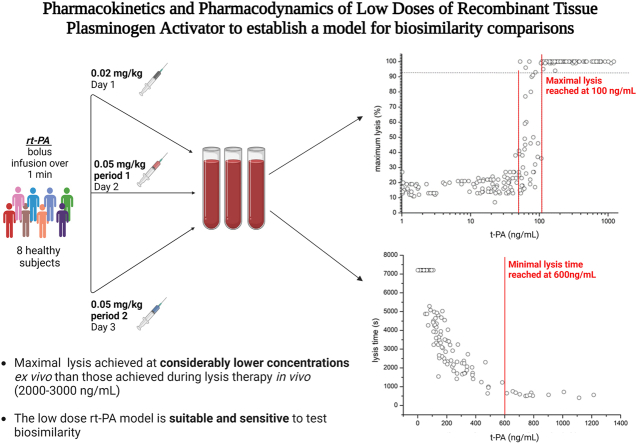
Abstract
Background
Recombinant tissue plasminogen activator (rt-PA) is a thrombolytic agent and essential in emergency medical care. Given recent supply shortages, the availability of biosimilar products is an urgent medical need. However, biosimilarity trials are difficult to perform in critically ill patients.
Objectives
The aim of this pilot study was to investigate the pharmacokinetics and pharmacodynamics of low rt-PA doses to establish a model for testing proposed biosimilars in healthy volunteers.
Methods
Eight healthy volunteers received 0.02 to 0.05 mg/kg rt-PA on 3 study days; blood samples were obtained every 4 minutes after the end of the bolus infusion to measure rt-PA antigen levels by enzyme immunoassay, and the pharmacodynamics were assessed with rotational thromboelastometry.
Results
Bolus infusion of low rt-PA doses was safe and well tolerated. Maximal plasma concentrations and the area under the curve increased dose-dependently. Time-concentration curves were clearly separated between the lower and the higher doses. As expected, the half-live of rt-PA was short (4.5-5 min), and representative for therapeutic doses. The intrasubject coefficient variations were moderate (<25%). Bolus infusion of rt-PA dose-dependently shortened lysis time and lysis onset time in both dose groups and caused maximum clot lysis of 100% in all participants.
Conclusion
In conclusion, the pharmacokinetics of rt-PA was dose linear and displayed limited intrasubject variability even at subtherapeutic doses. The half-life and thus clearance of rt-PA was representative of full therapeutic doses. The lysis time was shortened in a dose and time-dependent fashion and was clearly distinguishable between doses. Thus, the model appears to be suitable and sensitive to test biosimilarity.
Keywords: pharmacodynamics, pharmacokinetics, tissue plasminogen activator, clinical trial, thrombolytic therapy
Visual Abstract
Essentials
-
•
A model to test biosimilars of recombinant tissue plasminogen activator (rt-PA) was established.
-
•
The trial was conducted in 8 healthy volunteers receiving 2 subtherapeutic doses of rt-PA.
-
•
Low-dose rt-PA was safe, reproducibly and dose-dependently shortened clot lysis times.
-
•
This model is useful to test pharmacokinetic/pharmacodynamic biosimilarity and drug interactions.
1. Introduction
Recombinant tissue plasminogen activator (rt-PA; alteplase) is a thrombolytic agent that catalyzes the conversion of plasminogen to plasmin and is approved for the treatment of acute myocardial infarction, pulmonary embolism, and acute ischemic stroke. Increased global demand and challenging manufacturing processes led to a global shortage of rt-PA. Together with the relatively high treatment costs, the use of biosimilars may become attractive and even urgently necessary [[1], [2], [3]]. However, a biosimilarity trial with acute critically ill patients is difficult to perform. Study related procedures, such as the informed consent process, are time consuming and may interfere with emergency medical procedures and drug administration [[4], [5], [6], [7]]. In addition, pharmacokinetic (PK) studies in patients, in contrast to studies in healthy volunteers, showed a large intersubject variability [6,8,9]. Therefore, a well-defined model in healthy volunteers can be of great value for evaluating pharmacodynamic (PD) and PK properties of rt-PA.
There are only a few PK studies with rt-PA in healthy volunteers in the literature. Various doses and infusion schemes were examined in these studies [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19]]. However, PD effects of rt-PA could not be satisfactorily characterized by the coagulation parameters measured [10,17,19,20].
According to European Medicines Agency and Food and Drug Administration guidelines for development of biosimilars, evaluation of effects should be performed in the steep part of the dose-response curve [21,22]. For this and safety reasons, we wanted to test a low-dose model to evaluate PK and PD properties of rt-PA. We validated and used rotational thromboelastometry for PD evaluations, which can detect and quantify fibrinolysis [[23], [24], [25], [26], [27]].
Thus, the aim of this pilot study was to investigate the safety, PK, PD as well as the variability of PK/PD of low doses of alteplase. Finally, we wanted to determine whether this could be a valid model for testing biosimilar preparations of rt-PA in healthy volunteers.
2. Methods
2.2. Study population
The study was conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonization of Good Clinical Practice and was approved by the National Competent Authority and by the Ethics Committee of the Medical University of Vienna.
After informed consent, healthy male or female subjects aged 18 to 36 years were included. Key inclusion criteria were a healthy condition, a body weight between 65 to 100 kg and female subjects were either of nonchildbearing potential or had to practice reliable methods of contraception. Exclusion criteria comprised, among others, a history of anaphylaxis/allergy against the study drug, any known abnormality affecting the coagulation or fibrinolytic system, any history of disease or abnormal finding that was considered relevant by the investigator, trauma or surgery within the last 6 months, and elevated blood pressure values. Detailed information is provided in the study protocol which is available at the webpage of the European Union Drug Regulating Authorities Clinical Trials Database (www.eudract.ema.europa.eu), EudraCT No. 2017-001360-37.
2.2. Study design
This trial was a prospective, single-center, phase I study in healthy volunteers. The trial was performed in an open-label, dose escalation design.
Participants received 2 doses of alteplase (Actilyse, Boehringer Ingelheim, Ingelheim, FRG) as a bolus infusion: 0.02 mg/kg body weight (BW) on study day 0 and 0.05 mg/kg BW on study days 1 and 2. The third period repeating the higher dose was performed to determine the intrasubject variability. There had to be a washout period of at least 20 hours between the study days.
The dose of 0.02 mg/kg corresponds to approximately 1/40 and the dose of 0.05 mg/kg alteplase to approximately 1/20 of the recommended dose for the treatment of acute ischemic stroke (ie, 0.9 mg/kg, 10% of it as a bolus infusion).
2.3. Study procedures
All study days started at 08:00 in the morning and subjects were advised to keep an overnight fast. A baseline blood sample was obtained at time point −1 min after which the bolus infusion of alteplase was given over exactly 1 minute. Based on the expected short half-live of rt-PA, blood samples were taken at 4, 8, 10, 12, 14, 16, 18, and 20 minutes after the end of the bolus infusion. Further blood samples were obtained at 30 minutes, 50 minutes, 1 hour 10 minutes, and the last blood sample was taken 1 hour later at 4 hours 20 minutes. At day 0, 2 volunteers had slightly different blood sampling time points, just before the bolus infusion (−1 min), at 3, 7, 9, and 11 minutes after the end of the bolus infusion and the last blood sample was taken at 4 hours 20 minutes. All blood samples were obtained through an indwelling intravenous catheter from the contralateral arm, which was flushed after each blood sample. Vital parameters (oral temperature, heart rate, oxygen saturation, and systolic/diastolic blood pressure) were measured before and after the administration of the study drug, thereafter every hour until discharge.
The procedures were the same for all 3 study days. The end-of-study visit was scheduled at least 2 days after the last study day.
2.4. Safety and tolerability evaluation
The evaluation of safety and tolerability was based on adverse events, physical examinations, electrocardiograms (ECG), vital signs, and laboratory tests (clinical chemistry panel, blood counts, coagulation panel, urinalysis)
2.5. PK evaluation
Blood was collected into polypropylene tubes containing EDTA (1 mg/mL of blood; Vacuette Greiner Bio-One GmbH) at the time points described above. Thereafter, 1.5 mL sample was put immediately on ice and centrifuged (3000 rpm for 10 min at 4 °C). Samples were frozen at −80 °C before analysis by the TC12007, TECHNOZYM t-PA Ag EDTA ELISA (Diapharma), a commercially available ELISA for the quantitative detection of complexed and uncomplexed tissue plasminogen activator (tPA) in human plasma.
PK parameters were measured (maximal observed concentrations (Cmax), time of the maximal observed concentration (tmax) or calculated (area under the concentration-time curve [AUC0-30], half-life [t1/2]) according to a noncompartmental kinetic model, using the R-software package Perform Pharmacokinetic Non-Compartmental Analysis (https://cran.r-project.org/web/packages/PKNCA/index.html) and statistical software SAS 9.3.
2.6. PD evaluation
For the rotational thromboelastometry (ROTEM; Matel, Hausmannstätten, Austria) blood samples were collected into tubes containing 3.8% trisodium-citrate (0.129 mol/L buffered sodium citrate, Vacuette) at the time points described above. After collection, the tubes were gently inverted (3-6 times) and care was taken on the sample transport to avoid platelet agitation. Blood samples were kept at room temperature during the whole procedure. Specimen collection and processing were performed according to a recent review on platelet function testing [28]. Immediately after blood sampling, samples were brought to a technician who recalcified the samples and analyzed them using the extrinsic activation test (EXTEM) by ROTEM at 37 °C for 2 hours, as previously described [29]. Blood samples that were taken at the baseline and in the first 20 minutes after the end of the bolus infusion. The following ROTEM variables were collected: lysis time (LT; in seconds) lysis onset time (LOT; in seconds), and the maximum lysis (ML; percentages). LT is the time until 90% breakdown of maximal clot firmness is achieved in ROTEM, LOT is the time period from clotting time until 15% of maximal clot firmness is achieved and the maximum lysis represents the maximum fibrinolysis detected during the measurement.
The intra and intersubject variability of maximal lysis is < 20% in healthy volunteers [30].
2.7. Validation of the thromboelastometry method and investigation of rt-PA stability
We validated the thromboelastometry method focusing on investigating specificity, selectivity, precision, accuracy, and nonlinearity/linearity.
We also evaluated the influence of time on the ex vivo stability of rt-PA when spiked into whole blood (Supplementary Methods).
2.8. Statistical analysis
A formal sample size calculation was difficult to perform. Therefore, the sample size was chosen based on previous PK/PD studies, where a minimum of 6 up to 24 volunteers were included [6,7,[10], [11], [12], [13], [14], [15],17]. The data presented did not allow precise calculation of variation (which was one of our aims) but showed considerable variations in outcome parameters. Hence, a sample size of a minimum of 8 subjects was deemed adequate, because the gain in precision decreases considerably with higher number of subjects.
If necessary, after the interim analysis of the inter and intrasubject variability of 8 volunteers, additional subjects could have been included up to a maximum of 16.
We used descriptive statistics for demographics and safety evaluation (mean [SD]). Original data for the PK and PD endpoints are presented graphically and descriptive statistics are presented in tables. Per time point and rt-PA dose group the mean, SD, and geometric mean coefficient of variation (CV) were calculated as appropriate for quantitative PK and PD outcome parameters. Individual and mean curves (± SD at sampling times) indicating intersubject variability, were plotted.
A mixed model was used to explore dose-response relationships and to estimate the intra and intersubject variability. In the mixed model “subject” was included as random factor and “dose group” and “time” as factors. The intrasubject CV was calculated by CV = 100 × SQRT(EXP(VARerror − 1)). We performed a variance component analysis using the log-transformed values of AUC0-20, AUC0-30, AUC0-260, AUC0-inf, and Cmax of days 1 and 2 only in order to calculate the intrasubject CV of the 0.05 mg/kg dose.
2.8.1. Data imputation
The ROTEM device can only calculate maximal lysis, LOT, and LT if a sufficient degree of clot lysis occurs during the duration of analysis. In case of maximum lysis, values other than 100 are displayed by the software with a “ ∗ ” before the number indicating a preliminary (censored) result after 2 hours. In such cases, the values without “ ∗ ” stars are taken, ie, “13” instead of “13∗.” When LTs were not displayed by the software, because clot lysis did not occur within the 2-hour measurement period, a censured estimate of 7200 seconds was imputed for graphical presentation and statistical comparisons. No substitution for missing data was performed for LOTs.
All statistical analyses were done with SAS version 9.3 (SAS Institute Inc). A 2-sided P value of <.016 was considered significant to account for multiple comparisons between the 3 treatment periods.
For the validation of thromboelastometry and the evaluation of the ex vivo rt-PA stability descriptive statistics (mean, SD) was used and the CV(%) was calculated. Origin statistical software (OriginLab) and 4PL regression was used for evaluating linearity and to calculate the nonlinear fit equation.
3. Results
3.1. Baseline characteristics
Ten subjects were screened for this study, 2 screening failures occurred as exclusion criteria were met. One because of persistent hematuria and 1 female participant because her menstruation would have coincided with the study period.
All 8 dosed subjects were male (100%) with a mean age of 26 ± 6 years. The mean weight was 78.9 ± 12 kg and the body mass index 23.4 ± 2.6 kg/m2. Global coagulation tests were normal.
3.2. Safety
In total, 3 out of 8 subjects reported 3 adverse events (AEs). One local AE of mild intensity was related to the study drug (injection site bleeding), whereas 2 systemic AE’s of moderate intensity were not related (headache, common cold). No serious AEs or significant events occurred during this study.
3.3. PK evaluation
Table summarizes the key PK parameters of the 30-minute observation period.
Table.
Results from the noncompartmental analysis within the interval 0 to 30 minutes.
| Parameter | Day 0 | Day 1 | Day 2 |
|---|---|---|---|
| AUClast (ng ∗ min/mL) | 1910 (21.9) | 5250 (27.9) | 4420 (25.3) |
| Cmax (ng/mL) | 297 (27.8) | 819 (25.0) | 660 (28.3) |
| tmax (min) | 4.00 (3.00, 4.00) | 4.00 (4.00, 4.00) | 4.00 (4.00, 4.00) |
| Clall (mL/[kg∗min]) | 11.3 (18.4) | 9.73 (28.0) | 11.7 (25.1) |
| t1/2 (min) | 5.10 (1.17) | 4.51 (0.724) | 5.06 (1.25) |
AUClast, area under the concentration-time curve; Cmax, maximal observed concentrations; tmax, time of the maximal observed concentration calculated; t1/2, half-life.
Values are given with the geometric mean (geometric CV), except for half-life (mean [SD]) and tmax (median [range]).
On the first study day, volunteers received 0.02 mg/kg rt-PA, and on the second and third study days 0.05 mg/kg rt-PA. The absolute amount of dose per study day was calculated for each subject by dose (mg/kg) × BW (kg) which resulted in a mean amount of 1.58 ± 0.23 mg for day 0 and 3.94 ± 0.59 mg for days 1 and 2.
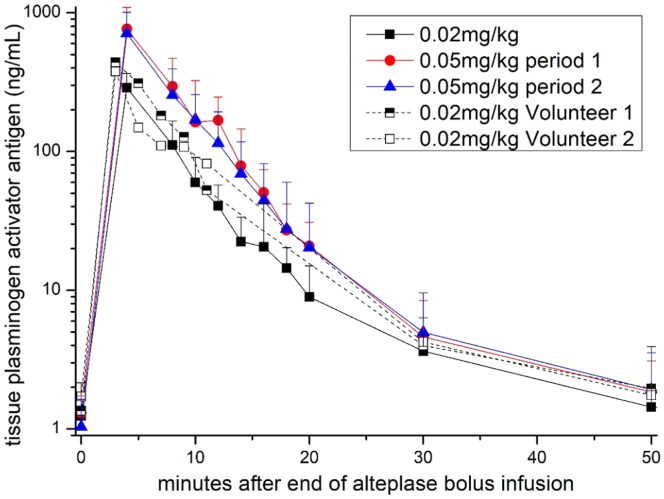
The basal plasma concentrations of t-PA Ag were 1.46 ± 0.67, 1.50 ± 0.71, and 1.27 ± 0.63 ng/mL for days 0, 1, and 2, respectively. Cmax increased dose-dependently and were 297 (geometric CV, 27.8) ng/mL on day 0 and 819 (25.0) and 660 (28.3) ng/mL on days 1 and 2 (Table and Figure 1). Figure 1 shows the time course of t-PA Ag concentrations over time and individual time-concentration curves are depicted in Supplementary Figure S1. The AUClast was 1910 (21.9), 5250 (27.9), and 4420 (25.3) ng × min/mL for days 0, 1, and 2, respectively (Table).
Figure 1.
Pharmacokinetics of tissue plasminogen activator antigen plasma concentrations. Eight healthy volunteers received bolus infusions of alteplase 0.02 mg/kg on day 0 (squares) and 0.05 mg/kg on days 1 (circles) and 2 (triangels). At day 0, volunteers 1 and 2 had different blood sampling time points and this is why we have depicted them separately. Data are given as medians (+ IQR).
After bolus infusion, rt-PA Ag levels peaked rapidly after 4 minutes in all groups and decreased with a mean half-life of 5.1 ± 1.17 minutes on day 0 and 4.51 ± 0.7 and 5.06 ± 1.25 minutes on days 1 and 2, respectively (Figure 1 and Table). The clearance based on the relative dose was 11.3 (18.4), 9.73 (28.0), and 11.7 (25.1) mL/(kg∗min), respectively (Table).
We used a variance component analysis to calculate the intrasubject CV of the 0.05 mg/kg dose. In the first model, we included the factors subject and day (Supplementary Table S1), and in the second model, the factor subject only (Supplementary Table S2). The intrasubject CV ranged from 14.8% to 20.9% (subject and day) and from 20.8% to 23.2% (subject).
Finally, we used mixed models to investigate whether AUC and Cmax values differed between study days. Both parameters were significantly different when day 0 was compared with day 1 or 2, respectively (P < .001 for all comparisons, Supplementary Tables S3 and S4). No significant difference was observed for both comparisons between study days 1 and 2 (P=not significant for all comparisons; Supplementary Tables S1 and S2).
3.4. PD evaluation
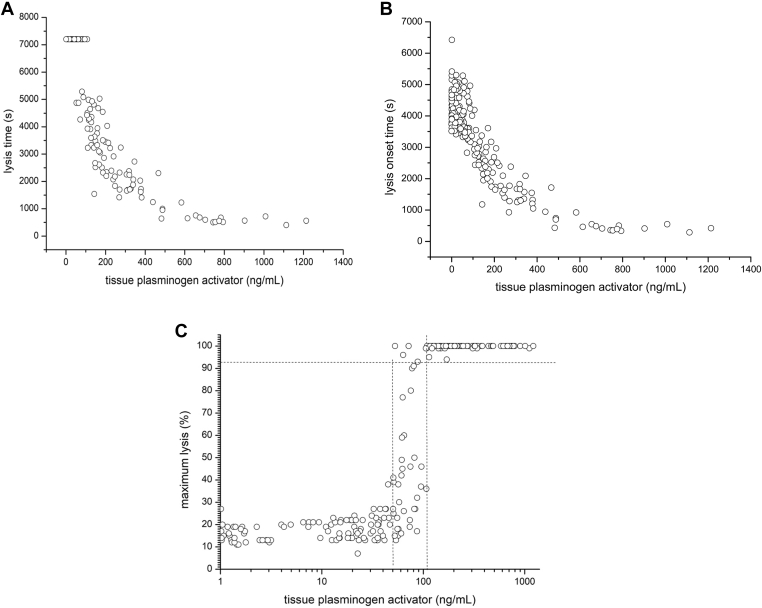
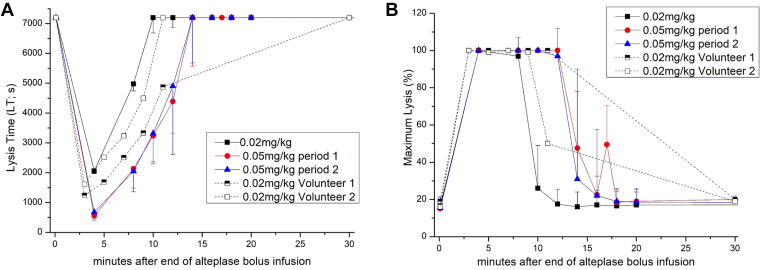
Bolus infusion of rt-PA dose-dependently shortened LT and LOT (Figures 2A, B and 3A; individual PD curves are depicted in Supplementary Fig S2). The rt-PA induced increase in the percentage of maximum lysis showed a sigmoid curve. After exceeding the threshold of 100 to 200 ng/mL of t-PA, maximum lysis invariably reached 100% (Figure 2C). Figure 3 presents PD values over time. The duration in which LT could be measured (< 7200 s) was dose-dependent and was about 1 half-life longer in the 0.05 mg/kg than in the 0.02 mg/kg dose group (Figure 3A). In both dose groups, 100% maximum lysis was seen in all subjects (Figure 3B). However, the duration of 100% maximum lysis was observed until approximately 8 min in the 0.02 mg/kg dose group and up to approximately 14 min in the 0.05 mg/kg dose group, which was again >1 half-life longer (Figure 3B).
Figure 2.
(A–C) Concentration-effect-curves of tissue plasminogen activator on lysis time, lysis onset time and maximum lysis. Eight healthy volunteers received 0.02 mg/kg alteplase on day 0 and 0.05 mg/kg on day 1 and on day 2, respectively. Datapoints are from all blood samples taken throughout individual observation periods. Lysis time (seconds), lysis onset time (seconds), maximum lysis (%) measured with rotational thromboelastometry; concentration of tissue plasminogen activator antigen (ng/mL).
Figure 3.
(A, B) Time course of lysis time and maximum lysis. Eight healthy volunteers received 0.02 mg/kg alteplase on day 0 (squares) and 0.05 mg/kg on day 1 (circles) and on day 2 (triangles), lysis time (seconds), maximum lysis (%) measured with rotational thromboelastometry. On day 0, volunteers 1 and 2 had different blood sampling time points and this is why we have depicted them separately. Data are given as medians (± IQR).
Post-hoc comparisons revealed that all PD parameters were significantly different when day 0 was compared with day 1 and day 2 (P < .001 for all evaluations). In contrast, no significant difference was observed in PD parameters between day 1 and day 2 (P = not significant for all comparisons).
3.5. Validation of the thromboelastometry method
The thromboelastometry method was validated successfully and results of the validation are available in the Supplementary Appendix. The overall CV values were <10.7 % when measuring the LT and all values obtained using commercially available controls fell within the specified ranges.
Lastly, the stability of rt-PA spiked into whole blood showed a decrease already 15 minutes post-spiking reflected by a significant increase in LT (Supplementary Appendix ; Figures 2A, B and 3).
4. Discussion
This pilot trial measured the variability of the PK/PD of low-dose rt-PA after an intravenous bolus infusion in healthy volunteers to establish a model for potential subsequent biosimilarity studies.
In patients with acute ischemic stroke, rt-PA is administered at a dose of 0.9 mg/kg BW (max dose 90 mg) over a 60-minute infusion period (10% as bolus infusion) [31,32]. Although lower doses (0.6 mg/kg) may be safer (less intracerebral or fatal bleeding), noninferiority to standard dose alteplase was not shown [33]. With the doses chosen in our study, ie, ∼1/40 and 1/20 of the recommended therapeutic dose, the measured maximal plasma concentrations were in the expected range based on the published volume of distribution (Table) [17]. To minimize exposure time, we chose a bolus infusion administered over 1 minute. Based on data from previous studies in healthy volunteers where even 10 times higher doses were used with various infusion schemes and even a 10 mg bolus of rt-PA was safely administered, we had no safety concerns regarding our significantly lower dose [15,[17], [18], [19],34].
Based on in vitro experiments, rt-PA concentrations from 250 to 333 ng/mL upwards were sufficient to achieve maximum lysis [29]. As expected, we observed maximum lysis in all subjects and were able to measure LT and LOT at the dose levels chosen. The EC50 for maximum lysis was estimated to fall within the range of 40 to 100 ng/mL and full clot lysis occurred at >100 ng/mL, However, the speed of clot lysis correlated with plasma concentrations of rt-PA in the range of 0 to 800 ng/mL and reached a plateau above those plasma levels. Hence, maximal ex vivo lysis is achieved at considerably lower concentrations than those (2-3 μg/mL) achieved during lysis therapy [9]. It would be interesting to examine a possible clinical impact of this finding in acute stroke.
We chose a sequential treatment within subjects for safety reasons. This is similar to a cross-over design, which is recommended for equivalence assessments [21] and allows lower numbers of participants to investigate intra and interindividual variability. We truncated the calculations of the AUC at 30 minutes, as endogenous t-PA levels will disproportionally increase the AUC the longer the observation period will last. This would decrease the assay sensitivity to detect putative differences in a biosimilar comparison. Our study revealed that the maximal t-PA concentrations and AUC were dose linear even at low doses (Figure 1, Table) and the time-concentration curves were clearly separated between the lower and the higher doses (Figure 1). Further, the observed short half-life of alteplase (3-5 min) fell within the range of previously reported data for full therapeutic doses [17,20]. This means that the clearance of rt-PA is constant over a wide dose range (0.02-1 mg/kg) which supports testing PK equivalence at low doses.
As expected, due to the short half-live of rt-PA, we observed no carry-over effect in our study. We repeated the higher dose to better characterize the intrasubject variability and to facilitate sample size calculations for later biosimilarity studies. Our results showed a moderate intrasubject variability (CV < 25%) for PK parameters (Supplementary Tables S3 and S4). This facilitates testing the biosimilarity of 2 rt-PA products in a reasonably low number of healthy volunteers, which is in contrast to huge sample sizes that would be required to test their putative biosimilarity using a clinical endpoint such as the numerical ranking scale in a stroke population.
Compared with other, plasma-based clot formation assays, the thrombelastometry system used in our study is a certified and commercially available system that measures the process of clot formation, clot strength, and fibrinolysis in whole blood, which is more physiologic, does not require the addition of an exogenous plasminogen activator and provides precise and reproducible results [[24], [25], [26], [27],35,36].
The assay requires only a small sample volume. Therefore, frequent blood sampling is only a minimal burden for the participants. Moreover, thromboelastometry has been tested in various clinical trials even with thrombolytic agents, providing a sound rationale for using this method for evaluating PD in this setting [[23], [24], [25],35,37]. It was recently shown, that sequential clot lysis analysis may predict early clinical outcomes in stroke patients with thrombolytic therapy [38].
We decided to use the EXTEM test for the main part of our study, although a protocol for using tissue factor with and without the addition of rt-PA was described by Kuiper et al. [39]. However, as we measured the fibrinolytic effects of IV rt-PA and no clinical conditions of hyperfibrinolysis compared with normal patients, this protocol was not considered optimal for our setting.
Interestingly, the technical validation in that study revealed higher CV values for the reproducibility of LTs (10%-20%), whereas our CV values were in the range of 4% to 11% (Supplementary Appendix). However, we investigated a range of different rt-PA concentrations from 50 to 600 ng/mL (Supplementary Appendix), whereas Kuiper et al. [39] used only 2 different rt-PA concentrations (125 and 175 ng/mL). Further, the different results may be explained by the fact that we used the commercially available tests and also that we have tested newer devices that may be more accurate.
In the main part of the study, LT and maximum lysis normalized within 8 to 12 minutes and within 10 to 18 minutes, respectively. LTs revert to baseline (ie, >7200 s) when plasma levels of tPA fall below ∼100 to 200 ng/mL, whereas maximum lysis normalizes once tPA concentrations fall below ∼50 ng/mL (Figure 2A). This corresponds well to the PKs of tPA-Ag (Figure 1). tPA-Ag levels are <10 ng/mL after 30 to 50 minutes, which does not have a measurable effect on clot lysis in the ROTEM system. The validation part of our trial and the results from the main part revealed that thromboelastometry using the EXTEM assay is a valid and accurate method, and thromboelastic parameters can be considered useful and valid surrogate parameters for PD effects of rt-PA in vivo.
Bolus infusion of alteplase shortened LT and LOT in a dose-dependent way in our model. The extent and duration of shortening were clearly distinguishable between dose groups (Figures 2 and 3). Further, 100% maximum lysis was reached in all subjects even in the lower rt-PA dose group (Figures 2 and 3). However, maximum lysis showed a steep sigmoid concentration-effect curve, which makes this parameter less sensitive for PD evaluation in this setting. LT of a clot is critical for reperfusion of organs including the brain or myocardium and is considered a good clinical surrogate parameter. Therefore, it may be used as the primary outcome parameter for PD biosimilarity.
Similar to PK, PD parameters were not different between days 1 and 2 (Supplementary Figure 2) and showed a moderate intrasubject variability (CV < 25%).
There was a clear dose-effect relationship in the steep ascending part of the dose-response curve as required by the guidelines of the European Medicines Agency [40]. This distinguishes our model from other studies with healthy subjects that could not establish clear dose-response relationships with conventional PD parameters, such as fibrinogen, fibrin degradation products, and plasminogen [10,17,19]. The reason for this finding was probably a lower sensitivity of these biomarkers.
A potential limitation is that we used bolus infusions in our study whereas a bolus-primed continuous infusion is used in clinical practice. Achieving a steady state almost immediately after the bolus infusion would provide advantages in healthy volunteers: there will be no overshoot in the plasma concentrations, and it can be expected that all plasma concentrations sampled during the continuous infusion, and therefore the PD effects are expected to be similar and constant. Having multiple PD measurements under steady state will also allow to identify any methodological or technical errors easily and will add to the credibility of the data. Thus, a bolus-primed continuous infusion scheme is planned for later biosimilarity studies.
A potential limitation of our study could be that the chosen parameters predominantly measure an immediate fibrinolytic activity and may not offer a comprehensive representation of a fibrinolytic drug’s efficacy in dissolving pre-existing thrombi over extended periods. As a thrombolytic reagent, however, the efficacy of dissolving preformed thrombi, if administered at later times, could be also important. Here differences in clot composition may mainly come into play, eg, whether it is an embolus from the heart or an atherosclerotic lesion of the cervical arteries, other assays may also be required but could be challenging to standardize. However, according to the guidelines biosimilar products may be authorized on basis of similar pharmacodynamics in humans [21,22]. Still, the quality of a reference product and proposed biosimilar are compared by multiple assays in vitro, and such data must be submitted to the regulatory agencies for approval of a trial application and eventual marketing authorization of the proposed biosimilar product.
Finally, 1 female participant had to be excluded because her menstruation coincided with the study, so our study was only conducted in men and future trials should also include women.
In conclusion, the results indicate that the pharmacokinetics of rt-PA are dose linear and displayed limited intrasubject variability (CV% < 25%). The half-life of rt-PA and therefore its clearance are representative of full therapeutic doses. LT was shortened in a dose and time-dependent fashion after rt-PA infusion and was clearly distinguishable between doses. Given the shortage of available alteplase concomitant with increasing thrombolysis rates in stroke, the development of a biosimilar rt-PA is an urgent medical need. The presented model appears suitable and sensitive to test its biosimilarity in the steep part of the dose-response curve in a timely manner. Lastly, this model may be used to investigate drug-drug interactions of tPA with other investigational medicinal products.
Acknowledgments
The authors would like to thank Sabine Schranz for her invaluable help throughout the trial.
Funding
This Investigator Initiated Trial was supported by a grant from Prediction Biosciences, Lyon, France.
Author contributions
N.B., M.M.S., G.F., C.S., G.G., and U.D. (the principal investigator) were responsible for conducting the trial. K.K.M. performed the validation of the thromboelastometry method and the investigation of rt-PA stability. C.D. performed the pharmacodynamic analyses. B.J. designed the trial, U.D. and B.J. wrote the protocol. F.K. performed the statistical analyses, all authors participated in data interpretation. All authors had full access to all the study data and contributed to both writing and critically reviewing the manuscript.
Relationship Disclosure
None declared.
Footnotes
Handling Editor: Dr Henri Spronk
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102518
Supplementary material
References
- 1.Marko M., Posekany A., Szabo S., Scharer S., Kiechl S., Knoflach M., et al. Trends of r-tPA (recombinant tissue-type plasminogen activator) treatment and treatment-influencing factors in acute ischemic stroke. Stroke. 2020;51:1240–1247. doi: 10.1161/STROKEAHA.119.027921. [DOI] [PubMed] [Google Scholar]
- 2.Weber R., Eyding J., Kitzrow M., Bartig D., Weimar C., Hacke W., et al. Distribution and evolution of acute interventional ischemic stroke treatment in Germany from 2010 to 2016. Neurol Res Pract. 2019;1:4. doi: 10.1186/s42466-019-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pensato U., Romoli M., Lazzarin S.M., Marcheselli S., Zini A. The shortage of thrombolytics for stroke: a call for action. Lancet Neurol. 2023;22:28. doi: 10.1016/S1474-4422(22)00477-X. [DOI] [PubMed] [Google Scholar]
- 4.Demarquay G., Derex L., Nighoghossian N., Adeleine P., Philippeau F., Honnorat J., et al. Ethical issues of informed consent in acute stroke. Analysis of the modalities of consent in 56 patients enrolled in urgent therapeutic trials. Cerebrovasc Dis. 2005;19:65–68. doi: 10.1159/000083250. [DOI] [PubMed] [Google Scholar]
- 5.Koster R.W., Cohen A.F., Kluft C., Kasper F.J., van der Wouw P.A., Weatherley B.C. The pharmacokinetics of recombinant double-chain t-PA (duteplase): effects of bolus injection, infusions, and administration by weight in patients with myocardial infarction. Clin Pharmacol Ther. 1991;50:267–277. doi: 10.1038/clpt.1991.136. [DOI] [PubMed] [Google Scholar]
- 6.Garabedian H.D., Gold H.K., Leinbach R.C., Yasuda T., Johns J.A., Collen D. Dose-dependent thrombolysis, pharmacokinetics and hemostatic effects of recombinant human tissue-type plasminogen activator for coronary thrombosis. Am J Cardiol. 1986;58:673–679. doi: 10.1016/0002-9149(86)90336-x. [DOI] [PubMed] [Google Scholar]
- 7.Topol E.J., Bell W.R., Weisfeldt M.L. Coronary thrombolysis with recombinant tissue-type plasminogen activator. A hematologic and pharmacologic study. Ann Intern Med. 1985;103:837–843. doi: 10.7326/0003-4819-103-6-837. [DOI] [PubMed] [Google Scholar]
- 8.Garabedian H.D., Gold H.K., Leinbach R.C., Johns J.A., Yasuda T., Kanke M., et al. Comparative properties of two clinical preparations of recombinant human tissue-type plasminogen activator in patients with acute myocardial infarction. J Am Coll Cardiol. 1987;9:599–607. doi: 10.1016/s0735-1097(87)80054-2. [DOI] [PubMed] [Google Scholar]
- 9.Tanswell P., Tebbe U., Neuhaus K.L., Glasle-Schwarz L., Wojcik J., Seifried E. Pharmacokinetics and fibrin specificity of alteplase during accelerated infusions in acute myocardial infarction. J Am Coll Cardiol. 1992;19:1071–1075. doi: 10.1016/0735-1097(92)90297-z. [DOI] [PubMed] [Google Scholar]
- 10.Chandler W.L., Alessi M.C., Aillaud M.F., Henderson P., Vague P., Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation. 1997;96:761–768. doi: 10.1161/01.cir.96.3.761. [DOI] [PubMed] [Google Scholar]
- 11.De Boer A., Kluft C., Kasper F.J., Kroon J.M., Schoemaker H.C., Breimer D.D., et al. Interaction study between nifedipine and recombinant tissue-type plasminogen activator in healthy subjects. Br J Clin Pharmacol. 1993;36:99–104. doi: 10.1111/j.1365-2125.1993.tb04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang Y.G., Liu P., Gao H.Z., Li H.Y., Hao G.T., Wang X.F., et al. Pharmacokinetics and safety of a new bioengineered thrombolytic agent, human tissue urokinase type plasminogen activator in Chinese healthy volunteers. Eur J Drug Metab Pharmacokinet. 2011;35:97–102. doi: 10.1007/s13318-010-0019-4. [DOI] [PubMed] [Google Scholar]
- 13.Martin U., von Mollendorff E., Akpan W., Kientsch-Engel R., Kaufmann B., Neugebauer G. Pharmacokinetic and hemostatic properties of the recombinant plasminogen activator bm 06.022 in healthy volunteers. Thromb Haemost. 1991;66:569–574. [PubMed] [Google Scholar]
- 14.Martin U., von Mollendorff E., Akpan W., Kientsch-Engel R., Kaufmann B., Neugebauer G. Dose-ranging study of the novel recombinant plasminogen activator BM 06.022 in healthy volunteers. Clin Pharmacol Ther. 1991;50:429–436. doi: 10.1038/clpt.1991.160. [DOI] [PubMed] [Google Scholar]
- 15.Seifried E., Tanswell P., Rijken D.C., Barrett-Bergshoeff M.M., Su C.A., Kluft C. Pharmacokinetics of antigen and activity of recombinant tissue-type plasminogen activator after infusion in healthy volunteers. Arzneimittelforschung. 1988;38:418–422. [PubMed] [Google Scholar]
- 16.Takada A., Hou P., Mori T., Takada Y. Changes in various parameters of fibrinolysis in persons infused with tissue plasminogen activator: special reference to plasminogen activator inhibitor. Thromb Res Suppl. 1988;8:23–33. doi: 10.1016/0049-3848(88)90151-x. [DOI] [PubMed] [Google Scholar]
- 17.Tanswell P., Seifried E., Su P.C., Feuerer W., Rijken D.C. Pharmacokinetics and systemic effects of tissue-type plasminogen activator in normal subjects. Clin Pharmacol Ther. 1989;46:155–162. doi: 10.1038/clpt.1989.120. [DOI] [PubMed] [Google Scholar]
- 18.Verstraete M., Bounameaux H., de Cock F., Van de Werf F., Collen D. Pharmacokinetics and systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator (rt-PA) in humans. J Pharmacol Exp Ther. 1985;235:506–512. [PubMed] [Google Scholar]
- 19.Verstraete M., Su C.A., Tanswell P., Feuerer W., Collen D. Pharmacokinetics and effects on fibrinolytic and coagulation parameters of two doses of recombinant tissue-type plasminogen activator in healthy volunteers. Thromb Haemost. 1986;56:1–5. [PubMed] [Google Scholar]
- 20.Glund S., Hoefler J., Lang B., Cafiero S., Panova-Noeva M., Place C., et al. Bioequivalence of intravenous alteplase from two different manufacturing processes in healthy male volunteers: results from a two-stage, adaptive-design study. Clin Pharmacokinet. 2023;62:1023–1030. doi: 10.1007/s40262-023-01253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agency E.M . 2014. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active-substance-quality-issues_en.pdf ((EMEA/CHMP/BMWP/42832/2005)) [Google Scholar]
- 22.FDA . Center for Drug Evaluation and Research (FDA-2011-D-0605); 2015. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product [Google Scholar]
- 23.Nilsson C.U., Tynngard N., Reinstrup P., Engstrom M. Monitoring fibrinolysis in whole blood by viscoelastic instruments: a comparison of ROTEM and ReoRox. Scand J Clin Lab Invest. 2013;73:457–465. doi: 10.3109/00365513.2013.801509. [DOI] [PubMed] [Google Scholar]
- 24.Solomon C., Asmis L.M., Spahn D.R. Is viscoelastic coagulation monitoring with ROTEM or TEG validated? Scand J Clin Lab Invest. 2016;76:503–507. doi: 10.1080/00365513.2016.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veigas P.V., Callum J., Rizoli S., Nascimento B., da Luz L.T. A systematic review on the rotational thrombelastometry (ROTEM(R)) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016;24:114. doi: 10.1186/s13049-016-0308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dibiasi C., Ulbing S., Bancher-Todesca D., Ulm M., Gratz J., Quehenberger P., et al. Concentration-effect relationship for tranexamic acid inhibition of tissue plasminogen activator-induced fibrinolysis in vitro using the viscoelastic ClotPro® TPA-test. Br J Anaesth. 2024;132:343–351. doi: 10.1016/j.bja.2023.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshii R., Sawa T., Kawajiri H., Amaya F., Tanaka K.A., Ogawa S. A comparison of the ClotPro system with rotational thromboelastometry in cardiac surgery: a prospective observational study. Sci Rep. 2022;12 doi: 10.1038/s41598-022-22119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiel A.O., Mayr F.B., Firbas C., Quehenberger P., Jilma B. Validation of rotation thrombelastography in a model of systemic activation of fibrinolysis and coagulation in humans. J Thromb Haemost. 2006;4:411–416. doi: 10.1111/j.1538-7836.2006.01715.x. [DOI] [PubMed] [Google Scholar]
- 29.Schoergenhofer C., Buchtele N., Schwameis M., Bartko J., Jilma B., Jilma-Stohlawetz P. The use of frozen plasma samples in thromboelastometry. Clin Exp Med. 2017;17:489–497. doi: 10.1007/s10238-017-0454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jilma-Stohlawetz P., Fritsche-Polanz S., Quehenberger P., Schorgenhofer C., Bartko J., Ristl R., et al. Evaluation of between-, within- and day-to-day variation of coagulation measured by rotational thrombelastometry (ROTEM) Scand J Clin Lab Invest. 2017;77:651–657. doi: 10.1080/00365513.2017.1394487. [DOI] [PubMed] [Google Scholar]
- 31.National Institute of Neurological D, Stroke rt PASSG Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 32.European commission . 2002. Live work travel in the EU.https://ec.europa.eu/health/documents/community-register/2002/200209305741/anx_5741_de.pdf [Google Scholar]
- 33.Anderson C.S., Robinson T., Lindley R.I., Arima H., Lavados P.M., Lee T.H., et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374:2313–2323. doi: 10.1056/NEJMoa1515510. [DOI] [PubMed] [Google Scholar]
- 34.Seifried E., Tanswell P., Ellbruck D., Haerer W., Schmidt A. Pharmacokinetics and haemostatic status during consecutive infusions of recombinant tissue-type plasminogen activator in patients with acute myocardial infarction. Thromb Haemost. 1989;61:497–501. [PubMed] [Google Scholar]
- 35.Ebinger T., Ruland A., Lakner M., Schwaiger M. Validity, regulatory registration and approval of ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2010;21:106–107. doi: 10.1097/MBC.0b013e3283306e28. [DOI] [PubMed] [Google Scholar]
- 36.Pieters M., Philippou H., Undas A., de Lange Z., Rijken D.C., Mutch N.J., et al. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:1007–1012. doi: 10.1111/jth.14002. [DOI] [PubMed] [Google Scholar]
- 37.Summaria L. Thromboelastographic study of fibrinolytic agents. Semin Thromb Hemost. 1995;21(Suppl 4):63–71. doi: 10.1055/s-0032-1313624. [DOI] [PubMed] [Google Scholar]
- 38.Tinchon A., Freydl E., Fitzgerald R.D., Duarte C., Weber M., Calabek-Wohinz B., et al. Real-time monitoring of intravenous thrombolysis in acute ischemic stroke using rotational thromboelastometry: a feasibility pilot study. J Neurol. 2022;269:6129–6138. doi: 10.1007/s00415-022-11271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuiper G.J., Kleinegris M.C., van Oerle R., Spronk H.M., Lance M.D., Ten Cate H., et al. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb J. 2016;14:1. doi: 10.1186/s12959-016-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.European Medicines Agency Guideline on the investigation of bioequivalence. 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.