Abstract
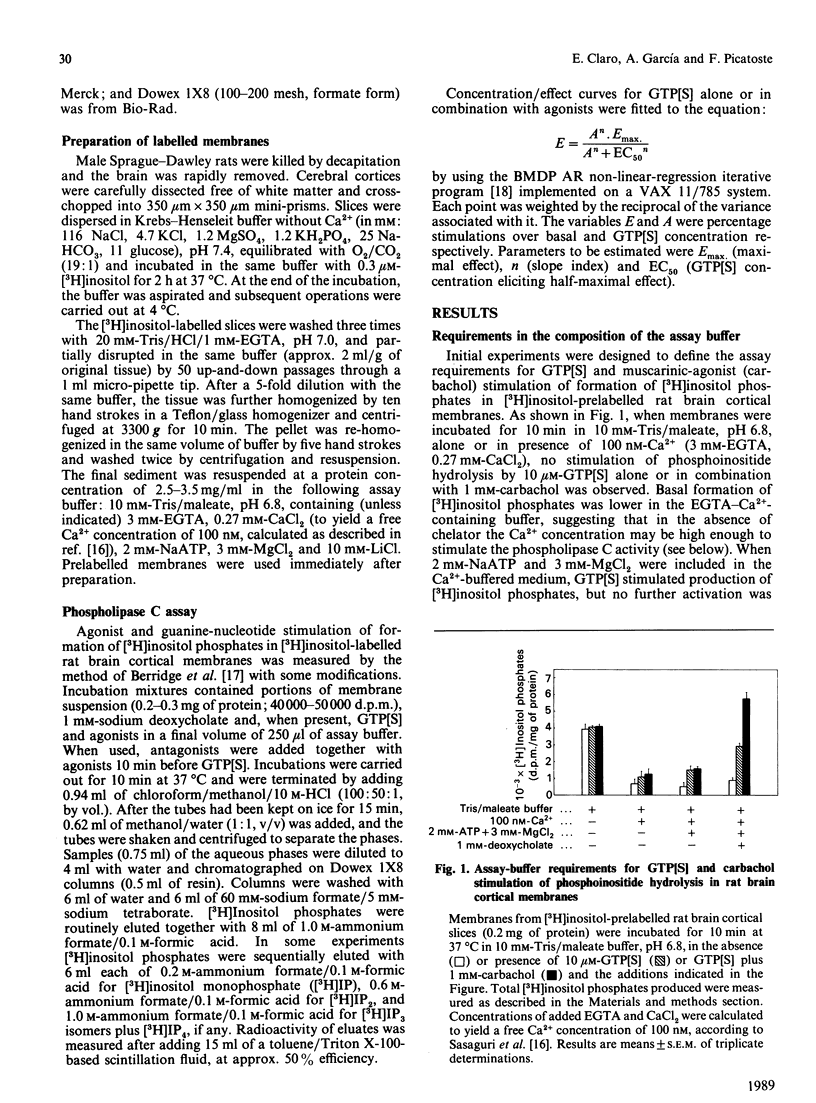
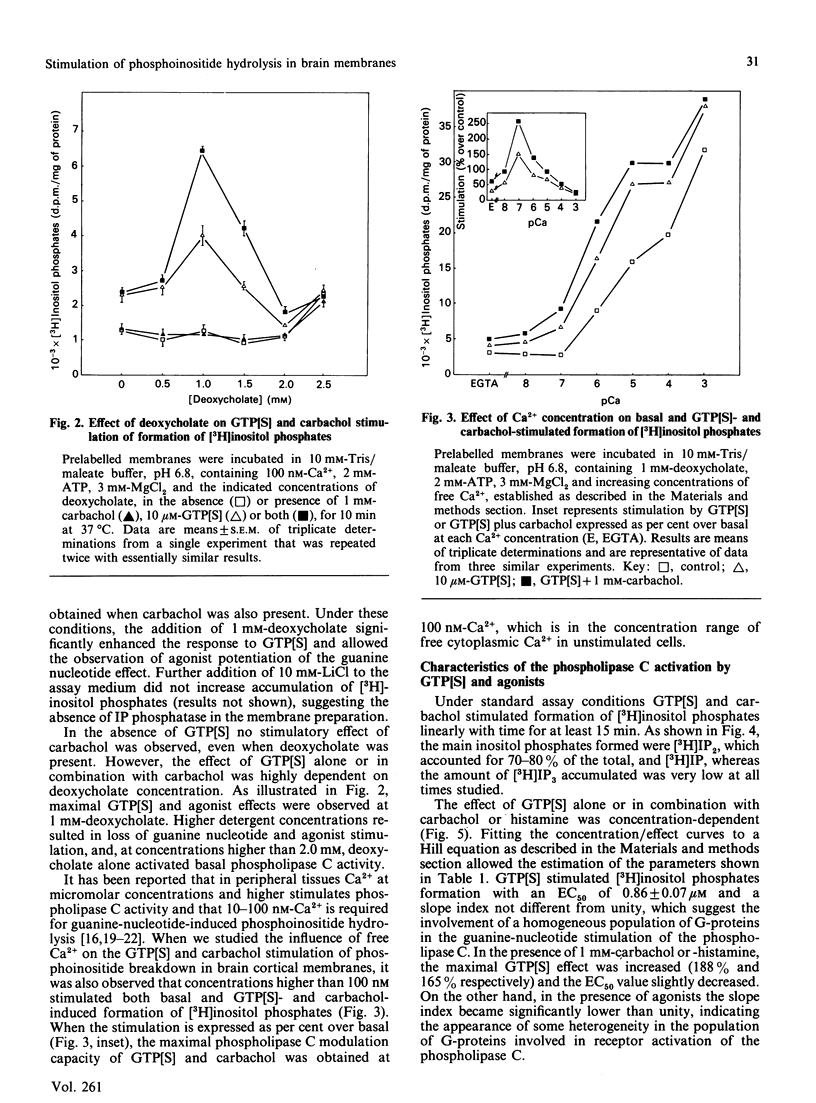
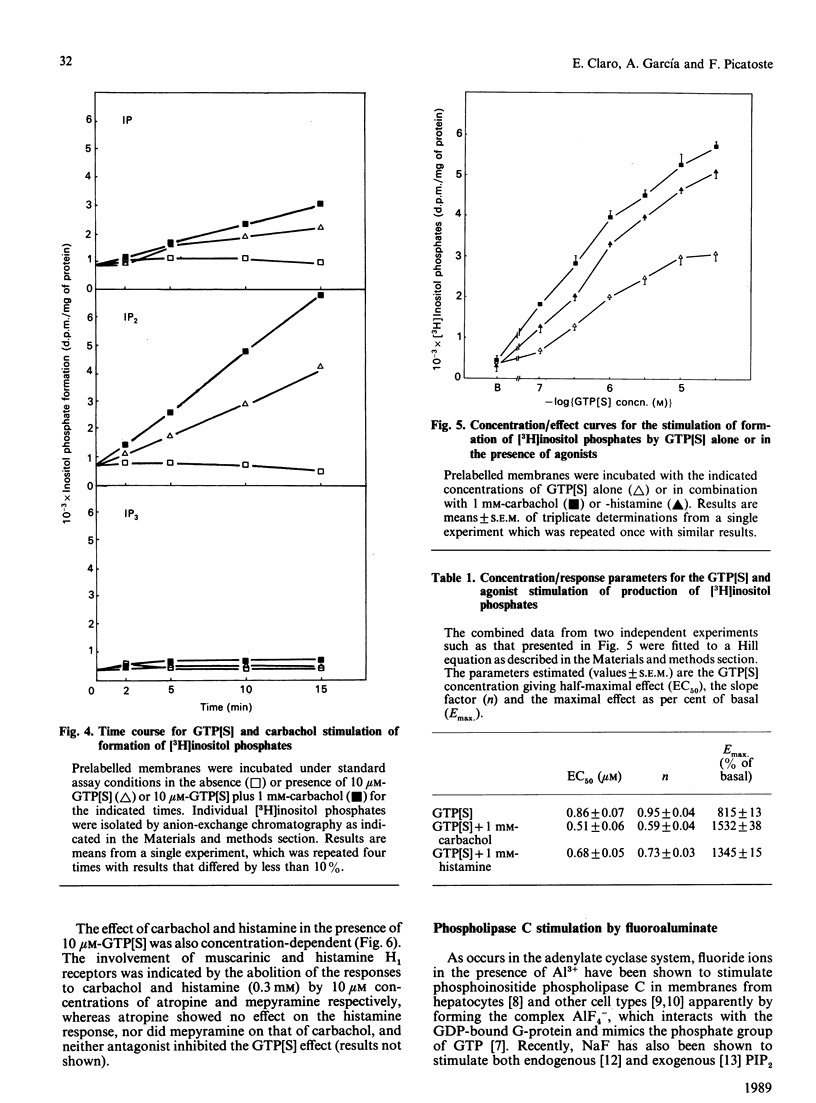
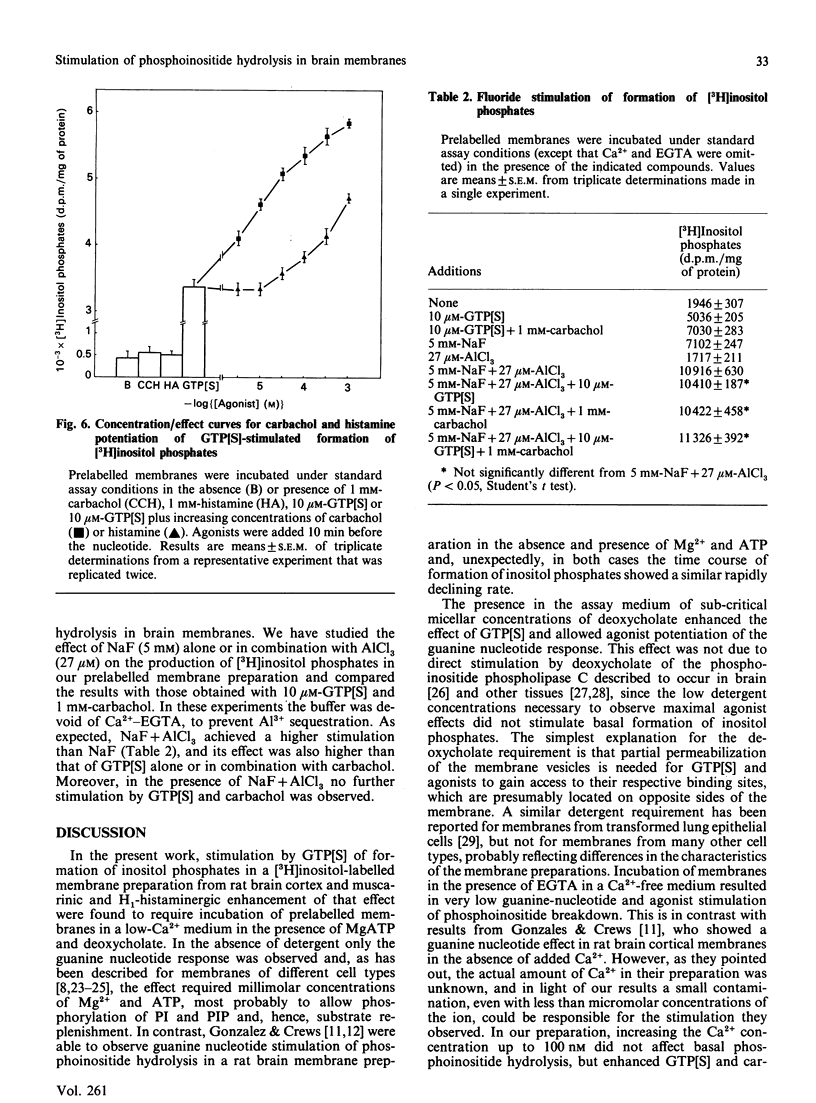
Guanine nucleotides have been shown to stimulate phosphoinositide breakdown in brain membranes, but no potentiation of such an effect by agonist was demonstrated. We have studied the effect of carbachol and histamine on guanosine 5'-[gamma-thio]triphosphate (GTP[S]) stimulation of inositol phosphates formation in [3H]inositol-labelled rat brain cortical membranes. In this preparation, GTP[S] enhancement of phosphoinositide hydrolysis required the presence of MgATP and low Ca2+ concentration (100 nM). Carbachol potentiation of the GTP[S] effect was only observed when 1 mM-deoxycholate was also added. Under these conditions, stimulated production of [3H]inositol phosphates was linear for at least 15 min, and [3H]inositol bisphosphate [( 3H]IP2) accounted for approx. 80%, whereas the amount of [3H]inositol trisphosphate [( 3H]IP3) was very low. Stimulation by GTP[S] was concentration-dependent (half-maximal effect at 0.86 microM), and its maximal effect (815% over basal) was increased by 1 mM-carbachol (1.9-fold) and -histamine (1.7-fold). Both agonists decreased the slope index of the GTP[S] concentration/effect curve to values lower than unity, suggesting the appearance of some heterogeneity in the population of guanine-nucleotide-binding proteins (G-proteins) involved. The carbachol and histamine effects were also concentration-dependent, and were inhibited by atropine and mepyramine respectively. Fluoroaluminate stimulated phosphoinositide hydrolysis to a higher extent than GTP[S] plus carbachol, and these stimulations were not additive, indicating that the same polyphosphoinositide phospholipase C-coupled G-protein mediates both effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Bojanic D., Wallace M. A., Wojcikiewicz R. J., Fain J. N. Guanine nucleotide and pyrophosphate activate exogenous phosphatidylinositol 4,5-bisphosphate hydrolysis in rat liver plasma membranes. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1088–1094. doi: 10.1016/s0006-291x(87)80182-1. [DOI] [PubMed] [Google Scholar]
- Brown E., Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: I. Receptor characterisation. J Neurochem. 1984 May;42(5):1379–1387. doi: 10.1111/j.1471-4159.1984.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Chiu A. S., Li P. P., Warsh J. J. G-protein involvement in central-nervous-system muscarinic-receptor-coupled polyphosphoinositide hydrolysis. Biochem J. 1988 Dec 15;256(3):995–999. doi: 10.1042/bj2560995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. M., Proia A. D., Klintworth G. K., Watson S. P., Lapetina E. G. Deoxycholate induces the preferential hydrolysis of polyphosphoinositides by human platelet and rat corneal phospholipase C. Biochem Biophys Res Commun. 1985 Jun 14;129(2):411–416. doi: 10.1016/0006-291x(85)90166-4. [DOI] [PubMed] [Google Scholar]
- Claro E., Arbonés L., García A., Picatoste F. Phosphoinositide hydrolysis mediated by histamine H1-receptors in rat brain cortex. Eur J Pharmacol. 1986 Apr 16;123(2):187–196. doi: 10.1016/0014-2999(86)90659-x. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987 Jan 15;241(2):409–414. doi: 10.1042/bj2410409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S. The dependence on Ca2+ of the guanine-nucleotide-activated polyphosphoinositide phosphodiesterase in neutrophil plasma membranes. Biochem J. 1986 Dec 1;240(2):503–507. doi: 10.1042/bj2400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Delvaux A., Moreau C., Dumont J. E. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat brain. Biochem Biophys Res Commun. 1986 Jan 14;134(1):351–358. doi: 10.1016/0006-291x(86)90570-x. [DOI] [PubMed] [Google Scholar]
- Evans S. W., Beckner S. K., Farrar W. L. Stimulation of specific GTP binding and hydrolysis activities in lymphocyte membrane by interleukin-2. Nature. 1987 Jan 8;325(7000):166–168. doi: 10.1038/325166a0. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Role of phosphoinositides in the regulation of liver function. Hepatology. 1988 Jan-Feb;8(1):152–166. doi: 10.1002/hep.1840080129. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gonzales R. A., Crews F. T. Characterization of the cholinergic stimulation of phosphoinositide hydrolysis in rat brain slices. J Neurosci. 1984 Dec;4(12):3120–3127. doi: 10.1523/JNEUROSCI.04-12-03120.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R. A., Crews F. T. Differential regulation of phosphoinositide phosphodiesterase activity in brain membranes by guanine nucleotides and calcium. J Neurochem. 1988 May;50(5):1522–1528. doi: 10.1111/j.1471-4159.1988.tb03039.x. [DOI] [PubMed] [Google Scholar]
- Gonzales R. A., Crews F. T. Guanine nucleotides stimulate production of inositol trisphosphate in rat cortical membranes. Biochem J. 1985 Dec 15;232(3):799–804. doi: 10.1042/bj2320799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler J. R., Harden T. K. Guanine nucleotide-dependent pertussis-toxin-insensitive stimulation of inositol phosphate formation by carbachol in a membrane preparation from human astrocytoma cells. Biochem J. 1986 Oct 1;239(1):141–146. doi: 10.1042/bj2390141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrbolich J. K., Culty M., Haslam R. J. Activation of phospholipase C associated with isolated rabbit platelet membranes by guanosine 5'-[gamma-thio]triphosphate and by thrombin in the presence of GTP. Biochem J. 1987 Apr 15;243(2):457–465. doi: 10.1042/bj2430457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Sherr C. J., Rock C. O. A guanine nucleotide-dependent phosphatidylinositol 4,5-diphosphate phospholipase C in cells transformed by the v-fms and v-fes oncogenes. J Biol Chem. 1986 Apr 15;261(11):4978–4985. [PubMed] [Google Scholar]
- Litosch I., Fain J. N. Regulation of phosphoinositide breakdown by guanine nucleotides. Life Sci. 1986 Jul 21;39(3):187–194. doi: 10.1016/0024-3205(86)90529-1. [DOI] [PubMed] [Google Scholar]
- Litosch I. Guanine nucleotide and NaF stimulation of phospholipase C activity in rat cerebral-cortical membranes. Studies on substrate specificity. Biochem J. 1987 May 15;244(1):35–40. doi: 10.1042/bj2440035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D. O., Bajjalieh S. M., Kowalchyk J. A., Martin T. F. Direct stimulation by thyrotropin-releasing hormone (TRH) of polyphosphoinositide hydrolysis in GH3 cell membranes by a guanine nucleotide-modulated mechanism. Biochem Biophys Res Commun. 1985 Oct 30;132(2):721–728. doi: 10.1016/0006-291x(85)91192-1. [DOI] [PubMed] [Google Scholar]
- Manning R., Sun G. Y. Detergent effects on the phosphatidylinositol-specific phospholipase C in rat brain synaptosomes. J Neurochem. 1983 Dec;41(6):1735–1743. doi: 10.1111/j.1471-4159.1983.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Pfeilschifter J., Bauer C. Different effects of phorbol ester on angiotensin II- and stable GTP analogue-induced activation of polyphosphoinositide phosphodiesterase in membranes isolated from rat renal mesangial cells. Biochem J. 1987 Nov 15;248(1):209–215. doi: 10.1042/bj2480209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M. J., Rosen O. M. Stimulation of polyphosphoinositide hydrolysis by thrombin in membranes from human fibroblasts. Biochem J. 1987 Jul 1;245(1):49–57. doi: 10.1042/bj2450049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri T., Hirata M., Kuriyama H. Dependence on Ca2+ of the activities of phosphatidylinositol 4,5-bisphosphate phosphodiesterase and inositol 1,4,5-trisphosphate phosphatase in smooth muscles of the porcine coronary artery. Biochem J. 1985 Nov 1;231(3):497–503. doi: 10.1042/bj2310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Hasegawa-Sasaki H. Activation of polyphosphoinositide phospholipase C by guanosine 5'-O-(3-thio)triphosphate and fluoroaluminate in membranes prepared from a human T cell leukemia line, JURKAT. FEBS Lett. 1987 Jun 22;218(1):87–92. doi: 10.1016/0014-5793(87)81024-4. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Straub R. E., Gershengorn M. C. Thyrotropin-releasing hormone and GTP activate inositol trisphosphate formation in membranes isolated from rat pituitary cells. J Biol Chem. 1986 Feb 25;261(6):2712–2717. [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. J., Exton J. H. Guanine-nucleotide and hormone regulation of polyphosphoinositide phospholipase C activity of rat liver plasma membranes. Bivalent-cation and phospholipid requirements. Biochem J. 1987 Dec 15;248(3):791–799. doi: 10.1042/bj2480791. [DOI] [PMC free article] [PubMed] [Google Scholar]


