Abstract
Controversy still exists regarding how much the inflow arterial percutaneous transluminal angioplasty (PTA) contributed to maintaining fistula function for hemodialysis. We aimed to analyze patency and risk factors after inflow arterial PTA. Hemodialysis patients with inflow arterial primary stenosis who were admitted to our institution from January 2017 to December 2022 were examined. One group had arterial-venous fistula with inflow artery stenosis alone (AVF + iAS) and another group had AVF with inflow artery stenosis and any vein stenosis (AVF + iAS + VS). The characteristics of patients, stenotic lesions, and PTA procedures were recorded. Kaplan–Meier analysis was used to compare primary patency, assisted primary patency, and secondary patency in the two groups. Cox proportional hazard analysis was used to identify risk factors associated with patency. We examined 213 patients, 53 in the AVF + iAS group (51 radial arterial stenosis and 2 ulnar arterial stenosis) and 160 in the AVF + iAS + VS group (159 radial arterial stenosis and 1 ulnar arterial stenosis). Kaplan–Meier analysis indicated the AVF + iAS group had better primary patency and assisted primary patency (both P < 0.05), but the groups had similar secondary patency. Cox proportional hazard analysis indicated that none of the analyzed clinical and biochemical indexes had clinically meaningful effects on primary patency, assisted primary patency, or secondary patency in either group. The patency and safety after PTA for inflow arterial stenosis were satisfactory, and none of the examined risk factors had a major clinical impact on patency. We recommend PTA as treatment for inflow stenosis of an AVF.
Keywords: Inflow arterial stenosis, Stenosis, Percutaneous transluminal angioplasty, Hemodialysis, Arteriovenous fistula
Subject terms: Outcomes research, Risk factors
Introduction
A vascular access with adequate function is essential for the successful hemodialysis (HD) of patients who have end-stage renal disease (ESRD)1,2. An arteriovenous fistula (AVF) is the preferred vascular access in these patients because it generally has good long-term patency and causes few complications3–6. Unfortunately, maintenance of vascular access is frequently hampered by the development of stenoses within the vascular conduit, and these can be adversely affected by problems elsewhere within the complete circuit7.
A venous problem is the most common cause of a dysfunctional fistula, and many studies have examined this topic8–11. A substantial percentage of stenoses occur in the outflow veins10,11, and a primary focus of HD access interventions has therefore been the identification and treatment of intimal hyperplastic stenoses in the outflow veins7. However, a functional AVF requires integrity in the entire vascular circuit, which consists of the heart, the body of the fistula, and the venous outflow tract leading back to the heart10.
Recent studies have devoted less attention to inflow arterial stenoses in AVFs12–15, although some studies reported the incidence of arterial inflow stenosis ranged from 4 to 40%12–19. The traditional approach when an access develops with poor inflow (i.e., insufficient blood flow for dialysis) is to abandon the access or change the location of the anastomosis to a more suitable segment of the same artery, but this approach consumes the patient’s vascular capital20. Previous studies found that an inflow arterial stenosis usually occurred in conjunction with venous stenotic lesions in the vascular access, and that percutaneous transluminal angioplasty (PTA) or stent placement were effective and safe methods to correct the inflow arterial and venous stenoses7,12–21. However, these studies provided no explanation regarding how much the inflow arterial PTA contributed to maintaining fistula function, and did not analyze factors associated with patency in inflow arterial PTA. The aim of this study was to analyze the patency of PTA for inflow arterial stenosis and identify the factors that affect patency after PTA.
Methods
Patient population
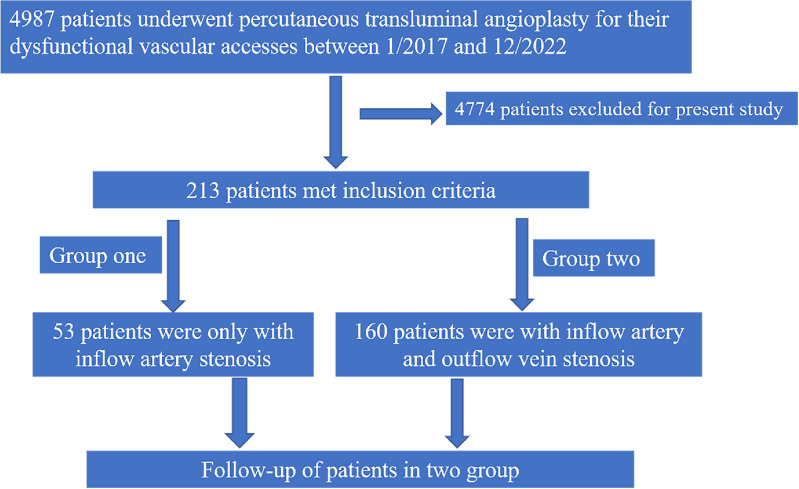
A total of 4987 ESRD patients who had upper extremity mature AVFs and attended at our hospital for PTA for treatment of dysfunctional vascular access because of stenosis or occlusion from January 2017 to December 2022 were examined (Fig. 1). This single-center retrospective cohort study collected clinical data, biochemical indexes, and personal data of all patients before the operation.
Fig. 1.
Disposition of study participants who received PTA for dysfunctional vascular access (n = 4987) and ultimately received AVF + iAS (n = 53) or AVF + iAS + VS (n = 160).
The inclusion criteria were: (i) at least one stenosis located at the inflow artery; and (ii) confirmation of stenosis based on (a) flow rates in brachial artery less than 500 mL/min and dialysis blood flow less than 200 mL/min and (b) luminal diameter of the stenosis less than 2 mm at the artery and less than 1.7 mm at the vein22; (iii) The inflow artery was defined as being comprised of that portion of the feeding artery(including brachial artery, radial artery or ulnar artery), artery adjacent to the arterio-venous anastomosis. The exclusion criteria were: (i) no inflow artery stenosis; (ii) subclavian artery stenosis; or (iii) inability to complete the follow-up or unavailable for follow-up.
For analysis, patients were divided into two groups: AVF with inflow artery stenosis alone (AVF + iAS) and AVF with inflow artery stenosis and any vein stenosis including the puncture site (AVF + iAS + VS). The institutional review board of the First Affiliated Hospital of Chongqing Medical University reviewed and approved the study protocol, and all patients provided informed consent. All methods were performed in accordance with Declarations of Helsinki.
Procedure
Before the operation, all patients were examined by ultrasound (US) using the Aplio 500 diagnostic system (18 MHz linear transducer probe, Toshiba, Tokyo, Japan). After the physical examination, the inflow artery, arteriovenous anastomosis, and draining vein(s) were visualized. The PTA was conducted in the operating room at the time of the operation, and after the patients or their families provided written informed consent.
For endovascular treatments, a brachial plexus block anesthesia was implemented using ropivacaine. Then, a 22-G needle was used to puncture the proximal part of the vein in a retrograde manner or, when combined with distal radial artery and brachial artery approach, in a prograde manner. A 6-Fr sheath (Terumo, Tokyo, Japan) was inserted into the draining vein or distal radial artery. After 3000 U of heparin was injected through the sheath, a 0.035-in hydrophilic wire (Terumo) was introduced into the sheath and crossed the lesion into the brachial artery. Angioplasty was performed using a balloon inflated to 12–24 atm for 30 s at least 3 times. Balloon size was based on the diameter of a normal vessel segment that was adjacent to the stenosis, utility time of the fistula, and the surgeon’s experience. US guidance was used during the entire procedure.
At the end of the procedure, US was used to examine vascular flow from the inflow artery to the draining vein and to ensure the absence of residual stenosis in the entire vascular region. The flow rate in the brachial artery, diameter of the arterial stenosis, and resistance index (RI) of the AVF were measured 24 h later using US.
Variables and definitions
The US variables of the two groups were diameter of the arterial stenosis, flow rate in the brachial artery, and resistance index (RI) before and after the PTA. The demographic and clinical variables were age, body mass index (BMI), sex, AVF type, AVF side (right or left arm), location of AVF, stenosis lesion of the artery, diabetes mellitus, history of hypotension (intradialytic hypotension, IDH), coronary heart disease (CHD), smoking, cause of ESRD, biochemical indexes before the operation, and PTA characteristics during the operation. History of hypotension was defined as the frequency of IDH within 3 months accounted for ≥ 20% of the total number of dialysis. Postintervention primary patency was defined as uninterrupted patency after intervention until the next intervention. Assisted primary patency was defined as uninterrupted patency of the inflow artery after intervention until the next intervention for the inflow artery. Postintervention secondary patency was defined as patency after the PTA until the AVF was surgically repaired, revised, or abandoned. The inflow arterial area was considered to include the artery from the subclavian artery to the arterial anastomosis. For follow-up, all patients were asked to return for vascular access US examinations and physical examinations every three months after the operation, or whenever there were abnormal clinical parameters (such as a decline in blood flow access during HD).
Technical success was defined as a residual stenosis of 30% or less after the procedure. Clinical success was defined as a resolution of pretreatment clinical indicators of access malfunction and the ability to provide adequate HD for at least one session23.
Statistical analysis
Data were analyzed using SPSS version 25.0 for Windows 10 (SPSS Inc, Chicago, Ill). Descriptive statistics were presented as means ± standard deviations, and categorical variables as numbers and percentages. US variables within each group before and after the procedure were compared using a paired t-test. Between-group comparisons of other clinical and biochemical variables were compared using a Chi-square test or a t-test. Kaplan–Meier survival analysis was used to compare post-intervention primary patency, assisted primary patency, and secondary patency rates in the two groups and a log-rank test is used to compare two groups for patency rates. Cox proportional hazard analysis was used to identify variables associated with patency. A P value less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the two groups
We examined 213 patients with autogenous arteriovenous fistula who were receiving long-term HD, 53 patients in the AVF + iAS group and 160 patients in the AVF + iAS + VS group (Tables 1, 2). The AVF + iAS group was younger, had a higher prevalence CHD, a higher BMI, a lower level of Ca × P product, a lower level of total cholesterol, and a lower level of parathyrin (all P < 0.05), but the groups did not differ in any of the other variables (all P > 0.05). These 213 patients all have no hemodialysis access-induced distal ischemia (HAIDI). Stenosis lesion of artery which have been operated included the proximal end of the radial artery or ulnar artery but there was no brachial inflow.
Table 1.
Demographic and clinical characteristics of the two groups.
| Variable | AVF + iAS | AVF + iAS + VS | P value |
|---|---|---|---|
| Age, years | 55.28 ± 18.77 | 58.74 ± 13.55 | 0.001 |
| Sex | 0.595 | ||
| Male | 20 (37.7) | 67 (41.9) | NA |
| Female | 33 (62.3) | 93 (58.1) | NA |
| Cause of ESRD | NA | ||
| Diabetes | 17 (32.1) | 69 (43.1) | 0.155 |
| Nephritides | 10 (18.9) | 50 (31.2) | 0.082 |
| Hypertension | 9 (17.0) | 16 (10) | 0.171 |
| ADPKD | 2 (3.8) | 3 (1.9) | 0.600 |
| SLE | 3 (5.7) | 6 (3.8) | 0.693 |
| Others | 12 (22.6) | 16 (10) | 0.038 |
| Diabetes mellitus | 26 (49.1) | 71 (44.4) | 0.553 |
| Smoking | 15 (28.3) | 33 (20.6) | 0.246 |
| History of hypotension | 10 (18.9) | 47 (29.4) | 0.13 |
| CHD | 12 (22.6) | 18 (11.3) | 0.039 |
| BMI | 23.68 ± 3.77 | 22.78 ± 3.77 | 0.001 |
| > 23.9 | 17 (32.1) | 47 (29.4) | 0.001 |
| 18.5–23.9 | 30 (56.6) | 98 (61.3) | 0.001 |
| < 18.5 | 6 (11.3) | 15 (9.4) | 0.001 |
Data are presented as n (%) or mean ± standard deviation.
ESRD, end stage renal disease; ADPKD, adult polycystic kidney disease; CHD, coronary heart disease; SLE, systemic lupus erythematosus; Hypotension, intradialytic hypotension (IDH).
Significant values are in bold.
Table 2.
Biochemical indexes in the two groups.
| Variable | AVF + iAS | AVF + iAS + VS | P value |
|---|---|---|---|
| Hemoglobin, g/L | 106.43 ± 24.07 | 111.31 ± 22.98 | 0.187 |
| D-dimer, mg/L | 1.13 ± 1.05 | 1.17 ± 3.14 | 0.917 |
| Albumin, g/L | 39.19 ± 5.37 | 40.74 ± 5.56 | 0.078 |
| Ca × P, mg/dL | 54.48 ± 15.01 | 56.91 ± 18.62 | < 0.001 |
| T-Chol, mmol/L | 2.11 ± 0.56 | 4.31 ± 1.15 | < 0.001 |
| Parathyrin, ng/L | 442.75 ± 194.40 | 804.93 ± 737.46 | < 0.001 |
Data are presented as mean ± standard deviation.
Ca × P, calcium-phosphorus product; T-Chol: total cholesterol.
Significant values are in bold.
The mean follow-up time among all 213 patients ranged from 1 to 54 months, and 25 patients died during follow-up (data not shown). Nine patients died from cerebral hemorrhage, 5 from myocardial infarction, 4 from heart failure, 1 from gastrointestinal hemorrhage, and 6 from unknown causes.
AVF and PTA characteristics of the two groups
We then compared the AVF and PTA characteristics of the two groups. Analysis of AVF characteristics (Table 3) indicated the two groups did not differ in AVF side, AVF type, AVF location, or stenosis of the ulnar artery (all P > 0.05). The most common location of inflow artery stenosis was the radial artery, and stenosis in this region was more common in the AVF + iAS + VS group, but no statistical difference has been detected between the two groups (P = 0.311).
Table 3.
AVF characteristics in the two groups.
| Variable | AVF + iAS | AVF + iAS + VS | P value |
|---|---|---|---|
| AVF side | 0.983 | ||
| Left arm | 41 (77.4) | 124 (77.5) | NA |
| Right arm | 12 (22.6) | 36 (22.5) | NA |
| AVF type | 0.437 | ||
| Radial-cephalic | 51 (96.2) | 159 (99.4) | NA |
| Ulnar-basilic | 1 (1.9) | 1 (0.06) | NA |
| Ulnar-cephalic | 1 (1.9) | 0 | NA |
| Location of AVF | 0.622 | ||
| Nasopharyngeal fossa | 7 (13.2) | 15 (9.4) | NA |
| Wrist | 43 (81.1) | 131 (81.9) | NA |
| Forearm | 3 (5.7) | 14 (8.7) | NA |
| Stenosis lesion of artery | 0.311 | ||
| Radial artery | 51 (96.2) | 159 (99.4) | NA |
| Ulnar artery | 2 (3.8) | 1 (0.06) | NA |
Data are presented as n (%). AVF in nasopharyngeal fossa is meant that arterio-venous anastomosis of fistula are located in a triangular area bounded by the Extensor Pollicis Longus and the Extensor Pollicis Brevis.
Analysis of PTA characteristics (Table 4) indicated a high-pressure balloon was the most common type of balloon used in both groups (92.5% vs. 90.6%, P = 0.788). The two groups did not differ in balloon diameter for the artery (P > 0.05), and the most common diameter was 4 mm in both groups (58.5% vs. 39.4%, P = 0.079). The two groups also had no statistical differences in specific complications (all P > 0.05), and hematoma was the most complication in both groups (58.5% vs. 50.6%, P = 0.635). When the hematoma and pseudoaneurysm occurred during the PTA, we immediately inflated the balloon to 2–6 atm while pressing from the outside for 1 min to stop the continuous bleeding, and then repeated this several times if needed. If the hematoma and pseudoaneurysm did not continue to increase, we stopped this procedure and performed careful follow-up.
Table 4.
PTA characteristics of the two groups.
| Variable | AVF + iAS | AVF + iAS + VS | P value |
|---|---|---|---|
| Balloon type for artery | NA | ||
| Plain | 1 (1.9) | 8 (5) | 0.456 |
| High pressure | 49 (92.5) | 145 (90.6) | 0.788 |
| Cutting | 3 (5.7) | 7 (4.4) | 0.713 |
| Balloon diameter for artery (mm) | |||
| 3 | 2 (1.9) | 2 (1.3) | 0.259 |
| 4 | 27 (58.5) | 63 (39.4) | 0.079 |
| 5 | 19 (32.1) | 60 (37.5) | 0.352 |
| 6 | 5 (22.6) | 35 (21.9) | 0.066 |
| Complication of artery | |||
| Hematoma | 31 (58.5) | 81 (50.6) | 0.635 |
| Pseudoaneurysm | 3 (5.7) | 12 (7.5) | 0.767 |
| Aortic dissection | 2 (3.8) | 4 (2.5) | 0.640 |
| None | 17 (32.1) | 67 (40.6) | 0.257 |
Data are presented as n (%).
Technical success, clinical success, complications, primary and secondary patency of the PTA, assisted primary patency for inflow arterial PTA
All PTA operations were successful, and we achieved clinical success in all patients (Table 5). In both groups, the results indicated that the operation led to increased flow rate in the brachial artery, decreased RI, and increased arterial diameter (all P < 0.001).
Table 5.
US variables of the two groups before and after PTA.
| Variable | AVF + iAS | AVF + iAS + VS | ||||||
|---|---|---|---|---|---|---|---|---|
| Before PTA | After PTA | t | P | Before PTA | After PTA | t | P | |
| Resistance index | 0.75 ± 0.13 | 0.49 ± 0.09 | 22.16 | < 0.001 | 0.74 ± 0.15 | 0.49 ± 0.10 | 11.03 | < 0.001 |
| Brachial artery flow rate, mL/min | 280.79 ± 141.62 | 962.39 ± 276.66 | 29.49 | < 0.001 | 364.08 ± 164.78 | 1041.13 ± 288.04 | 15.51 | < 0.001 |
| Artery diameter, mm | 1.24 ± 0.45 | 2.95 ± 0.65 | 16.63 | < 0.001 | 1.26 ± 0.40 | 2.94 ± 0.54 | 33.01 | < 0.001 |
Data are presented as mean ± standard deviation.
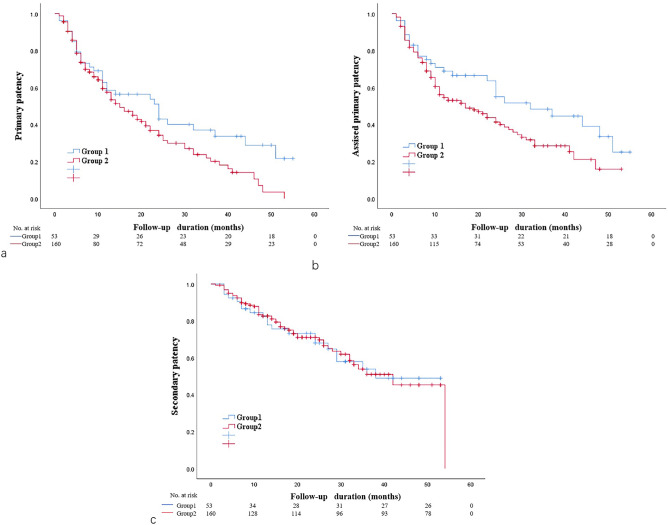
We then compared the patency in the two groups (Fig. 2). The AVF + iAS group had significantly better primary patency (P < 0.05; 6 months: 73.1% vs. 73.6%, 12 months: 58.6% vs. 45.1%, 18 months: 53.8% vs. 45.1%, 24 months: 43% vs. 34.2%) and significantly better assisted primary patency (P < 0.05; 6 months: 77.1% vs. 76%, 12 months: 68.9% vs. 54.7%, 18 months: 63.7% vs. 48.7%, 24 months:55.0% vs. 41.4%). However, the AVF + iAS and AVF + iAS + VS groups had similar rates of secondary patency (P > 0.05; 6 months: 90.5% vs. 92.4%, 12 months: 82.3% vs. 82.6%, 18 months: 73.2% vs. 75.0%, 24 months:68.0% vs. 69.6%).
Fig. 2.
Kaplan–Meier curves for primary patency (a), assisted primary patency (b), and secondary patency (c) of the AVF + iAS and AVF + iAS + VS groups.
Cox proportional hazard regression analysis for prognostic factors
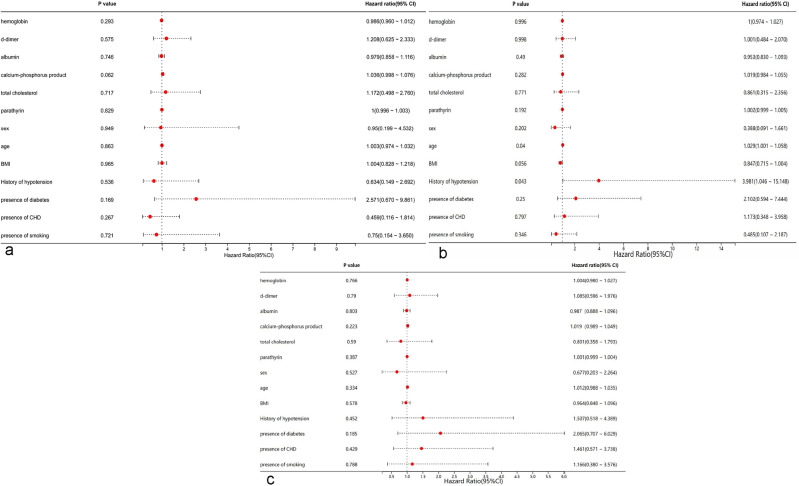
We then performed a multivariate Cox regression analysis to identify factors associated with each patency of two groups. Analysis of primary patency and secondary patency in the AVF + iAS group indicated that none of the examined variables had statistically significant effects (all P > 0.05, Fig. 3a,c). However, analysis of assisted primary patency in the AVF + iAS group showed that a history of hypotension (HR = 3.981, P = 0.042, Fig. 3b) was a statistically significant predictor, although its effect was weak and the confidence interval was wide (95% CI 1.046, 15.148).
Fig. 3.
Forest plot of factors associated primary patency (a), assisted primary patency (b), and secondary patency (c) in the AVF + iAS group based on multivariate Cox-regression.
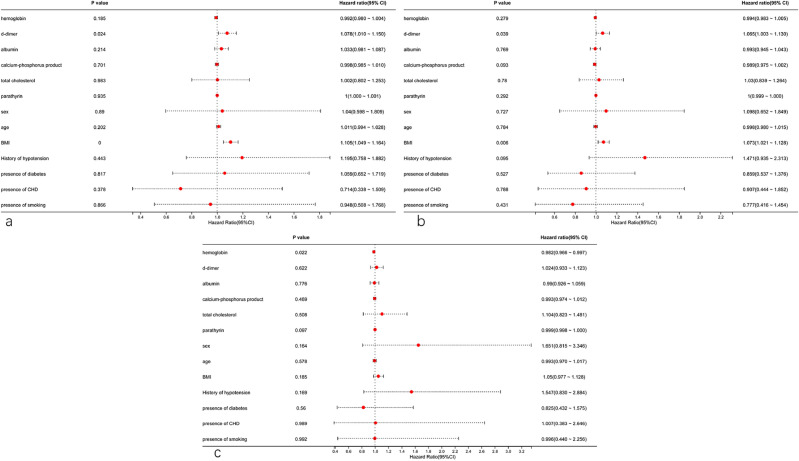
Analysis of primary patency and assisted primary patency in the AVF + iAS + VS group (Fig. 4a,b) indicated that only D-dimer (HR = 1.078, 95% CI 1.010, 1.150; P = 0.024) and BMI (HR = 1.105, 95% CI 1.049, 1.164; P < 0.001) were statistically significant predictors. However, both these effects were weak because the 95% confidence intervals were all close to 1. Analysis of secondary patency indicated that hemoglobin was the only statistically significant predictor (HR = 0.982, 95% CI 0.966, 0.997; P = 0.022, Fig. 4c), although this affect was also weak and probably not clinically relevant.
Fig. 4.
Forest plot of factors associated primary patency (a), assisted primary patency (b), and secondary patency (c) in the AVF + iAS + VS group based on multivariate Cox-regression.
Discussion
This retrospective study of patients undergoing HD who had native AVF dysfunction demonstrated that the primary patency and assisted primary patency of PTA treatment for inflow arterial stenosis was satisfactory throughout the follow-up period. The PTA treatments for inflow arterial stenosis also had relatively good safety. Our Cox proportional hazard regression analysis of factors related to patency indicated that none of the examined clinical or biochemical indexes had a major impact on patency.
We first compared the primary patency, assisted primary patency, and secondary patency of the AVF + iAS group and the AVF + iAS + VS group. Relative to the AVF + iAS + VS group, the AVF + iAS group had significantly better primary patency and assisted primary patency at 6, 12, 18, and 24 months after PTA. However, these two groups had similar secondary patency at 6, 12, 18, and 24 months after PTA. Previous studies7,12–15,19,20 reported PTA treatment that was guided by digital subtraction angiography (DSA) for inflow arterial stenosis was feasible, but these studies did not focus on patency. Duijm et al.7,15 reported the feasibility and technical success of endovascular treatment of inflow stenoses in AVFs and AV grafts (AVGs) guided by DSA through retrograde venous access puncture and catheter advancement into the central arterial inflow or aortic arch. Four other studies reported the incidence of inflow stenosis in dysfunctional HD access AVFs and AVGs using different definitions of inflow arterial stenosis12–14,19. However, these studies examined the incidence of inflow arterial stenosis and the success rate of the procedure, and provided no explanation about patency after PTA treatment for arterial inflow stenosis. Our results firstly showed that PTA treatment for inflow arterial stenosis provided good patency so far, suggesting this is an effective method for the AVF with inflow arterial stenosis for patients undergoing HD.
Although all of our patients received successful procedures, our incidence of complications of the inflow artery for hematoma during inflow arterial PTA were higher than reported in some previous studies7,12–15,19,20. This may be because these previous studies used DSA to guide the inflow arterial PTA, but we used US guidance solely during the procedure. Compared with US, DSA may be less likely to detect small hematomas caused by the tear of a vessel wall after balloon dilation24. US provides higher image resolution of superficial vessels than DSA, and the use of a high frequency probe allows easy detection of hematomas around the vessels. Although our approach may have increased the risk for complications, especially hematoma and pseudoaneurysm, these complications had no effect on the use of AVF in our HD patients.
There is some disagreement regarding the factors that influence primary patency after PTA treatment. Some studies reported different factors were related to patency, such as coronary artery disease, peripheral vascular disease, diabetes mellitus25,26, longer stenosis26–29, young age of fistula before the first failure23,28,30, older patient age31, albumin level less than 35 g/L31, hypertension31, and lesion at the arteriovenous anastomosis (a predictor of poorer long-term patency)32. Another study of postintervention secondary patency found that a primary renal disease (diabetes or renal vascular diseases) was significantly associated with AVF failure29. However, a different study reported none of many analyzed factors were significantly related to secondary patency after PTA, including patient age, sex, age of fistula at initial intervention, presence of diabetes, side and location of fistula, location of stenosis, and other factors33. We identified several factors that had statistically significant relationships with patency, but Cox analysis indicated their effects were very weak. Thus, we identified no obvious predictive factors that were related to post-intervention primary patency, assisted primary patency, or secondary patency of vascular access. To the best of our knowledge, our study was the first to thoroughly analyze the influence of multiple biochemical indexes on primary and second patency after PTA treatment on inflow arterial stenosis, but our results are not entirely consistent with previous studies about venous PTA (such as age, albumin etc.). Previous study also had shown that thrombophilia was independently associated with early post-angioplasty occlusion, hence early anti-aggregation/anticoagulation after PTA may improve the patency34. But this may reduce the incidence of thrombosis, whether it can improve the patency rate still needs to be further studied in a large sample. But our study was not including this factor because our patients have no routine anti-aggregation/anticoagulation for all patients. As a result, we suggest that further studies with larger sample sizes and in different populations should also examine factors related to patency after successful PTA.
There were some limitations in our study. First, the sample size was small, and we only examined 53 patients who received AVF + iAS. Second, our study was a single-center retrospective study, and was therefore subject to various biases that would not be present in a randomized prospective study. Third, we did not assess the causes of inflow arterial stenosis. All of these limitations should be addressed in future studies.
Conclusion
Inflow arterial PTA provides good outcomes for HD patients by maintaining the short-term and long-term patency rates of fistulas that have inflow arterial stenosis, without the need for dialysis catheters or surgical intervention. Current evidence suggests that no significant clinical or biochemical indexes were obviously associated with patency after angioplasty of an AVF that has inflow arterial stenosis.
Acknowledgements
We thank Medjaden Inc. for scientific editing of this manuscript.
Author contributions
B.C. and ZM. W. wrote the main manuscript text. B.T., L.C., XJ.G., Y.Z., Q.Q.L. and Q.L. collected the data. X.Z. did the statistics. All authors reviewed the manuscript.
Funding
This work was supported by Science-Health Joint Medical Research Project of Chongqing (Number: 2020FYYX064).
Data availability
All data can be made available by Ziming Wan upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daugirdas, J. T. et al. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am. J. Kidney Dis.66, 884–930 (2015). 10.1053/j.ajkd.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 2.Lacson, E. Jr. & Brunelli, S. M. Hemodialysis treatment time: A fresh perspective. Clin. J. Am. Soc. Nephrol.6(10), 2522–2530 (2011). 10.2215/CJN.00970211 [DOI] [PubMed] [Google Scholar]
- 3.Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am. J. Kidney Dis.48, S176–S322 (2006). 10.1053/j.ajkd.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 4.Tordoir, J. et al. EBPG on vascular access. Nephrol. Dial Transpl.22(Suppl. 2), ii88-117 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Sidawy, A. N. et al. The society for vascular surgery: Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J. Vasc. Surg.48(Suppl.5), 2S-25S (2008). 10.1016/j.jvs.2008.08.042 [DOI] [PubMed] [Google Scholar]
- 6.Lok, C. E. et al. KDOQI Clinical Practice Guideline for vascular access: 2019 update. Am. J. Kidney Dis.75(4 Suppl 2), S1–S164 (2020). 10.1053/j.ajkd.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Duijm, L. E. et al. Outpatient treatment of arterial inflow stenoses of dysfunctional hemodialysis access fistulas by retrograde venous access puncture and catheterization. J. Vasc. Surg.47(3), 591–598 (2008). 10.1016/j.jvs.2007.11.024 [DOI] [PubMed] [Google Scholar]
- 8.NATIONAL KIDNEY FOUNDATION. K/DOQI Clinical Practice Guidelines for vascular access: Update 2000. Am. J. Kidney Dis.37(Suppl1), S137–S181 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Beathard, G. A., Welch, B. R. & Maidment, H. J. Mechanical thrombolysis for the treatment of thrombosed hemodialysis access grafts. Radiology200(3), 711–716 (1996). 10.1148/radiology.200.3.8756920 [DOI] [PubMed] [Google Scholar]
- 10.Kanterman, R. Y. et al. Dialysis access grafts: Anatomic location of venous stenosis and results of angioplasty. Radiology195(1), 135–139 (1995). 10.1148/radiology.195.1.7892454 [DOI] [PubMed] [Google Scholar]
- 11.Doelman, C. et al. Stenosis detection in failing hemodialysis access fistulas and grafts: Comparison of color Doppler ultrasonography, contrast-enhanced magnetic resonance angiography, and digital subtraction angiography. J. Vasc. Surg.42(4), 739–746 (2005). 10.1016/j.jvs.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 12.Asif, A. et al. Inflow stenosis in arteriovenous fistulas and grafts: A multicenter, prospective study. Kidney Int.67(5), 1986–1992 (2005). 10.1111/j.1523-1755.2005.00299.x [DOI] [PubMed] [Google Scholar]
- 13.Khan, F. A. & Vesely, T. M. Arterial problems associated with dysfunctional hemodialysis grafts: Evaluation of patients at high risk for arterial disease. J. Vasc. Interv. Radiol.13(11), 1109–1114 (2002). 10.1016/S1051-0443(07)61952-6 [DOI] [PubMed] [Google Scholar]
- 14.Duijm, L. E. et al. Inflow stenoses in dysfunctional hemodialysis access fistulae and grafts. Am. J. Kidney Dis.48(1), 98–105 (2006). 10.1053/j.ajkd.2006.03.076 [DOI] [PubMed] [Google Scholar]
- 15.Duijm, L. E. et al. Retrograde catheterization of haemodialysis fistulae and grafts: Angiographic depiction of the entire vascular access tree and stenosis treatment. Nephrol. Dial. Transplant.24(2), 539–547 (2008). 10.1093/ndt/gfn526 [DOI] [PubMed] [Google Scholar]
- 16.Beathard, G. A., Arnold, P., Jackson, J., Litchfield, T., Physician Operators Forum of RMS Lifeline. Aggressive treatment of early fistula failure. Kidney Int.64(4), 1487–94 (2003). 10.1046/j.1523-1755.2003.00210.x [DOI] [PubMed] [Google Scholar]
- 17.Asif, A. et al. Percutaneous management of perianastomotic stenosis in arteriovenous fistulae: Results of a prospective study. Kidney Int.69(10), 1904–1909 (2006). 10.1038/sj.ki.5000358 [DOI] [PubMed] [Google Scholar]
- 18.Nassar, G. M. et al. Endovascular treatment of the “failing to mature” arteriovenous fistula. Clin. J. Am. Soc. Nephrol.1(2), 275–280 (2006). 10.2215/CJN.00360705 [DOI] [PubMed] [Google Scholar]
- 19.Wu, S. et al. Hemodynamically significant arterial inflow stenosis in dysfunctional hemodialysis arteriovenous fistulae and grafts. J. Vasc. Access13(4), 452–458 (2012). 10.5301/jva.5000081 [DOI] [PubMed] [Google Scholar]
- 20.Guerra, A. et al. Arterial percutaneous angioplasty in upper limbs with vascular access devices for haemodialysis. Nephrol. Dial. Transplant.17(5), 843–851 (2002). 10.1093/ndt/17.5.843 [DOI] [PubMed] [Google Scholar]
- 21.Chen, M. C. et al. Measuring the palpable pulsatility length as a physical examination test in defining the severity of inflow stenosis for hemodialysis fistulas. BMC Nephrol.20(1), 356 (2019). 10.1186/s12882-019-1536-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu, H. et al. Efficacy of ultrasound-guided percutaneous transluminal angioplasty for arteriovenous fistula stenosis or occlusion at juxta-anastomosis: A 3-year follow-up cohort study. J. Vasc. Surg.74(1), 217–224 (2021). 10.1016/j.jvs.2020.11.041 [DOI] [PubMed] [Google Scholar]
- 23.Heye, S. et al. Factors influencing technical success and outcome of percutaneous balloon angioplasty in de novo native hemodialysis arteriovenous fistulas. Eur. J. Radiol.81(9), 2298–2303 (2012). 10.1016/j.ejrad.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 24.Murphy, E. A. et al. Imaging in vascular access. Cardiovasc. Eng. Technol.8(3), 255–272 (2017). 10.1007/s13239-017-0317-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longwitz, D. et al. Angioplasty in the stenosed hemodialysis shunt: Experiences with 100 patients and 166 interventions. Rofo Fortschr. Geb. Rontgenstr. Neuen. Bildgeb. Verfahr.169, 68–76 (1998). 10.1055/s-2007-1015052 [DOI] [PubMed] [Google Scholar]
- 26.Clark, T. W. I. et al. Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J. Vasc. Interv. Radiol.13, 51–59 (2002). 10.1016/S1051-0443(07)60009-8 [DOI] [PubMed] [Google Scholar]
- 27.Aktas, A., Bozkurt, A., Aktas, B. & Kirbas, I. Percutaneous transluminal balloon angioplasty in stenosis of native hemodialysis arteriovenous fistulas: Technical success and analysis of factors affecting postprocedural fistula patency. Diagn. Interv. Radiol.21, 160–166 (2015). 10.5152/dir.2014.14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda, K., Furukawa, A., Yamasaki, M. & Murata, K. Percutaneous transluminal angioplasty for Brescia-Cimino hemodialysis fistula dysfunction: Technical success rate, patency rate and factors that influence the results. Eur. J. Radiol.54, 426–430 (2005). 10.1016/j.ejrad.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 29.Romann, A. et al. Risk factors associated with arteriovenous fistula failure after first radiologic intervention. J. Vasc. Access17, 167–174 (2016). 10.5301/jva.5000459 [DOI] [PubMed] [Google Scholar]
- 30.Turmel-Rodrigues, L. et al. Insufficient dialysis shunts: improved long-term patency rates with close hemodynamic monitoring, repeated percutaneous balloon angioplasty, an的stent placement. Radiology187, 273–278 (1993). 10.1148/radiology.187.1.8451428 [DOI] [PubMed] [Google Scholar]
- 31.Luo, Q., Liu, H. & Yang, Q. Analysis of factors influencing restenosis after percutaneous transluminal angioplasty. Blood Purif.51, 1–8 (2022). 10.1159/000524159 [DOI] [PubMed] [Google Scholar]
- 32.Manninen, H. I. et al. Endovascular treatment of failing Brescia-Cimino hemodialysis fistulae by brachial artery access: Initial success and long-term results. Radiology218, 711–718 (2001). 10.1148/radiology.218.3.r01mr38711 [DOI] [PubMed] [Google Scholar]
- 33.Rajan, D. K., Bunston, S., Misra, S., Pinto, R. & Lok, C. E. Dysfunctional autogenous hemodialysis fistulas: Outcomes after angioplasty—Are there clinical predictors of patency?. Radiology232(2), 508–515 (2004). 10.1148/radiol.2322030714 [DOI] [PubMed] [Google Scholar]
- 34.Chen, T. Y. et al. Thrombophilia associated with early post-angioplasty thrombosis of dialysis vascular access. Cardiovasc. Intervent. Radiol.41(11), 1683–1690 (2018). 10.1007/s00270-018-2046-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data can be made available by Ziming Wan upon request.