Abstract
Therapeutic cancer vaccines are valuable tools for educating the immune system to fight tumors precisely. Cancer cells are characterized with genetic instability and abundant somatic mutations, leading to the production of tumor specific antigens (TSA) called neoantigens. The main goal of neoantigen-based cancer vaccines is to activate the immune system and elicit effective tumor-specific T-cell responses. There have been no reports of advanced esophageal squamous cell carcinoma (ESCC) cases achieving partial remission after personalized mRNA (messenger RNA) vaccine treatment. As personalized neoantigen-based immunotherapies are emerging, here we report a 67-year-old male patient diagnosed with ESCC and multiple enlarged mediastinal lymph nodes, where mRNA vaccines were used for the first time. Tissue samples from the recurrence focus in the esophagus were subjected to whole transcriptome sequencing. The neoantigens were identified by bioinformatics analyses. The top 20 neoantigens were selected to compose the polyneoantigen vaccine, which were administered at 1 mg every 3 weeks for 4 cycles in combination with a PD-1 (programmed death-1) inhibitor. The patient was boosted with a single dose of the PD-1 inhibitor 8 weeks after the 4th cycle. In addition, immune responses were evaluated before and after the 4 cycles of vaccine therapy, and the lesions were evaluated by imaging examination. Our results revealed that neoantigen-based vaccines significantly activated the tumour-specific immune response. TCR (T cell receptor) V-J pairing analysis showed an increase in the abundance of oligoclonal TCRs, indicating improved homogeneity. No grade 3 or higher drug-related adverse events were observed, except for grade 4 thrombocytopenia caused by PD-1 inhibitor treatment. The patient achieved a partial response (PR), with a progression-free survival (PFS) time of 457 days, the OS (overall survival) time of 457 days, and DOR (duration of response) of 377 days. Our report suggests that combining the personalized mRNA vaccine therapy with PD-1 blockade therapy may be an effective treatment strategy for patient with advanced esophageal cancer. However, further clinical trials are necessary to confirm the efficacy and safety of personalized neoantigen-based immunotherapies in the treatment of advanced ESCC. This trial is registered with ClinicalTrials.gov, NCT03468244 on March 16, 2018, and is now complete.
Keywords: Personalized mRNA vaccine, advanced esophageal cancer, PD-1 inhibitor
Introduction
The prognosis of advanced esophageal squamous cell carcinoma (ESCC) remains extremely poor despite the use of chemotherapy and biological agents. The incidence of unresectable esophageal cancer, whether locally advanced or metastatic, ranges from 15 to 20% [1]. Due to the notable success of chemotherapy in combination with radiotherapy and/or immunotherapy, research on pharmaceutical therapy for esophageal cancer has expanded rapidly [2]. However, drug therapy has shown limited effects on alleviating disease and prolonging progression-free survival (PFS) and overall survival (OS) in patients with recurrent and metastatic esophageal cancer [3]. Therefore, there is an urgent need for effective therapeutics to improve the prognosis of metastatic esophageal cancer.
Therapeutic cancer vaccines serve as a valuable tool to educate the immune system to fight tumors precisely [4]. Cancer cells exhibit genetic instability and abundant somatic mutations, which can result in the production of tumour-specific antigens (TSAs) called neoantigens. The main goal of neoantigen-based cancer vaccines is to activate the immune system and elicit effective tumor-specific T-cell responses against cancer cells [5]. There have been no reports of patients with advanced ESCC achieving partial remission (PR) after personalized mRNA vaccine treatment. Here we present a case of personalized mRNA vaccine therapy based on polyneoantigens in an individual patient with advanced ESCC. Despite this microsatellite stabilized esophageal cancer patient not being an ideal candidate for PD-1 inhibitor therapy, the patient achieved a PR after 4 cycles of combined treatment with PD-1 inhibitor and personalized mRNA vaccine, with a PFS of 15.2. In our study, the neoantigens were identified by bioinformatics analyses and the top 20 were selected for the vaccine. Our results revealed that these neoantigens significantly activated the tumour-specific immune response. TCR CDR3 V-J pairing analysis showed that both the abundance of oligoclonal TCRs and clonal diversity of TCR libraries were increased. After treatment, the patient’s blood tumor mutation burden (bTMB) decreased from 7.63 to 1.53 Mut per Mb, indicating inhibited tumour cell growth. These findings demonstrate that the combination of the tumour vaccine and PD-1 inhibitor activates the immune response, enhancing antigen recognition and response abilities.
Materials and methods
Whole-exome sequencing (WES) and RNA sequencing (RNA-seq)
Esophageal cancer tissues were obtained by gastroscopy and then preserved for WES and RNA-seq. The WES-seq procedure included DNA quality control, DNA shearing, end repair, 3’ adenlylate, adapter ligation, PCR amplification, size selection, library hybridization with an exome array, PCR amplification, library quality assessment, bridging PCR, and sequencing. The RNA-seq procedure involved RNA extraction and detection using an Agilent 2100 bioanalyzer, library construction and quality inspection, followed by sequencing using Illumina NovaSeq 6000 with synthesis chemistry. The results of WES data analysis showed that this patient had an microsatellite instability-low (MSI-L) status. The mutations and major histocompatibility complex (MHC) types of the patient were further inferred from the sequencing data.
Identification of the neoantigen and preparation of vaccines
Sequencing quality control was guranteed using Trimmomatic (version 0.36) to trim the reads with an average Phred score of less than 20 remove standard adapters. Reads were aligned to the human genome (hg38) by employing Burrows-Wheeler Aligner (BWA) (version 0.7.12). The resulting mapping file in BAM format was sorted and generated with the Picard (version 2.3.0) tool SortSam, and duplicate reads were marked and removed using the Picard tool MarkDuplicates. Base recalibration was conducted with GATK (version 3.8.0) to reduce false-positive variant calls. Somatic mutations were called utilizing GATK Mutect2 (version 3.8.0). All the somatic mutations with allelic fractions < 0.05 or coverage < 10× were excluded to eliminate false-positive sites. Classical human leukocyte antigen (HLA) class I alleles (HLA-A, HLA-B, and HLA-C) in each sample were inferred from WES data using OptiType (version 1.3.4), while HLA class II alleles (HLA-DPB1) were detected by HLA-LA (version 1.0.1). Kallisto (version 0.46.2) was utilized to quantify the abundances of gene/isoforms in transcripts per million (TPM) based on the RNA-seq data. The reference transcriptome was downloaded from the Ensembl database for GRCh38 (version 89).
All the mutations were annotated with the Ensembl Variant Effect Predictor (VEP) to identify nonsynonymous mutations. For each missense mutation, the binding affinity of 8-11 mer peptides containing mutated amino acids for the patient’s HLA class I alleles was predicted by NetMHCpan-4.1, while the binding affinity of the corresponding 15-mer peptide to HLA class II alleles was predicted by NetMHCpanII-4.0. To identify the neoantigen candidates, various characteristics of the mutant peptides were evaluated, including the allele frequency of mutations, the binding affinity between HLA alleles and the mutant peptide, the affinity between HLA alleles and MHC molecules, and the corresponding wild-type peptides, as well as mutant peptide expression profiles. Mutations that produce mutant peptides with a corresponding gene expression level of >1 TPM and IC50 of < 500 nM for both HLA class I and HLA class II alleles were retained for further analysis. The remaining neoantigen candidates were further ranked using pTuneos (version 2.0.1) and the top 20 neoantigen sequences were selected for the downstream mRNA vaccine design and manufacturing.
Application of vaccines
The patient received an injection of this personalized vaccine combined with a PD-1 inhibitor, repeated every 3 weeks for a total of 4 cycles. The patient was then boosted with a single dose of PD-1 inhibitor 8 weeks after the 4th cycle. The treatment process is shown in Figure 1B. In each treatment cycle, a total dose of 1 mg vaccine (0.2 mg/injection site) was subcutaneously injected into the left upper limb, right upper limb, left lower limb, right lower limb, and abdomen within a 2-day period. Additionally, 240 mg of the PD-1 inhibitor toripalimab was injected one day before the mRNA vaccination. In addition, we separated the peripheral blood lymphocytes from the patient before and after treatment. Polymorphisms of CDR3 in the TCR (T-cell receptor) were analyzed by high-throughput sequencing. In addition, ctDNA (circulating tumour DNA) was obtained and the bTMB was determined through high-throughput sequencing of 605 genes.
Figure 1.
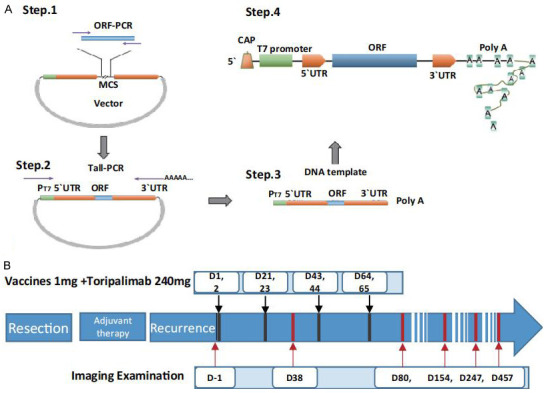
Design steps of vaccination and patient timeline. A. Schematic diagram of the mRNA vaccine structure. To design a suitable plasmid, the neoantigen and the target gene are subcloned into the 3 plasmids via the multiple cloning site. After the plasmids were linearized, the transcription template was obtained by tailing PCR. A complete linear DNA template containing the promoter, including the T7 promoter, 5’-UTR sequence, neoantigen or target gene, 3’-UTR, and poly (A) tail, was obtained. A complete mRNA structure for transcription in vitro, including a cap structure, T7 promoter, 5’-UTR sequence, neoantigen or target gene, 3’-UTR, and poly (A) tail was obtained. B. Timeline of events and summary of administered treatments.
Case report and treatment process
On January 7, 2011, a 63-year-old male patient underwent radical esophagectomy in the Thoracic Surgery Department of Shanghai Changhai Hospital. The postoperative pathological report indicated squamous cell carcinoma of the middle esophagus, with a pathological stage of T2N1M0. In July 2020, the patient was diagnosed with esophageal cancer recurrence with mediastinal lymph node metastasis after gastroscopy and mediastinal enhanced CT examination. In September 2020, he was enrolled in the clinical trial of an mRNA vaccine (NCT03468244). This clinical trial was approved by the Shanghai Changhai Hospital Ethics Committee of Naval Medical University.
To generate the mRNA vaccine, the designed sequence was synthesized and subcloned into an mRNA expression vector, which was then transcribed into a single mRNA chain before being coated with nanoliposome (Lipopolyplex, LPP), prepared and provided by StemiRNA Therapeutics LLC (Figure 1A). The treatment process of this case is shown in Figure 1B. During the first 4 cycles of vaccine therapy, the patient developed grade 1 fever without chills or headaches on the second day. The most common treatment-emergent adverse events (TEAEs) (grade 1) were redness, swelling, painful heat, and muscle induration at the injection sites, all of which subsided by Day 3 after each injection. A single dose of toripalimab was given in the 8th week after the 4th cycle. The platelet count continued to decline in the following days. Seven days after the PD-1 inhibitor administration, the grade 4 adverse events of thrombocytopenia occurred. According to the principle of dealing with adverse reactions related to PD-1 inhibitor treatment, daily administration of methylprednisolone at 2 mg per kilogram of body weight and Thrombopoietin (TPO) at 15000 U was initiated. Human immunoglobulin (pH 4, 20 g) was given by intravenous injection for 3 days. The platelet count recovered to 5×109/L 3 days later and 105×109/L 5 days later. TPO were then ceased. Glucocorticoids were orally taken daily for one week and were then gradually reduced and stopped 6 weeks later. There were no other grade 3 or higher drug-related adverse events related to vaccine treatment. After that, the patient did not receive further vaccine or PD-1 inhibitor treatment. However, 457 days after the first vaccine treatment, the patient died of pulmonary infection. A correlation between the pulmonary infection and the treatment with the vaccine and the PD-1 inhibitor was excluded.
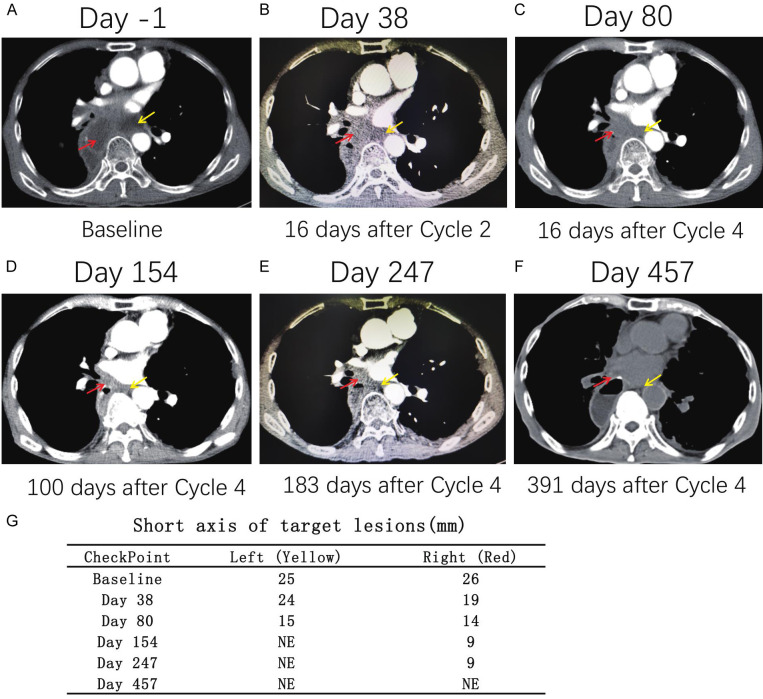
Lesions were evaluated by imaging after cycle 2 and cycle 4 and thereafter on Day 154, Day 247, and Day 457 according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guidelines. Two target lesions were identified in the mediastinal lymph nodes. Compared with that before vaccination, the sum of the short axis of the lesion was reduced by 36.4% (from 51 mm to 29 mm) after cycle 4, indicating a partial response (PR) (Figure 2A-G). Notably, the left lymph node could not be measured accurately on Day 154 and could not be clearly visualized on Day 247 and Day 457. Unfortunately, the patient died of lung infection 457 days after the first vaccine treatment. It should be noted that the tumour continued to shrink even though the vaccine treatment had been stopped for 38 weeks. The PFS time for this patient was 457 days, the OS time was 457 days, and the DOR was 377 days.
Figure 2.
Details of lesions before and after vaccination. A-F. There were two target lesions of the mediastinal lymph node. G. Compared with that before vaccination, the sum of the short axis of the lesion was reduced by 36.4% (from 51 mm to 29 mm) after cycle 4. The left lymph node could not be measured accurately on Day 154, and could not be clearly visualized on Day 247 and Day 457.
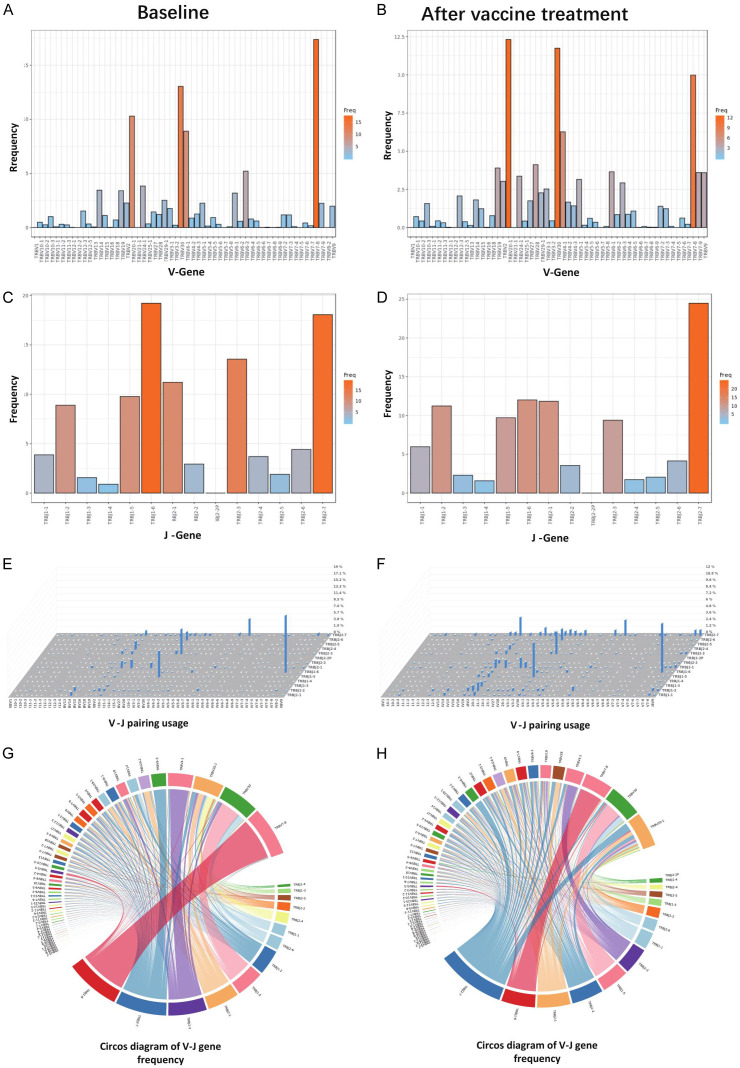
TCR CDR3 polymorphisms were analyzed by high throughput sequencing. The results showed that the types and frequency of TCR V and J genes did not change considerably (Figure 3A-D). However, V-J pairing analysis showed that the abundance of oligoclonals TCRs was increased (Figure 3E-H), indicating improved homogeneity of TCRs. This finding was also confirmed by the analysis of clonal diversity, with evenness increasing from 0.6426 on Day 1 to 0.7751 on Day 84. Moreover, the Shannon index also showed that the clonal diversity of the TCR library of the sample on Day 84 was higher than that on Day 1 (increasing from 5.9 to 7.0). This shows that the vaccine treatment activated the immune response, resulting in stronger antigen recognition and response abilities. In addition, after 4 cycles of treatment, the patient’s bTMB decreased from 7.63 to 1.53 mut/Mb, indicating inhibited tumour cell growth (Supplementary Table 1).
Figure 3.
Analysis of TCR CDR3 polymorphisms by high-throughput sequencing. A, B. The types of V genes in a single sample and the corresponding use frequency. C, D. The types of J genes in a single sample and the corresponding use frequency. Different colours represent different frequencies. The darker the colour, the higher is the frequency of use. We found that the types and frequency of use the V and J genes did not changed considerably. E, F. 3-D histogram of V-J gene frequency. The abscissa and ordinate on the planes how the V gene and J gene, respectively, and the height of the column represents the frequency of the V-J combination. G, H. Circos diagram of V-J gene frequency. The wider the colour block is, the higher the frequency. The line represents a V-J combination. The V-J combination represents a complete CDR3 sequence. We performed statistical analysis on the form and frequency of the V-J combinations in a single sample. V-J pairing analysis showed that the abundance of oligoclonal TCRs was increased.
Discussion
Personalized vaccine therapy has been one of the main hotspots in tumour immunotherapy research in recent years [6-8]. Therapeutic cancer vaccines, including peptide-, DNA-, RNA-, protein-, and tumour cell-based vaccines, aim to produce new tumour-specific T-cell responses against tumor cells [9-11]. Combinations treatments with checkpoint modulators and other novel drugs that reverse immunosuppression are rapidly advancing, although more research studies are needed to establish the best combinations and determine the optimum dose scheduling for each component [12]. mRNA-based approaches have become a promising platform for cancer immunotherapy. Tuning of the administration routes and codelivery of multiple mRNA vaccines with other immunotherapeutic agents (e.g., checkpoint inhibitors) have further boosted host antitumour immunity and increased the likelihood of tumour cell eradication [13].
ESCC is the most prevalent histological type of esophageal cancer. Despite the development of multidisciplinary therapeutic approaches, its prognosis remains unfavourable. Recently, the development of monoclonal antibodies inhibiting programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) has led to marked therapeutic responses in patients with multiple malignancies, including ESCC. However, only a few patients achieve clinical benefits due to resistance [14,15], especially those with MSI-L status and a low TMB [16,17].
Currently, personalized vaccine treatment, as a new method of tumour treatment, warrants more attention and deeper exploration. First, as cancer vaccines are based on neoantigen, it is crucial to analyse neoantigens quickly and accurately. WES (Whole exome sequencing) data alone does not reflect the transcription characteristics of genes. Compared to WES, integrated analyses combined with measurement of mRNA expression may provide a more accurate method for neoantigen analysis, for example, combined analysis of gene mutations, HLA affinity, wild-type gene profiles, HLA affinity, transcription of mutant genes, and mutant gene sharing, as mentioned above. Second, immune recognition of mutation-derived epitopes driven by MHC II molecules and CD4+ T cells is also very important [18-20]. Both MHC class I- and II-restricted neoantigens should be analyzed in designing mRNA vaccine therapies. Third, nanoliposomes, currently used as tumour vaccine carriers, is one of the most important vaccine delivery platforms. Nanoliposomes deliver vaccine components into antigen-presenting cells (APCs), especially dendritic cells (DCs). It is indisputable that an efficient and accurate drug delivery platform for delivering vaccines into APC cells is one of the important requirements for the success of vaccine treatment [21,22]. In addition, peptide vaccines combined with PD-1 immune checkpoint inhibitors have exhibited promising efficacy in patients with non-small cell lung cancer, melanoma and human papillomavirus 16-related cancer. Combination with PD-1 inhibitors has historically been a common method used in clinical trial for tumour vaccines. However, we must pay close attention to the adverse reactions caused by the application of PD-1 inhibitors.
This study inevitably has some limitations that should be noted. First, this study is a single case report, and further clinical trials are needed to confirm the efficacy and safety of personalized neoantigen-based immunotherapies in the treatment of advanced ESCC. Second, this patient had esophageal cancer with lymph node metastasis but no metastasis to distant organs. Patients with metastasis to lymph nodes, which are rich in immune cells, may be more likely to benefit from immunotherapy [23,24]. It remains unclear whether ESCC patients with distant organ metastasis can benefit from RNA vaccine treatment. Clinical studies need to include more patients with organ metastases to determine the potential benefits of RNA vaccine treatment for such patients. Third, antigen-specific TCR sequences activated by the 20 neoantigens could be better analysed separately to more accurately show the tumour-specific immune responses activated by the sequenced neoantigens in the future. The timing and dosage of PD-1 inhibitors could also be further explored to prevent the occurrence of moderate to severe adverse reactions related to immunotherapy in the future.
In conclusion, we report the first case of personalized vaccine therapy with polyneoantigen-coding mRNA in a patient with MSI-L and TMB-L advanced esophageal cancer, who achieved a partial response. This case suggests that combining the personalized mRNA vaccine with PD-1 blockade may be an effective treatment strategy for advanced esophageal cancer. The therapies combined with vaccine therapy in vivo remain to be further explored.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82072707), Shanghai Municipal Health Commission’s Clinical Research Project in the Health Industry (202040412), the Basic Research Project of Naval Medical University (grant no. 2023MS023), Changhai Hospital 234 and GUHAI Project (2019YXK019 and 2022009592), and Changhai Hospital Basic Medicine Research Fund (2023PY24, 2023PY29 and 2023QD04). The tests WES, RNA-seq, ctDNA, polymorphism of TCR CDR3 and preparation of Vaccines are all provided by StemiRNA therapeutics LLC.
The patients/participants provided their written informed consent to participate in this study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siddiqi A, Johnston FM. The perioperative and operative management of esophageal and gastric cancer. Surg Oncol Clin N Am. 2023;32:65–81. doi: 10.1016/j.soc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Cai Y, Hong Z, Duan H, Ke S. Comparison of efficacy and safety between neoadjuvant chemotherapy and neoadjuvant immune checkpoint inhibitors combined with chemotherapy for locally advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis. Int J Surg. 2024;110:490–506. doi: 10.1097/JS9.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao C, Zeng X, Zhang C, Yang Y, Xiao X, Luan S, Zhang Y, Yuan Y. Mechanisms of pharmaceutical therapy and drug resistance in esophageal cancer. Front Cell Dev Biol. 2021;9:612451. doi: 10.3389/fcell.2021.612451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briquez PS, Hauert S, de Titta A, Gray LT, Alpar AT, Swartz MA, Hubbell JA. Engineering targeting materials for therapeutic cancer vaccines. Front Bioeng Biotechnol. 2020;8:19. doi: 10.3389/fbioe.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamani P, Teymouri M, Nikpoor AR, Navashenaq JG, Gholizadeh Z, Darban SA, Jaafari MR. Nanoliposomal vaccine containing long multi-epitope peptide E75-AE36 pulsed PADRE-induced effective immune response in mice TUBO model of breast cancer. Eur J Cancer. 2020;129:80–96. doi: 10.1016/j.ejca.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Melief CJM, van der Gracht E, Wiekmeijer AS. Combination immunotherapy with synthetic long peptides and chemotherapy or PD-1 blocker for cancers caused by human papilloma virus type 16. Semin Immunopathol. 2023;45:273–277. doi: 10.1007/s00281-023-00986-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Zhao J, Wang W, Geng H, Wang Y, Gao B. Current advances in the application of nanomedicine in bladder cancer. Biomed Pharmacother. 2023;157:114062. doi: 10.1016/j.biopha.2022.114062. [DOI] [PubMed] [Google Scholar]
- 8.Sellars MC, Wu CJ, Fritsch EF. Cancer vaccines: building a bridge over troubled waters. Cell. 2022;185:2770–2788. doi: 10.1016/j.cell.2022.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA, dela Rosa C, Tietje K, Link J, Waisman J, Salazar LG. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J. Clin. Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acres B, Limacher JM. MUC1 as a target antigen for cancer immunotherapy. Expert Rev Vaccines. 2005;4:493–502. doi: 10.1586/14760584.4.4.493. [DOI] [PubMed] [Google Scholar]
- 12.Abd-Aziz N, Poh CL. Development of peptide-based vaccines for cancer. J Oncol. 2022;2022:9749363. doi: 10.1155/2022/9749363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Zhou WY, Luo XJ, Chen YX, Wong CW, Liu ZX, Bo Zheng J, Yu Mo H, Chen JQ, Li JJ, Zhong M, Xu YH, Zhang QH, Pu HY, Wu QN, Jin Y, Wang ZX, Xu RH, Luo HY. Long noncoding RNA Regulating ImMune Escape regulates mixed lineage leukaemia protein-1-H3K4me3-mediated immune escape in oesophageal squamous cell carcinoma. Clin Transl Med. 2023;13:e1410. doi: 10.1002/ctm2.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Sun L, Cai L, Guo M, Xu G, Liu S, Zheng G, Wang Q, Lian X, Feng F, Zhang H. Clinicopathological and prognostic values of PD-L1 expression in oesophageal squamous cell carcinoma: a meta-analysis of 31 studies with 5368 patients. Postgrad Med J. 2022;98:948–957. doi: 10.1136/postgradmedj-2021-140029. [DOI] [PubMed] [Google Scholar]
- 16.Thawani R, Agrawal N, Taflin NF, Kardosh A, Chen EY. Application of value framework to phase III trials of immune checkpoint inhibitors in esophageal and gastric cancer. Oncologist. 2023;28:40–47. doi: 10.1093/oncolo/oyac187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Nakamura J, Noshiro H. Promising immunotherapies for esophageal cancer. Expert Opin Biol Ther. 2017;17:723–733. doi: 10.1080/14712598.2017.1315404. [DOI] [PubMed] [Google Scholar]
- 18.Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, Behjati S, Velds A, Hilkmann H, El Atmioui D, Visser M, Stratton MR, Haanen JB, Spits H, van der Burg SH, Schumacher TN. Corrigendum: high-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2016;22:1192. doi: 10.1038/nm1016-1192d. [DOI] [PubMed] [Google Scholar]
- 19.Kreiter S, Vormehr M, van de Roemer N, Diken M, Löwer M, Diekmann J, Boegel S, Schrörs B, Vascotto F, Castle JC, Tadmor AD, Schoenberger SP, Huber C, Türeci Ö, Sahin U. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–6. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achinko DA, Dormer A, Narayanan M, Norman EF. Targeted immune epitope prediction to HHLA2 and MAGEB5 protein variants as therapeutic approach to related viral diseases. BMC Immunol. 2021;22:49. doi: 10.1186/s12865-021-00440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang J, Mei J, Yi S, Feng C, Ma Y, Liu Y, Liu Y, Chen C. Tumor associated macrophage and microbe: the potential targets of tumor vaccine delivery. Adv Drug Deliv Rev. 2022;180:114046. doi: 10.1016/j.addr.2021.114046. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Q, Xie M, Chen R, Yan F, Ye C, Li Q, Xu S, Wu W, Jia Y, Shen P, Ruan J. Cancer cell membrane-wrapped nanoparticles for cancer immunotherapy: a review of current developments. Front Immunol. 2022;13:973601. doi: 10.3389/fimmu.2022.973601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Deng L, Wang J, Zhang T, Wang W, Wang X, Liu W, Wu Y, Lv J, Feng Q, Zhou Z, Wang J, Wang L, Wang Z, Bi N. Induction PD-1 inhibitor toripalimab plus chemotherapy followed by concurrent chemoradiotherapy and consolidation toripalimab for bulky locally advanced non-small-cell lung cancer: protocol for a randomized phase II trial (InTRist study) Front Immunol. 2024;14:1341584. doi: 10.3389/fimmu.2023.1341584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C, Wang Z, Shi L, Liu W. Evaluation of neck control strategies for oral squamous cell carcinoma of stage I: neck dissection or potential immunotherapy. J Dent Sci. 2024;19:640–644. doi: 10.1016/j.jds.2023.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.