Abstract
Background
Cervicogenic dizziness is a clinical syndrome characterized by neck pain and dizziness, which has a rising incidence in recent years. In China, manual therapy has been widely used in the treatment of cervicogenic dizziness, but there is no high-quality medical evidence to support its effectiveness and safety. The purpose of this study was to assess the safety and efficacy of Shi's manual therapy (SMT) on the treatment of cervicogenic dizziness.
Methods
A multicenter randomized controlled trial (RCT) will perform on 106 patients (18≤ages≤65) who meet the diagnostic criteria of cervicogenic dizziness. Patients will be randomly allocated to the intervention group and the control group at a ratio of 1:1. Participants in the control group will be treated with Merislon (Betahistine Mesilate Tablets). Participants in the intervention group will be treated with SMT. The primary outcome is the response rate at week 2, which is defined as the proportion of patients who reduce their disability level measured by the Dizziness Handicap Inventory (DHI) score relative to baseline. Key secondary outcomes include DHI scores at weeks 1, 2, and 6 and changes from baseline, time to disappearance of dizziness symptoms, and recurrence rate of dizziness symptoms. Safety will be assessed by adverse events, physical examination and vital signs.
Discussion
This trial aims to provide high-quality evidence-based medical data to demonstrate that SMT can reduce dizziness in patients with cervicogenic dizziness effectively and safely.
Trial registration
Clinical Trial Registration Center NCT05604937. Registered on Nov 3, 2022.
Protocol version
1.0, November 20, 2022.
Keywords: Cervicogenic dizziness, Manipulation, Shi's manual therapy, DHI, Betahistine mesilate
1. Background
Cervicogenic dizziness is a clinical syndrome characterized by the presence of both neck pain and dizziness as symptoms [1,2]. However, there is currently a lack of definitive clinical or laboratory tests for the diagnosis of cervicogenic dizziness. As a result, the primary diagnostic approach involves excluding other potential pathologies and identifying false positives to reach an accurate diagnosis [3].
Cervicogenic dizziness can be triggered by consistent strain on the neck [4], such as prolonged use of smartphones, playing mobile games, and extended periods of desktop work. Because the equilibrium function is closely related to cervical receptors. Degenerative or traumatic changes in the spine and neck muscles can lead to the manifestation of dizziness symptoms [5]. In a multi-center, cross-sectional study about the prevalence of cervicogenic dizziness, it was found that 40.1 % of the patients with neck pain had obvious dizziness symptom. The most common etiology of neck pain was myofascial pain syndrome (MPS) with a percentage of 58.5 %. Among these patients, incidence rate of cervicogenic dizziness was 59.7 %. The females versus males ratio of cervicogenic dizziness was 1.641:1 [6]. According to a study conducted by Sho Takahashi et al., 89 % of patients with dizziness as chief complaint were diagnosed cervicogenic dizziness through imaging. These findings provide evidence for the existence of cervicogenic dizziness [7]. The present core mechanism of cervicogenic dizziness is vertebrobasilar insufficiency. Some local physiological structure changes can affect vertebral arterial blood flow through different pathways. Because blood supply for the vestibular system is mainly dependent on the vertebral artery. Insufficient vestibular blood supply will influence its function and produce dizziness symptom [8]. According to neck anatomy, the neck rotation mainly depends on the rotation of C1 and C2, so the atlantoaxial joint is the most common segment for vertebrobasilar artery insufficiency [9].
As a health problem, the incidence of cervicogenic dizziness is rising in recent years. With the change of living and working habits, the population of cervicogenic dizziness tend to be more and more younger. A singular effective treatment for cervicogenic dizziness is currently lacking. The importance of developing effective treatment methods for cervicogenic dizziness is highlighted. Manipulation can eliminate cervicogenic dizziness by relaxing local muscle tension, relieving muscle spasm, restoring the normal spinal sequence, removing stimulating factor to nerves and improving blood circulation. Although there is some evidence suggesting the effectiveness of manipulation in the treatment of cervicogenic dizziness [[10], [11], [12]], the support for this claim is still lacking high-quality randomized controlled trials [13]. Studies have demonstrated the significance of administering appropriate types and dosages of muscle relaxants in the management of cervicogenic dizziness characterized by stiffness in the neck and scapular muscles [7]. This highlights the importance of muscle relaxation in the treatment of cervicogenic dizziness [14]. In previous reports, routine drug therapy, such as mecobalamin, flunarizine hydrochloride, and betahistine mesilate, does not often have significant efficacy, so manual therapy may be an effective measure to relieve muscle tension in the neck and shoulder. Research has demonstrated that traction manipulation can result in symptom reduction, including pain and dizziness, along with improvements in mobility and stability [15]. Manual therapy has been found to be effective in enhancing cervical spine range of motion in all six physiological movement directions. However, it does not seem to have any effect on balance and repositioning accuracy. In comparison to passive joint movement, manual therapy has been shown to have better efficacy [16]. In cases where conservative intervention fails or open surgery is needed, coblation discoplasty can effectively alleviate the severity and frequency of cervicogenic dizziness [17]. This finding supports the notion that repairing the physiological structure of the cervical spine is a crucial aspect of treating cervical dizziness [18]. Manual therapy, a widely used physical therapy, offers a distinct advantage in restoring the physiological structure of the cervical spine [19].
Shi's manual therapy (SMT) is a method of manual therapy established based on Shi's traumatology Traditional Chinese Medicine (TCM) academic school with years of clinical experience summary and accumulation. It is one of the manual therapy methods, and the specific details of the manipulation of the SMT will be described below.
This study is a multicenter RCT study aimed at investigating the safety and efficacy and provide high-quality evidence-based medical research regarding the effectiveness of SMT for treating cervicogenic dizziness. In terms of effectiveness, we mainly focus on the advantage of SMT, compared with Merislon, in reducing disability level measured by DHI.
2. Methods/design
2.1. Study setting
In this study, a multicenter RCT will be conducted in four clinical institutions to objectively evaluate the safety and efficacy of SMT for the treatment of cervicogenic dizziness.
The four clinical institutions will be Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Yueyang traditional chinese and western medicine Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Baoshan traditional chinese and western medicine Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, and Shanghai General Hospital, which have widely used SMT in clinical and conducted a large number of related researches. They will be responsible to recruitment and intervention in this study.
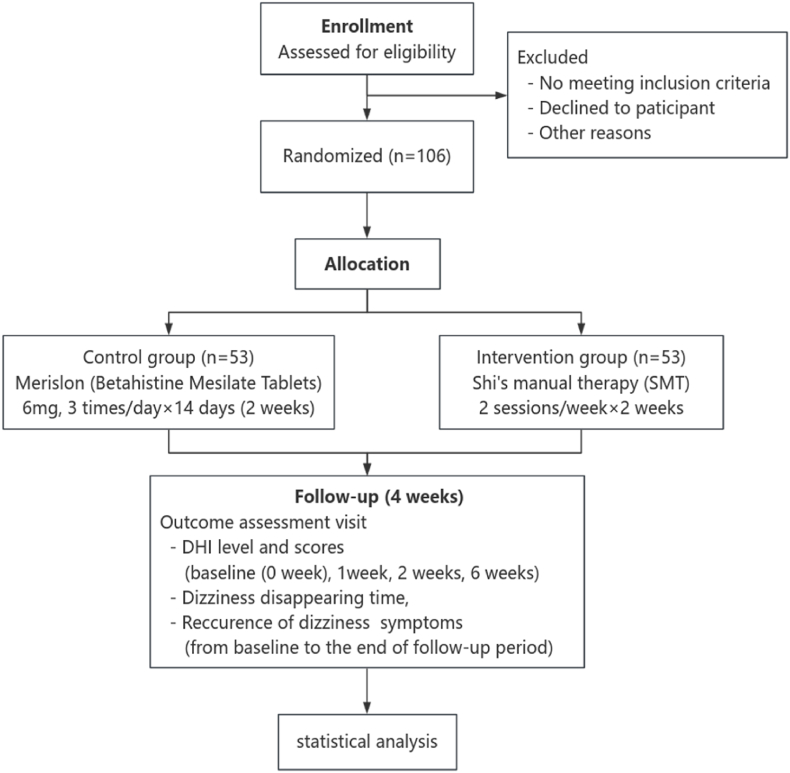
A total of 106 outpatients, aged between 18 and 65, who meet the diagnostic criteria for cervicogenic dizziness will be randomly assigned to either the intervention group or the control group in a 1:1 ratio. Participants in the intervention group will receive SMT, while participants in the control group will be treated with betahistine mesylate tablets. The response rate measured by DHI at week 2 is the primary outcome. The secondary outcomes of this trial include the difference in DHI score relative to baseline, the time to disappearance of dizziness symptoms, and the recurrence rate of dizziness symptoms. The safety outcomes encompass vital signs, physical examination, and adverse events (See Fig. 1).
Fig. 1.
The study procedure.
2.2. Sample size calculation
According to previous research reports [20], the response rate of dizziness patients to betahistine mesilate tablets is 60.1 %. In this trial, the response rate of the experimental group after manual therapy will be set to 85 %.
Sample size estimation was performed using a completely randomized design for a two population rates hypothesis test. The positive rate P1 of the control group is 60.1 % and positive rate P2 of the SMT group is 85 %, with a significance level of two-sided α = 0.05 and a power of β = 0.20. The calculation resulted in a requirement of 48 patients in each group, with an additional 10 % dropout rate taken into account. Therefore, a minimum of 53 patients per group, or 106 patients in total, were estimated to be necessary for the study.
Sample size calculation formula [21]:
2.3. Randomization and blinding
This trial was a randomized block design with center as a stratifying factor. The Interactive Web Response System (IWRS) will be used to allocate patients to the intervention group and the control group at a ratio of 1:1. Considering the large distinct of interventions in this trial, patients and operators are unblinded, while the efficacy evaluators are blinded.
2.4. Participants and recruitment
Participants will be recruited from outpatients of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Yueyang Traditional Chinese and Western Medicine Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Baoshan Traditional Chinese and Western Medicine Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, and Shanghai General Hospital. The researcher will provide both oral and written information about the trial to patients, including detailed information about interventions, procedures and potential adverse reactions. Prior to participation, written informed consent will be obtained from all participants. To protect the confidentiality of the participants, the researcher will maintain an anonymous state, and documents containing the identity of participants will be kept strictly confidential. Additionally, researchers at each clinical trial center will be responsible for the medical treatment of patients and will make medical decisions related to the clinical trial. Participants will get appropriate transportation allowance and receive CT examination, diagnosis and treatment freely.
2.4.1. Diagnostic criteria
The diagnosis of cervicogenic dizziness was made according to the strategy developed by Reiley AS et al. [22].
-
(1)
Symptoms: dizziness is the main clinical manifestation;
-
(2)
Signs: significant tenderness points in the neck, positive neck rotation test or dizziness symptoms associated with neck movement should be present;
-
(3)
History: a history of cervical spondylosis and neck pain or neck stiffness;
-
(4)
Imaging: imaging reveals cervical instability;
-
(5)
Excluding diseases: dizziness caused by other reasons should be excluded, such as otogenic, brain-derived, traumatic, ophthalmic or neurotic, intracranial tumors, and subclavian steal syndrome.
2.4.2. Inclusion criteria
-
(1)
Patients aged 18–65 years, including 18 and 65 years, male or female;
-
(2)
Met the diagnostic criteria of cervicogenic dizziness;
-
(3)
Cervical odontoid process deviation shown on CT three-dimensional reconstruction of cervical spine or CT plain scan of atlantoaxial joint cross-section;
-
(4)
Had dizziness symptoms at inclusion;
-
(5)
31 ≤ DHI ≤60;
-
(6)
The course of cervicogenic dizziness is more than 1 month;
-
(7)
Voluntarily participated in this trial and signed the informed consent form.
2.4.3. Exclusion criteria
-
(1)
Dizziness caused by other diseases (Otogenic, cerebral, traumatic, ophthalmogenic neurofunctional, intracranial tumor, subclavian steal syndrome);
-
(2)
Cervical vertebra fracture, dislocation, tuberculosis, acute cervical spine disc protrusion, infection or cancer;
-
(3)
Severe heart, liver, brain, kidney complications or other serious diseases;
-
(4)
Uncontrolled hypertension grade Ⅱ and above, or blood pressure≥160/100 mmHg under antihypertensive treatment;
-
(5)
Carotid plaque history;
-
(6)
Pregnancy, lactation, family planning, or patients judged not to use effective contraception;
-
(7)
History of mental diseases;
-
(8)
History of suspected or confirmed alcohol or drug abuse;
-
(9)
Patients are afraid of manual therapy;
-
(10)
Betahistine mesylate tablet allergy;
-
(11)
Those who participated in other clinical trials within 3 months;
-
(12)
Other conditions unsuitable to participate considered by researchers.
2.4.4. Drop out/withdrawal criteria
-
(1)
Participants revoke their informed consent;
-
(2)
Pregnancy event;
-
(3)
Serious adverse event;
-
(4)
Patients' conditions continue becoming worse and affect daily life seriously;
-
(5)
The subjects disobey the trial plan.
Upon a patient's withdrawal from the trial, it is imperative for researchers to document the reasons for the dropout in the medical record. Additionally, the evaluation items must be completed, and the record of the patient's last visit should be updated in the medical record. Any data collected from patients who withdraw should be entered into the CRF. It is important to note that patients who only undergo screening and do not receive a random number are not considered patients of withdrawal.
2.5. Intervention methods
2.5.1. Control group
Patients in the control group will be treated with Merislon (Betahistine Mesilate Tablets) for 2 weeks.
2.5.2. Intervention group
Patients in the intervention group will be treated with SMT for 2 weeks.
2.5.3. Betahistine mesilate
Merislon (Betahistine Mesilate Tablets), with specification 6 mg, will be manufactured by Sinopharm H20040130 and supplied by Eisai (China) Pharmaceutical Co., Ltd. The treatment duration will be 2 weeks, with a dosage of 6 mg each time, three times a day.
Merislon is a kind of antihistamine, which can be used to treat dizziness and Meniere's disease or syndrome. Although the effect is uncertain, Merislon is commonly used to treat cervicogenic dizziness in the clinic, and there are many related clinical research reports. It is ethically unacceptable to not intervene when patients have symptoms. Merislon is a symptomatic drug and no clear minimum course of treatment limit. Typically, a 2-week course of treatment is recommended in clinical practice.
2.5.4. Shi's manual therapy (SMT)
Qualified manual therapy doctors with a minimum of 5 years of experience in SMT will perform the interventions on the participants. Patients in the intervention group will be treated with SMT for 2 weeks, with the strength of manipulation adjusted based on the patient's tolerance. SMT will be performed twice a week and totally four times over a two-week period. Standard operating procedures (SOPs) will be established to ensure precise and consistent implementation [23].
The key procedures of SMT include the following steps.
-
(1)Relaxation manipulation: therapy doctor performs a 10-min soft-tissue manipulation, kneading and grasping manipulation, on the neck and shoulders to make them relaxed. The detail operations are as follow:
-
①Kneading manipulation: use the left or right palm root or the major thenar eminence to do rhythmic spiral movement on muscle of neck and shoulder from top to bottom.
-
②Grasping manipulation: exerts force between the thumb of both hands and the palmar surface of the other four fingers, pinches and lifts the muscles of neck up and down, and then slowly relaxes. Repeat the manipulation from the proximal end to the distal end.
-
①
-
(2)
Reconstruction manipulation: the patient lies in the supine position. The doctor sits on the patient's head side. Take rotation of C2 as an example. The doctor palpates the patient's C2 with obvious posterior protrusion and tenderness. The right hand is used as the manipulating hand, and the radial edge of the metacarpophalangeal joint of the right index finger is used to press the point on the posterior edge of the right transverse process of C2. Use the left palm to keep stable and support the patient's occipital bone. Slowly lift up and bend forward the head. Stop bending when the feeling of the posterior cervical muscle group being taut passes to the metacarpophalangeal joint of the indication finger. Exert a rotational force to the upper left on the contact point of the reconstructive hand, forming a relative force with the stable hand, and form a lateral bend to the left to the cervical spine. When the manipulating hand feels resistance, the lateral bending stops. Rotate the patient's head to right to the limit and lock the right transverse process of C2. The manipulating hand quickly gives a flash force on the contact point and the direction of the thumb (upper left). When hear the sound of the C2 joint, the manipulation is complete. Manipulation of C2 right rotation is contrary.
2.5.5. Concomitant medication and treatment
During the trial, other drugs and treatments for cervicogenic dizziness will be forbidden, except for drugs the patient had previously taken for other basic diseases.
Patients are permitted to use medications for concurrent diseases as needed, provided they are not contraindicated. All treatments and drugs utilized should be thoroughly documented, including the drug name, dosage, start and end dates, and indications.
2.5.6. Emergency and alternative treatment
Patinets will receive manual therapy combined with exercise therapy as an alternative treatment after the follow-up period, if intervention does not work [24].
Patients will be considered receiving emergency treatment, such as operation, if conditions of cervicogenic dizziness continue becoming worse and seriously affect daily life Table 1.
Table 1.
Trial schedule.
| stage |
screening period (day 0) |
treatment period (treatment day 1–14) |
follow-up period (4 weeks after treatment) |
|
|---|---|---|---|---|
| time | visit 1 (0 day) | visit 2 (7 days ± 1 day) | visit 3 (14 days ± 2 days) | visit 4 (6 weeks ± 4 days) |
| informed consent | × | |||
| dememographic dataa | × | |||
| living habitsb | × | |||
| medical history, treatment history, allergy historyc | × | |||
| condition of participanting in clinical trialsd | × | |||
| vital signse | × | × | × | |
| physical examinationf | × | × | × | |
| DHI socre | × | × | × | × |
| dizziness symptom reportg | × | × | × | × |
| standard treatment | × | × | ||
| randomization | × | |||
| journal card distribution | × | |||
| journal card collection | × | |||
| Shi's Manual Therapyh | × | × | ||
| control drug distribution | × | |||
| control drug collection | × | |||
| record drug combination | × | × | ||
| record adverse event | × | × | ||
Note.
Demographic data included date of birth (age), sex, and ethnicity.
Living habits include smoking and drinking history.
Medical history, treatment history, and allergy history included previous and treatment history of major illness, present medical history and treatment, allergy to drugs/food, etc.
Condition of participating in clinical trials included participating in clinical trials in the last 3 months.
Vital signs included body temperature, sitting blood pressure, 1-min pulse rate and respiratory rate.
Physical examination included the following: skin mucous membranes, lymph nodes, head and neck (eyes, oropharynx, thyroid), chest (heart, lungs), abdomen, musculoskeletal system, nervous system, and other sites.
Dizziness symptom reports should be filled in by the subject in the e-PRO system in a timely manner.
Treatment will be given twice a week, 4 times in 2 weeks.
2.6. Outcomes
2.6.1. Primary outcome
The primary outcome is the response rate at week 2, which is defined as the proportion of patients who reduce their disability level measured by the DHI score relative to baseline. The DHI scale is an internationally recognized and widely used measurement tool for dizziness. The DHI scale has been translated into multiple languages, including a Chinese version that has undergone rigorous research to establish its reliability and validity. Studies have demonstrated that the DHI scale is highly reliable, valid, and repeatable in assessing the severity of dizziness in patients with cervicogenic dizziness [25].
The DHI scale comprises 25 questions that assess the impact of dizziness or vertigo on patients across three dimensions: functional (F), emotional (E) and physical (P). Patients assign scores based on their subjective condition. The total score is calculated as the Dizziness Handicap Inventory Total (DHIT) score, with a maximum of 100 points. The functional score is represented by the DHIF (36 points), the emotional score by the DHIE (36 points), and the physical score by the DHIP (28 points). Each question is assigned a score of 4 points for a "Yes" response, 2 points for a "Sometimes" response, and 0 points for a "No" response. A lower score indicates less disability. The scoring range is as follows: 0–30 for mild dizziness, 31–60 for moderate dizziness, and 61–100 for severe dizziness.
2.6.2. Secondary outcome
The difference in DHI scores at 1, 2 and 6 weeks relative to baseline, the dizziness disappearing time, and the recurrence rate of dizziness symptoms within 4 weeks after the end of treatment will be the secondary efficacy outcomes. From the first day of treatment, the patient reported no dizziness symptoms for two consecutive days will be defined as the dizziness disappearing/treatment taking effect. The disappearing time will be the first day of these two consecutive days. Among patients who reported dizziness disappearing/treatment taking effect within 2 weeks of treatment, the patient reported dizziness symptoms for two consecutive days will be defined as recurrence. The recurrence rate will be calculated before executing statistical analysis.
2.6.3. Outcome assessments
Evaluation for DHI efficacy will be performed by an independent doctor in each center who will not be involved in the treatment. At 0, 1, 2, and 6 weeks, patients will report the DHI scale at the center. From treatment day one, they will report dizziness symptoms every day at home until the end of the follow-up period.
Electronic data capture (EDC) will be used to collect and manage the study data. The data of DHI scale will use an EDC system (Investigator-initiated Trial Electronic Data Capture system, IIT-EDC, V1.3) to collect. Patient dizziness symptoms, adverse events and medication situations will be collected by using an electronic patient-reported outcome (e-PRO) system (Trial Examinee/Patient Extramural Visit system, eVisit, V1.1.4.5). The e-PRO system is capable of collecting patient experience data and integrating it into the efficacy evaluation of this trial, which will play a crucial role in ensuring the smooth progression of the trial. Data collected by eVisit will be imported to EDC.
2.7. Safety monitoring
Researchers must evaluate each adverse event to determine whether it meets the criteria for serious adverse events and is relevant to the drug being tested. Patients who experience trial-related adverse events, including laboratory abnormalities that have clinical significance, should receive medical care and be informed truthfully with relevant information during the clinical trial and follow-up period. Researchers should also inform patients and be mindful of drug combinations that may affect the safety of patients or interfere with clinical trial results, especially for patients with combined diseases that require treatment. Researchers must regularly inquire about their occurrence with patients. They should then record this information truthfully in both the original medical records and the 'Adverse Event Record Form' within the CRF. This record should include details such as the time of occurrence, end time, duration, severity, measures taken, and outcome of the adverse event. Additionally, researchers must evaluate the relationship between the adverse event and the experimental drug. Meanwhile, a safety officer will be set to carry out data and safety monitoring during this trial and form a report of safety outcomes after 4 weeks of follow-up.
2.8. Quality control
To ensure the quality of the study, this trial intends to set safeguard measures for clinical trials, including setting a Clinical Research Associate (CRA) to monitor study quality, training manual therapy physicians, and making access and regular and irregular assessments consistent.
A monitor will be set up to monitor quality of each center throughout the study and generate a comprehensive quality report at the end of the trial. Clinical doctors responsible for performing SMT will attend a training on standard operating procedures of SMT. Regular or irregular manipulation standard spot checks will be performed on the manual therapy doctors at each center during the trial. The primary outcome, DHI, will be recorded through an electronic case report form (eCRF). The dizziness disappearing time, and the recurrence rate of dizziness symptoms will be recorded through an e-PRO system. The eCRF and the e-PRO system will be provided by an independent third-party company that can collect data in real time and retain data traces.
2.9. Statistical analysis
The statistical analysis will be conducted using SAS9.4 software. The analysis population for all efficacy analyses will be the full analysis set (FAS), comprising all subjects who are randomized and received at least one therapy and have at least one measurement of the outcome of interest. The per-protocol set (PPS) will be used for sensitivity analysis of the primary efficacy outcome. The PPS excludes patients with incomplete intervention, major protocol deviation, missing primary outcome, or other factors that are deemed to significantly affect efficacy evaluation by the review meeting. The response rate and 95 % CI will be compared between the two arms using a two-sided Cochran-Mantel Haenszel (CMH) test stratified by sites, and an associated odds ratio and 95 % CI will be calculated. An estimate of the difference in response rate and corresponding 95 % CI will be calculated using CMH methodology and adjusted by the same stratification factors. For the secondary efficacy outcome, the change in the DHI score at one, two, and four weeks relative to baseline between the two groups will be analyzed using the ANCOVA model or linear mixed model. The time to the disappearance of dizziness symptoms will be estimated using the Kaplan‒Meier method and the log-rank test or Cox model. Additionally, the recurrence rate of dizziness symptoms will be estimated using a chi-square test. Descriptive statistics (mean, median, standard deviation, quartile, min, max, and range) will be presented for continuous data, and frequencies and percentages will be presented for categorical data. Safety analysis will be performed based on safety set (SS), which is defined as the group of patients who received treatment at least once and had safety data collected. Adverse events will be coded according to the ICH MedDRA. This study will calculate the frequency and incidence of adverse events/reactions, serious adverse events/reactions, and adverse events/reactions leading to shedding during treatment. Additionally, a compliance analysis will be conducted to determine the percentage of patients who comply with treatment and medication within the range of 80 %–100 %. Missing data based on the ITT primary efficacy outcome analysis will be imputed using the last observation carried forward (LOCF) method and provide analysis results not carried forward. Other analyses will be performed using analysis results that are not carried forward.
3. Discussion and conclusion
This protocol outlines the rationale and design for a multicenter RCT that investigates the safety and efficacy of SMT compared to betahistine mesylate tablets as a positive control in the treatment of cervicogenic dizziness. The value of the trial is to provide evidence of high-quality evidence-based medical science to manual therapy and physical therapy.
The key factor affecting the quality of this trial is the quality of the SMT given by doctors from the 4 different clinical centers. Even though doctors will perform the same procedures of SMT, it is understandable that the optional details and personal habits might be different. The measures as follow will be used to minimized the potential difference.
First, before the trial starting, every participant in research group needs to obtain GCP certificates authorized by the China State Food and Drug Administration (SFDA), which can ensure all aspects of this research are standardized and reduce bias during the implementation process. Second, to make the manual therapy procedures as standardized and consistent as possible. All SMT doctors in the 4 clinical centers have to be trained and pass the assessment before the beginning of this trial. CRA will perform regular or irregular standard spot checks of manipulation in trial period. Third, only two experienced doctors will be appointed to perform the interventions in one clinical center. Them will be allowed to perform the interventions after they passed the training assessment and achieved the SOPs of SMT.
Meanwhile, this study has some other limitations. The follow-up time is relatively short to accurately evaluate the long-term effectiveness of massage in treating cervicogenic dizziness. In addition, it was not possible to perform a blind study due to the large difference between SMT and Merislon (Betahistine Mesilate Tablets). These limitations may lead to bias in interpreting the results. Therefore, Setting up CRA, independent efficacy evaluation and other quality control measures will be implemented by research group to ensure the reliability of study results.
Shi's orthopedics and traumatology academic school with manual therapy is well known in China, with a history of 150 years, and officially recognized by Chinese health care administration, which will contribute to improve the compliance of patients in this study. However, the risk associated with spinal manipulation still needs to be noted. The participants will be eligible for inclusion and exclusion criteria to minimize the occurrence of complications. During study process, doctors will immediately assess and take measures if trial-related serious adverse events appear.
In conclusion, this study will contribute to evidence-based manual therapy, and leading to improve the strategy of cervicogenic dizziness clinical treatment.
Ethics and dissemination
Ethical approval (Approval Number: 2022-1120-57-01) from the Institutional Review Board of Shuguang Hospital Affiliated with Shanghai University of TCM was obtained. All participants will be required to sign an informed consent before enrolment in the study. The results of the trial will be submitted for publication in a peer-reviewed journal.
Data management and oversight
To ensure protocol compliance, proper study management, and timely completion of study procedures, members of the research team from Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine will take responsibility for the conduct of all research staff and study participants.
Protocol and registration
The trial is registered with ClinicalTrials.gov, NCT05604937. Registered on Nov 3, 2022, https://clinicaltrials.gov/ct2/show/NCT05604937.
Data storage security and patient confidentiality
Patient's medical records (descriptive characteristics such as name initials, allocated study number, sex, age, BMI, outcome measures such as primary outcomes and secondary outcomes and laboratory results) will be kept in the respective hospital, and physicians will document the findings of the study in it, allowing researchers and ethics committees to access the data. Personal information of patients will not be revealed in the results of this study, and we will try everything we can to protect patients' privacy and medical data within Chinese law. According to medical research ethics, experimental data, especially personal privacy information, will not be allowed to be accessed and shared by the public and will be limited to web-based databases to ensure that personal privacy information is not disclosed.
Ethics and dissemination
The protocol for this trial was approved by the Independent Review Board of SGH (approval number: 2020-814-21-01). All participants will be required to sign an informed consent form before enrollment in this study. The model consent form and other related documentation given to participants can be provided upon request. (see online supplementary file).
Funding
This trial was funded by Shanghai Traditional Chinese Medicine Three-year Action Plan [ZY(2021–2023)-0211], Shanghai Education Commission Collaborative Innovation Center: Integrated Chinese and WesternMedicines - Chinese Patent Medicine Clinical Evaluation Platform (A1-U21-205-0103), Shanghai Shenkang Center Demonstration Research Ward Construction (SHDC2022CRW010), Shanghai Shenkang Center Medical EnterpriseIntegration Innovation Collaboration Project (SHDC2022CRT018) and Shanghai Chronic Osteopathy Clinical Medical Research Center Project (20MC1920600), National Nature Science Fundation of China (82374467).
Disclaimer
The funding organization has not played any role in the design and conduct of the study; collection, management, or analysis of the data; or preparation of the manuscript.
Patient consent for publication
Not required.
Availability of data and material
The datasets analyzed during the current study will be available from the corresponding author upon reasonable request.
Consent for publication
Not applicable.
Trial status
The protocol version is 1.0, November 20, 2022. This study is not yet recruiting. The recruitment will begin in December 2022 and finish in December 2023.
Ethics approval and consent to participate
The study design and procedures were approved by the Institutional Review Board of Shuguang Hospital Affiliated with Shanghai University of TCM (Approval Number: 2022-1120-57-01).
CRediT authorship contribution statement
Yunfan Zhan: Writing – review & editing, Writing – original draft. Yujie Zhang: Writing – review & editing, Writing – original draft. Kaoqiang Liu: Writing – original draft. Ye Zhao: Writing – original draft. Jiawei Ning: Writing – original draft. Yongli Chai: Writing – original draft. Lingjun Kong: Writing – review & editing, Writing – original draft, Methodology. Weian Yuan: Writing – review & editing, Writing – original draft, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments are given to the staff and students at Shuguang Hospital Affiliated with Shanghai University of Traditional Chinese Medicine for their contributions to the implementation of this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2024.101349.
Contributor Information
Lingjun Kong, Email: chunyong01@163.com.
Weian Yuan, Email: weian_1980@163.com.
Abbreviations:
- SMT
Shi's Manual Therapy
- RCT
randomized controlled trial
- DHI
Dizziness Handicap Inventory
- ITT
intention-to-treat
- MPS
myofascial pain syndrome
- eCRF
electronic case report form
- e-PRO
electronic patient-reported outcome
- CRA
Clinical Research Associate
- SOPs
standard operating procedures
- IWRS
Interactive Web Response System
- EDC
electronic data capture
- GCP
Good Clinical Practice,
- FAS
full analysis set,
- PPS
per-protocol set,
- SS
safety set,
- LOCF
Last Observation Carried Forward,
- TCM
Traditional Chinese Medicine,
- SFDA
State Food and Drug Administration
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ryan G.M., Cope S. Cervical vertigo. Lancet. 1955 Dec 31;269(6905):1355–1358. doi: 10.1016/s0140-6736(55)93159-7. [DOI] [PubMed] [Google Scholar]
- 2.Thompson-Harvey A., Hain T.C. Symptoms in cervical vertigo. Laryngoscope Investig Otolaryngol. 2018 Nov 28;4(1):109–115. doi: 10.1002/lio2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knapstad M.K., Nordahl S.H.G., Goplen F.K. Clinical characteristics in patients with cervicogenic dizziness: a systematic review. Health Sci Rep. 2019 Jul 26;2(9) doi: 10.1002/hsr2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu E.C.P., Lo F.S., Bhaumik A. Plausible impact of forward head posture on upper cervical spine stability. J Family Med Prim Care. 2020 May 31;9(5):2517–2520. doi: 10.4103/jfmpc.jfmpc_95_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu E.C.P., Chin W.L., Bhaumik A. Cervicogenic dizziness. Oxf Med Case Reports. 2019;2019(11):476–478. doi: 10.1093/omcr/omz115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vural M., Karan A., Albayrak Gezer İ., et al. Prevalence, etiology, and biopsychosocial risk factors of cervicogenic dizziness in patients with neck pain: a multi-center, cross-sectional study. Turk J Phys Med Rehabil. 2021 Dec 1;67(4):399–408. doi: 10.5606/tftrd.2021.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi S. Importance of cervicogenic general dizziness. J. Rural Med. 2018 May;13(1):48–56. doi: 10.2185/jrm.2958. doi:10.3390/medicina58121791. doi:10.2185/jrm.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y.W., Chen X.B., Zhu Z.F., et al. A review on the pathogenesis of cervical vertigo. Clinical Journal of Chinese Medicine. 2022;14(30):26–29. [Google Scholar]
- 9.Cai G., Zhu D., Chen J., et al. Effects of traction therapy on atlantoaxial joint dislocation-induced cervical vertigo. Braz. J. Med. Biol. Res. 2022;55 doi: 10.1590/1414-431X2022e11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill-Lussier J., Saliba I., Barthélemy D. Proprioceptive cervicogenic dizziness care trajectories in patient Subpopulations: a scoping review. J. Clin. Med. 2023 Feb 27;12(5):1884. doi: 10.3390/jcm12051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh H., Shin S., Lee E., et al. Chinese herbal medicine for cervicogenic dizziness: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2022 May 9;2022 doi: 10.1155/2022/2425851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu E.C., Lin A.F.C., Cheung G., et al. Cervicogenic dizziness after self-manipulation of the cervical spine. Cureus. 2023 Apr 3;15(4) doi: 10.7759/cureus.37051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaseen K., Hendrick P., Ismail A., et al. The effectiveness of manual therapy in treating cervicogenic dizziness: a systematic review. J. Phys. Ther. Sci. 2018 Jan;30(1):96–102. doi: 10.1589/jpts.30.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung Y.H. Suboccipital muscles, forward head posture, and cervicogenic dizziness. Medicina (Kaunas) 2022 Dec 5;58(12):1791. doi: 10.3390/medicina58121791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrasco-Uribarren A., Rodriguez-Sanz J., López-de-Celis C., et al. Short-term effects of the traction-manipulation protocol in dizziness intensity and disability in cervicogenic dizziness: a randomized controlled trial. Disabil. Rehabil. 2022 Jul;44(14):3601–3609. doi: 10.1080/09638288.2021.1872719. [DOI] [PubMed] [Google Scholar]
- 16.Reid S.A., Callister R., Katekar M.G., et al. Effects of cervical spine manual therapy on range of motion, head repositioning, and balance in participants with cervicogenic dizziness: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2014 Sep;95(9):1603–1612. doi: 10.1016/j.apmr.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 17.He L.L., Lai R.J., Leff J., et al. Cervicogenic dizziness alleviation after coblation discoplasty: a retrospective study. Ann. Med. 2021 Dec;53(1):639–646. doi: 10.1080/07853890.2021.1910336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamed A.A., Shendy W.S., Semary M., et al. Combined use of cervical headache snag and cervical snag half rotation techniques in the treatment of cervicogenic headache. J. Phys. Ther. Sci. 2019 Apr;31(4):376–381. doi: 10.1589/jpts.31.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-de-Celis C., Pérez-Guillen S., Tricás-Moreno J.M., et al. Short-term effects of the traction-manipulation protocol in dizziness intensity and disability in cervicogenic dizziness: a randomized controlled trial. Disabil. Rehabil. 2022 Jul;44(14):3601–3609. doi: 10.1080/09638288.2021.1872719. [DOI] [PubMed] [Google Scholar]
- 20.Murdin L., Hussain K., Schilder A.G. Betahistine for symptoms of vertigo. Cochrane Database Syst. Rev. 2016 Jun 21;2016(6) doi: 10.1002/14651858.CD010696.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Yongyong. High Education Press; Beijing: 2004. Medical Statistics (Version 2) p. 563. [Google Scholar]
- 22.Reiley A.S., Vickory F.M., Funderburg S.E., et al. How to diagnose cervicogenic dizziness. Arch Physiother. 2017 Sep 12;7:12. doi: 10.1186/s40945-017-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mingchai Z., Yinyu S., Dongyu Ch, et al. Technical specification of Shi's manipulation to correct cervical spine bone joint semidislocation and tendon off-position. Shanghai J. Tradit. Chin. Med. 2015;49(5):4–7. doi: 10.16305/j.1007-1334.2015.05.002. [DOI] [Google Scholar]
- 24.De Vestel C., Vereeck L., Reid S.A., et al. Systematic review and meta-analysis of the therapeutic management of patients with cervicogenic dizziness. J. Man. Manip. Ther. 2022;30(5):273–283. doi: 10.1080/10669817.2022.2033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei D., Chang L., Jiaxi W., et al. An evaluaiton of the dizziness Handicap inventory (Chinese version) Chinese Journal of Otology. 2013;11(2):228–230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study will be available from the corresponding author upon reasonable request.