Abstract
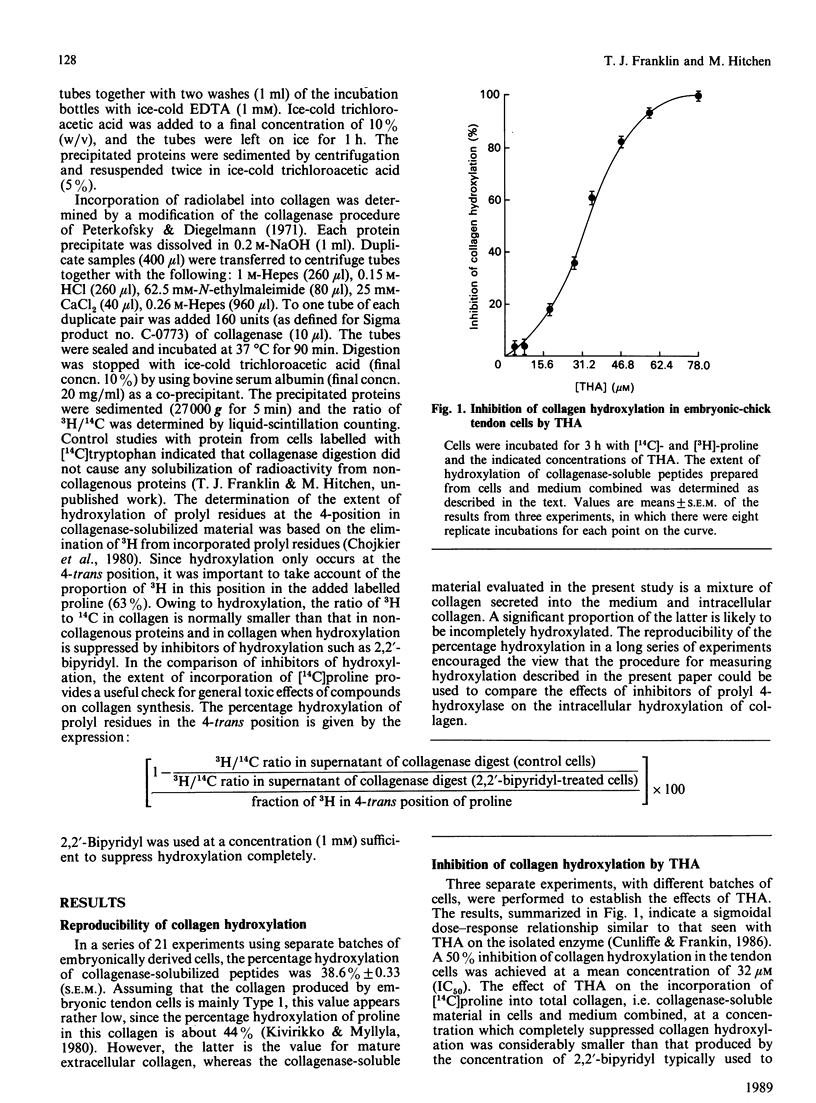
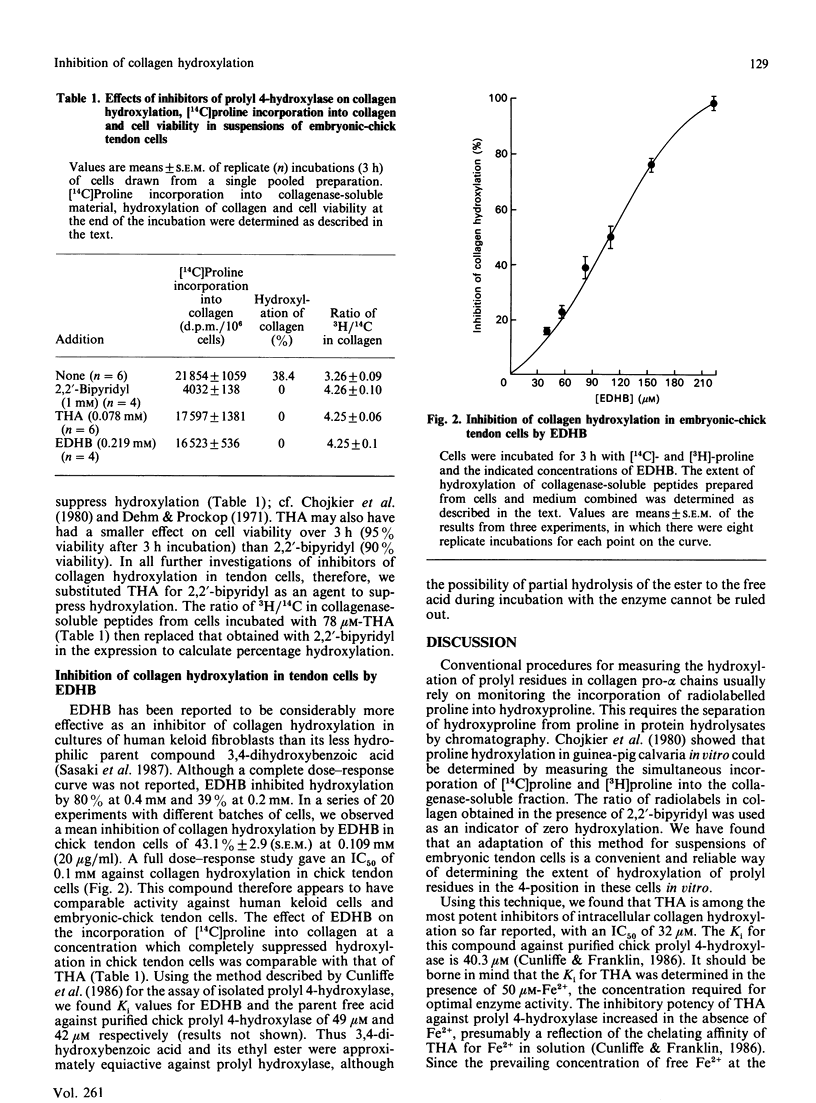
Prolyl 4-hydroxylase (EC 1.14.11.2) is an essential enzyme in the post-translational modification of collagen. Inhibitors of this enzyme are of potential interest for the treatment of diseases involving excessive deposition of collagen. 2,7,8-Trihydroxyanthraquinone (THA) is an effective inhibitor of prolyl 4-hydroxylase by virtue of its ability to compete with the co-substrate 2-oxoglutarate (Ki = 40.3 microM). Using a simple and reproducible assay for collagen hydroxylation, we show that THA inhibits the hydroxylation of collagen in embryonic-chick tendon cells in short-term culture, with an IC50 value (concn. giving 50% inhibition) of 32 microM. In comparison, the ethyl ester of 3,4-dihydroxybenzoic acid has an IC50 value of 0.1 mM against collagen hydroxylation by chick tendon cells, whereas its Ki versus isolated prolyl 4-hydroxylase is 49 microM.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttinen H., Ryhänen L., Oikarinen A. Effects of divalent cations on collagen biosynthesis in isolated chick embryo tendon cells. Biochim Biophys Acta. 1980 Sep 19;609(2):321–328. doi: 10.1016/0005-2787(80)90244-0. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Peterkofsky B., Bateman J. New method for determining the extent of proline hydroxylation by measuring changes in the ratio of [4-3H]:[14C]proline in collagenase digests. Anal Biochem. 1980 Nov 1;108(2):385–393. doi: 10.1016/0003-2697(80)90603-x. [DOI] [PubMed] [Google Scholar]
- Cunliffe C. J., Franklin T. J., Gaskell R. M. Assay of prolyl 4-hydroxylase by the chromatographic determination of [14C]succinic acid on ion-exchange minicolumns. Biochem J. 1986 Dec 1;240(2):617–619. doi: 10.1042/bj2400617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe C. J., Franklin T. J. Inhibition of prolyl 4-hydroxylase by hydroxyanthraquinones. Biochem J. 1986 Oct 15;239(2):311–315. doi: 10.1042/bj2390311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru T., Kanamaru T., Takahashi T., Ohta K., Okazaki H. Inhibition of prolyl hydroxylase activity and collagen biosynthesis by the anthraquinone glycoside, P-1894B, an inhibitor produced by Streptomyces albogriseolus. Biochem Pharmacol. 1982 Mar 15;31(6):915–919. doi: 10.1016/0006-2952(82)90320-3. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Rapaka R. S., Sorensen K. R., Lee S. D., Bhatnagar R. S. Inhibition of hydroxyproline synthesis by palladium ions. Biochim Biophys Acta. 1976 Mar 11;429(1):63–71. doi: 10.1016/0005-2744(76)90030-9. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Majamaa K., Uitto J. Reduction of collagen production in keloid fibroblast cultures by ethyl-3,4-dihydroxybenzoate. Inhibition of prolyl hydroxylase activity as a mechanism of action. J Biol Chem. 1987 Jul 5;262(19):9397–9403. [PubMed] [Google Scholar]
- Tschank G., Hanauske-Abel H. M., Peterkofsky B. The effectiveness of inhibitors of soluble prolyl hydroxylase against the enzyme in the cisternae of isolated bone microsomes. Arch Biochem Biophys. 1988 Mar;261(2):312–323. doi: 10.1016/0003-9861(88)90346-3. [DOI] [PubMed] [Google Scholar]
- Tschank G., Raghunath M., Günzler V., Hanauske-Abel H. M. Pyridinedicarboxylates, the first mechanism-derived inhibitors for prolyl 4-hydroxylase, selectively suppress cellular hydroxyprolyl biosynthesis. Decrease in interstitial collagen and Clq secretion in cell culture. Biochem J. 1987 Dec 15;248(3):625–633. doi: 10.1042/bj2480625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Synthesis and secretion of under-hydroxylated procollagen at various temperatures by cells subject to temporary anoxia. Biochem Biophys Res Commun. 1974 Sep 9;60(1):414–423. doi: 10.1016/0006-291x(74)90220-4. [DOI] [PubMed] [Google Scholar]


