Abstract
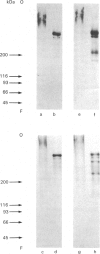
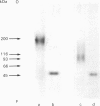
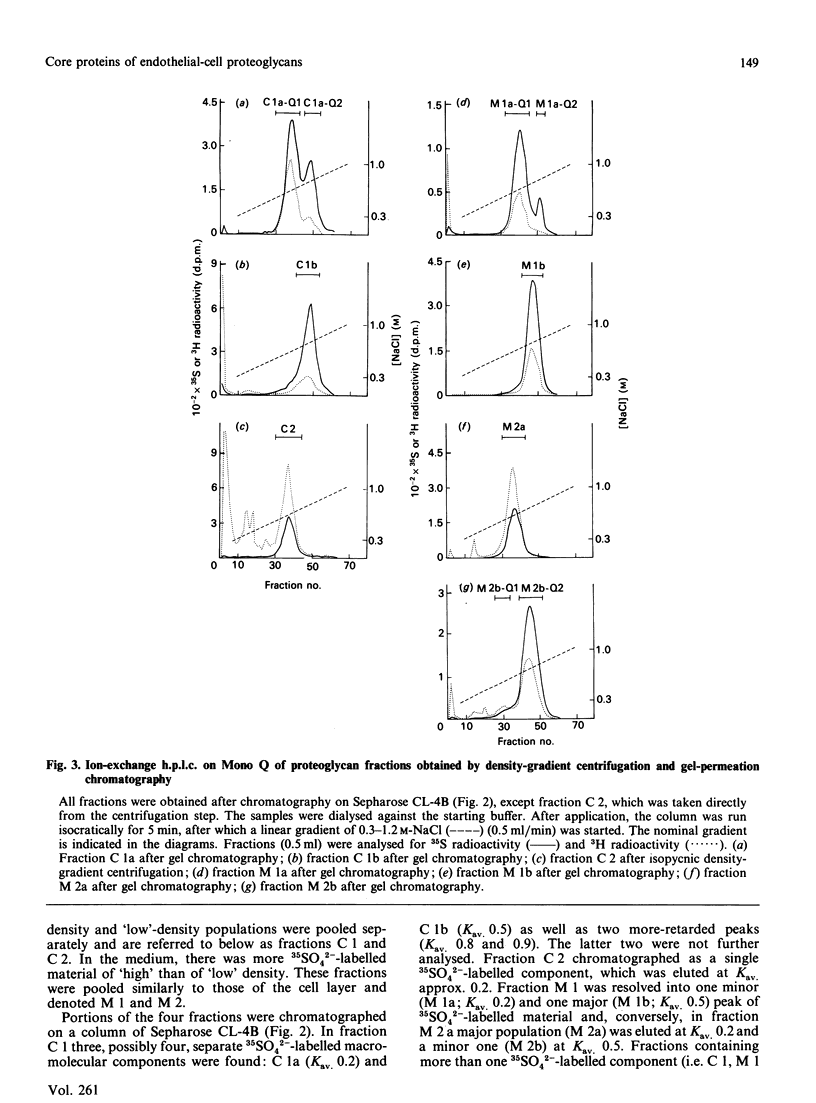
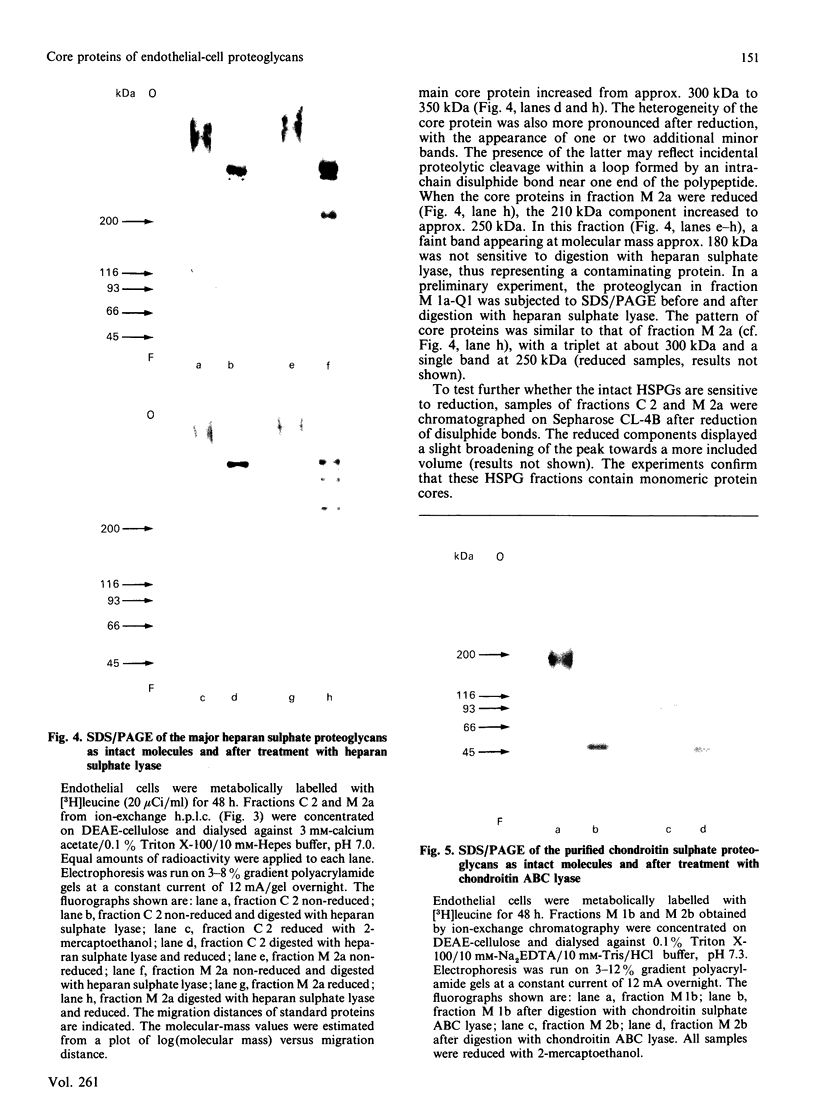
Proteoglycans, metabolically labelled with [3H]leucine and 35SO4(2-), were isolated from the spent media and from guanidinium chloride extracts of cultured human umbilical-vein endothelial cells by using isopycnic density-gradient centrifugation, gel filtration and ion-exchange h.p.l.c. The major proteoglycan species were subjected to SDS/polyacrylamide-gel electrophoresis before and after enzymic degradation of the polysaccharide chains. The cell extract contained mainly a heparan sulphate proteoglycan that has a buoyant density of 1.31 g/ml and a protein core with apparent molecular mass 300 kDa. The latter was heterogeneous and migrated as one major and one minor band. After reduction, the apparent molecular mass of the major band increased to approx. 350 kDa, indicating the presence of intrachain disulphide bonds. The proteoglycan binds to octyl-Sepharose and its polysaccharide chains are extensively degraded by heparan sulphate lyase. The proteoglycans of the medium contained 90% of all the incorporated 35SO4(2-). Here the predominant heparan sulphate proteoglycan was similar to that of the cell extract, but was more heterogeneous and contained an additional core protein with apparent molecular mass 210 kDa. Furthermore, two different chondroitin sulphate proteoglycans were found: one 200 kDa species with a high buoyant density (approx. 1.45 g/ml) and one 100 kDa species with low buoyant density (approx. 1.3 g/ml). Both these proteoglycans have a core protein of molecular mass approx. 47 kDa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourin M. C., Boffa M. C., Björk I., Lindahl U. Functional domains of rabbit thrombomodulin. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5924–5928. doi: 10.1073/pnas.83.16.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Carlstedt I., Kendall S., Malmström A., Schmidtchen A., Fransson L. A. Structure of proteoheparan sulfates from fibroblasts. Confluent and proliferating fibroblasts produce at least three types of proteoheparan sulfates with functionally different core proteins. J Biol Chem. 1986 Sep 15;261(26):12079–12088. [PubMed] [Google Scholar]
- Cöster L., Carlstedt I., Malmström A., Särnstrand B. Biosynthesis and secretion of dermatan sulphate proteoglycans in cultures of human skin fibroblasts. Biochem J. 1984 Jun 1;220(2):575–582. doi: 10.1042/bj2200575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L. Protein C activation. Semin Thromb Hemost. 1984 Apr;10(2):122–130. doi: 10.1055/s-2007-1004414. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Hassell J. R., Kimura J. H., Hascall V. C. Proteoglycan core protein families. Annu Rev Biochem. 1986;55:539–567. doi: 10.1146/annurev.bi.55.070186.002543. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y. Proteoglycans: an overview. Methods Enzymol. 1987;144:305–319. doi: 10.1016/0076-6879(87)44185-2. [DOI] [PubMed] [Google Scholar]
- Heremans A., Cassiman J. J., Van den Berghe H., David G. Heparan sulfate proteoglycan from the extracellular matrix of human lung fibroblasts. Isolation, purification, and core protein characterization. J Biol Chem. 1988 Apr 5;263(10):4731–4739. [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., Fritze L., Soff G., Rosenberg R. D. Human thrombomodulin gene is intron depleted: nucleic acid sequences of the cDNA and gene predict protein structure and suggest sites of regulatory control. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6425–6429. doi: 10.1073/pnas.84.18.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Structural characterization of heparan sulfate proteoglycan subclasses isolated from bovine aortic endothelial cell cultures. Biochemistry. 1988 Mar 22;27(6):2136–2144. doi: 10.1021/bi00406a048. [DOI] [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Oegema T. R., Brenchley P. E., Anderson J. C., Dickinson M. A., Horigan E. A., Hassell J. R. Glomerular basement membrane proteoglycans are derived from a large precursor. J Cell Biol. 1988 Mar;106(3):963–970. doi: 10.1083/jcb.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G., Nicolson G. L. Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem. 1982 Mar 10;257(5):2678–2686. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lories V., David G., Cassiman J. J., Van den Berghe H. Heparan sulfate proteoglycans of human lung fibroblasts. Occurrence of distinct membrane, matrix and secreted forms. Eur J Biochem. 1986 Jul 15;158(2):351–359. doi: 10.1111/j.1432-1033.1986.tb09758.x. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., Rosenberg R. D. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986 Jun 5;261(16):7507–7517. [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparan sulfate proteoglycan and the vascular endothelium. Semin Thromb Hemost. 1987 Oct;13(4):464–474. doi: 10.1055/s-2007-1003523. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry. 1984 Apr 10;23(8):1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Oohira A., Wight T. N., Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983 Feb 10;258(3):2014–2021. [PubMed] [Google Scholar]
- Rauch U., Glössl J., Kresse H. Comparison of small proteoglycans from skin fibroblasts and vascular smooth-muscle cells. Biochem J. 1986 Sep 1;238(2):465–474. doi: 10.1042/bj2380465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Gill P. J., Silbert J. E., Douglas W. H., Fanburg B. L. Involvement of cell surface heparin sulfate in the binding of lipoprotein lipase to cultured bovine endothelial cells. J Clin Invest. 1981 Oct;68(4):995–1002. doi: 10.1172/JCI110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Ozawa T. Evidence that cell surface heparan sulfate is involved in the high affinity thrombin binding to cultured porcine aortic endothelial cells. J Clin Invest. 1985 Apr;75(4):1308–1316. doi: 10.1172/JCI111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kusumoto H., Deyashiki Y., Nishioka J., Maruyama I., Zushi M., Kawahara S., Honda G., Yamamoto S., Horiguchi S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987 Jul;6(7):1891–1897. doi: 10.1002/j.1460-2075.1987.tb02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]