Abstract
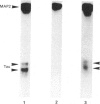
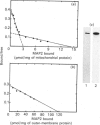
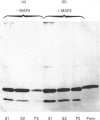
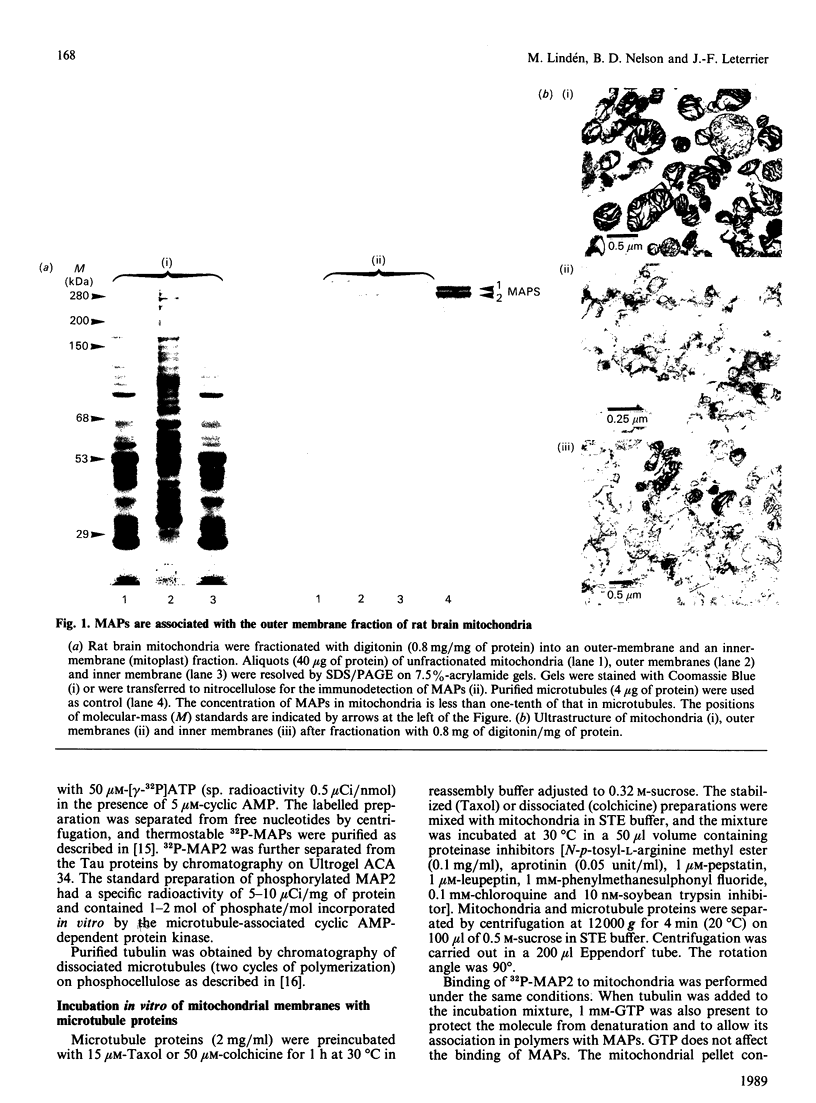
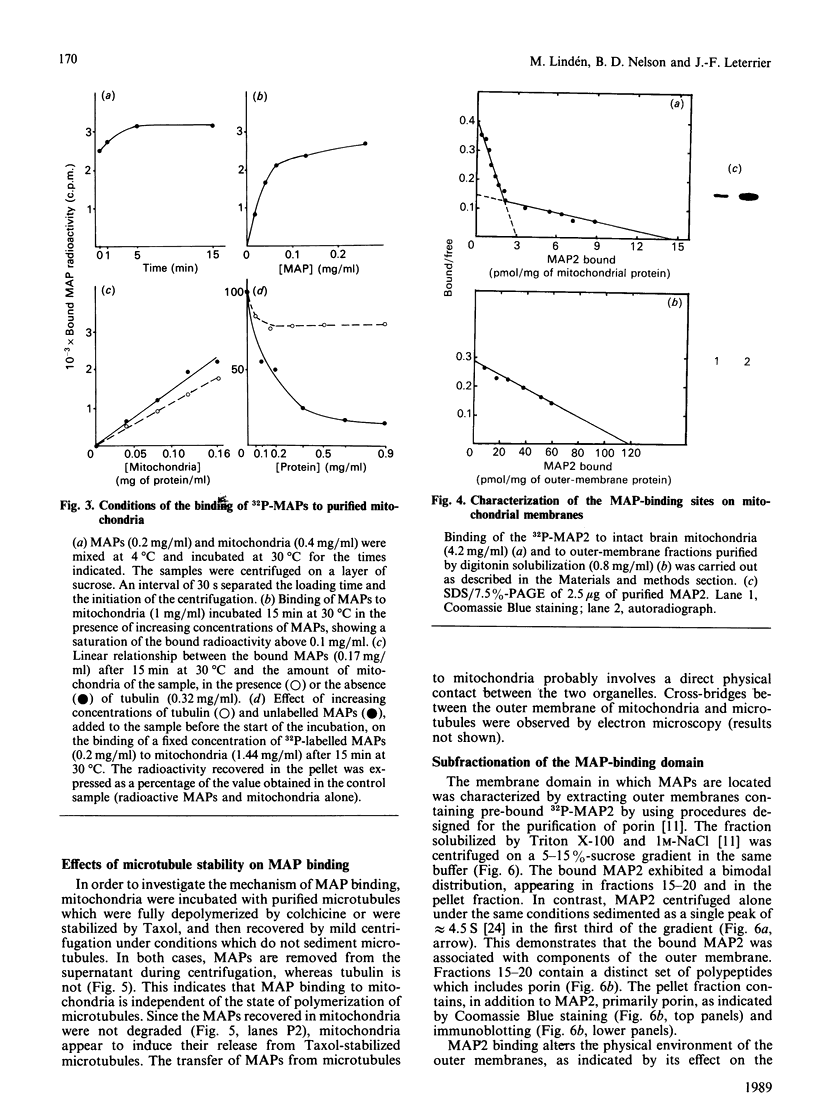
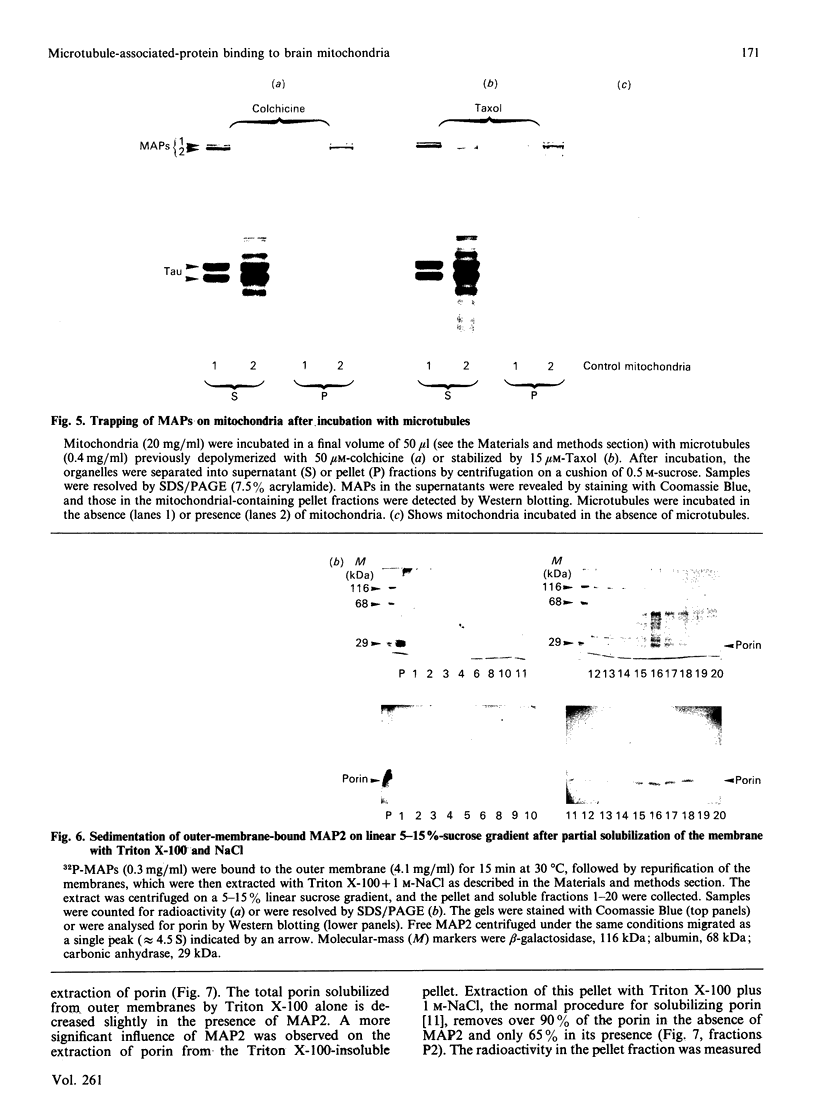
Purified mitochondria from rat brain contain microtubule-associated proteins (MAPs) bound to the outer membrane. Studies of binding in vitro performed with microtubules and with purified microtubule proteins showed that mitochondria preferentially interact with the high-molecular-mass MAPs (and not with Tau protein). Incubation of intact mitochondria with Taxol-stabilized microtubules resulted in the selective trapping of both MAPs 1 and 2 on mitochondria, indicating that an interaction between the two organelles occurred through a site on the arm-like projection of MAPs. Two MAP-binding sites were located on intact mitochondria. The lower-affinity MAP2-binding site (Kd = 2 x 10(-7) M) was preserved and enriched in the outer-membrane fraction, whereas the higher-affinity site (Kd = 1 x 10(-9) M) was destroyed after removing the outer membrane with digitonin. Detergent fractionation of mitochondrial outer membranes saturated with MAP2 bound in vitro showed that MAPs are associated with membrane fragments which contain the pore-forming protein (porin). MAP2 also partially prevents the solubilization of porin from outer membrane, indicating a MAP-induced change in the membrane environment of porin. These observations demonstrate the presence of specific MAP-binding sites on the outer membrane, suggesting an association between porin and the membrane domain involved in the cross-linkage between microtubules and mitochondria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E. H., Singer S. J. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1982 Jan;79(1):123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. D., Smith R. S. The movement of optically detectable organelles in myelinated axons of Xenopus laevis. J Physiol. 1974 Oct;242(1):77–97. doi: 10.1113/jphysiol.1974.sp010695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorbani L., Jancsik V., Linden M., Leterrier J. F., Nelson B. D., Rendon A. Subfractionation of the outer membrane of rat brain mitochondria: evidence for the existence of a domain containing the porin-hexokinase complex. Arch Biochem Biophys. 1987 Jan;252(1):188–196. doi: 10.1016/0003-9861(87)90023-3. [DOI] [PubMed] [Google Scholar]
- Fellous A., Francon J., Lennon A. M., Nunez J. Microtubule assembly in vitro. Purification of assembly-promoting factors. Eur J Biochem. 1977 Aug 15;78(1):167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M. A., Wandosell F., Avila J. Localization of the phosphorylation sites for different kinases in the microtubule-associated protein MAP2. J Neurochem. 1987 Jan;48(1):84–93. doi: 10.1111/j.1471-4159.1987.tb13130.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Bloom G. S., Vallee R. B. Cytoskeletal architecture and immunocytochemical localization of microtubule-associated proteins in regions of axons associated with rapid axonal transport: the beta,beta'-iminodipropionitrile-intoxicated axon as a model system. J Cell Biol. 1985 Jul;101(1):227–239. doi: 10.1083/jcb.101.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson L., Frey T., Zeeberg B., Dalldorf F., Caplow M. Inhibition of microtubule assembly by phosphorylation of microtubule-associated proteins. Biochemistry. 1980 May 27;19(11):2472–2479. doi: 10.1021/bi00552a027. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén M., Andersson G., Gellerfors P., Nelson B. D. Subcellular distribution of rat liver porin. Biochim Biophys Acta. 1984 Feb 29;770(1):93–96. doi: 10.1016/0005-2736(84)90077-4. [DOI] [PubMed] [Google Scholar]
- Lindén M., Gellerfors P., Nelson B. D. Purification of a protein having pore forming activity from the rat liver mitochondrial outer membrane. Biochem J. 1982 Oct 15;208(1):77–82. doi: 10.1042/bj2080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz D., Lasek R. J., Brady S. T., Allen R. D. Mitochondrial motility in axons: membranous organelles may interact with the force generating system through multiple surface binding sites. Cell Motil. 1984;4(2):89–101. doi: 10.1002/cm.970040203. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Lasek R. J. Cross-bridges mediate anterograde and retrograde vesicle transport along microtubules in squid axoplasm. J Cell Biol. 1985 Dec;101(6):2181–2193. doi: 10.1083/jcb.101.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose-Larsen P., Bravo R., Fey S. J., Small J. V., Celis J. E. Putative association of mitochondria with a subpopulation of intermediate-sized filaments in cultured human skin fibroblasts. Cell. 1982 Dec;31(3 Pt 2):681–692. doi: 10.1016/0092-8674(82)90323-3. [DOI] [PubMed] [Google Scholar]
- Nagy A., Delgado-Escueta A. V. Rapid preparation of synaptosomes from mammalian brain using nontoxic isoosmotic gradient material (Percoll). J Neurochem. 1984 Oct;43(4):1114–1123. doi: 10.1111/j.1471-4159.1984.tb12851.x. [DOI] [PubMed] [Google Scholar]
- Paschal B. M., Shpetner H. S., Vallee R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987 Sep;105(3):1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Ghetti B., Shelanski M. L. On the association between microtubules and mitochondria within axons. Brain Res. 1971 Nov;34(2):389–393. doi: 10.1016/0006-8993(71)90293-9. [DOI] [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cameron B. F. Morphological evidence for the participation of microtubules in axonal transport. Ann N Y Acad Sci. 1975 Jun 30;253:472–506. doi: 10.1111/j.1749-6632.1975.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Tobler H. J., Engel G. Affinity spectra: a novel way for the evaluation of equilibrium binding experiments. Naunyn Schmiedebergs Arch Pharmacol. 1983 Apr;322(3):183–192. doi: 10.1007/BF00500763. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem. 1978 Apr 25;253(8):2834–2845. [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. Structure and phosphorylation of microtubule-associated protein 2 (MAP 2). Proc Natl Acad Sci U S A. 1980 Jun;77(6):3206–3210. doi: 10.1073/pnas.77.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L. S., Nikaido H., Kagawa Y. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J Biol Chem. 1980 Mar 10;255(5):1771–1774. [PubMed] [Google Scholar]