Abstract
Introduction
Tumor genomic testing (TGT) is standard‐of‐care for most patients with advanced/metastatic cancer. Despite established guidelines, patient education prior to TGT is frequently omitted. The purpose of this study was to evaluate the impact of a concise 4 min video for patient education prior to TGT.
Methods
Based on a quality improvement cycle, an animated video was created to be applicable to any cancer type, incorporating culturally diverse images, available in English and Spanish. Patients undergoing standard‐of‐care TGT were enrolled at a tertiary academic institution and completed survey instruments prior to video viewing (T1) and immediately post‐viewing (T2). Instruments included: (1) 10‐question objective genomic knowledge; (2) 10‐question video message‐specific knowledge; (3) 11‐question Trust in Provider; (4) attitudes regarding TGT.
Results
A total of 150 participants were enrolled. For the primary objective, there was a significant increase in video message‐specific knowledge (median 10 point increase; p < 0.0001) with no significant change in genomic knowledge/understanding (p = 0.89) or trust in physician/provider (p = 0.59). Results for five questions significantly improved, including the likelihood of TGT impact on treatment decision, incidental germline findings, and cost of testing. Improvement in video message‐specific knowledge was consistent across demographic groups, including age, income, and education.
Conclusions
A concise, 3–4 min, broadly applicable video incorporating culturally diverse images administered prior to TGT significantly improved video message‐specific knowledge across all demographic groups. This resource is publicly available at http://www.tumor‐testing.com, with a goal to efficiently educate and empower patients regarding TGT while addressing guidelines within the flow of clinical practice.
Keywords: biomarkers, cancer education, cancer management, genomics
Tumor genomic testing is standard‐of‐care for most patients with advanced/metastatic cancer, yet despite established guidelines, patient education prior to TGT is frequently omitted. A concise 4 min video for patient education prior to tumor genomic testing was an effective tool to augment the provider‐patient discussion of tumor genomic testing. This video may facilitate awareness, enhance dialog, extend information from the clinic to the home setting, and aid in the shared decision‐making.
1. INTRODUCTION
Somatic next‐generation sequencing, also known as tumor genomic testing (TGT), has become increasingly adopted as part of standard cancer care for many cancers. 1 From 2017 to 2021, there was an estimated 3‐fold increase (9%–30%) in the proportion of tumors for which there was a TGT‐identified mutation with a disease‐matched, standard‐care, FDA‐approved therapy. 2 As a result of three molecular alterations each with tumor‐agnostic FDA‐approved therapies (NTRK fusion—larotrectinib; deficient mismatch repair—pembrolizumab; high tumor mutational burden—pembrolizumab), TGT is now viewed as a necessity by most oncologists in nearly all patients with metastatic cancer. 2 As a result, its use has become widespread in the metastatic setting and has increased in some early stage settings.
International guidelines all recommend that clinicians report incidental germline findings and likely germline findings (e.g., from tumor‐normal TGT or pathogenic variants in germline‐relevant genes at high allele frequencies) to their patients. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 The American Society of Clinical Oncology (ASCO) Policy Statement 3 notes: “(1) Oncology providers should communicate the potential for incidental/secondary germline information…before conducting somatic mutation profiling and should review potential benefits, limitations, and risks before testing; (2) Providers should carefully ascertain patient preferences regarding the receipt of germline information.” Despite these unified guidelines, evidence suggests that provider‐patient discussions around TGT are inconsistent, 11 , 12 which is complicated further by limited genetics/genomics literacy among patients, 13 particularly those who have lower income and/or those who are medically underserved. 14 This raises important ethical challenges including uncertainty of results, incidental germline findings, and disparities around TGT options and access. 11 , 15 , 16 , 17
Taken together, this evidence supports the need for consistent and improved communication between providers and patients about TGT. To address this need, we previously conducted a quality improvement (QI) initiative focused on patient education prior to TGT using a Plan‐Do‐Study‐Act (PDSA) approach. 18 , 19 Within that PDSA cycle, published guidelines related to pre‐TGT provider‐patient education were reviewed; a provider QI survey highlighted inconsistency in pre‐TGT discussion practice across providers; and patient focus groups and interviews revealed important themes and opportunities. Themes and opportunities were incorporated into a patient‐navigated, concise 3–4 min animated video for pre‐TGT education with content addressing 14/17 (82%) of key points described in the ASCO and ACMG guidelines. 3 , 6 , 19 The video is based on adult learning and communication theory and includes characters of varied races, ethnicities, and genders so that the images presented are relatable to patients of varied identities.
We report the primary outcome analysis of a prospective study evaluating the impact of this concise pre‐TGT educational video intervention on video message‐specific knowledge, general genomic knowledge/understanding, and trust in physician/provider.
2. METHODS
2.1. Patient eligibility and recruitment
The study protocol was approved by the Ohio State University Institutional Review Board (OSU#2021C0209). Participants were eligible for inclusion if they were age 18 or older at the time of study entry with biopsy‐confirmed cancer, spoke English or Spanish, and planned to undergo TGT. TGT could be from tumor tissue or blood‐based. Any commercial vendor of TGT or Ohio State University Molecular Pathology Lab was acceptable. Eligible patients receiving care in cancer care clinics at The Ohio State University's James Cancer Center facilities were identified by research staff through TGT order placement, screening, or provider referral. A total of 156 participants were consented to achieve the prespecified 150 enrolled subjects completing all required surveys to be eligible for inclusion in the primary analysis (CONSORT diagram Figure S1A) from March 2022 through May 2023 (Figure S1B).
2.2. Survey instruments
2.2.1. Video message‐specific knowledge (VMSK)
Message‐specific knowledge was measured 10 true/false statements that addressed key knowledge domains in the video intervention, with a final score reported as number correct multiplied by 100. Examples of statements include “My doctor might recommend that family members undergo genetic testing based on the result of this tumor test”, “The result of my tumor test might not change my treatment.”
2.2.2. Genomic knowledge/understanding
Objective knowledge of genes/genetics was measured with 10 true/false statements based on a published genetic knowledge instrument, with a final score reported as number correct multiplied by 100. 20 Examples of these statements include “It is possible to see a gene with the naked eye”, and “A person's race and ethnicity can affect how likely they are to get a disease”.
2.2.3. Trust in physician/provider (TIPP)
The 11‐item Trust in Physician/Provider Survey 21 uses a 5‐point Likert scale. TPS scores range from 11 to 55 with higher scores indicating greater trust in provider. 21
2.3. Study procedures
All study procedures, including informed consent, survey instruments, and video intervention, were completed through a single REDCap survey via tablet. Both English and Spanish versions of the survey instruments were provided as applicable. Participants were enrolled across three cohorts, with no mandated minimum or maximum number of patients per cohort: Cohort 1. Metastatic breast cancer (MBC); Cohort 2. Lung cancer (LC); Cohort 3. Other cancer of any type (OC). The video viewed by each cohort differed by the modular adaptation applied to the video: OC cohort participants viewed a 3.05‐min video; MBC cohort participants viewed the same video with the addition of a 16‐s clip indicating that at most four out of 10 patients with MBC receive a tumor genomic test result that determines their treatment; LC cohort participants also had an additional 16 s of video content indicating that three out of 10 patients with LC have a tumor genomic test result that determines their treatment. Survey instruments were completed at timepoint 1 (T1), immediately prior to video viewing, including: demographics, genomic knowledge/understanding, video message‐specific knowledge, trust in physician/provider, attitudes around genomic testing, and intentions regarding TGT. None of the knowledge‐related questions were cohort‐specific. With the exception of demographics, all instruments were repeated at timepoint 2 (T2), following video viewing and prior to discussion with the provider of the participant's own results. Additionally, at T2, participants completed an opinion assessment of the video itself. Survey questions are provided in Supplementary File 1.
2.4. Statistical analyses
Only patients who completed all T1 and T2 surveys were included for analyses (see CONSORT diagram Figure S1A). The primary objective was to assess change in video message‐specific knowledge from pre‐ to post‐exposure to the TGT educational video. Secondary endpoints included: (1) change in video message‐specific knowledge within each cohort (MBC, LC, or OC); (2) change in genomic knowledge/understanding in the overall study population and within each cohort; (3) change in Trust in Physician/Provider as a single score in the overall study population and within each cohort. Based on preliminary data, with a cohort of 150 patients, there would be 90% power to detect an effect size of 0.66 in change of recall accuracy from pre‐ (immediately prior) to post‐ (immediately after) video intervention, using a two‐sided Wilcoxon signed‐rank test with alpha of 0.05, corresponding to a large effect size (|r| > 0.5). All secondary outcomes were summarized using descriptive statistics and compared pre‐/post‐video using Wilcoxon signed‐rank test. Evaluation of change in proportion of individuals answering specific questions correctly within video message‐specific knowledge was assessed using McNemar's test for the whole population and within each cohort. The associations of video message‐specific knowledge, genomic knowledge/understanding, and trust in physician/provider with age were explored with Spearman correlations, and the associations of video message‐specific knowledge, genomic knowledge/understanding, and trust in physician/provider with other categorical demographics were explored with Kruskal–Wallis test; given the exploratory nature of these analyses no multiple test correction was used and nominal p‐values were reported.
3. RESULTS
3.1. Study participants
One hundred and fifty participants completed survey instruments at both T1 and T2 and were considered evaluable for the primary endpoint. Participant characteristics at baseline by cohort are provided in Table 1. In the total cohort, participant age ranged from 18 to 93 years at study entry. Most participants were female (94/150; 63%), of White race (132/150, 88%), and were married or in a domestic partnership (102/150, 62%). Education was relatively evenly distributed among high school or less, some college or technical school, and college or graduate degree, while income was similarly relatively evenly distributed from self‐reported annual income less than $25,000 to greater than $75,000 (Table 1). Among participants, eight distinct cancer types were represented, including breast cancer, lung cancer, and gastrointestinal cancer most common (Figure S1C). Genomic testing vendors included Tempus (n = 46), Foundation Medicine (n = 41), Guardant (n = 20), Caris Life Sciences (n = 13), Natera (n = 12), and Ohio State University Molecular Pathology (n = 18) (Figure S1D).
TABLE 1.
Participant characteristics at baseline.
| Variable | Category | MBC Arm (n = 53) | LC Arm (n = 38) | OC Arm (n = 59) | Total (n = 150) |
|---|---|---|---|---|---|
| Age at study entry | Median [IQR] (min, max) | 59 [48, 68] (32, 80) | 61.5 [54, 73] (37, 93) | 63 [48, 70] (18, 80) | 62 [50, 70] (18, 93) |
| Sex | Female | 53 (100%) | 17 (45%) | 24 (41%) | 94 (63%) |
| Male | 0 (0%) | 21 (55%) | 35 (59%) | 56 (37%) | |
| Education | Some high school or less | 2 (4%) | 3 (8%) | 2 (4%) | 7 (5%) |
| High school graduate | 12 (23%) | 11 (29%) | 19 (32%) | 42 (28%) | |
| Some college or technical school | 14 (26%) | 14 (37%) | 15 (25%) | 43 (29%) | |
| College or graduate | 25 (47%) | 10 (26%) | 23 (39%) | 58 (39%) | |
| Income | <$24,999 | 9 (17%) | 4 (11%) | 8 (14%) | 21 (14%) |
| $25,000–$34,999 | 6 (11%) | 2 (5%) | 7 (12%) | 15 (10%) | |
| $35,000–$49,999 | 3 (6%) | 9 (24%) | 3 (5%) | 15 (10%) | |
| $50,000–$74,999 | 7 (13%) | 9 (24%) | 10 (17%) | 26 (17%) | |
| $75,000–$99,999 | 7 (13%) | 6 (16%) | 10 (17%) | 23 (15%) | |
| >$100,000 | 3 (6%) | 0 (0%) | 5 (8%) | 8 (5%) | |
| Prefer not to answer | 4 (8%) | 4 (11%) | 6 (10%) | 14 (9%) | |
| Relationship/marital status | Married or domestic partnership | 35 (66%) | 22 (58%) | 45 (76%) | 102 (68%) |
| Divorced or separated | 11 (21%) | 5 (14%) | 5 (8%) | 21 (14%) | |
| Single, never married | 5 (9%) | 3 (8%) | 7 (12%) | 15 (10%) | |
| Widowed | 2 (4%) | 7 (18%) | 2 (3%) | 11 (7%) | |
| Prefer not to answer | 0 (0%) | 1 (3%) | 0 (0%) | 1 (1%) | |
| Self‐reported race | American Indian or Alaska Native | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Asian | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | |
| Black or African American | 3 (6%) | 4 (11%) | 6 (10%) | 13 (9%) | |
| Hispanic, Latino, or Spanish | 0 (0%) | 0 (0%) | 1 (2%) | 1 (1%) | |
| White | 48 (91%) | 32 (84%) | 52 (88%) | 132 (88%) | |
| Other race or ethnicity | 0 (0%) | 2 (5%) | 0 (0%) | 2 (1%) |
Abbreviations: LC, lung cancer; MBC, metastatic breast cancer; OC, other cancer of any type.
3.2. Video message‐specific knowledge, genomic knowledge and understanding, and trust in physician/provider
For the primary endpoint, there was a significant increase in video message‐specific knowledge score (sum of correct true‐false questions multiplied by 100) from T1 to T2 (median increase (interquartile range/IQR: 10 (0,10) Wilcoxon signed rank p < 0.0001)) (Figure 1A). Concurrently, genomic knowledge/understanding score did not significantly change (Wilcoxon signed rank p = 0.89 with median increase (IQR): 0 (−10, 10); Figure 1B) and trust in physician/provider score did not significantly change (Wilcoxon signed rank p = 0.59 with median increase (IQR): 0 (−1, 1); Figure 1C). There were significant increases in video message‐specific knowledge within each cohort (Wilcoxon signed rank p < 0.0001 MBC; p < 0.0001 LC; p < 0.0001 OC; Figure S2A) with no significant change within any cohort for genomic knowledge/understanding (Figure S2B; all p > 0.05) or trust in physician/provider (Figure S2B; all p > 0.05) (numerical data for Figure S2B provided as Table S2).
FIGURE 1.
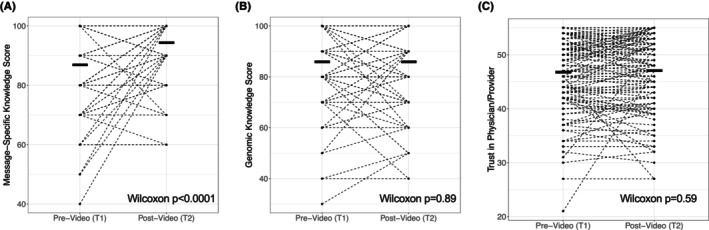
Change in knowledge and trust metrics pre‐ to post‐video. Participants completed survey assessments pre‐video viewing (T1) and post‐video viewing (T2) of the 3–4 min tumor genomic testing educational video intervention. Survey assessments included: 10‐question video message‐specific knowledge with score reported as number correct multiplied by 100 (VMSK; A), 10‐question general genomic knowledge and understanding with score reported as number correct multiplied by 100 (GKU; B), and 11‐question trust in physician/provider (TIPP; C). Paired scores for each participant are presented as dashed lines with mean indicated by thick line, and Wilcoxon signed rank p value in bottom right of each plot. Change in video message‐specific knowledge from T1 to T2 was the primary endpoint of the study.
3.3. Change in individual video message‐specific knowledge questions
To further understand what specific domains of knowledge related to the message changed over time, we evaluate the proportion of patients correctly answering each of the 10 true/false questions in the video message‐specific knowledge survey at T1 versus T2 (Table 2). The proportion correct significantly increased (McNemar's test p < 0.05) after viewing the video for five questions, including one general knowledge question (“We have genes in every cell of our bodies”), questions specifically addressing ASCO/ACMG guidelines (“Tumor tissue genomic results sometimes raise more questions that require more genetic testing”; “When my doctor has my results, they might recommend for me to see a genetics specialist”) and one question addressing cost of TGT (“The expense of TGT is not typically covered by health insurance”). The latter question addressing the cost of TGT demonstrated the greatest change, increasing from 51.3% (77/15) of participants answering correctly pre‐video/T1 to 80.1% (121/150) post‐video/T2, an improvement of 44 individuals answering the question correctly.
TABLE 2.
Change in video message‐specific knowledge responses by individual question.
| Video message‐specific question True or False | Addresses ASCO/ACMG guidelines | Participants correct at T1 # % | Participants correct at T2 # % | T1:T2 change | p Value | ||
|---|---|---|---|---|---|---|---|
| “We have genes in every cell of our bodies.” | N | 139 | 92.7 | 150 | 100 | +11 | 0.0009 |
| “TGT might help your doctor make decisions about your cancer treatment.” | Y | 149 | 99.3 | 150 | 100 | +1 | 0.3173 |
| “TGT always determines what treatment a person will have.” | Y | 115 | 76.7 | 130 | 86.7 | +15 | 0.0137 |
| “I must have TGT to continue with cancer treatment.” | Y | 133 | 88.7 | 138 | 92.0 | +5 | 0.2253 |
| “My doctor has other tools besides TGT to use to choose treatments for me.” | Y | 144 | 96.0 | 145 | 96.7 | +1 | 0.7630 |
| “Tumor tissue genomic results sometimes raise more questions that require more genetic testing.” | Y | 128 | 85.3 | 145 | 96.7 | +17 | 0.0011 |
| “The information that I get from tumor tissue genomic testing could be valuable to my children and other family members.” | Y | 145 | 96.7 | 149 | 99.3 | +4 | 0.1025 |
| “When my doctor has my results, they might recommend for me to see a genetics specialist.” | Y | 131 | 87.3 | 147 | 98.0 | +13 | 0.0006 |
| “The expense of TGT is not typically covered by health insurance.” | N | 77 | 51.3 | 121 | 80.1 | +44 | <0.0001 |
| “If you do not have health insurance, you cannot have TGT performed.” | N | 142 | 94.7 | 140 | 93.3 | ‐2 | 0.5930 |
Abbreviations: ACMG, American College of Medical Genetics; ASCO, American Society of Clinical Oncology; T1, time point 1 (pre‐video); T2, time point 2 (post‐video).
Bold indicates p < 0.05.
3.4. Association of patient characteristics with video message‐specific knowledge, genomic knowledge/understanding, and trust in physician/provider
As an exploratory objective, the association of patient characteristics with baseline video message‐specific knowledge, genomic knowledge/understanding, and Trust in Physician/Provider were assessed via Spearman correlation (Table 3). There was a significant association between education and baseline/T1 video message‐specific knowledge (nominal p < 0.0001) and baseline/T2 genomic knowledge/understanding (nominal p = 0.02) as well as income and baseline/T1 video message‐specific knowledge (nominal p = 0.002). There were no other significant associations between other patient characteristics and video message‐specific knowledge/genomic knowledge/understanding or any patient characteristics and Trust in Physician/Provider. To further evaluate these associations, we explored change in video message‐specific knowledge and genomic knowledge/understanding within individual patient characteristics; no formal statistical assessment was performed for these exploratory analyses. Among individuals with educational attainment of less than a college degree (some high school, high school completion, some college, or technical school; n = 92) there was a greater numerical increase in video message‐specific knowledge (mean 83.0–92.5) than among those with a college or graduate degree (mean 92.9–97.2) (Figure S3A). When evaluating age, there were similar increases in video message‐specific knowledge among those under age 70 at study entry (mean 87.5–93.9) and over age 70 at study entry (mean 85.0–95.5) suggesting similar knowledge receipt across the age continuum (Figure S3B). Evaluating income above/below $75,000 per year (Figure S3C) and race white/non‐white (Figure S3D) largely mirrored the overall population with numerical increase in video message‐specific knowledge but no change in genomic knowledge/understanding.
TABLE 3.
Association of participant characteristics with baseline survey metrics.
| Instrument | Demographic parameter | Breast p value* | Lung p value* | Agnostic p value* | Overall p value* |
|---|---|---|---|---|---|
| Video message‐specific knowledge at T1 | Age at entry | 0.72 | 0.09 | 0.96 | 0.14 |
| Sex | NA | 0.55 | 0.88 | 0.36 | |
| Education | 0.014 | 0.057 | 0.052 | <0.0001 | |
| Income | 0.22 | 0.10 | 0.083 | 0.002 | |
| Marital status | 0.83 | 0.089 | 0.95 | 0.54 | |
| Race | 0.40 | 0.63 | 0.045 | 0.16 | |
| Genomic knowledge and understanding at T1 | Age at entry | 0.63 | 0.74 | 0.74 | 0.78 |
| Sex | NA | 0.71 | 0.32 | 0.57 | |
| Education | 0.093 | 0.25 | 0.35 | 0.02 | |
| Income | 0.028 | 0.56 | 0.63 | 0.10 | |
| Marital status | 0.79 | 0.21 | 0.28 | 0.88 | |
| Race | 0.92 | 0.68 | 0.032 | 0.62 | |
| Trust in physician provider at T1 | Age at entry | 0.98 | 0.39 | 0.18 | 0.90 |
| Sex | NA | 0.41 | 0.15 | 0.55 | |
| Education | 0.46 | 0.28 | 0.15 | 0.33 | |
| Income | 0.30 | 0.77 | 0.14 | 0.42 | |
| Marital status | 0.68 | 0.17 | 0.48 | 0.49 | |
| Race | 0.35 | 0.09 | 0.40 | 0.45 |
Note: The associations of VMSK, GKU, and TIPP with age were explored with Spearman correlations. The associations of VMSK, GKU, and TIPP with other categorical demographics were explored with Kruskal–Wallis test.
Abbreviation: T1, time point 1 (pre‐video).
Nominal p value reported.
Bold indicates p < 0.05.
3.5. Patient assessment of and attitudes toward tumor genomic testing
In addition to objective metrics, participants also completed 10 descriptive questions assessing their perceived knowledge and knowledge insufficiency around TGT and perceptions of TGT, and an eight‐question assessment of the video itself. These variables are perceived knowledge and knowledge insufficiency, which are from the Risk Information Seeking and Processing Model. 22 Participants reported their perceived knowledge of TGT on a scale from zero to 10, with 10 meaning knowing everything that there was to know about TGT. Using the same scale, they also indicated how much they felt that they needed to know. There was a significant increase in participants' perceived knowledge (mean T1 2.7–T2 4.8; t‐test p < 0.001) while need for knowledge did not significantly increase (mean T1 5.8–T2 6.2; t‐test p = 0.23) (Figure S4A). As an attitudinal measure of participants' perception of TGT, patients responded to the statement “My having tumor genomic testing would have…” on a scale from negative three to three, where negative three indicated “a lot more negatives than positives” and three indicated “a lot more positives than negatives”, with zero representing an equivalent number of negatives and positives (Figure S4B). Average response value at T2 increased from 2.1 to 2.4, with 17% of participants having a more positive response.
To evaluate the clarity of the video message being delivered and to ensure that survey respondents had indeed viewed the video (manipulation check), participants were simply asked “what was the video about?” 148 of 150 respondents correctly identified that it was about tumor genomic testing, two respondents chose the option “screening for cancer,” both of which were considered acceptable. A Likert scale of agreement was used to measure participant's opinions of the video's utility, clarity, and engagement (Figure S4C), and most (>80%) of respondents agreed or strongly agreed that the video was helpful, easy to understand, and held their attention, with fewer but still most (60%) indicating that the graphics were helpful. Most participants (141 of 150) felt that the amount of information presented was adequate, while four participants felt the content was excessive, and five participants found the content insufficient.
4. DISCUSSION
TGT has increasingly become standard‐of‐care for most patients with advanced cancer and select patients with early‐stage cancer (including lung cancer). As TGT usage increases, there is an increasing burden on medical oncology providers to provide counseling, which is particularly challenging in community settings where access to genomic experts and genetic counseling may be more limited. 23 Further, there is significant variability in how frequently providers educate patients prior to TGT and the content of that education. 19 , 24 , 25 , 26 , 27 Despite consensus across international oncology and medical genetics societies around patient education prior to TGT, 3 , 4 , 5 , 6 , 7 , 8 , 9 few strategies have emerged that address key guidelines while also being feasible within the flow of busy oncology clinical settings. To address this critical gap, we demonstrate that our concise, 3–4 min animated video, now publicly released for widespread use, effectively conveys key information in an efficient timeframe to provide patient education prior to TGT.
Our results demonstrate a significant improvement in video message‐specific knowledge consistent across cohorts, with >80% of participants correctly answering the 10 video message‐specific knowledge questions after video viewing. As an internal control, genomic knowledge/understanding did not significantly change suggesting that knowledge gained was specifically related to the video. While individuals had a good overall baseline message‐specific knowledge, the ideal would be perfect knowledge prior to TGT and significant gaps improved with video viewing. Further, most of the improvement in video message‐specific knowledge score related to five questions encompassing diverse themes. The significant increase in the video message‐specific knowledge score indicates that the video is educating study participants beyond any discussion that participants had or may have had with their providers regarding TGT. Notably, there was no significant difference between the scores for all participants and the scores within each cohort. These primary outcome results are further substantiated by participants' self‐report of knowledge gain and a narrowing between the knowledge participants desired to have and the knowledge participants felt they needed to be effective partners in their own care.
Videos have been recognized as an effective form of patient education decision‐making tools, including specifically around genomic analyses. 28 , 29 , 30 The differences between more comprehensive pre‐TGT education (e.g., the Multimodality COMET eHealth Education Intervention) 31 and concise approaches such as this video 19 reflect a challenging balance between ensuring adequate content but also facilitating delivery within clinic flow. Shared decision‐making encompasses the need for patients to discuss their concerns with family members and supporters 28 , 32 and this video can be directly shared with family and friends with no need for the patient to synthesize information before sharing it with support persons. This video intervention clearly enhanced knowledge surrounding TGT as a publicly available resource.
In prior work, we had found that patients who underwent TGT without therapy change (representing the majority of patients) lost confidence in treatment. 33 Stronger patient‐provider therapeutic alliance results in improved adherence to therapy, 34 caregiver coping, 35 and cancer outcomes. 36 We did not see significant change in a ‘trust in physician/provider’ metric. 21 We hypothesize that TIPP may not change over the narrow time frame of pre‐/post‐video but may become evident later in the treatment course. We plan to assess TIPP again at 60–90 days post‐video. We did not specifically investigate other patient‐centered outcomes as our previous prospective decision analysis study demonstrated that validated metrics of depression scale (CES‐D), 37 anxiety (BAI), 38 and self‐efficacy (CASE‐cancer) 39 did not significantly change after TGT. This was similar to the results of the ECOG‐ACRIN NCI Community Oncology Research Program EAQ152 study, a randomized trial of web‐based genetic education versus usual care in advanced cancer patients undergoing tumor genetic testing, that found that a web‐based video intervention increased patient understanding but did not significantly reduce anxiety, depression, or cancer‐specific distress. 31 Future research should focus on defining the educational goal or PRO outcome regarding TGT which may include improving knowledge, shared decision‐making, reducing anxiety/distress, increasing use of genomically directed treatments, or some combination.
Patients with lower socioeconomic status and education have less understanding of genetic/genomic testing, emphasizing the need to equitably address patient understanding prior to TGT. 34 Less knowledge and confidence have been associated with lower rates of TGT and therefore less opportunity for TGT‐directed guideline concordant care. 23 Having a standardized video, using plain language to accommodate learners across health and genetic literacy levels, could result in more equitable enumeration of TGT benefits, limitations, possible outcomes, and risks. The exploratory analyses here demonstrated improvement in VMSK scores across demographics including income, education, and age with numerical narrowing of baseline knowledge gaps which may translate to more informed care and greater patient empowerment.
5. LIMITATIONS
Our racial demographic minority subsets were not robust enough to allow for meaningful data analysis independently; therefore, the 18 participants identifying as a race other than white were consolidated into one non‐white category. This is not ideal and did not reveal the disparity in genomic knowledge that was seen in previous work. 32 While a direct translation Spanish language video was available, only one Spanish‐speaking participant was accrued, thus no generalizations regarding the quality or effectiveness of the translated video can be made. This study was conducted at a single tertiary academic cancer center in a large city. Because provider genomics knowledge varies by setting, 40 we have expanded implementation of the intervention to community cancer centers. Overall, participants' opinions of the video affirmed that length and content were sufficient; however, a gap between the T1 need‐for‐knowledge response average and the T2 metric average suggested that patients' may in fact desire more knowledge. We are currently collecting data on T3 (60–90 days post‐TGT) which will be reported in the future to evaluate retention of knowledge.
6. CONCLUSIONS
This novel TGT video intervention is an effective tool to augment the provider‐patient discussion of TGT. It narrows the gap in equitable access to informed health care across several demographics: age, education, and income, but we have not demonstrated a substantial improvement in equitability across race and ethnicity. This video may be a valuable resource to facilitate awareness, enhance dialog, extend information from the clinic to the home setting, and aid in the shared decision‐making that is fundamental to patient‐centered care.
AUTHOR CONTRIBUTIONS
Deloris J. Veney: Data curation (supporting); investigation (equal); methodology (equal); project administration (equal); supervision (equal); writing – original draft (supporting); writing – review and editing (supporting). Lai Y. Wei: Conceptualization (equal); formal analysis (lead); investigation (equal); methodology (equal); writing – review and editing (supporting). Amanda E. Toland: Conceptualization (supporting); writing – review and editing (supporting). Carolyn J. Presley: Conceptualization (supporting); investigation (supporting); methodology (supporting); project administration (supporting); supervision (supporting); writing – review and editing (supporting). Heather L. Hampel: Conceptualization (supporting); methodology (supporting); writing – review and editing (supporting). Tasleem J. Padamsee: Conceptualization (supporting); writing – review and editing (supporting). Clara N. Lee: Conceptualization (supporting); methodology (supporting); writing – review and editing (supporting). William J. Irvin Jr: Investigation (supporting); resources (supporting); writing – review and editing (supporting). Michael J. Bishop: Investigation (supporting); project administration (supporting); writing – review and editing (supporting). James J. Kim: Investigation (supporting); project administration (supporting); writing – review and editing (supporting). Shelly R. Hovick: Conceptualization (equal); formal analysis (supporting); funding acquisition (equal); investigation (supporting); methodology (equal); project administration (supporting); resources (equal); writing – review and editing (equal). Leigha A. Senter: Conceptualization (equal); data curation (supporting); formal analysis (supporting); funding acquisition (equal); investigation (supporting); methodology (equal); project administration (supporting); resources (equal); supervision (supporting); writing – review and editing (supporting). Daniel G. Stover: Resources (equal); supervision (lead); visualization (lead); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
HH is on the scientific advisory board for Invitae Genetics, Genome Medical, and Promega. She has stock/stock options in Genome Medical and GI OnDemand. LS is a consultant and speaker for AstraZeneca. DS served on an advisory board for Novartis. CJP received payment for patient education material development through Jazz Pharmaceuticals.
ETHICS STATEMENT
The study protocol, OSU#2021C0209, was approved by the Ohio State University Institutional Review Board.
Supporting information
Figures S1–S4.
Table S1.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grant 1R21CA259985 (LW, LS, SRH, HLH, AET, CJP, and DGS), P30CA016058 (LW, TS, and AET), 1R01 CA215151 (AET), CJP is supported by 1K76AG074923‐01.
Veney DJ, Wei LY, Toland AE, et al. A video intervention to improve patient understanding of tumor genomic testing in patients with cancer. Cancer Med. 2024;13:e70095. doi: 10.1002/cam4.70095
Senter, MS and Daniel G. Stover co‐directed this work equally.
Clinical trial registration: ClinicalTrials.gov NCT05215769.
DATA AVAILABILITY STATEMENT
Raw data were generated at The Ohio State University. Derived data supporting the findings of this study are available from the corresponding author, DS, on request.
REFERENCES
- 1. Stover DG, Wagle N. Precision medicine in breast cancer: genes, genomes, and the future of genomically driven treatments. Curr Oncol Rep. 2015;17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakravarty D, Solit DB. Clinical cancer genomic profiling. Nat Rev Genet. 2021;22:483‐501. [DOI] [PubMed] [Google Scholar]
- 3. Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660‐3667. [DOI] [PubMed] [Google Scholar]
- 4. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15:465‐473. [DOI] [PubMed] [Google Scholar]
- 5. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high‐risk assessment: breast and ovarian, version 2.2017. J Natl Compr Cancer Netw. 2017;15:9‐20. [DOI] [PubMed] [Google Scholar]
- 6. Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciardiello F, Arnold D, Casali PG, et al. Delivering precision medicine in oncology today and in future—the promise and challenges of personalised cancer medicine: a position paper by the European Society for Medical Oncology (ESMO). Ann Oncol. 2014;25:1673‐1678. [DOI] [PubMed] [Google Scholar]
- 9. Bayle A, Bonastre J, Chaltiel D, et al. ESMO study on the availability and accessibility of biomolecular technologies in oncology in Europe. Ann Oncol. 2023;34(10):934‐945. [DOI] [PubMed] [Google Scholar]
- 10. Mandelker D, Donoghue M, Talukdar S, et al. Germline‐focussed analysis of tumour‐only sequencing: recommendations from the ESMO precision medicine working group. Ann Oncol. 2019;30:1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yabroff KR, Zhao J, de Moor JS, et al. Factors associated with oncologist discussions of the costs of genomic testing and related treatments. J Natl Cancer Inst. 2019;112:498‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pentz RD, Pocock RH, Pinheiro AM, Switchenko JM, Dixon MD. Physician communication and patient understanding of molecular testing of tumors. J Clin Oncol. 2017;35:e18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lea DH, Kaphingst KA, Bowen D, Lipkus I, Hadley DW. Communicating genetic and genomic information: health literacy and numeracy considerations. Public Health Genomics. 2011;14:279‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaphingst KA, Blanchard M, Milam L, Pokharel M, Elrick A, Goodman MS. Relationships between health literacy and genomics‐related knowledge, self‐efficacy, perceived importance, and communication in a medically underserved population. J Health Commun. 2016;21:58‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meric‐Bernstam F, Brusco L, Daniels M, et al. Incidental germline variants in 1000 advanced cancers on a prospective somatic genomic profiling protocol. Ann Oncol. 2016;27:795‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yusuf RA, Rogith D, Hovick SR, et al. Attitudes toward molecular testing for personalized cancer therapy. Cancer. 2015;121:243‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marron JM, Joffe S. Ethical considerations in genomic testing for hematologic disorders. Blood. 2017;130:460‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Senter L, Veney D, Surplus T, et al. Patient understanding of tumor genomic testing: a quality improvement effort. JCO Oncol Pract. 2023;19:e8‐e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fitzgerald‐Butt SM, Bodine A, Fry KM, et al. Measuring genetic knowledge: a brief survey instrument for adolescents and adults. Clin Genet. 2016;89:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson LA, Dedrick RF. Development of the Trust in Physician scale: a measure to assess interpersonal trust in patient‐physician relationships. Psychol Rep. 1990;67:1091‐1100. [DOI] [PubMed] [Google Scholar]
- 22. Griffin RJ, Dunwoody S, Neuwirth K. Proposed model of the relationship of risk information seeking and processing to the development of preventive behaviors. Environ Res. 1999;80:S230‐S245. [DOI] [PubMed] [Google Scholar]
- 23. Demeshko A, Pennisi DJ, Narayan S, Gray SW, Brown MA, McInerney‐Leo AM. Factors influencing cancer genetic somatic mutation test ordering by cancer physician. J Transl Med. 2020;18:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith‐Uffen M, Bartley N, Davies G, Best M. Motivations and barriers to pursue cancer genomic testing: a systematic review. Patient Educ Couns. 2021;104:1325‐1334. [DOI] [PubMed] [Google Scholar]
- 25. Blanchette PS, Spreafico A, Miller FA, et al. Genomic testing in cancer: patient knowledge, attitudes, and expectations. Cancer. 2014;120:3066‐3073. [DOI] [PubMed] [Google Scholar]
- 26. DiBiase JF, Scharnetzki E, Edelman E, et al. Urban‐rural and socioeconomic differences in patient knowledge and perceptions of genomic tumor testing. JCO Precis Oncol. 2023;7:e2200631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fenton AT, Anderson EC, Scharnetzki E, et al. Differences in cancer patients' and clinicians' preferences for disclosure of uncertain genomic tumor testing results. Patient Educ Couns. 2021;104:3‐11. [DOI] [PubMed] [Google Scholar]
- 28. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanderson SC, Suckiel SA, Zweig M, Bottinger EP, Jabs EW, Richardson LD. Development and preliminary evaluation of an online educational video about whole‐genome sequencing for research participants, patients, and the general public. Genet Med. 2016;18:501‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hyman DM, Solit DB, Arcila ME, et al. Precision medicine at memorial Sloan Kettering cancer center: clinical next‐generation sequencing enabling next‐generation targeted therapy trials. Drug Discov Today. 2015;20:1422‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bradbury AR, Lee J‐W, Gaieski JB, et al. A randomized study of genetic education versus usual care in tumor profiling for advanced cancer in the ECOG‐ACRIN cancer research group (EAQ152). Cancer. 2022;128:1381‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adams EJ, Asad S, Reinbolt R, et al. Metastatic breast cancer patient perceptions of somatic tumor genomic testing. BMC Cancer. 2020;20:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stover DG, Reinbolt RE, Adams EJ, et al. Prospective decision analysis study of clinical genomic testing in metastatic breast cancer: impact on outcomes and patient perceptions. JCO Precis Oncol. 2019;3:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trevino KM, Fasciano K, Prigerson HG. Patient‐oncologist alliance, psychosocial well‐being, and treatment adherence among young adults with advanced cancer. J Clin Oncol. 2013;31:1683‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trevino KM, Maciejewski PK, Epstein AS, Prigerson HG. The lasting impact of the therapeutic alliance: patient‐oncologist alliance as a predictor of caregiver bereavement adjustment. Cancer. 2015;121:3534‐3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the human connection scale. Cancer. 2009;115:3302‐3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385‐401. [Google Scholar]
- 38. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893‐897. [DOI] [PubMed] [Google Scholar]
- 39. Wolf MS, Chang CH, Davis T, Makoul G. Development and validation of the communication and Attudinal self‐efficacy scale for cancer (CASE‐cancer). Patient Educ Couns. 2005;57:333‐341. [DOI] [PubMed] [Google Scholar]
- 40. Chow‐White P, Ha D, Laskin J. Knowledge, attitudes, and values among physicians working with clinical genomics: a survey of medical oncologists. Hum Resour Health. 2017;15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S4.
Table S1.
Data Availability Statement
Raw data were generated at The Ohio State University. Derived data supporting the findings of this study are available from the corresponding author, DS, on request.


