Abstract
ADP-ribosylation (ADPRylation) is a post-translational modification (PTM) of proteins mediated by the activity of a variety of ADP-ribosyltransferase (ART) enzymes, such as the Poly (ADP-ribose) Polymerase (PARP) family of proteins. This PTM is diverse in both form and biological functions, which makes it a highly interesting modification, but difficult to study due to limitations in reagents available to detect the diversity of ADPRylation. Recently we developed a set of recombinant antibody-like ADP-ribose (ADPR) binding proteins using naturally occurring ADPR binding domains (ARBDs), including macrodomains and WWE domains, functionalized by fusion to the constant “Fc” region of rabbit immunoglobulin. Herein, we present an expansion of this biological toolkit, where we have replaced the rabbit Fc sequence with the sequence from two other species, mouse and goat. These new reagents are based on a previously characterized set of naturally occurring ARBDs with known specificity. Characterization of the new reagents demonstrates that they can be detected in a species-dependent manner by secondary immunological tools, recognize specific ADPR moieties, and can be used for simultaneous detection of mono ADPR and poly ADPR at single-cell resolution in various antibody-based assays. The expansion of this toolkit will allow for more multiplexed assessments of the complexity of ADPRylation biology in many biological systems.
Keywords: MARylation, PARylation, ADP-ribose, ADP-ribosylation, PARP, immunofluorescent staining
In recent years, there has been a substantial increase in our understanding of the biochemistry, molecular biology, normal physiology, and pathology of ADP-ribosylation (ADPRylation) of proteins (1, 2, 3). It is clear that this complex post-translational modification (PTM) plays important roles in many biological processes, including DNA repair, transcription, immune regulation, and condensate formation (among many others). This PTM is mediated by a variety of ADP-ribosyltransferase (ART) enzymes, including the Poly (ADP-ribose) Polymerase (PARP) family of proteins (with the exception of non-catalytic members). These enzymes mediate the transfer of ADP-ribose (ADPR) from nicotinamide adenine dinucleotide (NAD+) to a surprisingly diverse set of substrate proteins, on a wide range of amino acids. The substrates can be modified by ADPR chains of varying lengths and chemical structures, i.e. by either a single ADPR unit (monoADPRylation or mono ADPR [MAR]) or polymers of ADPR units (polyADPRylation or poly ADPR [PAR]), which serve to alter biochemical activities of the substrate protein or drive protein-protein interactions through new interaction surfaces (1, 2, 3, 4, 5).
ADPRylation “reader” proteins also exist in nature, with modules that can specifically recognize and bind to various forms of ADPR moieties. Some of the most well-characterized ADPR binding domains (ARBDs) include WWE domains, which recognize PAR (and oligo chains), and macrodomains, which recognize MAR or terminal ADPR moieties found in PAR (and oligo chains) (1, 3, 4, 6). Recently we developed a set of recombinant antibody-like ADPR binding proteins, using naturally occurring macrodomains and WWE domains, that were functionalized by fusion to the constant “Fc” region of rabbit immunoglobulin. These ARBD-Fc fusion proteins represent one example of tools developed for the detection of ADPR moieties. Our in vitro testing confirmed that PARP14 macrodomain 2/3 (Macro2/3) is selective for MAR by recognizing ADPR linked to the modified amino acid or surrounding protein sequence. The RNF146 WWE domain is selective for PAR by recognizing linkages between ADPR monomers, while the AF1521 macrodomain can detect both MAR and PAR by recognizing terminal ADPR units. By treating samples in solution or on membranes with hydroxylamine, which cleaves ADPR from acidic residues (e.g., glutamate and aspartate), we were able to determine that the bulk of the signal detected with these three reagents is from ADPRylated glutamate or aspartate residues (4). This was further confirmed with the PARP14 Macro2/3-based reagent, where depletion of the ADP-ribosyl hydrolase TARG1, which can specifically remove glutamate- or aspartate-linked ADPR, demonstrated specificity for these two amino acid residues (7).
Other examples of tools for the detection of ADPR include the development of monoclonal (8) and polyclonal (9, 10, 11, 12, 13, 14, 15, 16) antibodies to detect MAR and PAR, SpyTag-based modular antibodies to detect MAR (17, 18), and the use of the hydrolases ARH3 and TARG1 as tools to investigate ADPRylation specifically on serine and glutamate residues, respectively (19, 20). These reagents have all proven useful research tools in the field for the molecular recognition of various forms of ADPR (4), but significant limitations still exist. In particular, the distinct signaling mediated by MAR and PAR, the specific subcellular localization of PAR (nuclear) versus MAR (cytoplasmic), and their functional interplay represent a gap in our current knowledge. Therefore, given the complex nature of this modification, there is a need for the continued development of tools and refinement of existing ones, which will undoubtedly enhance our understanding of the intricate biological functions of ADPRylation.
Herein, we describe the generation and characterization of an expanded set of antibody-like ADPR binding proteins previously reported (4), in which these natural and specific ARBDs have been functionalized with the Fc region of mouse and goat immunoglobulin. This creates a more useful array of ADPR detection reagents that enables simultaneous detection of MAR and PAR at single-cell resolution (e.g., in immunofluorescent staining assays) to address the functional interplay between MAR and PAR signaling. Characterization of the new reagents indicates that they can be detected in a species-dependent manner, recognize specific ADPR moieties, and excitingly, can be used in various antibody-based assays by co-staining. The expansion of this tool will allow for more multiplexed assessments of the complexity of ADPRylation biology in many biological systems.
Results
Expression and purification of new recombinant mouse and goat ARBD-Fc fusion proteins
We previously described the generation of Fc fusion proteins using the macrodomain from H. sapiens PARP14 (Macro2/3), A. fulgidus AF1521, and WWE domain from H. sapiens RNF146, fused to the Fc region of rabbit IgG (4). We constructed six new vectors by replacing the rabbit-Fc with cDNA from mouse and goat Fc (Figs. 1A, S1 and S2). We expressed the ARBD-Fc fusion proteins in E. coli and purified them using Ni-NTA affinity chromatography as previously described (Tables S1 and S2). Purified proteins were analyzed by denaturing SDS-PAGE and stained with Coomassie (Fig. 1B; left panel). All fusion proteins migrated at the expected molecular weights (Fig. 1B; right panel). We observed that the new mouse and goat preparations of WWE had high purity, whereas some contaminating bands were present in the Macro2/3 (PARP14) and AF1521 preps, with a major contaminating band at ∼30 kDa, which did not seem to affect detection (Fig. 2A).
Figure 1.
Design, expression, and purification of new ARBD-Fc detection reagents.A, schematic diagram of the plasmid constructs used to express the ADPR binding domain-Fc (ARBD-Fc) fusion proteins in bacteria. The constructs contain DNA segments encoding (1) 10× His tag (blue), (2) an ADPR binding domain [Macro2/3 (PARP14; M2/3), Macro (AF1521), or WWE (RNF146); green] that detects a specific ADPR moiety, and (3) IgG constant fragment (rabbit, mouse or goat Fc; purple) that allows for assay detection. B, expression and purification of ADPR binding domain-Fc (ARBD-Fc) fusion proteins. ARBD-Fc fusion proteins were expressed in E. coli and purified using Ni-NTA agarose affinity resin. The purified proteins were separated by SDS-PAGE and stained with Coomassie brilliant blue (left panel). The asterisks indicate protein bands with the expected molecular weights of the ARBD-Fc fusion proteins. Molecular weight markers in kilodaltons (kDa) are indicated. The table is a list of 9 ARBD-Fc fusion proteins and their expected molecular weights (right panel). ADPR, ADP-ribose; Macro2/3, macrodomain 2/3; MAR, mono ADPR; PAR, poly ADPR.
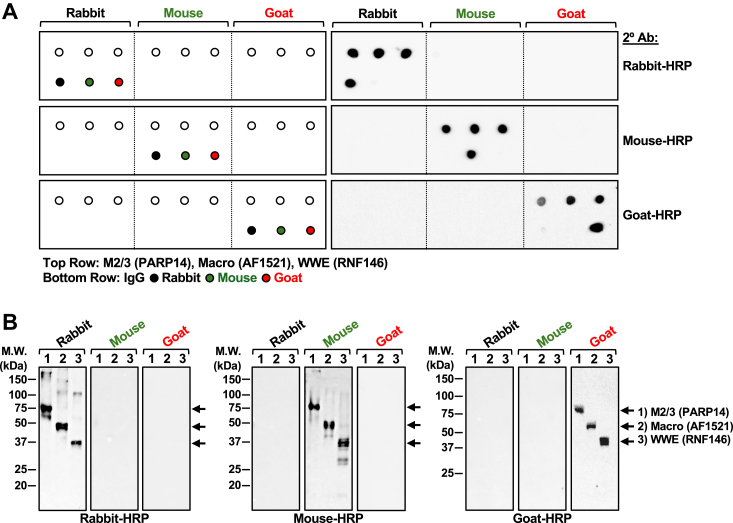
Figure 2.
Fc species-specificity of new ARBD-detection reagents.A, schematic of dot blot (left panel) and immunoblot analyses (right panel) of species specificity of rabbit, mouse, and goat Fc using ARBD-Fc fusion proteins. Nine purified ARBD-Fc fusion proteins, rabbit, mouse, and goat IgG protein were applied to a nitrocellulose membrane for dot blotting (left panel). Each spot contained approximately 500 ng of protein and was blotted using anti-rabbit, anti-mouse, and anti-goat HRP-conjugated antibodies, as indicated (right panel). B, immunoblot analyses of nine purified ARBD-Fc fusion proteins separated by SDS-PAGE, transferred to a nitrocellulose membrane, and subjected to immunoblotting using the anti-rabbit, anti-mouse, and anti-goat HRP-conjugated antibody, as indicated. Molecular weight markers in kilodaltons (kDa) are indicated.
ARBD-Fc fusion proteins can be detected in a species-specific manner
To test the ability of the new ARBD-Fc fusion proteins to be detected in a species-specific manner, we carried out dot blots using equal amounts of all nine purified fusion proteins and IgG from rabbit, mouse, and goat as controls (Fig. 2A; left panel), or SDS-PAGE analysis (Fig. 2B). Using species-specific HRP-conjugated secondary antibodies for detection of proteins, we show that each species of ARBD-Fc fusion proteins is detected exclusively by a single corresponding secondary antibody, indicating highly specific detection (Fig. 2, A and B).
ARBD-Fc fusion proteins can recognize specific forms of ADPR
To test the binding of the new ARBD-Fc fusion proteins and their ability to recognize exact forms of ADPR, we performed in vitro ADPRylation reactions to generate MAR or PAR chains. As previously described, purified recombinant PARP3 or PARP1 were used in biochemical reactions with NAD+ (ADPR donor) and sonicated salmon sperm DNA (activator) to generate automodified PARP3 (MAR) and PARP1 (PAR). Reactions lacking NAD+ were used as a control. As expected, in each species version, Macro2/3 (PARP14) was able to detect only MAR, WWE was able to detect PAR, and AF1521 was able to detect both forms of ADPR (Fig. 3A). These results confirm that each domain can detect a specific form of ADPR and exchanging the Fc region did not affect this detection.
Figure 3.
ADPRylation specificity of new ARBD-detection reagents.A, immunoblot of mono and poly ADPRylated PARP proteins using ARBD-Fc fusion proteins for detection. Purified recombinant PARP1 or PARP3 were incubated with or without NAD+ to promote auto ADPRylation. The yield was mono ADPRylated PARP3 (lane 2; blue), or poly ADPRylated PARP1 (lane 4; orange). The mono and poly ADPRylated PARP proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and subjected to immunoblotting using the nine ARBD-Fc fusion proteins, as indicated. Samples were diluted so that each lane contained approximately the same number of terminal ADPR units, to our best approximation. Molecular weight markers in kilodaltons (kDa) are indicated. B, immunoblot analyses of ADPRylation in 3T3-L1 mouse cells using ARBD-Fc fusion proteins. Cytosolic extracts were prepared from 3T3-L1 cells grown in culture and differentiated as described previously (Day 0/Undifferentiated; Day 3/Differentiated). Equal total protein levels were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and subjected to immunoblotting using the 3 Fc-species versions of Macro2/3 (PARP14)-Fc. Various unidentified MARylated proteins are detected (laddering pattern). Molecular weight markers in kilodaltons (kDa) are indicated. C, immunoblot analyses of ADPRylation in OVCAR3 human ovarian cancer cells using ARBD-Fc fusion proteins. Whole-cell extracts were prepared from OVCAR3 cells grown in culture following treatment with DMSO as control (Ctrl) or with 20 μM PARP inhibitor, nirparib. Equal total protein levels were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and subjected to immunoblotting using the 3 Fc-species versions of AF1521-Fc. Auto-PARylation of PARP1 is the major band detected. Molecular weight markers in kilodaltons (kDa) are indicated. D, immunoblot analyses of ADPRylation in AC16 human cardiomyocyte cells using ARBD-Fc fusion proteins. Whole cell extracts were prepared from AC16 cells grown in culture without any treatment or following treatment with 5 mM H2O2 and 10 μM PARP inhibitor, olaparib. Equal total protein levels were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and subjected to immunoblotting using the 3 Fc-species versions of WWE-Fc. Auto-PARylation of PARP1 is the major band detected at ∼110 kDa. The identity of lower ∼75 kDa PARylated protein is unknown. Molecular weight markers in kilodaltons (kDa) are indicated. ADPR, ADP-ribose; ADPRylation, ADP-ribosylation; Macro2/3, macrodomain 2/3.
To explore the utility of the new reagents in biological systems, we performed immunoblotting in three different cell-based models. First, we analyzed mono ADPRylation in 3T3-L1 mouse cells grown in culture and differentiated as previously described (21). Using the Macro2/3 (PARP14) detection reagent, we observe an increase in MARylation of various unidentified proteins upon differentiation with all three species versions (Day 0 vs Day 3; Fig. 3B; Table S3). Second, we analyzed poly ADPRylation in the OVCAR3 human ovarian cancer cell line treated in culture with and without the PARP inhibitor (PARPi), niraparib. Using the AF1521 detection reagent, we observe a clear reduction in auto-PARylation of the PARP1 signal upon treatment with PARPi (Fig. 3C). Third, we analyzed poly ADPRylation in AC16 human cardiomyocytes treated in culture with and without H2O2 and the PARPi, olaparib. Using the WWE detection reagent, we observe a clear increase in auto-PARylation of PARP1 (major band at ∼110 kDa) upon treatment with H2O2, which can be abrogated by pretreatment with PARPi (Fig. 3D). These results indicate that the detection reagents can recognize the expected specific forms of ADPR.
Expansion of species-specific ARBD-Fc fusion proteins allows for multiplexing capabilities
Given the reagent limitations in the field of PARP biology, we set out to create tools that would enable simultaneous detection of mono and poly ADPR and help tease out complexities of ADPR biology in biological systems. Thus, we tested various co-stains and assayed them by fluorescent immunocytochemistry and dual-color fluorescent Western blotting (Table S4). First, using OVCAR3 cell line treated with and without PARPi (veliparib or nirparib, as indicated), we co-stained to examine levels of PARylation and MARylation in cells by fluorescent immunocytochemistry. We observe a clear reduction in nuclear PARylation upon PARPi treatment, while cytosolic MARylation remained largely unchanged (Fig. 4, A and B). The same subcellular staining patterns for MAR and PAR were observed regardless of the fluorophore (Fig. 4A: PAR = green, MAR = red; Fig. 4B: PAR = red, MAR = green). The use of two different secondary fluorophores highlights the interchangeability and utility of the reagents. We also used 3T3-L1 cells in a differentiation protocol. We observe PARylation decrease, while MARylation increases, in response to differentiation signals (Fig. 4C).
Figure 4.
Testing new ARBD-detection reagents for fluorescent immunocytochemistry.A and B, immunofluorescent staining of ADPRylation in OVCAR3 cells using ARBD-Fc fusion proteins. OVCAR3 cells grown in chamber slides were treated with DMSO or PARP inhibitors veliparib (A) or niraparib (B), as indicated. Following treatment, the cells were fixed with paraformaldehyde and co-immunostained for ADPR using ARBD-Fc fusion proteins: Poly ADPRylation (WWE-rabbit) and Mono ADPRylation [Macro2/3 (PARP14)-mouse]. For each antibody, (r) and (m) denote the species rabbit or mouse, respectively. Different secondary fluorophores were used in each experiment to highlight the interchangeability and utility of the reagents. DAPI (blue) and merged images are shown. Scale bar = 10 μm. C, immunofluorescent staining of ADPRylation in 3T3-L1 cells using ARBD-Fc fusion proteins. 3T3-L1 cells were grown in chamber slides and differentiated as described previously. Following the indicated time, the cells were fixed with paraformaldehyde and co-immunostained for ADPR using ARBD-Fc fusion proteins: Poly ADPRylation (WWE-rabbit) and Mono ADPRylation [Macro2/3 (PARP14)-mouse]. For each antibody, (r) and (m) denote species rabbit or mouse, respectively. DAPI (blue) and merged images are shown. High PAR (green) level at Day 0 and high MAR (red) level at Day 3 is shown. Scale bar = 10 μm. ADPR, ADP-ribose; ADPRylation, ADP-ribosylation; Macro2/3, macrodomain 2/3; MAR, mono ADPR; PAR, poly ADPR.
Second, using the same 3T3-L1 differentiation protocol, we made whole-cell extracts to examine various co-stains by dual-color fluorescent Western blotting. For example, we are able to successfully co-stain using the Macro2/3 (PARP14)-rabbit reagent with RPS6-mouse (Fig. 5A; Table S4). Additionally, we demonstrate a clear overlap in signal using the goat and rabbit versions of WWE and AF1521, respectively (Fig. 5B). The signals are robust and comparable to what we usually observe using WWE-rabbit in an HRP-based detection system (Fig. 5B; far right panel). Altogether, these results indicate that having the species variety in ARBD-Fc reagents is a valuable tool for studying ADPR biology.
Figure 5.
Testing new ARBD-detection reagents for dual-color fluorescent Western blotting.A, dual-color immunofluorescence analyses of ADPRylation in 3T3-L1 cells using ARBD-Fc fusion proteins. Whole-cell extracts were prepared from 3T3-L1 cells grown in culture and differentiated as described previously. Equal total protein levels were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted for MAR: Macro2/3 (PARP14)-rabbit (green) and the 40S ribosomal protein S6 (RPS6)-mouse (red). For each antibody, (r) and (m) denote the species rabbit or mouse, respectively. B, dual-color immunofluorescence analyses showing overlap immunofluorescence signal (yellow) using WWE-goat (green) and WWE-rabbit (red) (left panel), AF1521-goat (green) and AF1521-rabbit (red) (middle panel), and immunoblotting using the WWE-rabbit and anti-rabbit HRP-conjugated antibody for comparison (right panel). For each antibody, (r) and (g) denote species rabbit or goat, respectively. ADPRylation, ADP-ribosylation; Macro2/3, macrodomain 2/3; MAR, mono ADPR.
Discussion
Herein, we have generated and characterized an expanded set of antibody-like ADPR binding proteins in which these natural and specific ARBDs have been functionalized with the Fc region of mouse and goat immunoglobulin. These reagents are based on a previously characterized set of naturally occurring ARBDs with known specificity (4), which we have confirmed here. In combination with the rabbit IgG Fc versions previously described (4), we have created a new set of ADPR detection reagents with expanded utility that allows for simultaneous detection of MAR and PAR at single-cell resolution. Characterization of the new reagents, which include mouse or goat IgG Fc regions, demonstrates that they can be detected by secondary immunological tools in a species-dependent manner and recognize specific ADPR moieties. Importantly, these new immunological reagents can be used in various antibody-based assays by co-staining. This new capability of multiplexed assessment will enhance our understanding of the complexity of ADPRylation biology across various biological systems.
In order to study the complex and diverse forms and functions of ADPR, immunological tools used for its detection and enrichment are necessary, but still quite limited. Considerable efforts have been made to develop antibodies to detect ADPR (MAR and PAR) (8, 9, 10, 11, 12, 13, 14, 15, 16). The original rabbit versions of the antibody-like ADPR binding reagents we previously generated have proven to be useful research tools in the field for the molecular recognition of various forms of ADPR (4). They are the first examples of functionalized ARBD-Fc fusion proteins that include all key features of a monoclonal antibody: 1) monospecificity, 2) binding to protein A and G, 3) binding to Ig-directed secondary antibodies, and 4) renewable production (4). Most importantly, for the first time, these reagents allowed for the distinct recognition of mono or oligo ADPR, which could not be resolved by the widely-used anti-PAR monoclonal antibody 10H (8). More recently, with improved methods for the chemical synthesis of ADPRylated substrates, coupled with phage display and SpyTag technology, the Matic laboratory has developed a new generation of site-specific, as well as broad-specificity antibodies to MAR (17, 18).
The expansion of the ARBD-Fc toolkit by including fusions with mouse or goat Fc greatly enhances their utility in a wide range of immunological assays. Particularly, the new reagents allow for multiplexed assessment of MAR, oligo ADPR, and PAR, which is diverse, rich, and complex, contributing to interesting biologies for ADPR in a variety of biological systems. The dual detection of MAR and PAR with these new reagents will also shed light on the interesting signaling interplay between these PTMs occurring simultaneously, in different subcellular compartments and distinct biological processes, providing a level of precision that has yet to be attained in the field. Hence, the continued development of tools and refinement of existing ones will undoubtedly enhance our understanding of the intricate biological functions of ADPRylation.
Experimental procedures
Construction of plasmid vectors for bacterial expression of ADPR binding domain−Fc fusion proteins
The cDNA sequences encoding the Mouse and Goat IgG are listed in Supporting information materials (Figs. S1 and S2). The cDNA fragments were synthesized as gene blocks by Integrated DNA Technologies (IDT), and designed with appropriate restriction sites for cloning. The gene blocks were amplified using polymerase chain reaction (PCR). For the new constructs, the Rabbit-Fc region in the pET19b vector (Novagen) containing Macro2/3 (PARP14), Macro (AF1521), or WWE (RNF146) (4) was excised using (1) the AgeI and EcoRI sites and replaced with PCR-amplified DNA encoding Mouse-Fc which was digested using AgeI and EcoRI or (2) the SalI and BamHI sites and replaced with PCR-amplified DNA encoding Goat-Fc which was digested using XhoI and BamHI. Sequences were verified using whole plasmid sequencing (Plasmidsaurus).
Expression and purification of the antibody-like ADPR binding reagents in bacteria
Expression
The ADPR binding reagents were expressed in bacteria using the pET19b-based vectors described above. Because of expression issues, a slightly modified protocol was used described in Table S1. E. coli strain BL21(DE3) Rosetta2 pLysS was made competent using a CaCl2 protocol and transformed with the pET19b-based plasmids encoding one of the ADPR binding reagents described above. For 37 °C induction, the transformed bacteria were grown in LB containing ampicillin and chloramphenicol at 37 °C until the OD595 reached 0.4–0.6. Recombinant protein expression was induced by the addition of isopropyl β-D-1-thiogala-ctopyranoside (IPTG) for 4 h at 37 °C. For 16 °C induction, the transformed bacteria were grown in LB containing ampicillin and chloramphenicol at 37 °C until the OD595 reached 0.2. The culture was then cooled to 16 °C and grown to an OD595 of <0.8. Recombinant protein expression was then induced by IPTG for 18 h at 16 °C. In all cases, the cells were collected by centrifugation, and the cell pellets were flash-frozen in liquid N2 and stored at −80 °C.
Purification
The frozen pellets were thawed on wet ice and lysed by sonication in Ni-NTA lysis buffer [10 mM Tris- HCl (pH 7.5), 0.5 M NaCl, 0.1 mM EDTA, 0.1% NP-40, 10% glycerol, 10 mM imidazole, 1 mM phenylmethanesulfonylfluoride fluoride (PMSF), and 1 mM β-mercaptoethanol]. The lysates were clarified by centrifugation at 20,000g using an SF14-50 rotor (Lynx 6000, Sorvall) at 4 °C for 30 min. The supernatant was incubated with 1 ml of Ni-NTA resin equilibrated in Ni-NTA equilibration buffer [10 mM Tris-HCl (pH 7.5), 0.5 M NaCl, 0.1% NP-40, 10% glycerol, 10 mM imidazole] at 4 °C for 2 to 3 h with gentle agitation. The resin was collected by centrifugation at 4 °C for 10 min at 800g, and the lysate (supernatant) was removed. The resin was washed four times with Ni-NTA wash buffer [10 mM Tris-HCl (pH 7.5), 1 M NaCl, 0.2% NP-40, 10% glycerol, 10 mM imidazole, and 1 mM PMSF]. The recombinant proteins were then eluted using Ni-NTA elution buffer [10 mM Tris-HCl (pH 7.5), 0.2 M NaCl, 0.1% NP-40, 10% glycerol, 500 mM imidazole, 1 mM PMSF, and 1 mM β-mercaptoethanol]. The eluates were collected by centrifugation (4 °C for 5 min at 800g) and dialyzed in Ni-NTA dialysis buffer [10 mM Tris-HCl (pH 7.5), 0.2 M NaCl, 10% glycerol, 10 mM imidazole, 0.1% NP-40, 1 mM PMSF, and 1 mM β-mercaptoethanol] (Table S2). The dialyzed proteins were collected and centrifuged at max speed (15,000 RPM) for 15 min at 4 °C. Clear supernatant was collected and quantified using a Bradford protein assay (Bio-Rad), aliquoted, flash-frozen in liquid N2, and stored at −80 °C. To assess the purity and quality of each purified ADPR binding reagent, 1 to 2 μg of purified protein was subjected to sodium dodecyl sulfate−polyacrylamide gel electrophoresis (SDS − PAGE) and stained with Coomassie brilliant blue.
In vitro auto ADPRylation reactions with PARP1 and PARP3 to generate mono-, and poly(ADPR) standards
Five hundred nanograms of purified recombinant mPARP1 or mPARP3 was incubated at 25 °C in a 100 μl reaction volume [20 mM HEPES (pH 8.0), 5 mM MgCl2, 5 mM CaCl2, 0.01% NP-40, 25 mM KCl, 1 mM DTT, 0.1 mg/ml sheared salmon sperm DNA (Invitrogen, AM9680), and 0.1 mg/ml BSA (Sigma)] under the following conditions: (1) PARP1 with 250 μM NAD+ for 5 min for poly(ADPR), and (2) PARP3 with 250 μM NAD+ for 30 min for mono(ADPR). All reactions were stopped by the addition of one-third of a reaction volume of 4× SDS-PAGE loading buffer, followed by heating to 95 °C for 5 min.
Dot blotting and immunoblotting
Preparation of nitrocellulose blotting membranes for dot blotting and immunoblotting
For dot blotting, 500 ng purified proteins were spotted in 5 μl amounts to a nitrocellulose membrane and dried to bind the ADPR binding reagents to the membrane. For immunoblotting, the aliquots of purified proteins, PARP1 or PARP3 ADPRylation reaction products, or 9 to 30 μg of nuclear or whole cell extract, were resolved on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane.
Dot blotting and immunoblotting
The membranes were blocked for 1 h at room temperature in Tris-buffered saline with 0.05% Tween (TBST) containing 5% nonfat dry milk. Primary antibodies and/or detection reagents (e.g., ADPR binding reagents; β-tubulin, Abcam ab6046) were diluted in TBST with 3% nonfat dry milk and incubated with membranes for 1 h at room temperature. As described in Table S3, after being extensively washed with TBST, the membranes were incubated with an appropriate HRP-conjugated secondary antibody (IgG Fc Goat anti-Rabbit, HRP, Invitrogen 31463; IgG Fc Rabbit anti-Mouse, HRP, Invitrogen 31455; IgG Fc Rabbit anti-Goat, HRP, Invitrogen 31433), and diluted in TBST with 3% nonfat dry milk for 1 h at room temperature. The membranes were washed extensively with TBST before chemiluminescent detection using SuperSignal West Pico substrate or femto substrate (Thermo Scientific) and a ChemiDoc imaging system (Bio-Rad).
Cell culture and treatments
3T3-L1 cells (22) were obtained from the American Type Cell Culture (ATCC, CL-173). They were maintained in DMEM (Cellgro, 10-017-CM) supplemented with 10% fetal bovine serum (Atlanta Biologicals, S11550) and 1% penicillin/streptomycin. For the induction of adipogenesis, the 3T3-L1 cells were grown to confluence and then cultured for 2 more days under contact inhibition. The cells were then treated for 2 days with an MDI adipogenic cocktail containing 0.25 mM IBMX, 1 μM dexamethasone, and 10 μg/ml insulin. Subsequently, the cells were cultured in a medium containing 10 μg/ml insulin for the indicated times before collection (21).
OVCAR3 cells were obtained from ATCC (HTB-161). The cells were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (Sigma), 1% penicillin/streptomycin, and 1% glutamax. The cells were grown to ∼80% confluence, and treated for 2 h with or without 20 μM PARP inhibitor, niraparib (MedChemExpress, HY-10619). Treated cells were gently washed and collected in ice-cold PBS and then pelleted by centrifugation at 450g for 5 min.
AC16 human adult ventricular cardiomyocyte cells were obtained from ATCC (CRL-3568). The cells were cultured in DMEM/F-12, no phenol red (Gibco, 11039021) supplemented with 12.5% fetal bovine serum (Sigma, F8067), 1% penicillin/streptomycin, and 0.025 mg/ml Gentamicin (Gibco, 15710064). The cells can be cultured for at least 10 passages after initial thawing. AC16 cells were grown to ∼80% confluence, treated for 2 h with or without 10 μM PARP inhibitor, olaparib (MedChemExpress, HY-10162), and then treated for an additional 10 min with or without 5 mM H2O2.
For all cell lines, cell stocks were regularly replenished from the original stocks, verified for cell type identity, and confirmed as mycoplasma-free every 3 months using a commercial testing kit.
Preparation of extracts from mammalian cells for immunoblotting
Preparation of whole cell extracts
The cell pellets were resuspended in 1× lysis buffer [50 mM Tris (pH 7.5), 0.5 M NaCl, 1 mM EDTA, 1% NP40, 10% glycerol, 1 M DTT, 10 μM PJ34 (Abcam, ab120981), 500 nM ADP-HPD (a PARG inhibitor; Sigma) and 1× protease inhibitor cocktail (Roche)] and incubated for 30 min on ice with gentile vortexing and then centrifuge at maximum speed for 15 min at 4 °C in microcentrifuge to remove the cell debris. The supernatant was collected as whole-cell protein extracts. Protein concentrations for the whole cell extracts were determined using a Bradford protein assay (Bio-Rad). The extracts were aliquoted, flash-frozen in liquid N2, and stored at −80 °C.
Preparation of nuclear extracts
The packed cell volume (PCV) was estimated, and the cell pellets were resuspended to homogeneity in 5× PCV of Lysis buffer [1× isotonic lysis buffer (10 mM Tris- HCl (pH 7.5), 2 mM MgCl2, 3 mM CaCl2, 0.3 M sucrose), 1 mM DTT, 1× protease inhibitor cocktail (Roche), 10 μM PJ34 (Sigma), and 500 nM ADP-HPD (a PARG inhibitor; Sigma)] and incubated on ice for 15 min. NP-40 was added from a 20% solution in lysis buffer to a final concentration of 0.6%, and the mixture was vortexed vigorously for 10 s. The lysate was subjected to a short burst of centrifugation for 30 s at 11000 RPM in a microcentrifuge at 4 °C to collect the nuclei. The pelleted nuclei were resuspended in 2/3× PCV of ice-cold nuclear extraction buffer C [20 mM HEPES (pH 7.6), 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, 25% (v/v) glycerol, 1 mM DTT, 1× protease inhibitor cocktail, 10 μM PJ34 inhibitor, and 500 nM ADP-HPD] and incubated while being gently mixed for 30 min at 4 °C. The mixture was subjected to centrifugation at maximum speed in a microcentrifuge for 10 min at 4 °C twice to remove the insoluble material. The supernatant was collected as the soluble nuclear extract followed by addition of an equal volume of buffer B [20 mM HEPES (pH 7.6), 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 1× protease inhibitor cocktail, 10 μM PJ34 inhibitor, and 500 nM ADP-HPD]. NP-40 was added from a 20% solution in lysis buffer to a final concentration of 0.5%. Protein concentrations for the nuclear extracts were determined using a Bradford protein assay (Bio-Rad). The extracts were aliquoted, flash-frozen in liquid N2, and stored at −80 °C.
Aliquots of nuclear or whole cell extracts were mixed with one-third of a volume of 4× SDS loading buffer [200 mM Tris- HCl (pH 6.8), 8% SDS, 40% glycerol, 4% β-mercaptoethanol, 50 mM EDTA, and 0.08% bromophenol blue], followed by heating to 70 °C for 10 min. The cell extracts were subjected to immunoblotting as described above.
Fluorescent immunocytochemistry
OVCAR3 cells were grown on a chamber slide (Invitrogen), and treated for 2 h with or without 20 μM PARP inhibitor [veliparib (MedChemExpress, HY-10129), niraparib]. 3T3-L1 cells were seeded into 4-well chambered slides (Thermo Fisher, 154534) and were grown and differentiated as described previously (21). The cells were washed twice with PBS on ice and fixed with 4% paraformaldehyde at room temperature for 15 min. The chamber slides were washed with ice-cold PBS and permeabilized with permeabilization buffer (1× PBS, 0.5% Triton X-100) for 5 min. The fixed cells on the chamber slides were washed with PBS and blocked with blocking solution for 1 h at room temperature. After being blocked, the samples were incubated with 20 μg/ml ADPR binding reagent in a blocking solution overnight at 4 °C. The samples were washed with PBST and incubated with fluorophore-conjugated secondary antibodies (Goat anti-Rabbit IgG (H+L) Alexa Fluor 594, A-11012; Goat anti-Rabbit IgG (H+L), Alexa Fluor 488, A-11008; Goat anti-Mouse IgG (H+L), Alexa Fluor 594, A-11005; Goat anti-Mouse IgG (H+L), Alexa Fluor 488, A-11001) diluted 1:500 in PBST for 1 h at room temperature in the dark. The samples were then washed with PBST, the coverslips were placed on cells coated with VectaShield Antifade Mounting medium with DAPI (Vector Laboratories), and the image was visualized using a Nikon confocal microscope.
Dual-color fluorescent Western blotting
The membranes were blocked for 1 h at room temperature in Tris-buffered saline with 0.05% Tween (TBST) containing 3% nonfat dry milk (Table S4). Primary antibodies and/or detection reagents (e.g., ADPR binding reagents; RPS6, Santa Cruz sc-74459) were diluted in TBST with 3% nonfat dry milk and incubated with membranes for 1 h at room temperature. After being extensively washed with TBST, the membranes were incubated with secondary antibodies (IRDye 800CW Donkey anti-Rabbit IgG Secondary Antibody, Li-Cor 926-32213; IRDye 680RD Donkey anti-Rabbit IgG Secondary Antibody, Li-Cor 926-68073; IRDye 680RD Donkey anti-Mouse IgG Secondary Antibody, Li-Cor 926-68072; IRDye 800CW Donkey anti-Goat IgG Secondary Antibody, Li-Cor 926-32214) diluted in TBST with 3% nonfat dry milk for 1 h at room temperature. The membranes were washed extensively with TBST. The image was developed using the ChemiDoc imaging system (Bio-Rad).
Data availability
All data and reagents presented within this article are available upon request. The generated plasmids will be available through EMD Millipore.
Supporting information
This article contains supporting information (23).
Conflict of interest
W. L. Kraus is a founder, consultant, and Science Advisory Board member for ARase Therapeutics, Inc. W. L. Kraus is a co-holder of U.S. Patent 9,599,606 covering the ADPR detection reagents described herein. The rabbit ARBD-Fc fusions have been licensed to and are sold by EMD Millipore.
Acknowledgments
We thank members of the Kraus lab Aarin Jones and MiKayla S. Stokes for help in cloning new constructs; MiKayla S. Stokes, Palak Ahuja, Marwa W. Aljardali, and Xinrui Tan for providing cell lysates for screening the detection reagents; Dan Huang for providing purified protein preparations and Charles W. Renshaw for help with purifications and immunoblotting assays.
Author contributions
W. L. K., C. V. C. writing–review & editing; W. L. K., C. V. C. supervision; W. L. K. project administration; W. L. K. funding acquisition; W. L. K. conceptualization. C. V. C. and S-P. C. writing–original draft; C. V. C. and S-P. C. validation; C. V. C. and S-P. C. methodology; C. V. C. and S-P. C. formal analysis; C. V. C. and S-P. C. data curation.
Funding and additional information
This work was supported by grants from the NIH/National Cancer Institute (R01 CA251943 to W. L. K.), NIH/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK069710 to W. L. K.), Cancer Prevention and Research Institute of Texas (CPRIT; RP220325 and RP240225 to W. L. K.), U.S. Deparment of Defense Ovarian Cancer Research Program (DOD-OCRP; OC200311 to W. L. K.), and funds from the Cecil H. and Ida Green Center for Reproductive Biology Sciences Endowment to W. L. K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Brian D. Strahl
Contributor Information
Cristel V. Camacho, Email: Cristel.Camacho@utsouthwestern.edu.
W. Lee Kraus, Email: Lee.Kraus@utsouthwestern.edu.
Supporting information
References
- 1.Huang D., Kraus W.L. The expanding universe of PARP1-mediated molecular and therapeutic mechanisms. Mol. Cell. 2022;82:2315–2334. doi: 10.1016/j.molcel.2022.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupte R., Liu Z., Kraus W.L. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 2017;31:101–126. doi: 10.1101/gad.291518.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson B.A., Kraus W.L. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 4.Gibson B.A., Conrad L.B., Huang D., Kraus W.L. Generation and characterization of recombinant antibody-like ADP-ribose binding proteins. Biochemistry. 2017;56:6305–6316. doi: 10.1021/acs.biochem.7b00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasovich M., Leung A.K.L. PARPs and ADP-ribosylation: deciphering the complexity with molecular tools. Mol. Cell. 2023;83:1552–1572. doi: 10.1016/j.molcel.2023.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challa S., Ryu K.W., Whitaker A.L., Abshier J.C., Camacho C.V., Kraus W.L. Development and characterization of new tools for detecting poly(ADP-ribose) in vitro and in vivo. Elife. 2022;11 doi: 10.7554/eLife.72464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Challa S., Nandu T., Kim H.B., Gong A., Renshaw C.W., Li W.C., et al. A PARP14/TARG1-regulated RACK1 MARylation cycle drives stress granule dynamics in ovarian cancer cells. bioRxiv. 2023 doi: 10.1101/2023.10.13.562273. [preprint] [DOI] [Google Scholar]
- 8.Kawamitsu H., Hoshino H., Okada H., Miwa M., Momoi H., Sugimura T. Monoclonal antibodies to poly(adenosine diphosphate ribose) recognize different structures. Biochemistry. 1984;23:3771–3777. doi: 10.1021/bi00311a032. [DOI] [PubMed] [Google Scholar]
- 9.Bredehorst R., Ferro A.M., Hilz H. Production of antibodies against ADP-ribose and 5'-AMP with the aid of N6-carboxymethylated ADP-ribose conjugates. Eur. J. Biochem. 1978;82:105–113. doi: 10.1111/j.1432-1033.1978.tb12001.x. [DOI] [PubMed] [Google Scholar]
- 10.Eide B., Gierschik P., Spiegel A. Immunochemical detection of guanine nucleotide binding proteins mono-ADP-ribosylated by bacterial toxins. Biochemistry. 1986;25:6711–6715. doi: 10.1021/bi00369a058. [DOI] [PubMed] [Google Scholar]
- 11.Kanai Y., Miwa M., Matsushima T., Sugimura T. Studies on anti-poly(adenosine diphosphate ribose) antibody. Biochem. Biophys. Res. Commun. 1974;59:300–306. doi: 10.1016/s0006-291x(74)80206-8. [DOI] [PubMed] [Google Scholar]
- 12.Kanai Y., Miwa M., Matsushima T., Sugimura T. Comparative studies on antibody and antibody production to poly(ADP-ribose) in mice. Immunology. 1978;34:501–508. [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai Y., Sugimura T., Matsushima T. Induction of specific antibodies to poly(ADP-ribose) in rabbits by double-stranded RNA, poly(A)-poly(U) Nature. 1978;274:809–812. doi: 10.1038/274809a0. [DOI] [PubMed] [Google Scholar]
- 14.Meyer T., Hilz H. Production of anti-(ADP-ribose) antibodies with the aid of a dinucleotide-pyrophosphatase-resistant hapten and their application for the detection of mono(ADP-ribosyl)ated polypeptides. Eur. J. Biochem. 1986;155:157–165. doi: 10.1111/j.1432-1033.1986.tb09471.x. [DOI] [PubMed] [Google Scholar]
- 15.Osago H., Terashima M., Hara N., Yamada K., Tsuchiya M. A new detection method for arginine-specific ADP-ribosylation of protein -- a combinational use of anti-ADP-ribosylarginine antibody and ADP-ribosylarginine hydrolase. J. Biochem. Biophys. Methods. 2008;70:1014–1019. doi: 10.1016/j.jprot.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Sakura H., Miwa M., Kanai Y., Matsushima T., Sugimura T. Formation and characterization of antibody against 2'-(5"-phosphoribosyl)-5' AMP, the monomer form of poly(adenosine diphosphate ribose) Nucleic Acids Res. 1978;5:4025–4038. doi: 10.1093/nar/5.11.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longarini E.J., Dauben H., Locatelli C., Wondisford A.R., Smith R., Muench C., et al. Modular antibodies reveal DNA damage-induced mono-ADP-ribosylation as a second wave of PARP1 signaling. Mol. Cell. 2023;83:1743–1760.e11. doi: 10.1016/j.molcel.2023.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonfiglio J.J., Leidecker O., Dauben H., Longarini E.J., Colby T., San Segundo-Acosta P., et al. An HPF1/PARP1-based chemical biology strategy for exploring ADP-ribosylation. Cell. 2020;183:1086–1102.e23. doi: 10.1016/j.cell.2020.09.055. [DOI] [PubMed] [Google Scholar]
- 19.Fontana P., Bonfiglio J.J., Palazzo L., Bartlett E., Matic I., Ahel I. Serine ADP-ribosylation reversal by the hydrolase ARH3. Elife. 2017;6 doi: 10.7554/eLife.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharifi R., Morra R., Appel C.D., Tallis M., Chioza B., Jankevicius G., et al. Deficiency of terminal ADP-ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. EMBO J. 2013;32:1225–1237. doi: 10.1038/emboj.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo X., Ryu K.W., Kim D.S., Nandu T., Medina C.J., Gupte R., et al. PARP-1 controls the adipogenic transcriptional Program by PARylating C/EBPbeta and modulating its transcriptional activity. Mol. Cell. 2017;65:260–271. doi: 10.1016/j.molcel.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz J.C., Philp R.L., Bickhart D.M., Smith T.P.L., Hammond J.A. The antibody loci of the domestic goat (Capra hircus) Immunogenetics. 2018;70:317–326. doi: 10.1007/s00251-017-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and reagents presented within this article are available upon request. The generated plasmids will be available through EMD Millipore.