Abstract
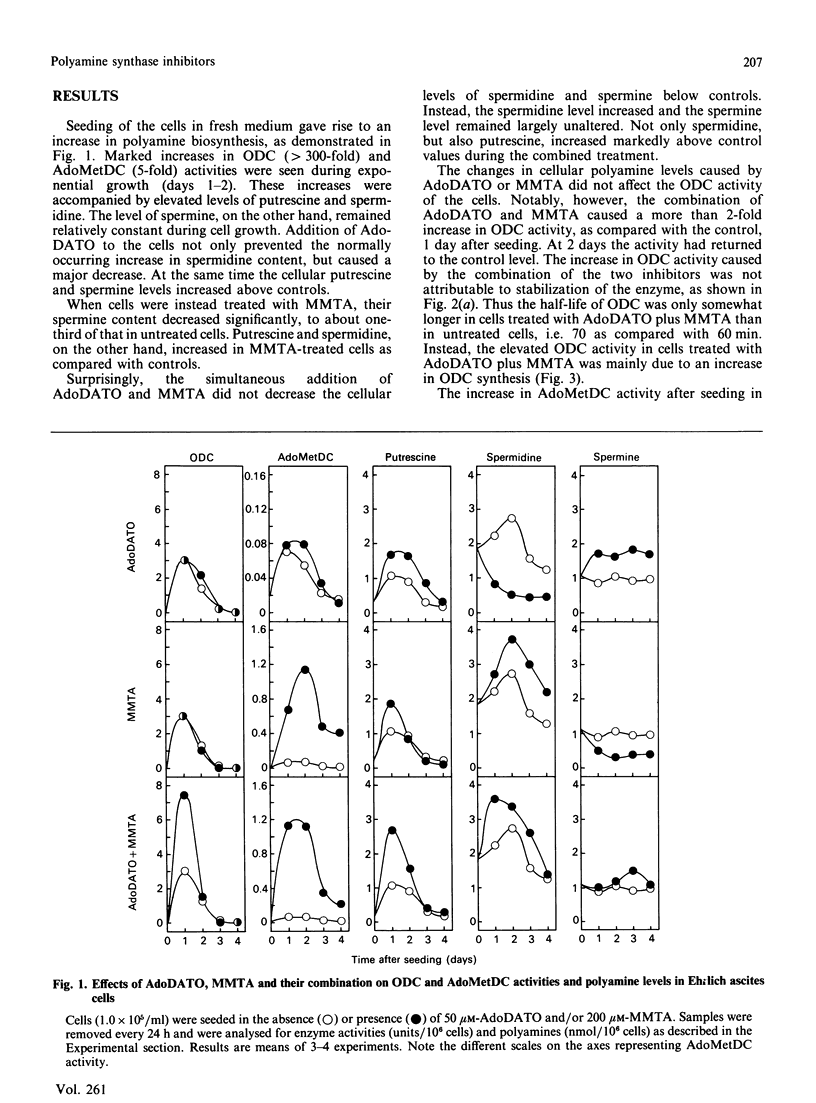
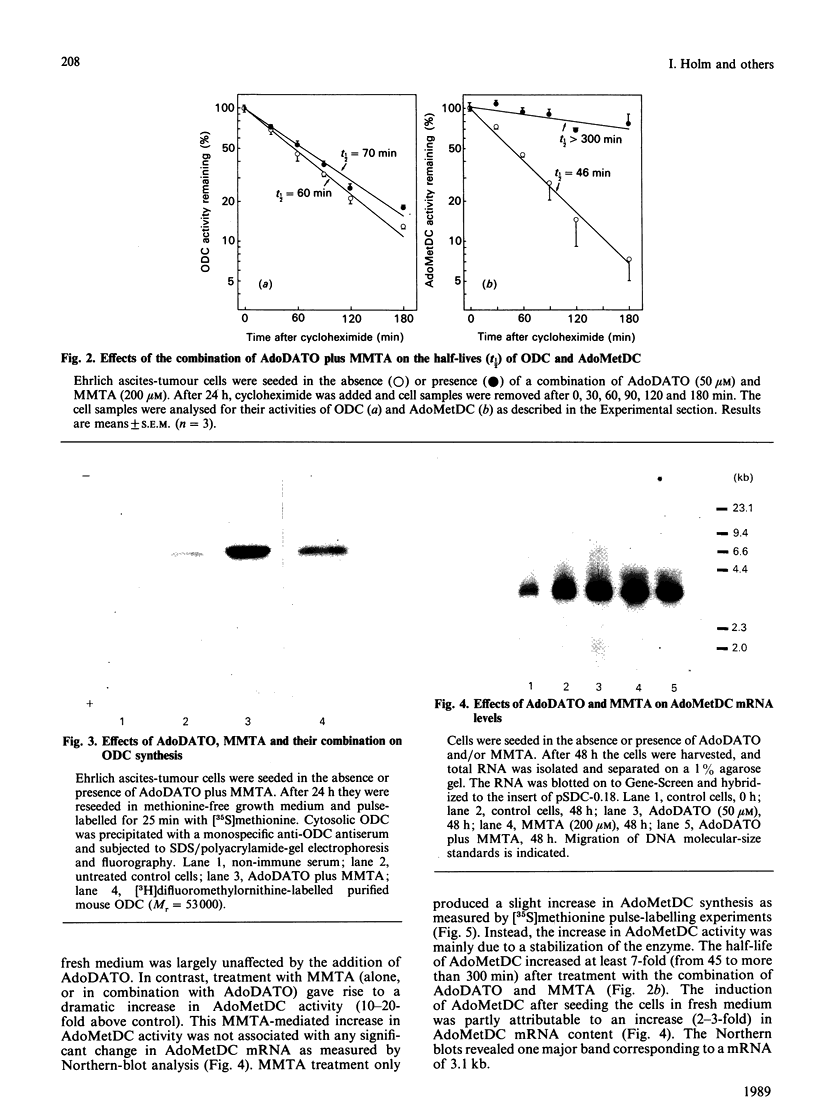
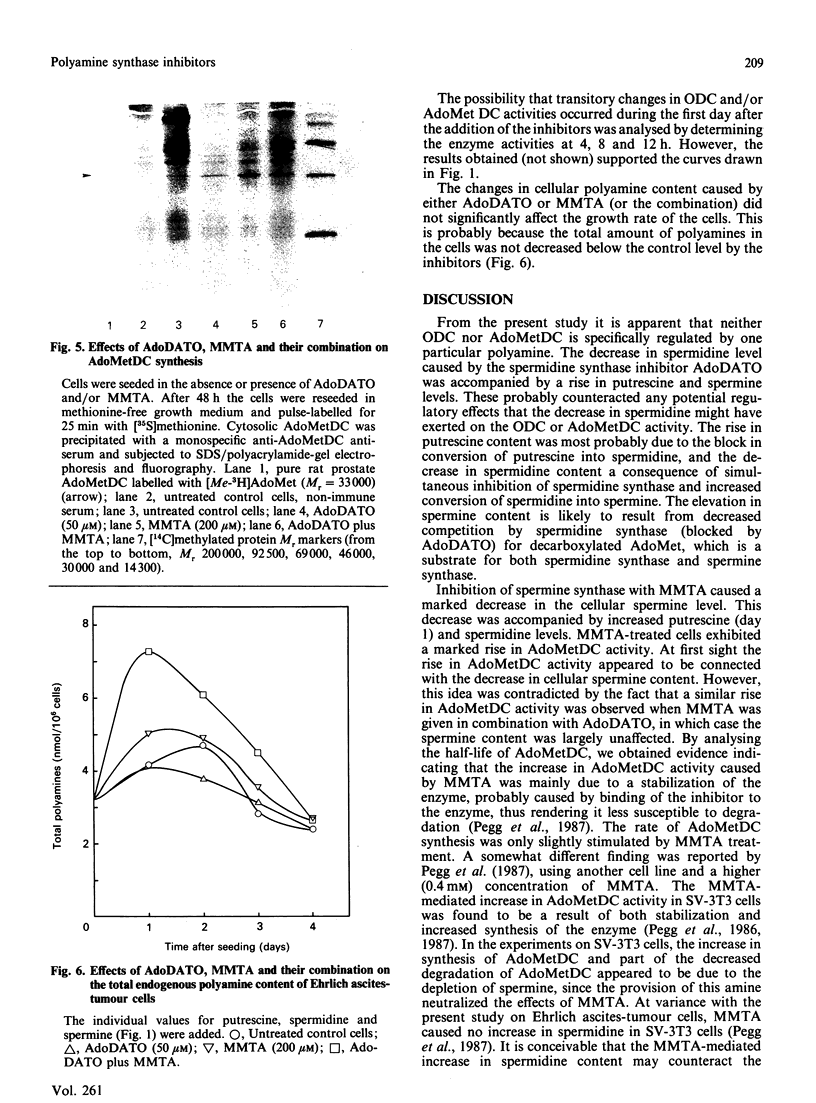
The rate-limiting enzymes in polyamine biosynthesis, ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC), are negatively regulated by the polyamines spermidine and spermine. In the present work the spermidine synthase inhibitor S-adenosyl-1,8-diamino-3-thio-octane (AdoDATO) and the spermine synthase inhibitor S-methyl-5'-methylthioadenosine (MMTA) were used to evaluate the regulatory role of the individual polyamines. Treatment of Ehrlich ascites-tumour cells with AdoDATO caused a marked decrease in spermidine content together with an accumulation of putrescine and spermine. Treatment with MMTA, on the other hand, gave rise to a marked decrease in spermine, with a simultaneous accumulation of spermidine. A dramatic increase in the activity of AdoMetDC, but not of ODC, was observed in MMTA-treated cells. This increase appears to be unrelated to the decrease in spermine content, because a similar rise in AdoMetDC activity was obtained when AdoDATO was given in addition to MMTA, in which case the spermine content remained largely unchanged. Instead, we show that the increase in AdoMetDC activity is mainly due to stabilization of the enzyme, probably by binding of MMTA. Treatment with AdoDATO had no effects on the activities of ODC and AdoMetDC, even though it caused a precipitous decrease in spermidine content. The expected decrease in spermidine-mediated suppression of ODC and AdoMetDC was most probably counteracted by the simultaneous increase in spermine. The combination of AdoDATO and MMTA caused a transient rise in ODC activity. Concomitant with this rise, the putrescine and spermidine contents increased, whereas that of spermine remained virtually unchanged. The increase in ODC activity was due to increased synthesis of the enzyme. There were no major effects on the amount of AdoMetDC mRNA by treatment with the inhibitors, alone or in combination. However, the synthesis of AdoMetDC was slightly stimulated in cells treated with MMTA or AdoDATO plus MMTA. The present study demonstrates that regulation of neither ODC nor AdoMetDC is a direct function of the polyamine structure. Instead, it appears that the biosynthesis of the polyamines is feedback-regulated by the various polyamines at many different levels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler A. P., McDonald F. F. Transient induction of ornithine decarboxylase mRNA in rat hepatoma cells treated with 12-O-tetradecanoylphorbol-13-acetate. Biochem Biophys Res Commun. 1987 Sep 15;147(2):809–817. doi: 10.1016/0006-291x(87)91002-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Elliott R. W., Berger F. G. DNA sequence polymorphism in an androgen-regulated gene is associated with alteration in the encoded RNAs. Proc Natl Acad Sci U S A. 1983 Jan;80(2):501–504. doi: 10.1073/pnas.80.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Effect of putrescine on the synthesis of S-adenosylmethionine decarboxylase. Biochem J. 1987 Apr 1;243(1):285–288. doi: 10.1042/bj2430285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Kahana C. Transcriptional activation of mammalian ornithine decarboxylase during stimulated growth. Mol Cell Biol. 1987 Jul;7(7):2641–2643. doi: 10.1128/mcb.7.7.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontula K. K., Torkkeli T. K., Bardin C. W., Jänne O. A. Androgen induction of ornithine decarboxylase mRNA in mouse kidney as studied by complementary DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):731–735. doi: 10.1073/pnas.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D. L., Sufrin J. R., Porter C. W. Modulation of polyamine-biosynthetic activity by S-adenosylmethionine depletion. Biochem J. 1988 Jan 15;249(2):581–586. doi: 10.1042/bj2490581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach M., White M. W., Neubauer M., Degen J. L., Morris D. R. Isolation of a cDNA clone encoding S-adenosylmethionine decarboxylase. Expression of the gene in mitogen-activated lymphocytes. J Biol Chem. 1986 Sep 5;261(25):11697–11703. [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Mitogens and protein synthesis inhibitors induce ornithine decarboxylase gene transcription through separate mechanisms in the BC3H1 muscle cell line. Mol Cell Biol. 1986 Aug;6(8):2792–2799. doi: 10.1128/mcb.6.8.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Coward J. K., Talekar R. R., Secrist J. A., 3rd Effects of certain 5'-substituted adenosines on polyamine synthesis: selective inhibitors of spermine synthase. Biochemistry. 1986 Jul 15;25(14):4091–4097. doi: 10.1021/bi00362a016. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Wechter R., Pajunen A. Increase in S-adenosylmethionine decarboxylase in SV-3T3 cells treated with S-methyl-5'-methylthioadenosine. Biochem J. 1987 May 15;244(1):49–54. doi: 10.1042/bj2440049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Pajunen A. Detection of proenzyme form of S-adenosylmethionine decarboxylase in extracts from rat prostate. Biochem Biophys Res Commun. 1988 Jan 29;150(2):788–793. doi: 10.1016/0006-291x(88)90460-3. [DOI] [PubMed] [Google Scholar]
- Persson L. Antibodies to ornithine decarboxylase. Immunochemical cross-reactivity. Acta Chem Scand B. 1982;36(10):685–688. doi: 10.3891/acta.chem.scand.36b-0685. [DOI] [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Regulation of ornithine decarboxylase mRNA translation by polyamines. Studies using a cell-free system and a cell line with an amplified ornithine decarboxylase gene. J Biol Chem. 1988 Mar 5;263(7):3528–3533. [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Translational regulation of ornithine decarboxylase by polyamines. FEBS Lett. 1986 Sep 15;205(2):175–178. doi: 10.1016/0014-5793(86)80892-4. [DOI] [PubMed] [Google Scholar]
- Persson L., Oredsson S. M., Anehus S., Heby O. Ornithine decarboxylase inhibitors increase the cellular content of the enzyme: implications for translational regulation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):239–245. doi: 10.1016/0006-291x(85)91794-2. [DOI] [PubMed] [Google Scholar]
- Persson L., Seely J. E., Pegg A. E. Investigation of structure and rate of synthesis of ornithine decarboxylase protein in mouse kidney. Biochemistry. 1984 Jul 31;23(16):3777–3783. doi: 10.1021/bi00311a033. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Rincke G., Marks F. The induction of ornithine decarboxylase by the tumor promoter TPA is controlled at the post-transcriptional level in murine Swiss 3T3 fibroblasts. Biochem Biophys Res Commun. 1987 Aug 31;147(1):219–225. doi: 10.1016/s0006-291x(87)80109-2. [DOI] [PubMed] [Google Scholar]
- Sertich G. J., Pegg A. E. Polyamine administration reduces ornithine decarboxylase activity without affecting its mRNA content. Biochem Biophys Res Commun. 1987 Mar 13;143(2):424–430. doi: 10.1016/0006-291x(87)91371-4. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Christman K. L., Pegg A. E. Quantitation of S-adenosylmethionine decarboxylase protein. Biochemistry. 1985 Jul 30;24(16):4417–4423. doi: 10.1021/bi00337a024. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Increased content of mRNA for a precursor of S-adenosylmethionine decarboxylase in rat prostate after treatment with 2-difluoromethylornithine. J Biol Chem. 1986 Oct 15;261(29):13833–13837. [PubMed] [Google Scholar]
- Tang K. C., Pegg A. E., Coward J. K. Specific and potent inhibition of spermidine synthase by the transition-state analog, S-adenosyl-3-thio-1,8-diaminooctane. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1371–1377. doi: 10.1016/0006-291x(80)90102-3. [DOI] [PubMed] [Google Scholar]