Key Points
Question
Were there changes in preventable risk factors, causes of preterm birth, and delivery rates during the 3 COVID-19 pandemic phases in Germany in 2020 compared with previous years?
Findings
In this cohort study including all 184 827 births in Hesse, Germany, from 2017 through 2020, reduction of preterm births increased with the ongoing lockdown measures. While quality markers of prenatal care remained unchanged, decreases were observed in high-risk pregnancies with history of maternal serious disease and deliveries for pathologic cardiotocography and intrauterine infection.
Meaning
The findings of this cohort study highlight opportunities for prevention measure programs intended to reduce potentially modifiable risks for preterm delivery and to intensify financial and research investments to reduce preterm deliveries.
This cohort study assesses changes in perinatal care, causes of preterm delivery, and rates of preterm delivery before and during the COVID-19 pandemic in Hesse, Germany.
Abstract
Importance
Population-based analyses provided divergent data on the changes in preterm birth rates during the COVID-19 pandemic, and there is a gap of knowledge on the variations in birth characteristics.
Objective
To study changes in perinatal care, causes of preterm delivery, and very preterm (VPT; defined as <32 weeks’ gestation) birth rates before and during the COVID-19 pandemic.
Design, Setting, and Participants
This population-level cohort study used data from the quality assurance registry, which covers all births in Hesse, Germany. Deliveries during the COVID-19 pandemic (2020) were compared with the corresponding grouped prepandemic time intervals (2017 to 2019). Analyses were executed between August 2023 and July 2024.
Exposures
Analyses were directed to study differences in preterm births before and during 3 pandemic phases: first (March 14 to May 15, 2020) and second (October 19 to December 31, 2020) lockdowns and a period of less-vigorous restrictions between them (May 16 to October 18, 2020).
Main Outcomes and Measures
Outcomes of interest were variations in preterm birth rates in the context of baseline characteristics and causes of preterm births during vs before the first year of the COVID-19 pandemic.
Results
From the total cohort of 184 827 births from 2017 to 2020, 719 stillbirths occurred and 184 108 infants were liveborn. Compared with the prepandemic period, medical care characteristics did not differ during the COVID-19 period. The odds of VPT births were lower during the pandemic period (odds ratio [OR], 0.87; 95% CI, 0.79-0.95) compared with the prepandemic period, with the greatest reduction observed during the second lockdown period (OR, 0.69; 95% CI, 0.55-0.84). Reduction in VPT births was attributed to fewer births in pregnancies among individuals with a history of serious disease (OR, 0.64; 95% CI, 0.50-0.83), pathologic cardiotocography (OR, 0.66; 95% CI, 0.53-0.82), and intrauterine infection (OR, 0.82; 95% CI, 0.72-0.92) while incidences of history of preterm birth, multiple pregnancies, serious or severe psychological distress, and preeclampsia, eclampsia, or hemolysis, elevated liver enzymes, and low platelet count syndrome as cause for preterm delivery remained unchanged.
Conclusions and Relevance
In this population-based cohort study on the COVID-19 pandemic and preterm birth rates, the duration of exposure to mitigation measures during pregnancy was associated with accelerated reductions in preterm births. The findings of lower rates of baseline risks and causes of preterm deliveries support efforts to intensify health care prevention programs during pregnancy to reduce the preterm birth burden. These findings of this study put particular focus on hygiene measures to reduce the rate of deliveries for intrauterine infection and highlight the potential of expanding strategies to the different risks and causes of preterm delivery.
Introduction
The COVID-19 pandemic resulted in dramatic restrictions for daily life. The lockdown measures incidentally changed the access to health care services, as well as personal attitudes to use of medical care.1,2 The change in perception of health-related risks for pregnant individuals might have impacted contact behavior among the individuals in general.3,4,5,6 Several studies have evaluated the associations between the onset of the COVID-19 pandemic and preterm birth rates based on patient registries and found diverse results, with unchanged or reduced preterm birth rates. While early reports from Denmark and Ireland observed dramatic reductions, no decline was found for Norway, Sweden, or Denmark when the observation period was expanded beyond the initial lockdown period.7,8,9,10,11 Two systematic meta-analyses concluded that no overall reduction in preterm birth rates occurred during the COVID-19 pandemic, but any effects might have been restricted to areas with more restrictive lockdown measures and high-income countries.12,13 The decline in preterm birth rates in the US with the 2020 lockdown was attributed to decreases in cesarean deliveries and induced deliveries, while pregnancy risk-factors of self-pay status, history of premature delivery, and maternal diabetes or arterial hypertension were associated with an increased risk of preterm birth in Colorado.14,15
Reports on changes in stillbirth rates provided divergent results, and most analyses did not consider the potential trade-offs between stillbirth and preterm birth rates.2,6,10,16,17,18,19,20,21,22 A more detailed analysis from Australia detected fewer deliveries for fetal compromise but an increase in stillbirths.22
While most studies focused on maternal and neonatal outcomes, few studies detailed the associations of the COVID-19 pandemic with changes in maternal baseline characteristics, use of routine health care services during pregnancy, perinatal care in impeding preterm delivery, and causes of preterm birth.3,4,12,22 These aspects were addressed as objectives of our population-level descriptive epidemiological cohort study in the federal state of Hesse, Germany, with more than 6.2 million inhabitants and more than 60 000 births annually between 2017 and 2020.
Methods
This cohort study was approved by the ethics committee of the Justus-Liebig-University Giessen. The requirement for informed consent was waived for the retrospective analysis of anonymous data. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Source and Study Population
The quality assurance registry of Hesse constitutes an obligatory medical reporting system for all deliveries of stillbirths at 500 g or more and liveborn infants with at least 22 0/7 weeks’ gestation in the state. Deliveries are recorded within the documentation system in obstetric and neonatal departments from patient records after discharge by the medical staff of each center based on predefined definitions of the database, which partly differ from International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. Data were reviewed for plausibility during data entry and export. Assessments of completeness of datasets were executed by the quality assurance body based on the accounting data of the hospitals and were consistently greater than 99%. All items remained unchanged during the study period and were prospectively collected into the database before start of the analyses.
The analyses were directed toward very preterm (VPT) infants delivered at less than 32 weeks’ gestation, and results were put into the context of maternal baseline characteristics and causes of VPT birth. Additionally, outcomes were reassessed among the more mature infants born between 32 0/7 and 36 6/7 weeks. COVID-19 pandemic restrictions in Hesse became effective on March 14, 2020. Due to the reported seasonal variations in preterm birth rates, the observation periods from January 1 to March 13 were excluded from the datasets in the COVID-19 and control collectives.23 The COVID-19 pandemic was further separated into 3 phases: the first lockdown (March 14 to May 15, 2020), a period of less vigorous restrictions (May 16 to October 18, 2020), and the second lockdown (October 19 to December 31, 2020) that differed from the first lockdown mainly by the continued personal school attendance across all educational levels but comparable restrictions at the workplace and in private life. Data were compared with the grouped prepandemic corresponding time intervals during 2017 to 2019 (eFigure 1 and eFigure 2 in Supplement 1).
Definition of Outcomes and Covariates
Our primary outcomes were preterm birth rates before compared with after the onset of the COVID-19 pandemic and changes during the different phases of the pandemic. Maternal baseline characteristics included maternal age, history of maternal serious disease or family history of serious disease, history of preterm birth, history of previous cesarean delivery or uterine surgery, complications during previous pregnancies, and singleton or multiple pregnancy. Pregnancy risks included diabetes during pregnancy, maternal obesity, serious or severe psychological burden during pregnancy, preexisting hypertension, bleeding at less than 28 weeks’ gestation or at 28 weeks’ gestation or greater, and placenta previa. Prenatal care items included the number of routine medical check-ups during pregnancy, gestational age at admission, period between hospital admission and delivery, antenatal steroid (ANS) application, and mode of delivery as spontaneous birth or cesarean delivery, with separate analysis of emergency cesarean delivery and stillbirth. ANS administration was counted when at least 1 dose was given, irrespective of the interval to delivery.
Categories for preterm deliveries were separated into spontaneous onset with preterm labor, amniotic infection, and premature rupture of membranes, which were summarized under the term delivery for intrauterine infection that fall into the category of nonindicated deliveries; indicated births included preeclampsia, eclampsia, hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, placental insufficiency with intrauterine growth restriction, placental abruption, and pathologic cardiotocography (CTG). Intrauterine infection was counted when at least 1 clinical criterion was fulfilled. Premature rupture of membranes was defined as occurring more than 18 hours before onset of labor.
Gestational age was determined by routine obstetric assessment based on the date of the last menstrual period and routine ultrasonographic measurements. Further items included sex, multiple births, pH and base excess from the arterial umbilical cord blood, and Apgar scores at 1, 5, and 10 minutes of life.
Statistical Analysis
All statistical analyses were performed with R software package version 4.2.2 (R Project for Statistical Computing). The study used exploratory and descriptive statistical methods. As our retrospective descriptive epidemiological study was not driven by prespecified hypotheses, no predefined cutoff P value was used to indicate statistical significance; instead, calculated P values are reported.
Birth rates were presented as absolute and relative frequencies for all births, segmented by gestational age at birth and lockdown phase. Odds ratios (OR) with 95% CIs were calculated, with CIs based on Fisher exact test. Contingencies between birth rate and birth year were assessed using the Pearson χ2 test or Fisher exact test for variables with a small number of cases. Two logistic regression models were analyzed to assess the association between birth year and the incidence of VPT births. First, the trend variable was calculated with year as numeric variable but without 2020, then the trend was calculated with 2020. Models were compared using Akaike Information Criterion. Contingency tables were created to compare the counts of premature and term births in each year category. Fisher exact test was used to determine the statistical significance of differences. Furthermore, the distribution of indicated and nonindicated deliveries was studied in 2020 compared with 2017 to 2019.
Maternal baseline and pregnancy characteristics, along with neonatal characteristics, were described using absolute and relative frequencies. Qualitative variables, like mode of delivery or sex, were analyzed using the Pearson χ2 test for contingency tables, and quantitative variables were summarized using medians and IQRs, using the 2-sided Wilcoxon rank-sum test for 2-sided hypotheses.
For pregnancy and birth risks, which involve multiple answer sets and result in a large number of parameters tested, P values were adjusted for multiple testing using the Holm method.24 In addition to ORs, Fisher exact test P values, 95% CIs, and adjusted P values were reported.
To investigate the association of the lockdown with VPT births, logistic regression models were constructed. Stratified models were included as an exploratory analysis to account for potential confounding factors (eg, maternal age >35 years, pathological CTG, and the occurrence of either amniotic infection syndrome, preterm labor, or premature rupture of membranes). Models were compared using Akaike Information Criterion, and the Tjur pseudo R2.25 Analyses were executed between August 2023 and July 2024.
Results
Live Births, Preterm Births, and Stillbirths Before and During the COVID-19 Pandemic
Our analyses sample covered 184 827 births overall from 2017 to 2020, including 719 stillbirths and 184 108 liveborn infants. A total of 901 infants were not considered in analyses because the gestational age at birth had not been documented. Pandemic-era enrollment was restricted to 44 481 births from March 14 to December 31, 2020, when lockdown restrictions were effective, and this period was further separated into the first (9207 births) and second (10 204 births) lockdowns and a period between them with less vigorous restrictions (25 070 births) (eFigure 1 in Supplement 1). Further details on the numbers of births are detailed within the flowchart (eFigure 2 in Supplement 1).
There was a 4.9% reduction in overall live births in 2020 compared with the prepandemic period (Table 1). There was also a lower rate of VPT births during the COVID-19 pandemic, with 572 preterm births (1.29%) documented in 2020, compared with 2064 preterm births (1.47%) during the control periods (Table 1). Odds of preterm birth were significantly lower during 2020 compared with the control period (OR, 0.87; 95% CI, 0.79-0.95) (Table 2; eFigure 3 in Supplement 1). We included a time-series analysis of the rates of total births and VPT births during 2017 to 2019 and 2017 to 2020 and observed an accelerated decrease of VPT births if 2020 was included (eTable 1 in Supplement 1). Analysis segregating VPT births into the different phases of the COVID-19 pandemic in 2020 found that the most prominent reduction in VPT births occurred in the third period, which corresponded with an equivalent reduction in total births (OR, 0.69; 95% CI, 0.55-0.85) (Table 2). Comparable changes were observed in the analyses of all preterm infants born at less than 37 weeks’ gestation and when considering preterm infants born 32 0/7 to 36 6/7 weeks (Table 1; eTable 2 in Supplement 1). Analysis of stillbirth rates during the COVID-19 pandemic did not find differences between the pandemic and the prepandemic eras or for the different COVID-19 periods; the combined consideration of live births and stillbirths did not change the degree of reduction in births (Table 3; eFigure 3 in Supplement 1).
Table 1. Changes in Birth Rates and Preterm Delivery Rates in 2017-2019 vs 2020.
| Period | Births, No. (%) | P valuea | ||
|---|---|---|---|---|
| 2017-2019 | 2020 | Total | ||
| Total births | ||||
| Overall | 140 346 (100) | 44 481 (100) | 184 827 (100) | .06 |
| March 14 to May 15 | 28 975 (20.65) | 9207 (20.7) | 38 182 (20.66) | .81 |
| May 16 to October 18 | 78 417 (55.87) | 25 070 (56.36) | 103 487 (55.99) | .07 |
| October 19 to December 31 | 32 954 (23.48) | 10 204 (22.94) | 43 158 (23.35) | .02 |
| Full term born infants (≥37 wk) | ||||
| Overall | 128 262 (91.39) | 40 943 (92.05) | 169 205 (91.55) | .18 |
| March 14 to May 15 | 26 457 (18.85) | 8472 (19.05) | 34 929 (18.9) | .78 |
| May 16 to October 18 | 71 721 (51.1) | 23 049 (51.82) | 94 770 (51.27) | .18 |
| October 19 to December 31 | 30 084 (21.44) | 9422 (21.18) | 39 506 (21.37) | .07 |
| Late preterm infants (32-36 wk) | ||||
| Overall | 9334 (6.65) | 2751 (6.18) | 12 085 (6.54) | .15 |
| March 14 to May 15 | 1927 (1.37) | 551 (1.24) | 2478 (1.34) | .48 |
| May 16 to October 18 | 5202 (3.71) | 1589 (3.57) | 6791 (3.67) | .06 |
| October 19 to December 31 | 2205 (1.57) | 611 (1.37) | 2816 (1.52) | .12 |
| Very preterm infants (<32 wk) | ||||
| Overall | 2064 (1.47) | 572 (1.29) | 2636 (1.43) | .01 |
| March 14 to May 15 | 445 (0.32) | 144 (0.32) | 589 (0.32) | .07 |
| May 16 to October 18 | 1097 (0.78) | 316 (0.71) | 1413 (0.76) | .37 |
| October 19 to December 31 | 522 (0.37) | 112 (0.25) | 634 (0.34) | .005 |
Calculated with Pearson test.
Table 2. Odds Ratios and Relative Risks for Very Preterm Birth at Less Than .
| Phase | Births, No. | OR (95% CI) | RR | P valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2017-2019 | ||||||||
| <32 wk | ≥37 wk | <32 wk | ≥37 wk | ||||||
| All | 572 | 40 943 | 2064 | 128 262 | 0.87 (0.79-0.95) | 0.87 (0.79-0.95) | .003 | ||
| March 14 to May 15 | 144 | 8472 | 445 | 26 457 | 1.01 (0.83-1.22) | 1.01 (0.84-1.21) | .92 | ||
| May 16 to October 18 | 316 | 23 049 | 1097 | 71 721 | 0.90 (0.79-1.02) | 0.90 (0.79-1.02) | .09 | ||
| October 19 to December 31 | 112 | 9422 | 522 | 30 084 | 0.69 (0.55-0.84) | 0.06 (0.56-0.84) | <.001 | ||
Abbreviations: OR, odds ratio; RR, relative risk.
Calculated with Fisher exact test.
Table 3. Maternal Baseline and Pregnancy Characteristics in 2017-2019 and 2020.
| Characteristic | Births, No. (%) | P value | ||
|---|---|---|---|---|
| 2017-2019 | 2020 | Total | ||
| All births | ||||
| No. | 140 346 | 44 481 | 184 827 | NA |
| Routine medical check-ups during pregnancy | ||||
| Median (IQR) | 11 (10-13) | 11 (9-13) | 11 (10-13) | .15a |
| Missing, No. | 35 496 | 10 931 | 46 427 | NA |
| Maternal age, median (IQR), y | 31 (28-35) | 32 (28-35) | 31 (28-35) | <.001a |
| GA at admission | ||||
| Median (IQR), wk | 39 4/7 (38 3/7 to 40 2/7) | 39 4/7 (38 4/7 to 40 3/7) | 39 4/7 (38 3/7 to 40 2/7) | <.001a |
| Missing, No. | 686 | 215 | 901 | NA |
| Admission to birth interval, median (IQR), d | 0 (0-1) | 0 (0-1) | 0 (0-1) | .28a |
| Antenatal steroids | 6399 (4.56) | 1481 (3.33) | 7880 (4.26) | <.001b |
| Mode of delivery | ||||
| Spontaneous | 93 711 (66.77) | 29 736 (66.85) | 123 447 (66.79) | .003b |
| Cesarean | 46 635 (33.23) | 14 745 (33.15) | 61 380 (33.21) | .76b |
| Emergency cesarean deliveryc | 2071 (4.44) | 708 (4.80) | 2779 (4.53) | .07b |
| Stillbirth | 548 (0.39) | 171 (0.38) | 719 (0.39) | .86b |
| Full term infants (≥37 wk) | ||||
| No. | 128 262 | 40 943 | 169 205 | NA |
| Routine medical check-ups during pregnancy | ||||
| Median (IQR) | 11 (10-13) | 11 (10-13) | 11 (10-13) | .02a |
| Missing, No. | 32 725 | 10 217 | 42 942 | NA |
| Maternal age, median (IQR), y | 31 (28-35) | 32 (28-35) | 31 (28-35) | <.001a |
| GA at admission, median (IQR), wk | 39 5/7 (38 5/7 to 40 3/7) | 39 5/7 (38 6/7-40 3/7) | 39 5/7 (38 5/7 to 40 3/7) | .01a |
| Admission to birth interval, , median (IQR), d | 0 (0-1) | 0 (0-1) | 0 (0-1) | .85a |
| Antenatal steroids | 2024 (1.58) | 330 (0.81) | 2354 (1.39) | <.001b |
| Mode of delivery | ||||
| Spontaneous | 88 419 (68.94) | 28 164 (68.79) | 116 583 (68.9) | <.001b |
| Cesarean | 39 843 (31.06) | 12 779 (31.21) | 52 622 (31.10) | .57b |
| Emergency cesarean deliveryc | 1560 (3.92) | 540 (4.23) | 2100 (3.99) | .12b |
| Stillbirth | 136 (0.11) | 53 (0.13) | 189 (0.11) | .22b |
| Late preterm infants (32-36 wk) | ||||
| No. | 9334 | 2751 | 12 085 | NA |
| Routine medical check-ups during pregnancy | ||||
| Median (IQR) | 9 (8-11) | 9 (8-11) | 9 (8-11) | .49a |
| Missing, No. | 1751 | 439 | 2190 | NA |
| Maternal age, median (IQR), y | 32 (29-36) | 32 (29-36) | 32 (29-36) | .65a |
| GA at admission, median (IQR), wk | 35 4/7 (34 1/7 to 36 2/7) | 35 4/7 (34 1/7-36 2/7) | 35 3/7 (34 0/7-36 2/7) | .12a |
| Admission to birth interval, median (IQR), d | 0 (0-2) | 1 (0-2) | 1 (0-2) | .44a |
| Antenatal steroids | 2738 (29.33) | 707 (25.70) | 3445 (28.51) | <.001b |
| Mode of delivery | ||||
| Spontaneous | 4272 (45.79) | 1280 (46.53) | 5554 (45.96) | .17b |
| Cesarean | 5060 (54.21) | 1471 (53.47) | 6531 (54.04) | .49b |
| Emergency cesareanc | 291 (5.75) | 104 (7.07) | 395 (6.05) | .06b |
| Stillbirth | 137 (1.47) | 41 (1.49) | 178(1.47) | .93b |
| Very preterm infants (<32 wk) | ||||
| No | 2064 | 572 | 2636 | NA |
| Routine medical check-ups during pregnancy | ||||
| Median (IQR), No. | 6 (5-8) | 6 (5-8) | 6 (5-8) | .90a |
| Missing, No. | 44 | 96 | 544 | NA |
| Maternal age, median (IQR), y | 32 (28-36) | 32 (29-36) | 32 (28-36) | .65a |
| GA at admission, median (IQR), wk | 27 2/7 (24 3/7 to 29 6/7) | 27 2/7 (24 5/7 to 29 5/7) | 27 2/7 (24 4/7 to 29 6/7) | .54a |
| Admission to birth interval, d | 3 (1-9) | 2 (1-8) | 3 (1-9) | .18a |
| Antenatal steroids | 1624 (78.67) | 441 (77.10) | 2065 (78.34) | .42b |
| Mode of delivery | ||||
| Spontaneous | 550 (26.65) | 147 (25.70) | 697 (26.44) | .04b |
| Cesarean | 1514 (73.35) | 425 (74.30) | 1939 (73.56) | .65b |
| Emergency cesarean deliveryc | 206 (13.60) | 61 (14.35) | 267 (13.77) | .69b |
| Stillbirth | 267 (12.94) | 76 (13.29) | 343 (13.01) | .83b |
Abbreviations: GA, gestational age; NA, not applicable.
Test used: Wilcoxon test.
Test used: Pearson test.
Relative frequency is calculated in relation to all cesarean deliveries.
Maternal and Neonatal Characteristics Before and During the COVID-19 Pandemic
There were no clinically relevant differences between the pandemic and prepandemic eras for the total cohort or among preterm births in prenatal care characteristics, including the number of routine medical check-ups during pregnancy, gestational age at admission, period between admission to hospital and delivery, ANS application, mode of delivery, and frequency of emergency cesarean delivery (Table 3). Among neonatal characteristics, there were no differences in gestational age, birth weight and head circumference at birth, sex, or singleton vs multiple birth between the COVID-19 pandemic era and the corresponding periods in 2017 to 2019. Furthermore, blood gas parameters of pH and negative base excess and Apgar scores at 1, 5, and 10 minutes after birth did not differ. Separate consideration of the preterm infant population rendered the same results (Table 4).
Table 4. Neonatal Characteristics in 2017-2019 and 2020.
| Characteristic | 2017-2019 | 2020 | Total | P value | |
|---|---|---|---|---|---|
| All births | |||||
| No. | 140 346 | 44 481 | 184 827 | NA | |
| Gestational age at birth | |||||
| Median (IQR), wk | 39 4/7 (38 4/7 to 40 3/7) | 39 4/7 (38 4/7 to 40 3/7) | 39 4/7 (38 4/7 to 40 3/7) | <.001a | |
| Missing, No. | 686 | 215 | 901 | NA | |
| Birth weight, g | 3380 (3040 to 3700) | 3400 (3065 to 3710) | 3380 (3050 to 3700) | <.001a | |
| Head circumference at birth | |||||
| Median (IQR), cm | 35.00 (34.00 to 36.00) | 35.00 (34.00 to 36.00) | 35.00 (34.00 to 36.00) | .52a | |
| Missing, No. | 51 880 | 24 249 | 76 129) | NA | |
| Sex, No. (%) | |||||
| Male | 72 215 (51.45) | 22 831 (51.33) | 95 046 (51.42) | .09c | |
| Female | 68 109 (48.53) | 21 641 (48.65) | 89 750 (48.56) | ||
| Diverseb | 0 | 2 (<0.01) | 2 (<0.01) | ||
| Undefined | 22 (0.02) | 7 (0.02) | 29 (0.02) | ||
| Multiples, No. (%) | 5451 (3.88) | 1662 (3.74) | 7113 (3.85) | .16c | |
| Umbilical cord blood gas | |||||
| pH | |||||
| Median (IQR), | 7.28 (7.22 to 7.34) | 7.28 (7.21 to 7.33) | 7.28 (7.22 to 7.33) | <.001a | |
| Missing, No. | 1485 | 483 | 1986 | NA | |
| Base excess | |||||
| Median (IQR) | −3.70 (−6.30 to −1.40) | −3.80 (−6.40 to −1.50) | −3.70 (−6.30 to −1.50) | <.001a | |
| Missing | 1834 | 484 | 2318 | NA | |
| Apgar score | |||||
| 1 Min | |||||
| Median (IQR) | 9 (9 to 9) | 9 (9 to 9) | 9 (9 to 9) | .19a | |
| Missing | 563 | 185 | 748 | NA | |
| 5 Min | |||||
| Median (IQR) | 10 (10 to 10) | 10 (10 to 10) | 10 (10 to 10) | <.001a | |
| Missing | 627 | 193 | 820 | NA | |
| 10 Min | |||||
| Median (IQR) | 10 (10 to 10) | 10 (10 to 10) | 10 (10 to 10) | .02a | |
| Missing | 733 | 216 | 949 | NA | |
| Full term infants (≥37 wk) | |||||
| No. | 128 262 | 40 943 | 169 205 | ||
| Gestational age at birth, median (IQR), wk | 39 5/7 (38 6/7 to 40 4/7) | 39 5/7 (38 6/7 to 40 4/7) | 39 5/7 (38 6/7 to 40 4/7) | .02a | |
| Birth weight, median (IQR), g | 3420 (3130 to 3725) | 3440 (3145 to 3740) | 3425 (3130 to 3730) | <.001a | |
| Head circumference at birth | |||||
| Median (IQR), cm | 35.00 (34.00 to 36.00) | 35.00 (34.00 to 36.00) | 35.00 (34.00 to 36.00) | .89a | |
| Missing, No. | 46 125 | 22 037 | 68 162 | NA | |
| Sex, No. (%) | |||||
| Male | 65 562 (51.12) | 20 893 (51.03) | 86 455 (51.09) | .06c | |
| Female | 62 682 (48.87) | 20 045 (48.96) | 82 727 (48.89) | ||
| Diverseb | 0 | 2 (<0.01) | 2 (<0.01) | ||
| Undefined | 18 (0.01) | 3 (0.01) | 21 (0.01) | ||
| Multiples, No. (%) | 2457 (1.92) | 748 (1.83) | 3205 (1.89) | .25c | |
| Umbilical cord blood gas | |||||
| pH | |||||
| Median (IQR) | 7.28 (7.22 to 7.33) | 7.27 (7.21 to 7.33) | 7.28 (7.22 to 7.33) | <.001a | |
| Missing, No. | 815 | 291 | 1106 | NA | |
| Base excess | |||||
| Median (IQR) | −3.80 (−6.40 to −1.50) | −3.90 (−6.50 to −1.60) | −3.80 (−6.40 to −1.50) | <.001a | |
| Missing, No. | 1132 | 292 | 1424 | NA | |
| Apgar score | |||||
| 1 Minute | |||||
| Median (IQR) | 9 (9 to 9) | 9 (9 to 9) | 9 (9 to 9) | .13a | |
| Missing, No. | 223 | 75 | 298 | NA | |
| 5 Minutes | |||||
| Median (IQR) | 10 (10 to 10) | 10 (10 to 10) | 10 (10 to 10) | <.001a | |
| Missing, No. | 234 | 69 | 303 | NA | |
| 10 Minutes | |||||
| Median (IQR) | 10 (10 to 10) | 10 (10 to 10) | 10 (10 to 10) | <.001a | |
| Missing, No. | 280 | 84 | 364 | NA | |
| Late preterm infants (32 to 36 wk) | |||||
| No. | 9334 | 2751 | 12 085 | ||
| Gestational age at birth, median (IQR), wk | 35 5/7 (34 4/7 to 36 3/7) | 35 5/7 (34 4/7 to 36 3/7) | 35 5/7 (34 4/7 to 36 3/7) | .45a | |
| Birth weight, median (IQR), g | 2500 (2150 to 2830) | 2490 (2142 to 2800) | 2500 (2150 to 2820) | .32a | |
| Head circumference at birth | |||||
| Median (IQR), cm | 33.00 (32.00 to 34.00) | 33.00 (32.00 to 34.00) | 33.00 (32.00 to 34.00) | .68a | |
| Missing, No. | 4055 | 1606 | 5661 | NA | |
| Sex, No. (%) | |||||
| Male | 5175 (55.44) | 1534 (55.76) | 6709 (55.52) | .13c | |
| Female | 4157 (44.54) | 1214 (44.13) | 5371 (44.54) | ||
| Undefined | 2 (0.02) | 3 (0.11) | 5 (0.04) | ||
| Multiples, No. (%) | 2333 (24.99) | 757 (27.52) | 3090 (25.57) | .008c | |
| Umbilical cord blood gas | |||||
| pH | |||||
| Median (IQR) | 7.30 (7.24 to 7.35) | 7.30 (7.24 to 7.34) | 7.30 (7.24 to 7.34) | .006a | |
| Missing, No. | 232 | 72 | 304 | NA | |
| Base excess | |||||
| Median (IQR) | −2.90 (−5.40 to −0.90) | −3.00 (−5.50 to −1.00) | −2.90 (−5.40 to −1.00) | .18a | |
| Missing, No. | 262 | 72 | 334 | NA | |
| Apgar score | |||||
| 1 Minute | |||||
| Median (IQR) | 9 (8 to 9) | 9 (8 to 9) | 9 (8 to 9) | .005a | |
| Missing, No. | 118 | 44 | 162 | NA | |
| 5 Minutes | |||||
| Median (IQR) | 10 (9 to 10) | 10 (9 to 10) | 10 (9 to 10) | .003a | |
| Missing, No. | 143 | 51 | 194 | NA | |
| 10 Minutes | |||||
| Median (IQR) | 10 (10 to 10) | 10 (9 to 10) | 10 (9 to 10) | .16a | |
| Missing, No. | 261 | 54 | 216 | NA | |
| Very preterm infants (<32 wk) | |||||
| No. | 2064 | 572 | 2636 | NA | |
| Gestational age at birth, median (IQR), wk | 28 5/7 (26 1/7 to 30 4/7) | 28 5/7 (25 6/7 to 30 4/7) | 28 5/7 (26 0/7 to 30 4/7) | .80a | |
| Birth weight, median (IQR), g | 1090 (740 to 1440) | 1091 (739 to 1446) | 1090 (740 to 1440) | .83a | |
| Head circumference at birth, cm | |||||
| Median (IQR), cm | 26.50 (23.50 to 28.15) | 26.00 (23.00 to 29.00) | 26.30 (23.00 to 28.50) | .49a | |
| Missing, No. | 1257 | 410 | 1667 | NA | |
| Sex, No. (%) | |||||
| Male | 1132 (54.84) | 292 (51.05) | 1424 (54.02) | .25c | |
| Female | 930 (45.06) | 279 (48.78) | 1209 (45.86) | ||
| Undefined | 2 (0.10) | 1 (0.17) | 3 (0.11) | ||
| Multiples, No. (%) | 646 (31.3) | 157 (27.4) | 803 (30.5) | .08c | |
| Umbilical cord blood gas | |||||
| pH | |||||
| Median (IQR) | 7.32 (7.26 to 7.36) | 7.31 (7.25 to 7.36) | 7.32 (7.26 to 7.36) | .05a | |
| Missing, No. | 418 | 119 | 537 | NA | |
| Base excess | |||||
| Median (IQR) | −2.70 (−5.10 to −0.80) | −2.60 (−4.70 to −0.60) | −2.70 (−5.10 to −0.80) | .28a | |
| Missing, No. | 419 | 119 | 538 | NA | |
| Apgar score | |||||
| 1 Minute | |||||
| Median (IQR) | 7 (5 to 8) | 7 (4 to 8) | 7 (5 to 8) | .07a | |
| Missing, No. | 216 | 65 | 361 | NA | |
| 5 Minutes | |||||
| Median (IQR) | 8 (7 to 9) | 8 (6 to 9) | 8 (7 to 9) | .25a | |
| Missing, No. | 243 | 72 | 315 | NA | |
| 10 Minutes | |||||
| Median (IQR) | 9 (8 to 9) | 9 (8 to 9) | 9 (8 to 9) | .05a | |
| Missing, No. | 284 | 77 | 361 | NA | |
Abbreviation: NA, not applicable.
Tests used: Wilcoxon test.
Diverse is a legal option for sex assignment at birth in Germany for intersex infants when assignment to either male or female is not completely clear.
Tests used: Pearson test.
Maternal Risk Factors for Preterm Delivery
When comparing maternal baseline characteristics for the total cohort before and during the COVID-19 pandemic and applying adjustment for multiple testing, no differences were detected for maternal age older than 35 years, history of preterm birth, history of previous cesarean delivery or uterine surgery, or complications during previous pregnancies. However, the odds of VPT birth were decreased in pregnancies with a history of maternal serious disease (OR, 0.64; 95% CI, 0.50-0.83), while other factors, such as a family history of serious disease, diabetes during pregnancy, maternal obesity, serious or severe psychological burden during pregnancy, preexisting hypertension, bleeding at less than 28 weeks or at 28 weeks or later, placenta previa, and multiple pregnancies, remained unchanged (Figure, A; eTable 3 in Supplement 1).13 Analyses of the total cohort of preterm infants born at less than 37 weeks found the same results, aside from maternal age older than 35 years. When only considering preterm infants born at 32 0/7 to 36 6/7 weeks, maternal age older than 35 years remained a risk factor but not a history of maternal serious disease (eTable 4 and eTable 5 in Supplement 1).
Figure. Changes of Maternal Baseline Risks and Causes of Preterm Deliveries During the COVID-19 Pandemic.
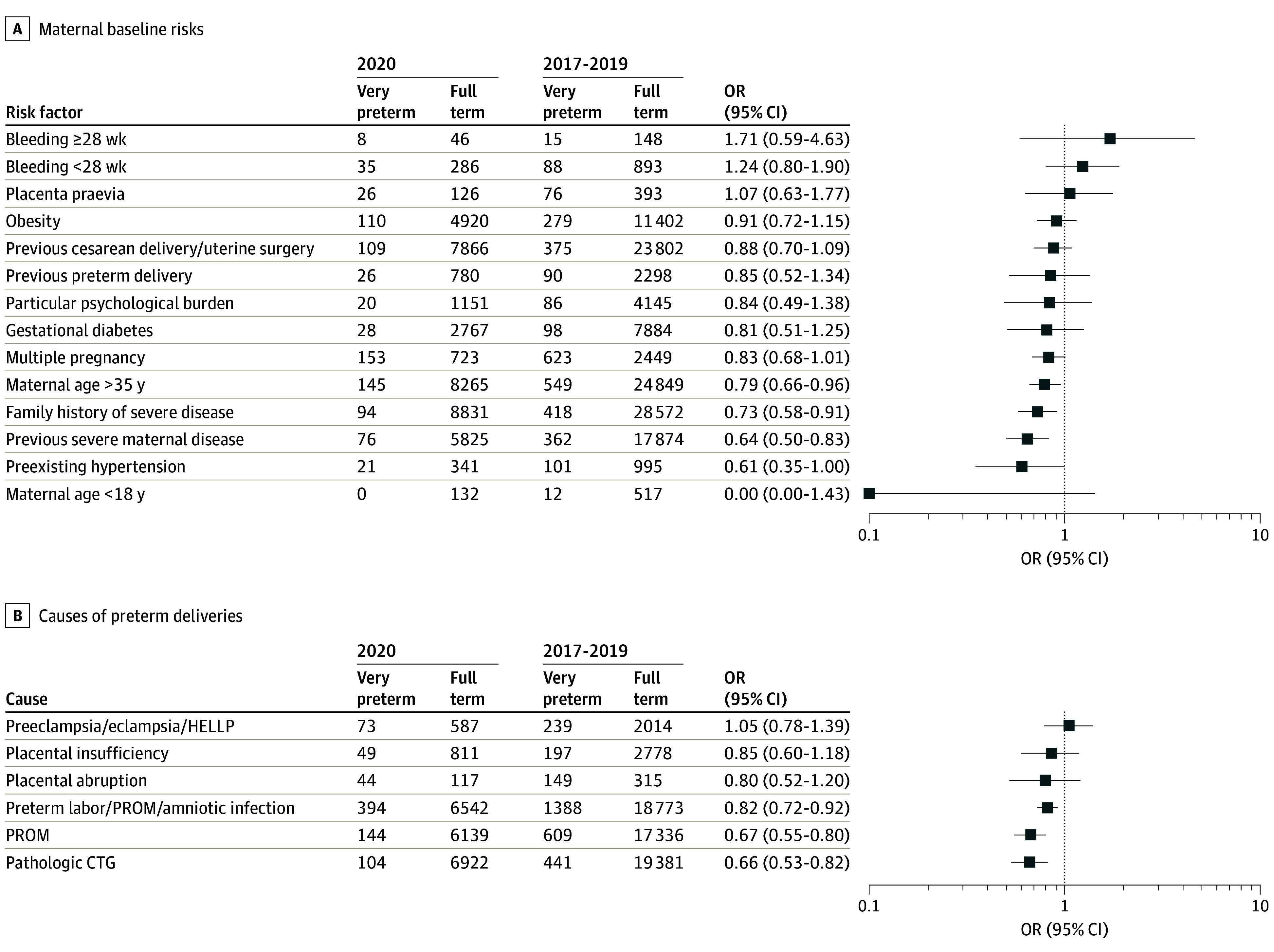
Analyses of maternal baseline risks (A) and causes of preterm deliveries (B) from the quality-assurance registry of Hesse, Germany, comparing the prepandemic period (2017-2019) and the COVID-19 pandemic (2020) in preterm births at less than 32 weeks are presented as odds ratios (ORs) and 95% CIs. CTG indicates cardiotocography; HELLP, hemolysis, elevated liver enzymes, low platelet count; PROM, premature rupture of membranes
Causes of Preterm Births Before and During the COVID-19 Pandemic
Lastly, we evaluated the variations in causes of preterm birth before and during the pandemic. While preterm birth for maternal hypertension; preeclampsia, eclampsia, or HELLP syndrome; placental insufficiency with intrauterine growth restriction; and placental disruption did not change, the frequency of births for intrauterine infection was reduced among VPT infants during the pandemic. Furthermore, odds of births for pathologic CTG were lower (OR, 0.66; 95% CI, 0.53-0.82) (Figure, B; eTable 3 in Supplement 1). The reductions of preterm deliveries at less than 32 weeks were comparable in the categories of all indicated and nonindicated births (eFigure 4 in Supplement 1). Lastly, we studied the associations of maternal age, pathological CTG, and indicated birth with our outcomes of interest (eTable 6 in Supplement 1). Again, the evaluation of all preterm infants born at less than 37 weeks and of those delivered at 32 0/7 weeks to 36 6/7 weeks rendered congruent results for intrauterine infection, while pathologic CTG was not a significant cause of birth in this patient population (eTable 4 and eTable 5 in Supplement 1).
Discussion
This population-based, descriptive, epidemiological cohort study of more than 180 000 births from the federal state of Hesse, Germany, had 2 key findings that are of importance for future studies on the variations in VPT birth rates during the COVID-19 pandemic and for all future research efforts intended to reduce the rate of preterm births. First, we mirrored the findings from previous studies that strict lockdown measures were associated with a reduction of preterm birth rates, but we found changing dynamics of this association and increased associations with later stages of the pandemic, including restrictions of normal work and private life.12 This finding is relevant, as the longer duration of altered exposure might have implications for future research strategies to prevent preterm birth; furthermore, this finding argues for taking into consideration the total duration of pregnancy and not solely the third trimester. This consideration has not been fully examined by other studies that compared premature birth rates during the COVID-19 pandemic period with the prepandemic period. Additionally, we did not observe the previously reported shift toward higher gestational ages within the population of preterm infants.26 Second, we observed relevant changes in maternal risks and causes for preterm birth, including lower rates of history of own serious disease and delivery for intrauterine infection, while other factors, like bleeding during pregnancy, multiple pregnancy, psychological distress, and preeclampsia, eclampsia, or HELLP syndrome, were not altered. This was reflected by the comparable reduction in indicated and nonindicated preterm deliveries. We postulate that measures enacted during the pandemic to mitigate the spread of COVID-19 had differing levels of impact for the different risk factors and causes of preterm birth. Our registry-based analyses do not allow us to assess variations in clinicians’ attitudes toward delivery in nonemergency situations, but maternal and fetal emergency situations should not have changed the indication for delivery. Overall, these results demand separate consideration of the different risks and causes of preterm birth.
Data Interpretation in the Context of the Hygiene Hypothesis
Our finding of decreased incidence of preterm births for intrauterine infection might indicate that the contact and hygiene restrictions during the COVID-19 pandemic also reduced preterm births. Alternatively, the very strict lockdown measures and stay-at-home orders might have reduced individual stress levels during pregnancy, particularly with respect to work and leisure activity stressors, and the association of this potentially reduced stress with preterm birth has not been evaluated thoroughly.27,28 As the number of routine medical check-ups during pregnancy in the ambulatory sector was unchanged, our results argue against lower utilization of preventive health care services. The unchanged time intervals from admission to birth, the constant rate of ANS application, and the constant distribution of modes of delivery argue against altered preclinical medical care use, delayed hospital admissions, or changes in hospital care practices as observed in other disease entities and emergency situations during the COVID-19 pandemic. This might be due to individuals’ own estimation of their vulnerability related to pregnancy, COVID-19, and other health factors, which did not lead to a change in behaviors for seeking medical care.29,30,31,32 It is encouraging that the direction of health care resources toward the treatment of patients with COVID-19 infection did not result in neglect or lower prioritization of pregnant individuals admitted for immanent preterm birth by hospital staff. In these points, our results are broadly in line with other publications on this topic.10,33,34
Measurements for Preterm Birth Prevention
While improvements in postnatal preterm care have dramatically improved survival and outcomes for preterm infants during the last decades, efforts to reduce the rate of preterm deliveries have not resulted in comparable improvements, and rates of preterm births continue to increase.35,36,37 While baseline risk factors, including maternal age older than 35 years, multiple pregnancies, and the use of medical reproductive measures, particularly assisted reproductive technologies, are nonmodifiable except by increasing awareness and education on the heightened risks, secondary obstetric preventive measures so far have failed to reduce the risk of preterm births. Particularly disappointingly, all the secondary prevention measures and strategies to prolong the duration of pregnancy in patient populations at risk of preterm birth, including cervical pessary or cerclage, vaginal progesterone, and the use of tocolytic agents, have had low efficacy in reducing rates of preterm birth.38,39,40 Therefore, this obstetric challenge remains unresolved. Our findings of a reduction in the overall rate of preterm birth at less than 37 weeks’ gestation for high-risk pregnancies, including maternal age older than 35 years and history of serious maternal disease, during the COVID-19 pandemic might be indicative of a reduced preparedness of women with baseline risk factors to get pregnant or lower frequency of pregnancies following artificial fertilization. This suggests that primary preventative measures could be effective in reducing the risk of premature birth. While our datasets do not allow to specify which lockdown measures in the working environment and private life may account for this reduction, our results justify further elaboration within future prospective studies. Primary preventive measures might be more effective than prolonging the gestation in pregnancies with evident risks. It remains speculative how the drop in pathologic CTG rates during the COVID-19 pandemic can be explained. An Australian analysis provides a potential explanation: there was a decrease in iatrogenic preterm deliveries for fetal compromise, including pathologic CTG cases, and a simultaneous increase in stillbirths, and we cannot exclude underdocumentation of such events during the COVID-19 pandemic. One further explanation might be that pathologic CTG is a part of a larger risk constellation and thereby might reflect pregnancies in which no underlying baseline risks were documented. Our divergent results for the different risk categories and causes for preterm births indicate that there will be no one-size-fits-all strategy. Different approaches and focus on prespecified relevant clinical outcomes will be necessary, as is already established other clinical practices, such as in oncologic therapies.41,42 The COVID-19 lockdown measures were not associated with reduced rates of prematurity for other baseline risk constellations, such as preeclampsia, eclampsia, and HELLP syndrome, as reported elsewhere.13 These risks are not predetermined by modifiable baseline characteristics that may have been impacted by the pandemic, suggesting more work is needed to find efficient screening and prevention measures that can be applied in the general public.
Strengths and Limitations
Our descriptive epidemiological cohort study has several important strengths, including the use of a population-level study cohort from a region that is broadly representative of routine medical care during pregnancy and the risks and preventive measures for preterm birth in Germany and Europe. Due to the obligatory documentation within the quality assurance registry, the database has complete coverage, and the sample size of preterm births is larger than in most previous reports on this topic.2,7,8,9 Furthermore, a broad range of clinically relevant and patient-focused measures were available that were collected based on prespecified and pretested items. Additionally, we were able to exclude carry-over effects by changes in the stillbirth rate.
This study has several limitations. First, while the patient data collection was complete, we cannot exclude underreporting of pregnancy risks, as these items were not individually queried but were collected within 1 section of the documentation system. We also cannot exclude individuals who had more than 1 preterm delivery during the COVID-19 pandemic, but this should have been a rare event. Second, the introduction of a national guideline on the prevention and management of preterm birth in 2019 may have affected outcomes of this study, but it is unlikely that the categories of causes of preterm deliveries with declines were influenced to such an extent, and the implementation of these new guidelines may have been delayed due to the pandemic. Most importantly, the rate of preterm births at less than 32 weeks returned to the prepandemic level after the official end of the pandemic in Germany. Third, changes in psychological states are well described for the COVID-19 pandemic. In our analysis, we did not see an increased psychological stress burden, which might be due to underreporting from patients themselves.43 Fourth, we cannot exclude residual confounding by undocumented characteristics, such as physical activity, maternal diet, nicotine or alcohol consumption during pregnancy, maternal body mass index, and availability of prevention programs of preterm delivery before and during the pandemic. Fifth, we were not able to assess variations in societal stressors during pregnancy or in SARS-CoV-2 infection rates during pregnancy. Sixth, the sample did not allow us to examine outcomes by monthly intervals to describe changes over time. Furthermore, we cannot assess whether and how increasing concern regarding the COVID-19 pandemic impacted the behavior of pregnant individuals before the lockdown measures were enacted. Therefore, we decided not to introduce further subcategories. Additionally, we studied pregnancies and preterm births from 1 region, so our findings should be assessed against other independent cohorts during the COVID-19 pandemic. Undocumented changes in lifestyle, exposure to infectious agents besides COVID-19, and societal behavior change associated with the COVID-19 pandemic might also have contributed to the results.
Conclusions
The findings of this cohort study suggest that there was no deterioration of medical care during pregnancy or immediately before preterm delivery, which allowed us to observe the reduction in preterm birth rates during the COVID-19 pandemic. Importantly, the duration of exposure to the strict lockdown measures and stay-at-home orders was associated with the degree of reduction in preterm births. The combined analyses of all available datasets revealed that primary care measures were associated with reduced risk for preterm birth in several categories of high-risk pregnancies. The unchanged level of psychological burden documented during pregnancy suggests that it may be worthwhile to study measures to reduce maternal stress in efforts to decrease risk of preterm births for intrauterine infection and that research should focus on reducing rates of preterm deliveries in pregnancies among individuals with a history of serious disease. Our findings add novel information on the epidemiology of the COVID-19 pandemic in association with preterm birth prevention and suggest that further research efforts on primary preventive measures and different causes of preterm delivery are justified and promising.
eFigure 1. Diagram of the Pandemic Phases and Corresponding Prepandemic Time Periods
eFigure 2. Flowchart of the Total Sample and Patient Subpopulations Included in the Study
eFigure 3. Livebirths, Preterm Births <32 Weeks’ Gestation, and Stillbirths by Year (2017-2020)
eFigure 4. Indicated and Nonindicated Preterm Births (<32 Weeks) Before and During the COVID-19 Pandemic
eTable 1. Time Effect With and Without the Year 2020 (COVID-19 Lockdown)
eTable 2. Odds Ratios for Prematurity (<37 Weeks) in Different Phases of the Year
eTable 3. Pregnancy and Birth Risks in 2017-2019 and 2020 in Very Preterm (<32 Weeks) Infants
eTable 4. Pregnancy and Birth Risks in 2017-2019 and 2020 in Preterm (<37 Weeks) Infants
eTable 5. Pregnancy and Birth Risks in 2017-2019 and 2020 in Late Preterm (32 to 36 Weeks) Infants
eTable 6. Logistic Regression Models for Very Preterm Births <32 Weeks vs Term-Born Infants With Covariates
Data Sharing Statement
References
- 1.Dema E, Gibbs J, Clifton S, et al. Initial impacts of the COVID-19 pandemic on sexual and reproductive health service use and unmet need in Britain: findings from a quasi-representative survey (Natsal-COVID). Lancet Public Health. 2022;7(1):e36-e47. doi: 10.1016/S2468-2667(21)00253-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil A, von Dadelszen P, Kalafat E, et al. ; PregnaCOVID3 study group . Change in obstetric attendance and activities during the COVID-19 pandemic. Lancet Infect Dis. 2021;21(5):e115. doi: 10.1016/S1473-3099(20)30779-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allotey J, Stallings E, Bonet M, et al. ; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchand G, Patil AS, Masoud AT, et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to June 3, 2021. AJOG Glob Rep. 2022;2(1):100049. doi: 10.1016/j.xagr.2021.100049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vousden N, Bunch K, Morris E, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One. 2021;16(5):e0251123. doi: 10.1371/journal.pone.0251123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324(7):705-706. doi: 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedermann G, Hedley PL, Bækvad-Hansen M, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):93-95. doi: 10.1136/archdischild-2020-319990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip RK, Purtill H, Reidy E, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health. 2020;5(9):e003075. doi: 10.1136/bmjgh-2020-003075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5(11):e604-e611. doi: 10.1016/S2468-2667(20)30223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurol-Urganci I, Waite L, Webster K, et al. Obstetric interventions and pregnancy outcomes during the COVID-19 pandemic in England: a nationwide cohort study. PLoS Med. 2022;19(1):e1003884. doi: 10.1371/journal.pmed.1003884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakley LL, Örtqvist AK, Kinge J, et al. Preterm birth after the introduction of COVID-19 mitigation measures in Norway, Sweden, and Denmark: a registry-based difference-in-differences study. Am J Obstet Gynecol. 2022;226(4):550.e1-550.e22. doi: 10.1016/j.ajog.2021.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawco S, Rolnik DL, Woolner A, et al. The impact of mitigation measures on perinatal outcomes during the first nine months of the COVID-19 pandemic: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2022;274:117-127. doi: 10.1016/j.ejogrb.2022.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(6):e759-e772. doi: 10.1016/S2214-109X(21)00079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dench D, Joyce T, Minkoff H. United States preterm birth rate and COVID-19. Pediatrics. 2022;149(5):e2021055495. doi: 10.1542/peds.2021-055495 [DOI] [PubMed] [Google Scholar]
- 15.Hwang SS, Weikel BW, Hannan KE, Bourque SL. Impact of coronavirus disease-19 “stay-at-home” orders on preterm birth in Colorado. J Pediatr. 2022;242:238-241.e1. doi: 10.1016/j.jpeds.2021.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson AN, Snelgrove JW, Sutradhar R, Everett K, Liu N, Baxter NN. Perinatal outcomes during the COVID-19 pandemic in Ontario, Canada. JAMA Netw Open. 2021;4(5):e2110104. doi: 10.1001/jamanetworkopen.2021.10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedley PL, Hedermann G, Hagen CM, et al. Preterm birth, stillbirth and early neonatal mortality during the Danish COVID-19 lockdown. Eur J Pediatr. 2022;181(3):1175-1184. doi: 10.1007/s00431-021-04297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litman EA, Yin Y, Nelson SJ, Capbarat E, Kerchner D, Ahmadzia HK. Adverse perinatal outcomes in a large United States birth cohort during the COVID-19 pandemic. Am J Obstet Gynecol MFM. 2022;4(3):100577. doi: 10.1016/j.ajogmf.2022.100577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Curtis M, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(4):456. doi: 10.1136/archdischild-2020-320682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey EM, McNeer E, McDonald MF, et al. Association of preterm birth rate with COVID-19 statewide stay-at-home orders in Tennessee. JAMA Pediatr. 2021;175(6):635-637. doi: 10.1001/jamapediatrics.2020.6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handley SC, Mullin AM, Elovitz MA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March-June 2020. JAMA. 2021;325(1):87-89. doi: 10.1001/jama.2020.20991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui L, Marzan MB, Potenza S, et al. Increase in preterm stillbirths in association with reduction in iatrogenic preterm births during COVID-19 lockdown in Australia: a multicenter cohort study. Am J Obstet Gynecol. 2022;227(3):491.e1-491.e17. doi: 10.1016/j.ajog.2022.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hviid A, Laksafoss A, Hedley P, et al. Assessment of seasonality and extremely preterm birth in Denmark. JAMA Netw Open. 2022;5(2):e2145800. doi: 10.1001/jamanetworkopen.2021.45800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat Theory Appl. 1979;6(2):65-70. [Google Scholar]
- 25.Tjur T. Coefficients of determination in logistic regression models—a new proposal: the coefficient of discrimination. Am Stat. 2009;63(4):366-372. doi: 10.1198/tast.2009.08210 [DOI] [Google Scholar]
- 26.Shukla VV, Carper BA, Ambalavanan N, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Social distancing and extremely preterm births in the initial COVID-19 pandemic period. J Perinatol. 2024;44(7):1050-1057. doi: 10.1038/s41372-024-01898-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKee KS, Tang X, Tung I, et al. ; program collaborators for Environmental influences on Child Health Outcomes . Perinatal outcomes during versus prior to the COVID-19 pandemic and the role of maternal depression and perceived stress: a report from the ECHO Program. Am J Perinatol. 2024;41(S 01):e1404-e1420. doi: 10.1055/a-2033-5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladstone ME, Paquin V, McLean MA, et al. Prenatal maternal stress was not associated with birthweight or gestational age at birth during COVID-19 restrictions in Australia: the BITTOC longitudinal cohort study. Aust N Z J Obstet Gynaecol. 2023;63(4):509-515. doi: 10.1111/ajo.13673 [DOI] [PubMed] [Google Scholar]
- 29.Glance LG, Joynt Maddox KE, Shang J, et al. The COVID-19 pandemic and associated inequities in acute myocardial infarction treatment and outcomes. JAMA Netw Open. 2023;6(8):e2330327. doi: 10.1001/jamanetworkopen.2023.30327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burus T, Lei F, Huang B, et al. Undiagnosed Cancer cases in the US during the first 10 months of the COVID-19 pandemic. JAMA Oncol. 2024;10(4):500-507. doi: 10.1001/jamaoncol.2023.6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llanos AAM, Ashrafi A, Ghosh N, et al. Evaluation of inequities in cancer treatment delay or discontinuation following SARS-CoV-2 infection. JAMA Netw Open. 2023;6(1):e2251165. doi: 10.1001/jamanetworkopen.2022.51165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGowan EC, McGrath M, Law A, et al. ; program collaborators for Environmental Influences on Child Health Outcomes (ECHO) . Health care utilization during the COVID-19 pandemic among individuals born preterm. JAMA Netw Open. 2023;6(4):e2310696. doi: 10.1001/jamanetworkopen.2023.10696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gemmill A, Casey JA, Catalano R, Karasek D, Margerison CE, Bruckner T. Changes in preterm birth and caesarean deliveries in the United States during the SARS-CoV-2 pandemic. Paediatr Perinat Epidemiol. 2022;36(4):485-489. doi: 10.1111/ppe.12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fresson J, Bruckner TA, Ray CL, et al. Decreases in preterm birth during the first COVID-19 lockdown in France by gestational age sub-groups and regional COVID-19 incidence. Ann Epidemiol. 2022;72:74-81. doi: 10.1016/j.annepidem.2022.05.004 [DOI] [PubMed] [Google Scholar]
- 35.Cao G, Liu J, Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990-2019. JAMA Pediatr. 2022;176(8):787-796. doi: 10.1001/jamapediatrics.2022.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel RM, Kandefer S, Walsh MC, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331-340. doi: 10.1056/NEJMoa1403489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell EF, Hintz SR, Hansen NI, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327(3):248-263. doi: 10.1001/jama.2021.23580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EPPPIC Group . Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397(10280):1183-1194. doi: 10.1016/S0140-6736(21)00217-8 [DOI] [PubMed] [Google Scholar]
- 39.Yamaji N, Suzuki H, Saito K, et al. Tocolytic therapy inhibiting preterm birth in high-risk populations: a systematic review and meta-analysis. Children (Basel). 2023;10(3):443. doi: 10.3390/children10030443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman MK, Clifton RG, Biggio JR, et al. ; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network . Cervical pessary for prevention of preterm birth in individuals with a short cervix: the TOPS randomized clinical trial. JAMA. 2023;330(4):340-348. doi: 10.1001/jama.2023.10812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger TR, Wen PY, Lang-Orsini M, Chukwueke UN. World Health Organization 2021 classification of central nervous system tumors and implications for therapy for adult-type gliomas: a review. JAMA Oncol. 2022;8(10):1493-1501. doi: 10.1001/jamaoncol.2022.2844 [DOI] [PubMed] [Google Scholar]
- 42.Petty WJ, Paz-Ares L. Emerging strategies for the treatment of small cell lung cancer: a review. JAMA Oncol. 2023;9(3):419-429. doi: 10.1001/jamaoncol.2022.5631 [DOI] [PubMed] [Google Scholar]
- 43.Madigan S, Racine N, Vaillancourt T, et al. Changes in depression and anxiety among children and adolescents from before to during the COVID-19 pandemic: a systematic review and meta-analysis. JAMA Pediatr. 2023;177(6):567-581. doi: 10.1001/jamapediatrics.2023.0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Diagram of the Pandemic Phases and Corresponding Prepandemic Time Periods
eFigure 2. Flowchart of the Total Sample and Patient Subpopulations Included in the Study
eFigure 3. Livebirths, Preterm Births <32 Weeks’ Gestation, and Stillbirths by Year (2017-2020)
eFigure 4. Indicated and Nonindicated Preterm Births (<32 Weeks) Before and During the COVID-19 Pandemic
eTable 1. Time Effect With and Without the Year 2020 (COVID-19 Lockdown)
eTable 2. Odds Ratios for Prematurity (<37 Weeks) in Different Phases of the Year
eTable 3. Pregnancy and Birth Risks in 2017-2019 and 2020 in Very Preterm (<32 Weeks) Infants
eTable 4. Pregnancy and Birth Risks in 2017-2019 and 2020 in Preterm (<37 Weeks) Infants
eTable 5. Pregnancy and Birth Risks in 2017-2019 and 2020 in Late Preterm (32 to 36 Weeks) Infants
eTable 6. Logistic Regression Models for Very Preterm Births <32 Weeks vs Term-Born Infants With Covariates
Data Sharing Statement


