Summary
This study aimed to determine the optimal high-sensitivity cardiac troponin I (hs-cTnI)-based algorithm for early diagnosis of non-ST-elevation myocardial infarction (NSTEMI) in Chinese patients. We prospectively enrolled 1,606 patients with suspected NSTEMI from three emergency departments across China, collecting blood samples at 0, 1, and 3 h post-admission. Patients were classified using the 0/1-h and 0/3-h algorithms. The 2015 and 2020 ESC 0/1-h algorithms rapidly triaged 70% of patients with high negative predictive value (NPV) (99.7%) and sensitivity (99.5%). The 0/3-h algorithm showed higher specificity (93.8%) but lower NPV (96.8%) and sensitivity (91.2%). An optimized 0/1-h algorithm improved specificity to 92.1% while maintaining high NPV (99.7%) and sensitivity (99.2%). Low 30-day and 180-day all-cause mortality and major adverse cardiac event (MACE) rates were observed in rule-out groups for all algorithms. The ESC 0/1-h algorithm is a safe and efficient triage method for patients with suspected NSTEMI, with optimization further enhancing specificity and efficiency for the Chinese population.
Subject areas: Cardiovascular medicine, Bioinformatics
Graphical abstract
Highlights
-
•
ESC 0/1-h algorithms rapidly triage 70% of Chinese chest pain patients with high NPV
-
•
ESC 0/3-h algorithm has higher specificity but lower sensitivity and NPV than 0/1h
-
•
Optimal 0/1h algorithm improves specificity and efficiency, keeping NPV and sensitivity
-
•
Low 30-day and 180-day mortality and MACE risk in rule-out groups for all algorithms
Cardiovascular medicine; Bioinformatics
Introduction
Chest pain is one of the most common reasons patients visit the emergency department (ED).1 Acute myocardial infarction (AMI) is a potentially life-threatening disease that is accompanied by chest pain but accounts for only a small proportion of patients with chest pain in the ED.2 Non-ST-segment elevation myocardial infarction (NSTEMI) poses a particular diagnostic challenge due to nonspecific electrocardiography (ECG) findings and clinical symptoms. It is critical to safely and accurately triage suspected AMI patients by employing a clinical treatment course based on biomarkers.
High-sensitivity cardiac troponin (hs-cTn) has been recommended as a preferred cardiac biomarker in the universal definition of myocardial infarction, enabling the detection or exclusion of AMI more precisely.3,4 The 2015 and 2020 European Society of Cardiology (ESC) Guidelines for managing acute coronary syndrome without persistent ST-segment elevation endorse the use of the hs-cTn-based 0/1-h algorithm for ruling in or ruling out NSTEMI.5,6 The 2015 and 2020 ESC 0/1-h algorithms facilitate rapid triage based on hs-cTn levels at baseline and after 1 h. Specifically, patients with very low baseline hs-cTn levels, or low baseline hs-cTn levels with very small changes within 1 h, can be ruled out as low-risk. Conversely, patients with high baseline hs-cTn levels or who exhibit significant changes within 1 h can be considered at high risk. Patients who do not meet these criteria require further observation.
The influence of race on clinical decision limits for hs-cTn patients is well established, despite the use of consistent methodologies.7 However, more robust evidence is needed to support the 2015 ESC 0/1-h and 0/3-h algorithms in the Chinese population. The 2020 ESC 0/1-h algorithm introduced significant threshold changes, but its suitability for the Chinese population remains uncertain. Additionally, although other cohort studies demonstrate the safety of the 2015 and 2020 ESC 0/1-h algorithms, the associated specificity and positive predictive value (PPV) are not ideal, with approximately 25% of patients still needing observation after 1 h. Optimizing multiple thresholds within the 0/1-h algorithm is challenging.8 Previous studies have indicated that the CART model can enhance diagnostic performance by effectively partitioning datasets, managing complex relationships, generating interpretable decision trees, and optimizing diagnostic metrics.9,10,11 Therefore, we implemented the CART model to optimize the 0/1-h algorithm and identify the most suitable thresholds for the Chinese population.
The primary objective of this study was to validate the ESC 0/1-h and 0/3-h algorithm in a prospective multicenter cohort study involving patients with chest pain admitted to the ED in China. Additionally, we aimed to determine optimal thresholds for the Chinese population using universally defined myocardial infarction criteria and the validated CART model, creating an optimized 0/1-h algorithm. Finally, we conducted a head-to-head comparison of the 2015 ESC 0/1-h algorithm, 2020 ESC 0/1-h algorithm, optimal 0/1-h algorithm, and 2015 ESC 0/3-h algorithm in this cohort of Chinese patients.
Results
Baseline characteristics of patients
A total of 1,032 patients (67.2%) presented to the ED more than 3 h after the onset of chest pain. According to the third myocardial infarction (MI) definition, 402 patients (26.2%) were diagnosed with NSTEMI (Table 1).
Table 1.
Baseline characteristics of the patients
| Non-NSTEMI N = 1,133 |
NSTEMI N = 402 |
Total N = 1,535 |
p value | |
|---|---|---|---|---|
| Male sex | 657 (58.0%) | 296 (73.4%) | 953 (62.1%) | <0.001 |
| Age—years | 63 (55, 70) | 63 (55, 70) | 63 (55, 70) | 0.987 |
| Chest pain characteristics | ||||
| Early presenters (≤3 h) | 402 (35.5%) | 101 (25.1%) | 503 (32.3%) | <0.001 |
| Pain present ED | 666 (58.8%) | 205 (50.9%) | 871 (56.7%) | 0.006 |
| ECG findings | ||||
| ST-segment depression | 34 (3.0%) | 36 (8.9%) | 70 (4.6%) | <0.001 |
| T-wave inversion | 19 (1.7%) | 20 (5.0%) | 39 (2.5%) | 0.001 |
| Other ECG changes | 306 (27.0%) | 197 (48.9%) | 503 (32.7%) | <0.001 |
| No significant ECG changes | 783 (69.1%) | 164 (40.7%) | 947 (61.7%) | <0.001 |
| Risk factors | ||||
| Current smoking | 233 (20.6%) | 132 (32.8%) | 365 (23.8%) | <0.001 |
| History of smoking | 115 (10.2%) | 41 (10.2%) | 156 (10.2%) | <0.001 |
| Current alcohol intake | 116 (10.2%) | 62 (15.4%) | 178 (11.6%) | <0.001 |
| History of alcohol intake | 47 (4.1%) | 18 (4.5%) | 65 (4.2%) | <0.001 |
| History | ||||
| Coronary artery disease | 517 (45.6%) | 191 (47.5%) | 708 (46.1%) | 0.759 |
| Previous MI | 142 (12.5%) | 75 (18.7%) | 217 (14.1%) | 0.010 |
| PCI | 215 (19.0%) | 66 (16.4%) | 281 (18.3%) | 0.631 |
| CABG | 31 (2.7%) | 8 (2.0%) | 39 (2.5%) | 0.701 |
| Hypertension | 674 (59.5%) | 252 (62.7%) | 926 (60.3%) | 0.630 |
| Diabetes | 311 (27.4%) | 122 (30.3%) | 433 (28.2%) | 0.583 |
| Hypercholesterolemia | 569 (50.2%) | 218 (54.2%) | 787 (51.3%) | 0.499 |
| Previous stroke | 89 (7.9%) | 36 (9.0%) | 125 (8.1%) | 0.797 |
| Previous heart failure | 13 (1.1%) | 2 (0.5%) | 15 (1.0%) | 0.588 |
| Kidney dysfunction | 19 (1.7%) | 15 (3.7%) | 34 (2.2%) | 0.047 |
| Peripheral artery disease | 14 (1.2%) | 2 (0.5%) | 16 (1.0%) | <0.001 |
| Treatment | ||||
| Medication | 941 (83.1%) | 193 (47.9%) | 1,134 (73.8%) | <0.001 |
| PTCA | 13 (1.2%) | 16 (3.9%) | 29 (1.9%) | <0.001 |
| PCI | 115 (10.2%) | 173 (42.9%) | 288 (10.8%) | <0.001 |
| CABG | 19 (1.7%) | 25 (6.2%) | 44 (2.9%) | <0.001 |
| Not necessary | 46 (4.1%) | 0 (0.0%) | 46 (3.0%) | <0.001 |
| Vital signs | ||||
| Body mass index (kg/m2) | 25 (22, 25) | 25 (23, 27) | 25 (23, 27) | 0.016 |
| Heart frequency (bpm) | 72 (65, 83) | 73 (65, 83) | 73 (65, 83) | 0.347 |
| Systolic blood pressure (mmHg) | 140 (126, 157) | 140 (124, 156) | 140 (125, 157) | 0.734 |
| Diastolic blood pressure (mmHg) | 78 (70, 88) | 80 (70, 90) | 79 (70, 88) | 0.107 |
| RBC (1012/L) | 4.6 (4.2, 5.0) | 4.8 (4.3, 5.0) | 4.6 (4.3, 5.0) | 0.001 |
| WBC (109/L) | 6.9 (5.7, 8.3) | 7.8 (6.5, 9.4) | 7.1 (5.9, 8.6) | <0.001 |
| PLT (109/L) | 215 (179, 253) | 214 (180, 255) | 215 (179, 254) | 0.592 |
| Hb (g/L) | 140 (128, 151) | 144 (131, 155) | 141 (129, 152) | 0.001 |
| ALT (IU/L) | 22 (16, 35) | 24 (17, 35) | 22 (16, 35) | 0.105 |
| AST (IU/L) | 25 (20, 32) | 32 (24, 46) | 26 (21, 35) | <0.001 |
| Creatinine clearance mL/min/1.73m2 | 90 (75, 103) | 86 (68, 98) | 89 (73, 102) | <0.001 |
| Glu (mmol/L) | 6.7 (5.9, 8.7) | 7.2 (6.2, 10.4) | 6.8 (5.9, 9.2) | <0.001 |
| K (mmol/L) | 3.9 (3.7, 4.2) | 3.9 (3.6, 4.2) | 3.9 (3.7, 4.2) | 0.099 |
| Na (mmol/L) | 139 (137, 141) | 138 (137, 140) | 139 (137, 141) | <0.001 |
| Cl (mmol/L) | 104 (102, 106) | 104 (101, 106) | 104 (100, 106) | 0.176 |
| LA (mm) | 36.0 (34.0, 39.0) | 37.0 (34.0, 40.0) | 36.0 (34.0, 39.8) | 0.125 |
| LV (mm) | 47.0 (45.0, 51.0) | 48.0 (45.0, 52.0) | 48.0 (45.0, 51.0) | 0.002 |
| LVEF (%) | 60 (59, 63) | 60 (56, 63) | 60 (59, 63) | <0.001 |
| GRACE score | 101 (83, 122) | 116 (99, 139) | 105 (64, 111) | <0.001 |
Continuous variables are described as medians with interquartile ranges (25th, 75th), and categorical variables are described as numbers and percentages. ED, emergency department; NSTEMI, non-ST-segment elevation myocardial infarction; ECG, electrocardiography; PTCA, percutaneous transluminal coronary angioplasty; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; RBC, red blood cell; WBC, white blood cell; PLT, platelet; Hb, hemoglobin; ALT, alanine transaminase; AST, aspartate transaminase; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction. Creatinine clearance was calculated using the CKD epidemiology collaboration (CKD-EPI) formula. Differences in baseline characteristics were assessed using the Mann-Whitney U test for continuous variables and the Pearson chi-square test for categorical variables, as appropriate. p values represent differences between non-NSTEMI and NSTEMI patients.
Concentrations of serial hs-cTnI
The median intervals for the first-to-second and first-to-third blood draws for serial hs-cTnI measurements were 60 min (10th–90th range: 57–65 min) and 180 min (10th–90th range: 168–185 min), respectively (Table S1). The hs-cTnI concentrations at presentation and at 1 h and 3 h were significantly greater in patients with NSTEMI than in non-NSTEMI patients. The changes in 0/1-h and 0/3-h hs-cTnI levels in NSTEMI patients were more pronounced than those in non-NSTEMI patients (Table S2). This significant change was observed in patients with chest pain durations of less than 3 h and more than 3 h. The receiver operating characteristic (ROC) curves revealed a progressive increase in the area under the curve (AUC) for the hs-cTnI level at presentation and at 1 h and 3 h, with values of 0.961, 0.975, and 0.981, respectively. Notably, the change in the AUC from 0 h to 1 h was not significantly different from the change observed at 3 h (Figure S2).
Diagnostic performance of the 2015 ESC 0/1-h algorithm using hs-cTnI levels
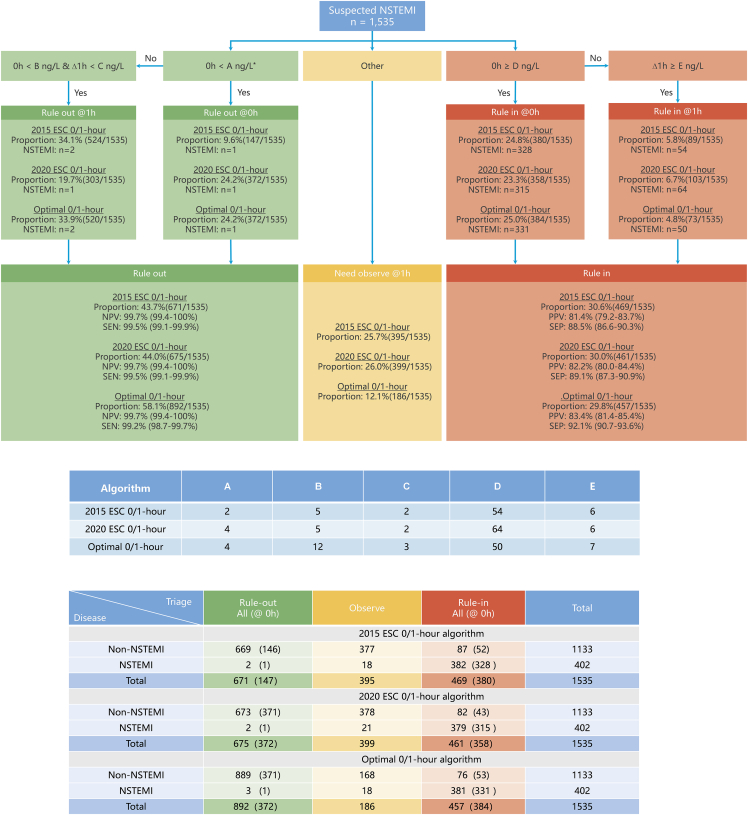
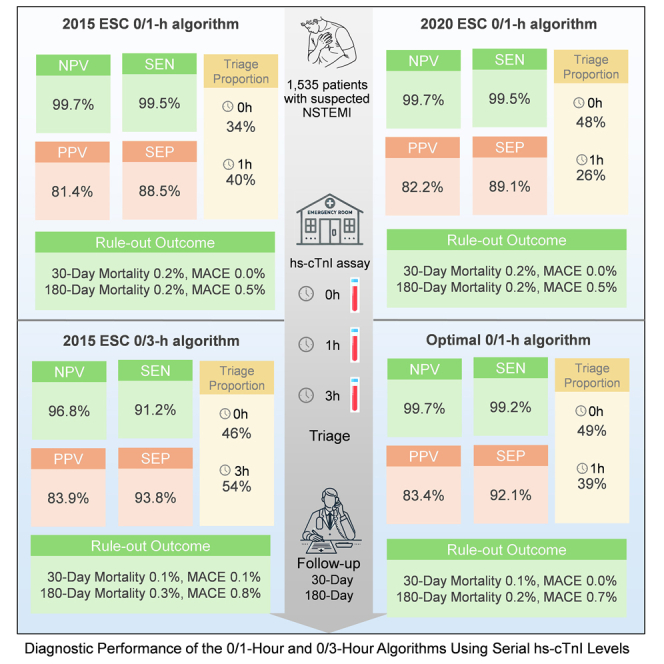
Using the 2015 ESC algorithm,5 9.6% of suspected MI patients (147 of 1,535) were directly triaged to rule-out group (0 h hs-cTnI <2 ng/L) upon presentation at the ED. After monitoring hs-cTnI 1 h post-admission, an additional 34.1% of suspected MI cases (524 of 1,535) were ruled out based on a 0- to 1-h hs-cTnI change <2 ng/L. Details about the two missed females (0.3%, 2 of 670) are shown in Table S3. The negative predictive value (NPV) (99.7% [95% confidence interval (CI) 99.4%–100%]) and sensitivity (99.5% [95% CI 99.1%–99.9%]) values of the 2015 ESC 0/1-h algorithm were exceptionally high. A total of 24.8% of suspected MI cases (380 of 1,535) were directly ruled in (0 h hs-cTnI ≥52 ng/L) at 0 h. After monitoring hs-cTnI at the 1-h time point, 5.8% of suspected MI patients (89 of 1,535) were ruled in based on a 0/1-h hs-cTnI change ≥6 ng/L. Overall, 30.6% of suspected MI patients (469 of 1,535) were triaged toward NSTEMI. The PPV and specificity were 81.4% (95% CI 79.2%–83.7%) and 88.5% (95% CI 86.6%–90.3%), respectively. A total of 25.7% of the patients (395 of 1,535) required observation (Figure 1).
Figure 1.
Diagnostic performance of the 0/1-h algorithm using hs-cTnI
NPV, negative predictive value; SEN, sensitivity; PPV, positive predictive value; SEP, specificity.
In suspected MI patients who presented later to the ED (Chest Pain Onset [CPO] >3 h and ≤6 h), the 2015 ESC 0/1-h algorithm was safe and accurate. However, the PPV of diagnosis among patients with CPO ≤3 h, <2 h, and <1 h significantly decreased to 73.4%, 68.8%, and 63.2%, respectively. The concordance was significantly impacted in patients who presented very early (CPO <2 h and <1 h) (Table 2).
Table 2.
Diagnostic performance according to time between CPO and the first blood draw
| Time since CPO | Rule-out all (direct) | Observe all | Rule-in all (direct) | Concordance | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| 2015 ESC 0/1-h Algorithm | |||||||||
| Very late presenters >6 h | Non-NSTEMI | 255 (97) | 164 | 35 (25) | 92.8% (90.6%–95.1%) | 99.5% (98.9%–100%) | 87.9% (85.1%–90.8%) | 85.7% (82.7%–88.8%) | 99.6% (99.1%–100%) |
| NSTEMI | 1 (1) | 6 | 210 (202) | ||||||
| Late presenters >3 h | Non-NSTEMI | 416 (146) | 262 | 53 (35) | 92.9% (91.0%–94.7%) | 99.7% (99.2%–100%) | 88.7% (86.4%–91.0%) | 84.4% (81.8%–87.0%) | 99.8% (99.4%–100%) |
| NSTEMI | 1(1) | 13 | 287(263) | ||||||
| Early presenters ≤3 h | Non-NSTEMI | 255 (98) | 113 | 34 (17) | 90.6% (87.7%–93.6%) | 97.9% (96.5%–99.3%) | 88.2% (85.0%–91.5%) | 73.4% (69.0%–77.8%)a | 99.2% (98.3%–100%) |
| NSTEMI | 2 (2) | 5 | 94 (65) | ||||||
| Very early presenters <2 h | Non-NSTEMI | 122 (56) | 44 | 15 (6) | 80.0% (71.5%–88.5%)a | 94.3% (89.4%–99.2%)a | 70.0% (60.3%–79.7%)a | 68.8% (58.9%–78.6%)a | 94.6% (89.8%–99.4%)a |
| NSTEMI | 2 (2) | 1 | 33 (22) | ||||||
| Extremely early presenters <1 h |
Non-NSTEMI | 35 (20) | 14 | 7 (5) | 66.7% (46.5%–86.8%)a |
100% (100%–100%) |
22.2% (4.4%–40.0%)a |
63.2% (42.5%–83.8%)a |
100% (100%–100%) |
| NSTEMI | 0 (0) | 0 | 12 (8) | ||||||
| 2020 ESC 0/1-h Algorithm | |||||||||
| Very late presenters >6 h | Non-NSTEMI | 258 (229) | 166 | 30 (19) | 93.8% (91.6%–95.9%) | 99.5% (98.9%–100%) | 89.6% (86.9%–92.3%) | 87.4% (84.5%–90.3%) | 99.6% (99.1%–100%) |
| NSTEMI | 1 (1) | 8 | 208 (197) | ||||||
| Late presenters >3 h | Non-NSTEMI | 420 (371) | 263 | 48 (27) | 93.5% (91.7%–95.3%) | 99.7% (99.2%–100%) | 89.7% (87.6%–91.9%) | 85.6% (83.1%–88.1%) | 99.8% (99.4%–100%) |
| NSTEMI | 1 (1) | 15 | 285 (256) | ||||||
| Early presenters ≤3 h | Non-NSTEMI | 257 (237) | 112 | 33 (16) | 90.6% (87.7%–93.6%) | 96.8% (95.1%–98.6%) | 88.6% (85.4%–91.8%) | 73.6% (69.2%–78.0%)a | 98.8% (97.8%–99.9%) |
| NSTEMI | 3 (3) | 6 | 92 (59) | ||||||
| Very early presenters <2 h | Non-NSTEMI | 124 (113) | 43 | 14 (6) | 90.2% (85.7%–94.6%) | 91.4% (87.3%–95.6%)a | 89.9% (85.4%–94.4%) | 69.6% (62.7%–76.4%)a | 97.6% (95.4%–99.9%)a |
| NSTEMI | 3 (3) | 1 | 32 (20) | ||||||
| Extremely early presenters <1 h |
Non-NSTEMI | 35 (33) | 14 | 7 (5) | 85.2% (75.7%–94.7%)a |
91.7% (84.3%–99.0%) |
83.3% (73.4%–93.3%) |
61.1% (48.1%–74.1%)a |
97.2% (92.8%–100%) |
| NSTEMI | 1 (1) | 0 | 11 (8) | ||||||
| Optimal 0/1 h Algorithm | |||||||||
| Very late presenters >6 h | Non-NSTEMI | 355 (229) | 69 | 30 (25) | 94.8% (93.0%–96.6%) | 99.5% (99.0%–100%) | 92.2% (90.1%–94.4%) | 87.4% (84.8%–90.1%) | 99.7% (99.3%–100%) |
| NSTEMI | 1 (1) | 7 | 209 (202) | ||||||
| Late presenters >3 h | Non-NSTEMI | 565 (371) | 121 | 45 (35) | 94.9% (93.4%–96.3%) | 99.7% (99.3%–100%) | 92.6% (90.9%–94.3%) | 86.4% (84.2%–88.6%) | 99.8% (99.5%–100%) |
| NSTEMI | 1 (1) | 14 | 286 (264) | ||||||
| Early presenters ≤3 h | Non-NSTEMI | 327 (237) | 45 | 30 (18) | 92.5% (90.1%–94.9%) | 95.9% (94.0%–97.7%)a | 91.6%(89.0%–94.1%) | 75.6% (71.7%–79.6%)a | 98.8% (97.8%–99.8%) |
| NSTEMI | 4(3) | 4 | 93(67) | ||||||
| Very early presenters <2 h | Non-NSTEMI | 147 (113) | 22 | 12 (7) | 92.2% (88.5%–96.0%) | 91.2% (87.2%–95.2%)a | 92.5% (88.7%–96.2%) | 72.1% (65.8%–78.4%)a | 98.0% (96.0%–100%)a |
| NSTEMI | 3 (3) | 2 | 31 (22) | ||||||
| Extremely early presenters <1 h | Non-NSTEMI | 41 (33) | 9 | 6 (5) | 88.1% (79.9%–96.4%) | 91.7% (84.6%–98.7%) | 87.2% (78.7%–95.7%) | 64.7% (52.5%–76.9%)a | 97.6% (93.7%–100%) |
| NSTEMI | 1 (1) | 0 | 11 (8) | ||||||
Indicates p < 0.05, compared with CPO >3 h by chi-squared test or Fisher’s exact test. CPO, Chest Pain Onset.
Diagnostic performance of the 2020 ESC 0/1-h algorithm using hs-cTnI
According to the 2020 ESC algorithm,6 24.2% (372 of 1,535) and 19.7% (303 of 1,535) of suspected MI patients were triaged to rule out at 0 h and 1 h, respectively. The NPV and sensitivity of the 2020 ESC 0/1-h algorithm were 99.7% (95% CI 99.4%–100%) and 99.5% (95% CI 99.1%–99.9%), respectively. Additionally, 23.3% (358 of 1,535) and 6.7% (103 of 1,535) of suspected MI patients were directly ruled in at 0 h (hs-cTnI ≥64 ng/L) and 1 h (0/1-h hs-cTnI change ≥6 ng/L). A total of 26.0% of patients (399 of 1,535) required observation. The PPV and specificity were 82.2% (95% CI 80.0%–84.4%) and 89.1% (95% CI 87.3%–90.9%), respectively (Figure 1). Similar to the optimal 0/1-h algorithm, the diagnostic PPVs among subgroups with CPO <3 h, <2 h, and <1 h were impaired (Table 2).
Diagnostic performance of the 2015 ESC 0/3-h algorithm using hs-cTnI
According to the ESC 0/3-h algorithm,5 26.4% (394/1,526) and 19.1% (292/1,526) of suspected MI patients were triaged to rule out and rule in, respectively, upon admission to the ED. After retesting hs-cTnI at 3 h, 45.2% (689/1,526) and 9.3% (142/1,526) of suspected MI patients were triaged to rule out and rule in, respectively. The ESC 0/3-h algorithm had an NPV of 96.8% (95% CI 95.9%–97.7%), a sensitivity of 91.2% (95% CI 89.8%–92.6%), a PPV of 83.9% (95% CI 82.0%–85.7%), and a specificity of 93.8% (95% CI 92.6%–95.0%) (Figure S3).
Optimal 0/1-h algorithm using hs-cTnI
The sensitivity and NPV of each hs-cTnI concentration at presentation for NSTEMI patients are shown in Figure S4. A baseline hs-cTnI <4 ng/L was determined to be the optimal cutoff, with a sensitivity of 99.0% (95% CI 97.5%–99.7%) and an NPV of 99.2% (95% CI 97.9%–99.7%). For ruling out NSTEMI, combining cutoff values for baseline (B) < 12 ng/L and a 0/1-h absolute change (C) < 3 ng/L resulted in a sensitivity of >99% and an NPV of >99.5%. The optimal rule-in thresholds for the 0/1-h algorithm in this Chinese cohort were discovered using the CART model. The results showed that the direct rule-in cutoff value (D) at baseline was ≥50 ng/L and the rule-in cutoff value after 1 h was a 0- to 1-h change ≥ 7 ng/L (E). According to the optimal 0/1-h algorithm, the NPV, sensitivity, PPV, and specificity were 99.7% (95% CI 99.4%–100%), 99.2% (95% CI 98.7%–99.7%), 83.4% (95% CI 81.4%–85.4%), and 92.1% (95% CI 90.7%–93.6%), respectively (Figure 1).
The diagnostic concordance of the optimal 0/1-h algorithm [94.1% (95% CI 92.9%–95.4%)] was slightly greater than that of the 2015 ESC 0/1-h algorithm [92.2% (95% CI 90.6%–93.8%)], although this difference was not statistically significant (p = 0.053). Overall the proportion of patients ruled out at 1 h was 58.1%, representing a 14% increase compared to the 2015 and 2020 ESC 0/1-h algorithms. Additionally, the observation zone was reduced to 12.1%, which was represented a reduction of 53% compared to those of the 2015 and 2020 algorithms.
Similarly, in suspected MI patients who presented later to the ED (CPO >3 h and ≤6 h), the optimal 0/1-h algorithm was safe and accurate. However, the diagnostic PPVs among patients with CPO ≤3 h, <2 h, and <1 h were significantly lower, at 75.6%, 72.1%, and 64.7%, respectively (Table 2).
Comparison of the diagnostic performance of multiple algorithms using hs-cTnI
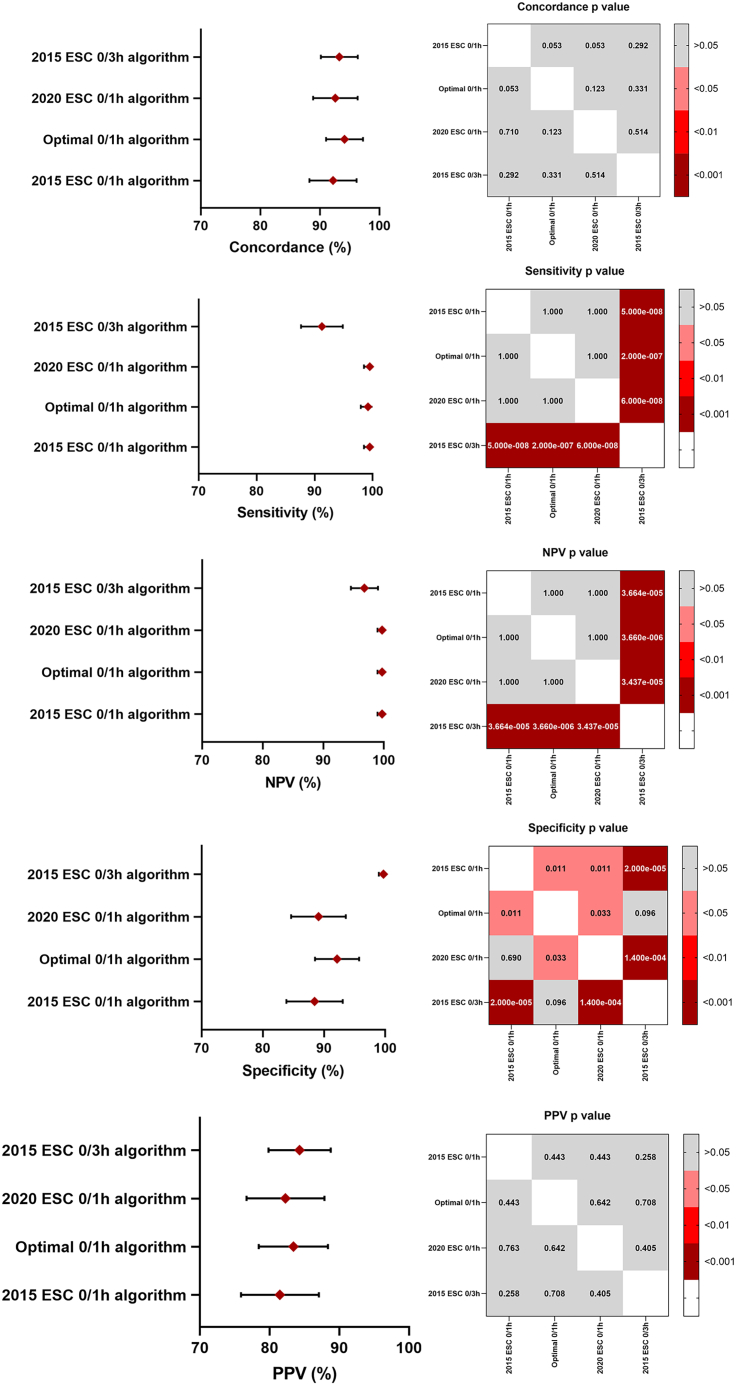
The three 0/1-h algorithms were safer than the 0/3-h algorithm. Among the 2015 ESC 0/1 h, 2020 ESC 0/1 h, optimal 0/1 h, and 2015 ESC 0/3 h algorithms, the three 0/1 h algorithms showed higher NPVs (all 99.7% vs. 96.8%) and sensitivities (99.2%–99.5% vs. 91.2%) than did the 0/3 h algorithm. Moreover, the specificity of the 0/3 h algorithm (93.8%) was greater than that of the ESC 0/1 h algorithms (88.5% and 89.7%). No difference was reported in the PPV and concordance of all algorithms (Figure 2).
Figure 2.
Comparing the diagnostic performance of the different algorithms
Note: Pearson’s chi-squared test.
Subgroup analyses of the performance of different 0/1-h algorithms
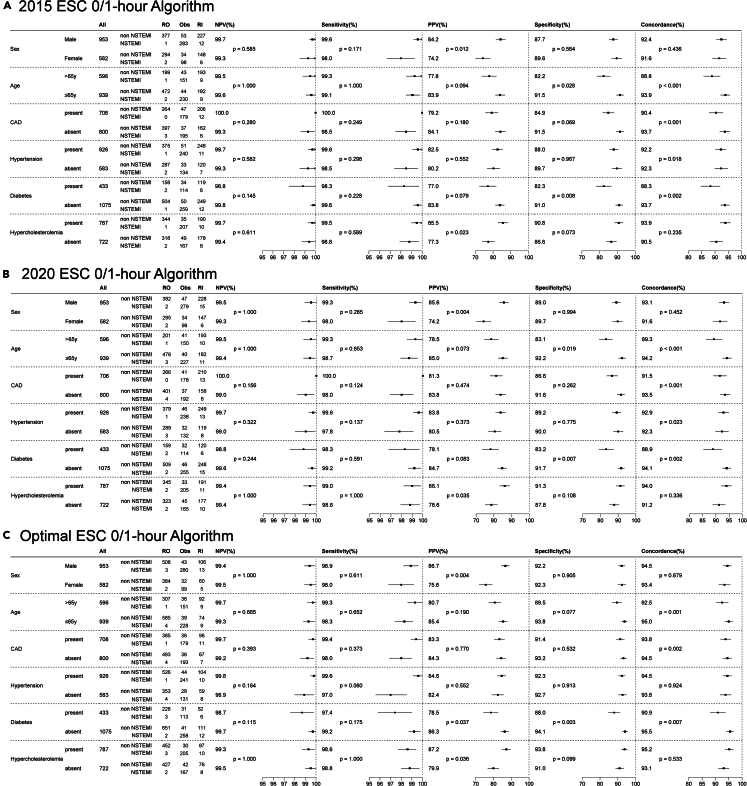
Upon analyzing the subgroups based on sex, age, and history of coronary artery disease, hypertension, diabetes, and hypercholesterolemia, the classification performance of the 0/1-h algorithm showed no significant differences in NPV or sensitivity across these subgroups. Notably, the PPV was greater in male patients and those with a history of hypercholesterolemia. Specificity was reduced among elderly patients older than 65 years and those with diabetes. The concordance of the algorithm was adversely affected by advanced age and a history of coronary artery disease, hypertension, and diabetes (Figure 3).
Figure 3.
Subgroup analyses of the performance of different 0/1-h algorithms
(A) 2015 ESC 0/1-h algorithm.
(B) 2020 ESC 0/1-h algorithm.
(C) Optimal ESC 0/1-h algorithm.
RO, rule out; Obs, observe; RI, rule in; NPV, negative predictive value; SEN, sensitivity; PPV, positive predictive value. Concordance refers to the agreement or consistency with clinical diagnosis.
Comparison of the prognostic performance of multiple algorithms using hs-cTnI
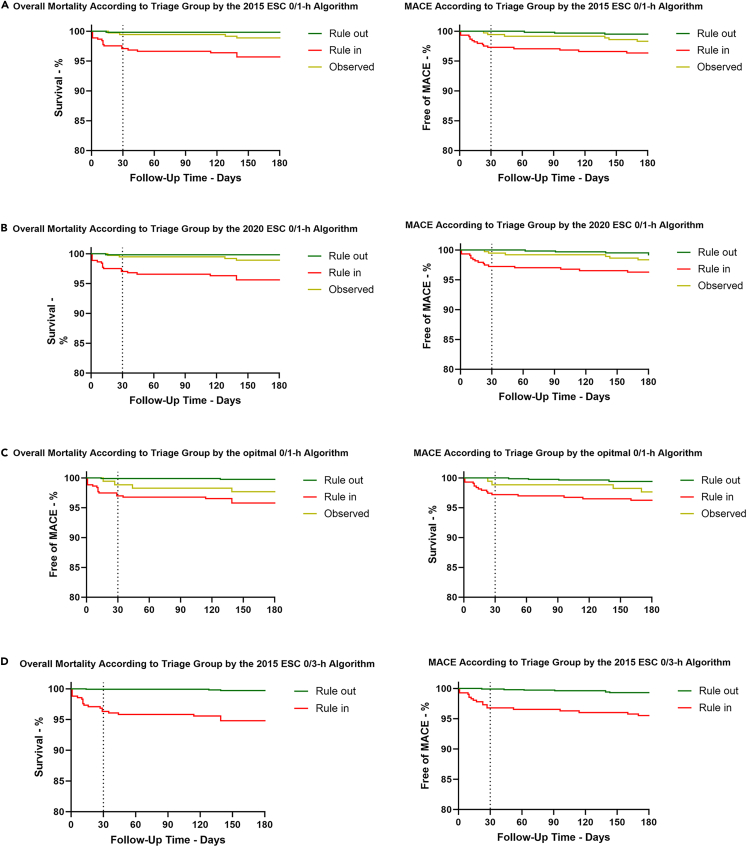
A total of 1,461 (95.1%) patients completed 30-day and 180-day follow-ups, with 24 deaths and 26 major adverse cardiac events (MACEs). Patients triaged to the rule-in group according to the four algorithms had significantly greater overall mortality and MACEs than did those in the rule-out group (Figure 4).
Figure 4.
Cardiac events according to triage group by different algorithms
(A) According to the 2015 ESC 0/1-h algorithm, the 30-day all-cause mortality rates were 0.2%, 0.5%, and 2.9% (log rank, p = 0.029) in the rule-out, observe, and rule-in groups, respectively. The 180-day all-cause mortality rates were 0.6%, 1.1%, and 4.3% (log rank, p = 0.002) in the rule-out, observe, and rule-in groups, respectively. The MACE rates of the rule-out group were significantly lower at 30 days and 180 days (0% and 0.5%, respectively). The rule-in group showed an extremely high risk of MACEs at 30 days and 180 days (2.4% and 3.6%, respectively). The observational group showed an intermediate risk of MACEs at 30 days and 180 days (0.5% and 1.9%, respectively).
(B) According to the 2020 ESC 0/1-h algorithm, the cumulative 30-day overall mortality was 0.2%, 0.5%, and 2.9% (log rank, p = 0.026) in the rule-out, observe, and rule-in groups, respectively. The cumulative 180-day all-cause death rates were 0.2%, 1.1%, and 4.3% (log rank, p < 0.001) in the rule-out, observe, and rule-in groups, respectively. The cumulative 30-day MACE was 0%, 0.5%, and 2.5% (log rank, p < 0.001) in the rule-out, observe, and rule-in groups, respectively. At 180 days, the percentage of MACE was 0.5%, 1.8%, and 3.6% (log rank, p < 0.001) in the rule-out, observe, and rule-in groups, respectively.
(C) According to the optimal 0/1-h algorithm, the cumulative 30-day overall mortality was 0.1%, 1.1%, and 2.8% (log rank, p < 0.001) in the rule-out, observe, and rule-in groups, respectively. The cumulative 180-day all-cause death rates were 0.2%, 2.2%, and 3.9% (log rank, p < 0.001) in the rule-out, observe, and rule-in groups, respectively. The cumulative 30-day MACE was 0%, 1.1%, and 2.4% (log rank, p < 0.001) in the rule-out, observe, and rule-in groups, respectively. At 180 days, the percentage of MACE was 0.7%, 2.2%, and 3.5% (log rank, p < 0.001) in the rule-out, observe, and rule-in groups, respectively.
(D) According to the 2015 ESC 0/3-h algorithm, within 30 days, all-cause mortality was 0.2% and 3.4% in the rule-out and rule-in groups (log rank, p < 0.001), respectively. At 180 days, the cumulative death rates were 0.4% and 4.9% in the rule-out and rule-in groups (log rank, p < 0.001), respectively. The MACE rates of the rule-out group were significantly lower at 30 days and 180 days (0.1% and 0.8%, respectively). The rule-in group showed an extremely high risk of MACEs at 30 days and 180 days (2.9% and 4.4%, respectively). All log rank p values were <0.001.
A comparison of the rule-out and observed groups among the four algorithms revealed that the 30-day and 180-day overall mortality and MACEs were almost identical (Figures S5 and S6). In all the rule-in groups, although the follow-up overall mortality and MACEs according to the 2015 ESC 0/3-h algorithm were slightly greater than those according to the 0/1-h algorithms, no statistically significant differences were observed (Figure S7).
Discussion
This prospective multicenter study is the first to demonstrate that the ESC 0/1-h and 0/3-h algorithms using hs-cTnI are safe, accurate, and effective in Chinese patients, particularly among suspected MI patients who present late to the ED (CPO >3 h). In our study, the ESC 0/1-h algorithm demonstrated strong diagnostic performance. The optimal 0/1-h algorithm developed using the CART method improved the efficiency. All three 0/1-h algorithms (2015 ESC 0/1 h, 2020 ESC 0/1 h, and optimal 0/1 h) demonstrated higher NPVs and sensitivities than did the 0/3-h algorithm. The rule-out efficacy of the optimal 0/1-h algorithm at presentation to the ED was superior to that of the 2015 and 2020 ESC 0/1-h algorithms, due to the use of optimal rule-out cutoffs at baseline and 1 h.
The 2015 and 2020 ESC 0/1-h algorithms based on hs-cTnI were safe and showed high sensitivity for detecting NSTEMI in Chinese patients, with an NPV and sensitivity of 99.7% and 99.5%, respectively. The safety level was comparable to that reported in previous studies in different cohorts.8,12,13,14,15 According to a meta-analysis of 32 studies (20 cohorts) involving 30,066 patients, the NPV and sensitivity of the ESC 0/1-h triage based on Architect hs-cTnI were 99.8% (99.5–99.9%) and 99.3% (98.4–99.7%), respectively.8 In our study, only two females were incorrectly triaged to the rule-out group; one presented very early (70 min) to the ED and the other presented with left ventricular motion incoordination. These findings underscore to pay attention to CPO and ischemia evidence in clinical settings. Notably, all-cause mortality and MACEs in the rule-out group were extremely low (0.2% and 0%, respectively) at the 30-day follow-up. Similarly, a real-world study conducted by Twerenbold et al. found that MACEs occurring in the rule-out group at 30 day was 0.2%.16 Another meta-analysis involving 10 cohorts with 11,014 patients reported 30-day and 1-year all-cause mortality rates of 0.1% and 0.8%, respectively, in the rule-out group.15
A head-to-head comparison between the 2015 and 2020 ESC 0/1-h algorithms was performed in the Chinese patients included in this study. Our comparative results show that the 2015 and 2020 ESC 0/1-h algorithms are very similar in terms of sensitivity, NPV, specificity, PPV, and diagnostic accuracy. Although the proportion of patients excluded after 1 h was the same for both the 2015 and 2020 algorithms, the 2020 algorithm increased the baseline exclusion threshold from 2 ng/L to 4 ng/L, significantly increasing the proportion of patients excluded at baseline from 9% to 23.6%, which greatly enhances the efficiency of triage based on baseline hs-cTnI levels. Additionally, setting 4 ng/L as the baseline exclusion threshold resembles the 10% CV LoQ of Architect hs-cTnI, resulting in less variability in laboratory test results.17
The optimal 0/1-h algorithm based on hs-cTnI showed promising potential for improved diagnostic performance compared to the ESC 0/1-h algorithm. As previous studies demonstrate, hs-cTnI levels vary significantly among different racial populations. For instance, a large cohort study of healthy Chinese individuals showed that the 99th percentile URL for males (17.8 ng/L) and females (13.7 ng/L) was lower than the values from the AACC universal sample bank (males: 35.2 ng/L, females: 15.6 ng/L).18,19 Consequently, the ESC 0/1-h algorithm thresholds should be optimized for the Chinese population, as the current thresholds are primarily derived from European and American cohorts. In this study, we used the CART algorithm, which was previously employed to determine thresholds for European and American populations, to optimize the thresholds for the Chinese population. The new baseline rule-in threshold is 50 ng/L, lower than the ESC 0/1-h algorithm thresholds (52 ng/L in 2015 and 64 ng/L in 2020 ESC), increasing the specificity of the 0/1-h algorithm to 92.1%, which is greater than the ESC specificity (88.5% in 2015 and 89.1% in 2020, p < 0.05). Meanwhile, the overall rule-in proportion remains at 30%. This new approach, which involved combining baseline and 1-h change thresholds, increased the 1-h rule-out proportion by approximately 14% (58% vs. 44%), while maintaining sensitivity and NPV above 99%. According to the optimized algorithm, only 12% of patients required observation, whereas previous studies indicated that 16%–32% patients required observation after triage using the ESC 0/1-h algorithm.16,20,21,22
Early diagnosis of patients presenting with chest pain in China requires attention to the duration reported. The release of troponin in acute MI patients is a continuous, dynamic process closely related to the duration of chest pain.23 For patients in the early stages of chest pain, due to the short onset time or the phenomenon of “delayed release” in some patients, even the lowest detection limit of high-sensitivity troponin may still result in missed diagnoses. This finding was further confirmed in our study. Our results show that for patients reporting a chest pain duration of less than 3 h, the NPV and sensitivity of the three 0/1-h algorithms are significantly reduced to below 99%, which fails to meet the recognized safety standards. This suggests that when applying the 0/1-h algorithm to rule out patients in clinical practice, the duration of chest pain should be accounted for. Continued measurement of hs-cTnI could help distinguish NSTEMI patients who experience a delayed release of hs-cTnI.24
The three 0/1-h algorithms demonstrated high NPVs and sensitivities among patients with different sex and ages and histories of coronary artery disease, hypertension, diabetes, and hypercholesterolemia. A study by Twerenbold et al., which included 4,368 clinical cases, reported that the ESC 0/1-h algorithm achieved an NPV of 99% for women, elderly patients (over 65 years), and those with coronary artery disease during the initial diagnosis.12 However, a recent study by Ashburn et al. indicated that the 30-day safety of patients in the rule-out group who were classified using hs-cTnT-based ESC 0/1-h triage did not meet the accepted 99% safety standard in patients with a history of coronary artery disease.25 This discrepancy may be due to differences in the inclusion criteria and detection methods used.
Conclusion
The ESC algorithm using hs-cTnI was well validated in this prospective multicenter study. Although the three 0/1-h algorithms did not significantly differ in terms of safety or accuracy, the 0/1-h algorithms were more effective at diagnosing Chinese patients with suspected MI presenting to the ED than the 2015 and 2020 ESC.
Limitations of the study
There are several limitations to this study. First, most enrolled subjects presented to the ED after a CPO of less than 2 h, leading to a high number of very early presenters. Second, we did not differentiate between type I and type II AMI among NSTEMI patients. Future research should investigate the diagnostic performance of these methods separately in type I and type II MI patients. Third, the small sample sizes of certain subgroups, such as patients with mild heart failure (15 patients) and chronic kidney disease (35 patients with an estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), resulted in limited statistical power for these analyses. Fourth, the threshold values of the optimal 0/1-h algorithm have not yet undergone external validation, despite employing a rigorous 10-fold cross-validation approach. This method allows performance to be robustly assessed by ensuring that each data point is used for both training and validation; however, it may not fully capture the generalizability of the model to other populations or clinical settings.26 In the future, we plan to conduct a larger prospective study with a cohort of patients with acute chest pain to externally validate the optimized algorithm developed in this study. This cohort will include patients with heart failure, arrhythmias, and kidney disease, as well as a larger sample size of patients presenting with chest pain very early. This will allow us to determine the diagnostic performance of the 0/1-h algorithm in these specific subgroups.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| 1606 patients' plasma | Fuwai Hospital | https://clinicaltrials.gov/ct2/show/NCT03734796 |
| Critical commercial assays | ||
| Architect STAT hs-cTnI assay | Abbott Laboratories | G5-6634/R01 |
| Software and algorithms | ||
| IBM SPSS Statistics version 23.0 | IBM Corp., Armonk, NY | https://www.ibm.com |
| GraphPad Prism version 8.0.0 | GraphPad Software | https://www.graphpad.com/ |
| R version 4.1.2 | R Project | https://www.r-project.org/ |
| Python version 3.12.3 | Python Software Foundation | https://www.python.org |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Zhou Zhou (zhouzhou@fuwaihospital.org).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Pertinent data reported in this paper will be shared by the lead contact upon reasonable request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request
-
•
All original code is available in this paper’s supplementary files “Data S1”
Experimental model and study participant details
Study design
The prospective study examined changes in high-sensitivity cardiac troponin I levels and the impact on the diagnosis of suspected acute coronary syndrome patients in China (ClinicalTrials.gov Identifier: NCT03734796) at 3 centers located in East China, South China and North China. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.12 Briefly, from January 2017 to October 2020, patients aged 18 years or older with a high suspicion of NSTEMI were included. Patients with STEMI, recent surgery within four weeks, malignancy, or pregnancy were excluded. Patients with confirmed diagnoses of acute myocarditis, chronic heart failure at NYHA class III-IV, severe arrhythmias, endocarditis, anemia, or recent thrombolytic therapy at the time of presentation were also excluded.
Study population
A total of 1,606 patients with suspected MI were recruited from three hospital EDs in Beijing, Nanjing, and Guangzhou between January 2017 and June 2020. Seventy-one patients were excluded due to missing serial hs-cTnI measurements and records, or because they presented with other cardiac diseases. Overall, 1,535 patients were included in the final analysis (Figure S1). The median age of the patients was 63 years (range 27–93), and the study population included 953 males (62.1%).
Ethics statement
This study complied with the Declaration of Helsinki and approved by the ethics committee of Fuwai Hospital (permission number 2016-809). All subjects provided written informed consent.
Clinical diagnosis
All patients underwent a routine clinical assessment that included clinical presentation, physical examination, 12-lead ECG, serial measurements of local (hs)-cTn, echocardiography, cardiac exercise test, and coronary computed tomography or angiography. According to the third MI definition4, patients were classified as NSTEMI and non-NSTEMI by two independent cardiologists. Discrepancies in diagnosis were resolved by a third senior cardiologist. In brief, NSTEMI was diagnosed when an increase and/or decrease in cardiac troponin with at least one value above the 99th percentile URL combined with at least one clinical setting consistent with myocardial ischemia was detected.
Follow-up and clinical outcomes
Patients were followed up via telephone calls, outpatient visits, and medical records at 30 and 180 days to collect information on all-cause death and major adverse cardiovascular events (MACEs), including cardiac death, AMI (excluding the index event), and unplanned coronary revascularization.
Method details
Hs-cTnI measures
Blood samples were collected in tubes with EDTA-K2 at 0, 1 and 3 h after admission to the ED. Freshly isolated plasma was immediately measured using an Architect STAT hs-cTnI assay (Abbott Laboratories, Lake Bluff, Illinois). The hs-cTnI assay has a sex-specific 99th percentile upper reference limit (URL) of 15.6 ng/L for females and 34.2 ng/L for males with corresponding coefficients of variation (CVs).13 The limit of blank (LoB) and limit of detection (LoD) were 0.7 ng/L and 1.1 ng/L, respectively. The limit of quantitation (LoQ) for a 10% CV and a 20% CV were estimated to be 4.7 ng/L and 1.3 ng/L, respectively.14
ESC algorithm
All patients were triaged toward rule-out, observe and rule-in groups by an independent senior cardiologist who was familiar with the 2015 and 2020 ESC algorithms. Another senior cardiologist randomly reviewed 10% of the results. Briefly, according to the 0/1-h algorithm, suspected AMI patients were directly ruled out when baseline levels of hs-cTnI were very low (2015 ESC algorithm <2 ng/L, or 2020 ESC algorithm <4 ng/L) and chest pain onset(CPO) was more than 3 h. The thresholds for direct rule-in were defined as ≥ 52 ng/L (2015 ESC algorithm) or ≥64 ng/L (2020 ESC algorithm) at baseline. The rule-out and rule-in thresholds within the first hour were an absolute change <2 ng/L and ≥6 ng/L, respectively. According to the 2015 ESC 0/3-h algorithm, if the hs-cTnI at 0 h was below the sex-specific 99th percentile URL, patients with a chest pain duration of more than 6 h were ruled out. If hs-cTnI at 0 h was above five times sex-specific 99th percentile URL, patients were ruled in. The other patients were retested for hs-cTnI at 3 h. If the baseline level of hs-cTnI was below the sex-specific 99th percentile URL, patients were ruled in when the 0–3 h relative change was ≥50% of the sex-specific 99th percentile URL and if the hs-cTnI at 3 h was above the 99th percentile URL. If the baseline level of hs-cTn was above the sex-specific 99th percentile URL, a 0/3-h relative change of ≥20% compared with baseline was considered significant. Otherwise, patients were considered to be ruled out for having NSTEMI.
Optimal thresholds of the 0/1-h algorithm
The optimal algorithm for detecting hs-cTnI was developed based on a classification and regression tree (CART) analysis of the entire cohort.15 Gini impurity was selected to evaluate the quality of node splitting, the maximum depth of the decision tree was set to 4, and the minimum sample number for leaf nodes was set to 10. The training process was executed using the DecisionTreeClassifier method from scikit-learn.16 The optimal rule-out thresholds of baseline and 0/1-h absolute levels of hs-cTnI were determined by calculating diagnostic sensitivities ≥99% and negative predictive values (NPVs) ≥99%. The optimal rule-in thresholds for the baseline and 0/1-h absolute levels of hs-cTnI were determined by PPVs ≥80%. We employed 10-fold cross-validation to internally validate our model, for which the dataset was randomly partitioned into 10 equal-sized folds.17 The CART code used in the Data S1.
Quantification and statistical analysis
Continuous variables are expressed as medians with interquartile ranges (25th, 75th), and differences between groups were analyzed using the Mann-Whitney U test. Categorical variables are expressed as percentages and analyzed using the Pearson chi-square test. The NPV and sensitivity (SEN) were used to evaluate the safety of assigning patients to the rule-out group. PPV, specificity (SEP), and concordance were used to assess the accuracy of the rule-in assignment. The efficacy proportions for rule-out and rule-in were also calculated. Diagnostic performance comparisons among subgroups were performed with the Pearson chi-square test or Fisher’s exact test. Survival and event-free outcomes during the 30-day and 180-day follow-ups were analyzed using Kaplan–Meier curves and the log rank test. p values <0.05 (two-tailed) were considered to indicate statistical significance. Statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY), GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, California, USA), R version 4.1.2 and Python version 3.12.3.
Acknowledgments
This study was supported by National Key R&D Program of China (Grant No. 2023YFC2413000) and Abbott Laboratories. The data were collected and analyzed by authors. We sincerely thank Prof. Yang Wang, Dr. Yan-yan Zhao, Dr. Xiao-yu Zhang, and Dr. Ye Lu for data analysis and data interpretation, from Medical Research and Biometrics Centre, National Centre for Cardiovascular Diseases, Beijing, China.
Author contributions
Z.Z., Y.L., J.Z., and Y.H.L. conceived the study and designed the investigation. Funding was acquired by Z.Z. and Y.L. Patient enrollment was conducted by H.Z., S.K.W., G.Z.Z., Z.Y., C.D.L., D.W., G.X.F., and M.L. Clinical diagnosis and event adjudication were performed by Y.L., Z.Y., G.X.F., and Y.M.Y. Patient follow-up was managed by H.Z., S.K.W., Z.Y., D.F.G., and Q.Y. The manuscript was drafted by Y.H.L., with revisions provided by Y.H.L., Z.Z., Y.L., and J.Z. Data collection and cleaning were handled by G.Z.Z., C.D.L., D.F.G., Q.Y., and Z.B.G. Data analysis was performed by Y.H.L. and W.K.L. Results were interpreted by Y.H.L., Y.L., Z.Z., and J.Z. All authors reviewed and approved the final version of the manuscript.
Declaration of interests
All authors declare no conflicts relevant to the contents of this article.
Published: August 5, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110643.
Contributor Information
Zhou Zhou, Email: zhouzhou@fuwaihospital.org.
Yan Liang, Email: fwliangyan2016@163.com.
Supplemental information
References
- 1.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., Blankstein R., Boyd J., Bullock-Palmer R.P., Conejo T., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 2.Bandstein N., Ljung R., Johansson M., Holzmann M.J. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J. Am. Coll. Cardiol. 2014;63:2569–2578. doi: 10.1016/j.jacc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., ESC Scientific Document Group Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D., Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Thygesen K., Alpert J.S., White H.D., et al. Third universal definition of myocardial infarction. Eur. Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 5.Roffi M., Patrono C., Collet J.P., Mueller C., Valgimigli M., Andreotti F., Bax J.J., Borger M.A., Brotons C., Chew D.P., et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 6.Collet J.P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 7.Clerico A., Zaninotto M., Ripoli A., Masotti S., Prontera C., Passino C., Plebani M., on the behalf of the Study Group on Cardiovascular Risk Biomarkers of the Italian Society of Clinical Biochemistry SIBioC The 99th percentile of reference population for cTnI and cTnT assay: methodology, pathophysiology and clinical implications. Clin. Chem. Lab. Med. 2017;55:1634–1651. doi: 10.1515/cclm-2016-0933. [DOI] [PubMed] [Google Scholar]
- 8.Chiang C.-H., Chiang C.-H., Pickering J.W., Stoyanov K.M., Chew D.P., Neumann J.T., Ojeda F., Sörensen N.A., Su K.-Y., Kavsak P., et al. Performance of the European Society of Cardiology 0/1-Hour, 0/2-Hour, and 0/3-Hour Algorithms for Rapid Triage of Acute Myocardial Infarction : An International Collaborative Meta-analysis. Ann. Intern. Med. 2022;175:101–113. doi: 10.7326/M21-1499. [DOI] [PubMed] [Google Scholar]
- 9.Reichlin T., Schindler C., Drexler B., Twerenbold R., Reiter M., Zellweger C., Moehring B., Ziller R., Hoeller R., Rubini Gimenez M., et al. One-Hour Rule-out and Rule-in of Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin T. Arch. Intern. Med. 2012;172:1211–1218. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- 10.Nestelberger T., Boeddinghaus J., Greenslade J., Parsonage W.A., Than M., Wussler D., Lopez-Ayala P., Zimmermann T., Meier M., Troester V., et al. Two-Hour Algorithm for Rapid Triage of Suspected Acute Myocardial Infarction Using a High-Sensitivity Cardiac Troponin I Assay. Clin. Chem. 2019;65:1437–1447. doi: 10.1373/clinchem.2019.305193. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow G.C., Adams K.F., Abraham W.T., Yancy C.W., Boscardin W.J., ADHERE Scientific Advisory Committee, Study Group, and Investigators Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 12.Twerenbold R., Neumann J.T., Sörensen N.A., Ojeda F., Karakas M., Boeddinghaus J., Nestelberger T., Badertscher P., Rubini Giménez M., Puelacher C., et al. Prospective Validation of the 0/1-h Algorithm for Early Diagnosis of Myocardial Infarction. J. Am. Coll. Cardiol. 2018;72:620–632. doi: 10.1016/j.jacc.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Twerenbold R., Badertscher P., Boeddinghaus J., Nestelberger T., Wildi K., Puelacher C., Sabti Z., Rubini Gimenez M., Tschirky S., du Fay de Lavallaz J., et al. 0/1-Hour Triage Algorithm for Myocardial Infarction in Patients With Renal Dysfunction. Circulation. 2018;137:436–451. doi: 10.1161/circulationaha.117.028901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeddinghaus J., Nestelberger T., Twerenbold R., Neumann J.T., Lindahl B., Giannitsis E., Sörensen N.A., Badertscher P., Jann J.E., Wussler D., et al. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur. Heart J. 2018;39:3780–3794. doi: 10.1093/eurheartj/ehy514. [DOI] [PubMed] [Google Scholar]
- 15.Chiang C.-H., Chiang C.-H., Lee G.H., Gi W.-T., Wu Y.-K., Huang S.-S., Yeo Y.H., Giannitsis E., Lee C.-C. Safety and efficacy of the European Society of Cardiology 0/1-hour algorithm for diagnosis of myocardial infarction: systematic review and meta-analysis. Heart. 2020;106:985–991. doi: 10.1136/heartjnl-2019-316343. [DOI] [PubMed] [Google Scholar]
- 16.Twerenbold R., Costabel J.P., Nestelberger T., Campos R., Wussler D., Arbucci R., Cortes M., Boeddinghaus J., Baumgartner B., Nickel C.H., et al. Outcome of Applying the ESC 0/1-hour Algorithm in Patients With Suspected Myocardial Infarction. J. Am. Coll. Cardiol. 2019;74:483–494. doi: 10.1016/j.jacc.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 17.Krintus M., Kozinski M., Boudry P., Capell N.E., Köller U., Lackner K., Lefèvre G., Lennartz L., Lotz J., Herranz A.M., et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin. Chem. Lab. Med. 2014;52:1657–1665. doi: 10.1515/cclm-2014-0107. [DOI] [PubMed] [Google Scholar]
- 18.Liu C., Deng Z., Wu W., Li Y., Yang F., Ge R., Ge M., Niu S., Liu H., Ji L., et al. Ethnicity and sex-specific 99th percentile upper reference limits of high-sensitivity cardiac troponin I among adults in Xinjiang, China. Clin. Biochem. 2023;116:94–99. doi: 10.1016/j.clinbiochem.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Apple F.S., Ler R., Murakami M.M. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin. Chem. 2012;58:1574–1581. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 20.Pickering J.W., Greenslade J.H., Cullen L., Flaws D., Parsonage W., Aldous S., George P., Worster A., Kavsak P.A., Than M.P. Assessment of the European Society of Cardiology 0-Hour/1-Hour Algorithm to Rule-Out and Rule-In Acute Myocardial Infarction. Circulation. 2016;134:1532–1541. doi: 10.1161/circulationaha.116.022677. [DOI] [PubMed] [Google Scholar]
- 21.Rubini Gimenez M., Twerenbold R., Jaeger C., Schindler C., Puelacher C., Wildi K., Reichlin T., Haaf P., Merk S., Honegger U., et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am. J. Med. 2015;128:861–870.e4. doi: 10.1016/j.amjmed.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Stoyanov K.M., Hund H., Biener M., Gandowitz J., Riedle C., Löhr J., Mueller-Hennessen M., Vafaie M., Katus H.A., Giannitsis E. RAPID-CPU: a prospective study on implementation of the ESC 0/1-hour algorithm and safety of discharge after rule-out of myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care. 2020;9:39–51. doi: 10.1177/2048872619861911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mair J., Lindahl B., Hammarsten O., Müller C., Giannitsis E., Huber K., Möckel M., Plebani M., Thygesen K., Jaffe A.S. How is cardiac troponin released from injured myocardium? Euro. Heart J. Acute Cardiovas. Care. 2018;7:553–560. doi: 10.1177/2048872617748553. [DOI] [PubMed] [Google Scholar]
- 24.Chenevier-Gobeaux C., Sebbane M., Meune C., Lefebvre S., Dupuy A.M., Lefèvre G., Peschanski N., Ray P. Is high-sensitivity troponin, alone or in combination with copeptin, sensitive enough for ruling out NSTEMI in very early presenters at admission? A post hoc analysis performed in emergency departments. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburn N.P., Snavely A.C., O’Neill J.C., Allen B.R., Christenson R.H., Madsen T., Massoomi M.R., McCord J.K., Mumma B.E., Nowak R., et al. Performance of the European Society of Cardiology 0/1-Hour Algorithm With High-Sensitivity Cardiac Troponin T Among Patients With Known Coronary Artery Disease. JAMA Cardiol. 2023;8:347–356. doi: 10.1001/jamacardio.2023.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steyerberg E.W. Validation in prediction research: the waste by data splitting. J. Clin. Epidemiol. 2018;103:131–133. doi: 10.1016/j.jclinepi.2018.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Pertinent data reported in this paper will be shared by the lead contact upon reasonable request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request
-
•
All original code is available in this paper’s supplementary files “Data S1”