Summary
Background
We aimed to assess real-world efficacy of the PARP inhibitor, olaparib, in US Veterans with metastatic prostate cancer (mPC) by leveraging the national data repository and evaluate a novel approach to assess treatment efficacy in tumors considered rare or harboring rare mutations.
Methods
Included Veterans had 1) mPC with somatic or germline alterations/mutations in genes involved in homologous recombination repair (HRR), 2) received olaparib monotherapy as well as a novel hormonal therapy/androgen receptor pathway inhibitors (NHT/ARPI), and/or chemotherapy, and 3) estimable rates of tumor growth (g-rate) using PSA values obtained while receiving treatment. Previous work has shown an excellent inverse correlation of g-rate with survival. Using g-rate, we determined tumor doubling time (DT) and DT ratios (DT on olaparib/DT on prior medication). We postulated that a DT ratio 1 was associated with benefit.
Findings
We identified 139 Veterans, including 42 Black males with tumors harboring mutations/alterations in HRR genes who received olaparib: BRCA2 (50), ATM (32), BRCA1 (10), other mutations (47). 62/139 (45%) of all and 21/42 (50%) of Black Veterans had DT ratios ≥1, including 31, 10, 2, and 19 with BRCA2, ATM, BRCA1, and other mutations, respectively (p = 0.006). Median survival with DT ratios ≥1 was superior, being 24.5 vs. 11.4 months for DT ratio <1 (p = 0.01, HR 0.50, 95% CI 0.29–0.85). Benefit from olaparib, defined as DT ratio ≥1, was not observed for germline status, starting PSA value, number of prior therapies, or immediate prior therapy. Compared to matched cohorts, tumors in the olaparib cohort had shorter DTs with enzalutamide in first line (367 vs. 884 days; p = 0.0043).
Interpretation
Using equations indifferent to timing of assessments ideal for real-world efficacy analyses, we showed DT ratio ≥1 representing slower tumor growth on olaparib relative to the prior therapy correlates with improved survival. Olaparib efficacy in Veterans with mPC harboring mutations/alterations in HRR genes emulates clinical trial results. Black men had comparable results. Compared to matched cohorts, in first line, enzalutamide was less efficacious in tumors harboring mutations/alterations in HRR genes.
Funding
American Society of Clinical Oncology Conquer Cancer Foundation (ASCO CCF), the Blavatnik Family Foundation and the Prostate Cancer Foundation (PCF).
Keywords: Homologous recombination repair, Olaparib, Real-world data, g-rate, Efficacy evaluation, Prostate cancer
Research in context.
Evidence before this study
Alterations in the genes implicated in homologous recombination repair (HRR) have been reported in 20–25% of patients with metastatic prostate cancer (mPC). The TOPARP-B and PROfound clinical trials reported composite overall response rates between 22% and 54% depending on various preplanned cohorts and olaparib doses, with higher responses possibly seen in tumors with BRCA1/2 alterations. Data regarding PARP inhibitor responses in Black males is lacking due to limited clinical trial enrollment. While PSA is most commonly used to monitor the progression of mPC in the real-world, it's uncommonly the lone clinical trial endpoint. We have employed a method to estimate rates of tumor growth (g) and regression (d) using measurements of tumor burden obtained radiographically or with serum markers, including PSA values obtained while a patient undergoes treatment, and have shown robust inverse correlation with overall survival (OS).
Added value of this study
We report on olaparib efficacy utilizing real-world data in US Veterans with PCs harboring different mutations in the genes implicated in HRR. This study also informs the efficacy of olaparib in Black Veterans as it includes more Black patients than all olaparib registration clinical trials combined. Importantly, we present a novel method to assess tumor response using doubling time (DT) ratio. We first estimate the tumor growth rates (g-rates) using serial PSA values while receiving olaparib and the medication received just prior to olaparib and calculate a DT (0.693/g). DT ratio is calculated by dividing the DT on olaparib by the DT on the prior medication. We demonstrate a DT ratio ≥1 representing slower tumor growth on olaparib relative to the prior therapy correlates with improved OS.
Implications of all the available evidence
Olaparib is an effective medication in patients with prostate cancers harboring diverse HRR alterations, albeit slowing of tumor growth with prolongation of the DT was more likely in tumors with certain alterations, such as BRCA2 and PALB2. Black Veterans show similar response and survival outcomes. Anemia is the most common grade III cytopenia noted in our real-world analysis and was more pronounced in those who had a greater benefit from olaparib, possibly due to a longer duration of therapy. The data suggest a difference in response to novel hormonal therapies in patients with mPC whose tumors harbor HRR alterations, which should be studied prospectively. Equations indifferent to the timing of assessments that can be used to estimate g-rates and DTs, are ideal for real-world efficacy analyses. This method of determining efficacy has broad applicability and can be used in clinical trialsto assess the efficacy of any experimental therapy in patients with tumors considered rare or harboring rare mutations since it uses a patient as their own control.
Introduction
Metastatic prostate cancer (mPC) continues to be lethal, with 5-year survival rates of 31% and cancer-specific mortality of 78%.1,2 Advances in tumor molecular characterization are driving precision medicine strategies. Alterations in the genes implicated in homologous recombination repair (HRR) — BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, and RAD54L — have been reported in 20–25% of patients with mPC,3, 4, 5 and implicated in conferring increased sensitivity to poly-ADP ribose polymerase (PARP) inhibitors, including olaparib. The TOPARP-B and PROfound clinical trials reported composite overall response rates between 22% and 54% depending on various preplanned cohorts and olaparib doses, with higher responses possibly seen in tumors with BRCA1/2 alterations.5,6 This led to FDA approval in May 2020 of olaparib for “patients with deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC), who have progressed following prior treatment with enzalutamide or abiraterone.” Data regarding PARP inhibitor responses in Black males is limited. PROfound enrolled only 3 Black men, and race stratification was not reported in TOPARP-B, but likely enrolled very few Black males.5,6
While PSA is most commonly used to monitor the progression of mPC in the real-world, it's uncommonly the lone clinical trial endpoint. We have employed a method to estimate rates of tumor growth (g) and regression (d) using measurements of tumor burden obtained radiographically or with serum markers, including CA19-9 and PSA values obtained while a patient undergoes treatment.7, 8, 9, 10, 11, 12, 13 Across more than one dozen cancers treated with chemotherapy, targeted therapies, and immunotherapy, we have reported inverse correlations of g-rate values with overall survival, including published data in more than 20,000 patients with a diagnosis of mPC patients, with more than 5000 Veterans treated in Veterans Administration Medical Centers (VAMCs).7,8
The US Veterans Health Administration (VHA) is the largest integrated healthcare system in the United States, where Veterans receive equal care, decreasing the impact of socioeconomic factors. All data is stored in the VA Corporate Data Warehouse (CDW) and made available to researchers via a VA Informatics and Computing Infrastructure (VINCI). We aimed to assess real-world responses to olaparib in Veterans diagnosed with mPC harboring alterations/mutations in genes implicated in HRR, including responses in Black men. We report efficacy as tumor doubling time (DT), assessing olaparib efficacy by comparing DTs on olaparib and the medication administered before olaparib.
Methods
We have had a VA IRB-approved prospective observational study to assess outcomes in Veterans with prostate cancer since 2015. We mine the VA Corporate Data Warehouse (CDW) using VA Informatics and Computing Infrastructure (VINCI) to identify Veterans with mPC based on ICD-9/ICD-10 codes. Data is cross-referenced with the CDW Oncology registry (VACCR) and VA Prostate Data Core to confirm the diagnosis.14 Oral and intravenous (IV) medication dispensing details are obtained from the CDW pharmacy database. For this project, we first identified Veterans who received olaparib utilizing the pharmacy database and then conducted a manual review of the Veteran's chart. The mutation data was identified in scanned reports from FoundationOne [the large majority] or other companies. For a few in whom a report could not be found, we conducted a second manual review, looking at the notes from their oncologists to identify the mutation for which olaparib was prescribed.
Patient cohort
Included Veterans had 1) somatic or germline alterations/mutations in genes involved in HRR, 2) received olaparib monotherapy as well as at least one regimen that included either a novel hormonal therapy/androgen receptor pathway inhibitors (NHT/ARPI) either abiraterone or enzalutamide, and/or chemotherapy (docetaxel, cabazitaxel or a platinum compound) before olaparib, and 3) estimable rates of tumor growth (g-rate) using PSA values obtained while receiving olaparib and the drug administered prior to olaparib.
Covariates
We extracted data regarding demographics (age, race), Gleason scores, genetic mutations (somatic, germline if available), prior and subsequent lines of therapy and laboratory results (PSA, hemoglobin, WBC, absolute neutrophil count, platelets) while on medication. Supplemental Figure S1 illustrates important known confounders and potential bias using a directed acyclic graph (DAG).
Outcomes
Overall survival (OS) was the primary outcome of interest; death dates were collected from VA vital status files. Overall survival was defined as the time from the start of the treatment to death from any cause. Patients who were not deceased or were lost to follow-up at the cut off time of analysis, February 20, 2022, were censored.
g-rate method of data analysis
The regression-growth models describe changes in tumor quantity during therapy resulting from simultaneous exponential decay/regression, termed d, and exponential growth/regrowth of the tumor, termed g. This basic mathematical model is:
| f (t) = exp (−d ∗ t) + exp (g ∗ t) – 1 |
At the time (t), the total tumor burden (f) is the sum of the therapy-resistant part of the tumor growing exponentially at rate of g/day and the therapy-sensitive part of the tumor regressing/decaying at the rate of d/day.7, 8, 9, 10, 11, 12, 13,15 Both rates are calculated using the TUMGr package for R using serial PSA values while on a drug (https://cran.r-project.org/web/packages/tumgr/index.html).16 This approach has been validated in patients with prostate cancer using clinical trials and VINCI data.7,8,12,13
Doubling time (DT) ratio calculation
The doubling time (DT) can be readily calculated by dividing 0.693, the natural logarithm of 2, by the g-rate [DT = 0.693/g-rate].7, 8, 9, 10, 11, 12, 13,15 Furthermore, a DT ratio was calculated by dividing the DT on olaparib by the DT on the treatment just prior to olaparib. Emulating prior work that noted decrements in efficacy of successive treatment regimens,8,17 we postulated a DT ratio ≥1 would indicate clinical benefit from olaparib demonstrated by improved OS.
Matching
Using VINCI data, we have developed a large reference cohort that includes 38,122 unique Veterans who received medications for mPC as of June 2022. This includes 12,328, 9,529, and 7899 Veterans who received abiraterone, enzalutamide, and docetaxel, respectively and had estimable g values while on the medications. In this analysis, we matched patients in the olaparib cohort who received the abiraterone, enzalutamide or docetaxel as first-line treatment to those who received the same first-line treatment in the reference cohort. Matching variables included race, age in fixed 5-year categories or age 10 years, PSA value at time of medication start (<1, 1–10, >10), prior lines of therapy (0, 1, 2), and Gleason score (<8, 8).
Statistical analysis
The absolute and relative frequencies of patients' characteristics were calculated separately for patients with a doubling time (DT) ratio <1 and 1. A chi-square test was performed to compare the differences between the two groups. A Fisher's exact test was used when any expected cell count was less than 1, or more than 20% of the expected cell counts were less than 5. Comparisons of the g-rates were made by a two-sided Wilcoxon rank-sum test. For the matching analysis, a Wilcoxon signed-rank test was used. Survival probability was estimated using the Kaplan–Meier method. A log-rank test was used to compare the difference between the survival curves. The Cox proportional hazards model was used to estimate the hazard ratio for OS. The proportional hazards assumption for the Cox model was checked using a test for independence between Schoenfeld residuals and time, and no violation of the assumption was observed. A p-value <0.05 was considered statistically significant. All analyses were performed using R Statistical Software (version 4.2.0).
Ethics
The study was approved by the James J Peters (Bronx) VA Medical Center Institutional Review Board (protocol ID:1606682-9). The informed consent is waived for using VA CDW data via VINCI. All data has been anonymized.
Role of funders
ASCO Conquer Cancer Foundation provided a grant to protect principal investigator's (PI) time to conduct the research. Generous support was also provided by the Blavatnik Family Foundation and the Prostate Cancer Foundation (PCF). None of the Foundations had any role in study design, data collection, data analyses, interpretation or writing of report.
Results
We identified 139 Veterans who received olaparib between 2018 and 2022 and met the study criteria. The median and IQR of the follow-up time for the overall cohort from the start of olaparib was 282 [174–449] days (9.4 [1.6–15.0] months), with 23 Veterans still receiving olaparib at the time of data extraction. The censor rate for survival was 52%. The median age of the cohort is 74 years and includes 42 (30%) Black Veterans. The tumors of 50 (36%) Veterans had a BRCA2 mutation/alteration. ATM, CDK12, BRCA1, CHEK2, and PALB2 mutations/alterations were seen in 32 (23%), 13 (9%), 10 (7%), 8 (6%), and 4 (3%) of tumors, respectively. In fourteen tumors (10%), mutations were identified in more than one of the genes implicated in HRR. Sixty-two Veterans had germline testing found on chart review, and 37 of these Veterans had a germline mutation in an HRR gene. Gleason scores were available for 128 Veterans, with 87/128 (68%) Veterans having a Gleason score ≥ 8.125 Veterans had 2 prior lines of therapy. An NHT/ARPI and chemotherapy were administered right before olaparib to 81 and 54 Veterans, respectively (Table 1).
Table 1.
Patient characteristics.
| Characteristic | Patient (N = 139) no. (%) | DT 1 (N = 62) no. (%) | DT < 1 (N = 77) no. (%) | Chi-Square/Fisher's Exact—p-value |
|---|---|---|---|---|
| Age group | ||||
| <65 years | 12 (9) | 6 (50) | 6 (50) | 0.41 |
| 65–69 years | 12 (9) | 8 (67) | 4 (33) | |
| 70–74 years | 56 (40) | 26 (46) | 30 (54) | |
| 75–79 years | 28 (20) | 11 (39) | 17 (61) | |
| >80 years | 31 (22) | 11 (35) | 20 (65) | |
| Race | ||||
| White | 90 (65) | 40 (44) | 50 (56) | 0.22 |
| Black | 42 (30) | 21 (50) | 21 (50) | |
| Other/unknown | 7 (5) | 1 (14) | 6 (86) | |
| Mutation | ||||
| ATM | 32 (23) | 10 (31) | 22 (69) | 0.0058 |
| BRCA1 | 10 (7) | 2 (20) | 8 (80) | |
| BRCA2 | 50 (36) | 31 (62) | 19 (38) | |
| CDK12 | 13 (9) | 3 (23) | 10 (77) | |
| CHEK2 | 8 (6) | 3 (38) | 5 (62) | |
| PALB2 | 4 (3) | 3 (75) | 1 (25) | |
| Other | 8 (6) | 6 (75) | 2 (25) | |
| Multiple | 14 (10) | 4 (29) | 10 (71) | |
| Number of lines before olaparib | ||||
| 1 | 14 (10) | 4 (29) | 10 (71) | 0.45 |
| 2 | 53 (38) | 27 (51) | 26 (49) | |
| 3 | 37 (27) | 15 (41) | 22 (59) | |
| 4 | 23 (17) | 12 (52) | 11 (48) | |
| 5 | 12 (9) | 4 (33) | 8 (67) | |
| Treatment right before olaparib | ||||
| Abiraterone | 32 (23) | 14 (44) | 18 (56) | 0.88 |
| Cabazitaxel | 26 (19) | 14 (54) | 12 (46) | |
| Darolutamide | 5 (4) | 2 (40) | 3 (60) | |
| Docetaxel | 24 (17) | 8 (33) | 16 (67) | |
| Enzalutamide | 44 (32) | 20 (45) | 24 (55) | |
| Platinum | 4 (3) | 2 (50) | 2 (50) | |
| Radium-223 | 4 (3) | 2 (50) | 2 (50) | |
| Gleason grade | ||||
| 8 | 87 (63) | 38 (44) | 49 (56) | 0.96 |
| <8 | 41 (29) | 19 (46) | 22 (54) | |
| Unknown | 11 (8) | 5 (45) | 6 (55) | |
| Starting PSA value (ng/ml) | ||||
| <1 | 39 (28) | 15 (38) | 24 (62) | 0.66 |
| 1–9.9 | 45 (32) | 21 (47) | 24 (53) | |
| >10 | 55 (40) | 26 (47) | 29 (53) | |
| Number of Veterans who had taken following medication at any time point before olaparib | |||||
|---|---|---|---|---|---|
| Enzalutamide | 120 (86%) | Abiraterone | 116 (85%) | Docetaxel | 80 (58%) |
| Cabazitaxel | 34 (24%) | Darolutamide | 6 (4%) | Apalutamide | 1 (<1%) |
Assessing olaparib efficacy by calculating the ratio of the DT on olaparib to the DT on the therapy prior to olaparib
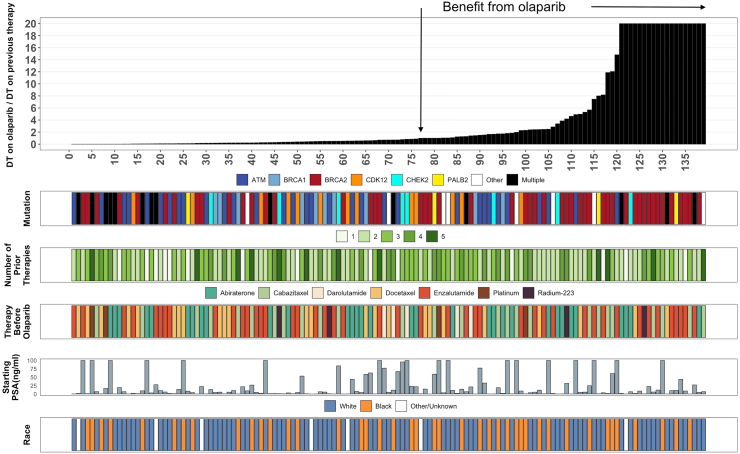
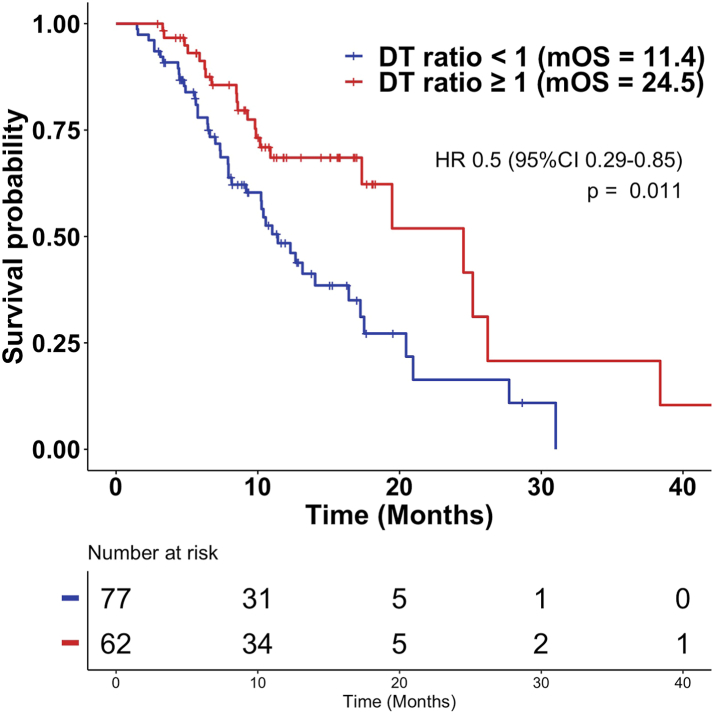
Comparing the DT on olaparib to that on the prior therapy, 62 of 139 patients (45%) had DT ratio ≥1 including 31 (62%), 2 (20%), 10 (31%), 3 (23%), 3 (38%), 3 (75%), 6 (75%), and 4 (29%) Veterans with tumors harboring BRCA2, BRCA1, ATM, CDK12, CHEK2, PALB2, other and multiple alterations/mutations, respectively. There was no statistically significant difference in the DT ratio <1 and DT ratio ≥1 group regarding age, race, number of prior lines of therapy, treatment right before olaparib, Gleason score, and starting PSA value (Table 1). The downward pointing arrow in Fig. 1 identifies a DT ratio of 1. Veterans shown to the right of the arrow (DT ratio ≥1) had a g-rate on olaparib that was not faster than on the previous therapy. The median OS was 24.5 months (95% CI 17.3-NR) in the group with a DT ratio ≥1 compared to 11.4 months (95% CI 9.2–17.2) for those with a DT ratio <1 (p = 0.011, HR 0.50, 0.29–0.85), demonstrating a better outcome, measured as a longer DT, with olaparib compared to the prior therapy in the cohort with a DT ratio ≥1 (Fig. 2). The median duration of therapy in the group with a DT ratio ≥1 was 204 days (127–319) vs. 103 days (72–158) in the group with a DT ratio <1 (p < 0.0001). At the time of data cutoff, 15 Veterans had received more than 12 months of therapy, primarily those whose tumors harbored BRCA2 mutations but also tumors with other mutations—BRCA2 (n = 8), ATM (n = 2), CHEK2 (n = 1), CDK12 (n = 2), PALB2 (n = 1) and 1 other mutation.
Fig. 1.
Figure depicting the assessment of olaparib efficacy in 139 Veterans who received olaparib as part of their treatment regimen. We began by estimating the rate of tumor growth or g-rate in the 139 Veterans while they received olaparib and the treatment before olaparib. Next, we divided 0.693, the natural logarithm of 2, by the rate of tumor growth, g-rate, and estimated the tumor's doubling time (DT). Finally, the tumor DT while receiving olaparib was divided by the DT on the therapy prior to olaparib, allowing us to estimate the ratio of DTs. We chose a ratio of 1 as the threshold for activity and confirmed its contribution [see Fig. 2]. The downward pointing arrow identifies a DT ratio of 1. All those to the right of this had a DT ratio 1 meaning olaparib efficacy was superior to the efficacy of the previous therapy evidenced by slower tumor growth and a prolonged DT. Note that many of these ratios were very high indicative of marked slowing of tumor growth by olaparib in Veterans who received the drug often as their third or fourth line of treatment, as shown by the green bars describing the number of prior therapies (see Table 1). Also shown are the HRR genes harboring mutations, the drug used just prior to olaparib, the PSA value when olaparib was started, and the race of the Veteran.
Fig. 2.
Kaplan Meier plots of overall survival support the choice of a DT ratio of 1 as the discriminating DT ratio. The median overall survival of those with a DT ratio <1 was 11.4 months, and those with a DT ratio 1 was 24.5 months. Cutoffs less than 1 did not discriminate and had comparable overall survivals.
Efficacy in Black Veterans
Comparing the DT on olaparib to that on the prior therapy, 21 of 42 (50%) Black Veterans had a DT ratio ≥ 1. A DT ratio 1 was observed in 9 (60%) and 12 (44%) of tumors harboring BRCA and non-BRCA alterations/mutations, respectively (p = 0.52). Looking at the DT ratio 1 group, the median OS was comparable in Black (38.4 months, 95% CI 9.3–NR) compared to White Veterans [24.5 months, 95% CI 17.3–NR; HR 1.09, 95% CI 0.35–3.38; p = 0.88) (Supplemental Figure S2). Amongst Black Veterans, the median duration of therapy was 145 days (113–268) in the group with a DT ratio 1 vs. 108 days (93–164) in the group with a DT ratio <1 (p = 0.10).
Occurrence of cytopenia and effects of prior chemotherapy on response to olaparib
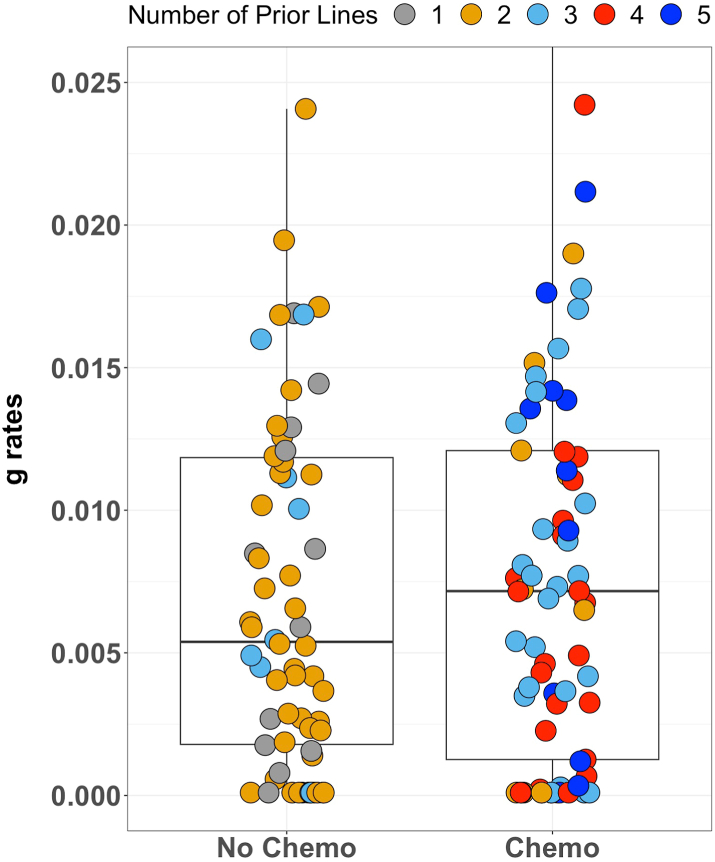
We compared g-rates while on olaparib of Veterans who received chemotherapy any time before olaparib (n = 73) to those who did not receive chemotherapy (n = 66). The median prior lines of therapy before olaparib were three for those who received prior chemotherapy and two for those who did not. The median g-rate was faster (0.007167/d; DT 97 days) in Veterans who had received chemotherapy before olaparib compared to those who did not (0.005385/d; DT 129 days), but the difference was statistically insignificant (p = 0.56). (Fig. 3).
Fig. 3.
Box plot demonstrating comparable distribution of g values while receiving olaparib in Veterans who had not or had received chemotherapy prior to olaparib. Slight differences in g values were not statistically different. Note that a majority of those who had received prior chemotherapy had received 3, 4, or 5 prior therapies, while those who did not receive chemotherapy before olaparib had, for the most part, only 1 or 2 prior therapies, with a few having had 3. The difference is likely explained by the treatment with chemotherapy. This figure shows what data analysis found: olaparib efficacy was indifferent to the number of prior therapies, evidenced here by the comparable distribution of the different color dots.
Common Terminology Criteria for Adverse Events (CTCAE) grade III anemia (hemoglobin <8 g/dL) was noted in 22 (36%) of Veterans in the DT ratio ≥1 group compared to 12 (16%) of Veterans in the group with a DT ratio <1 (p = 0.01). CTCAE grade III decreases in neutrophil and platelet counts were not statistically different (Table 2). There was no significant difference in the occurrence of dose reduction in the two groups. Additionally, CTCAE occurrence of grade III cytopenias in Veterans who had received chemotherapy (n = 73) before olaparib compared to those who had not received chemotherapy (n = 65) were not statistically different (Supplemental Table S1).
Table 2.
Comparison of tolerability and duration of therapy in DT 1 and DT < 1 cohorts.
| N = 138 [blood tests not available for one patient] | N | DT 1 (N = 61) | DT < 1 (N = 77) | Chi-square/Fisher's Exact p value |
|---|---|---|---|---|
| Hemoglobin g/dl nadir | ||||
| <8 | 34 | 22 (36%) | 12 (16%) | 0.010 |
| 8 | 104 | 39 (64%) | 65 (84%) | |
| Absolute neutrophil count (ANC)/dl nadir | ||||
| <1000 | 10 | 7 (11%) | 3 (4%) | 0.10 |
| 1000 | 128 | 54 (89%) | 74 (96%) | |
| Platelet/dl nadir | ||||
| <50,000 | 6 | 4 (3%) | 2 (3%) | 0.41 |
| 50,000 | 132 | 57 (93%) | 75 (93%) | |
| Olaparib dose reduction | ||||
| Yes | 30 | 14 (23%) | 16 (21%) | 0.96 |
| No | 109 | 48 (77%) | 61 (79%) | |
| Duration of olaparib therapy | ||||
| Days (median, IQR) | 204 (127–319) | 103 (72–158) | <0.0001 | |
NHT/ARPI and chemotherapy efficacy in patients with HRR mutations via matching analysis
Amongst the 139 Veterans in the olaparib cohort, 102, 112, and 81 had received abiraterone, enzalutamide, and docetaxel at some point in their treatment history, respectively. Additionally, 59/102 (58%), 41/112 (37%), and 16/81 (20%) of the abiraterone, enzalutamide, and docetaxel cohorts had received the cohort-defining drug in first line. These numbers defined the size of a 3:1 reference cohort—177 (59 × 3) for the abiraterone cohort, 123 (41 × 3) for the enzalutamide cohort, and 48 (16 × 3) for the docetaxel cohort. These cohorts then allowed us to compare the median g-rates while receiving abiraterone, enzalutamide, and docetaxel in the first line in Veterans with tumors harboring alterations/mutations in genes implicated in HRR with the median g-rates of the reference cohorts (Table 3, Supplemental Table S2). The median g-rates of those in the olaparib cohort who had received abiraterone or docetaxel in first line were similar to those of the matched reference cohort of Veterans without known mutations/alterations in HRR. However, the median g-rate while receiving enzalutamide in first line as the first systemic therapy for mPC was faster in the Veterans with HRR alterations/mutations who comprise our olaparib cohort — g = 0.001889 (DT 367 days) in the olaparib cohort and g = 0.000784 (DT 884 days) in the reference cohort — a statistically significant difference (p = 0.0043) and consistent with reduced enzalutamide efficacy in those with HRR alterations/mutations.
Table 3.
Comparison of g-rates of drugs used in 1st line in the olaparib cohort and in a matched reference cohort.a
| Abiraterone |
Enzalutamide |
Docetaxel |
||||
|---|---|---|---|---|---|---|
| Olaparib cohort [N = 59]b | Reference cohort [N = 175]b | Olaparib cohort [N = 41]b | Reference cohort [N = 117]b | Olaparib cohort [N = 16]b | Reference cohort [N = 45c]b | |
| Age, median, IQR, years | 71 [67–75] | 73 [71–78] | 72 [70–80] | 74 [71–81] | 68 [60–69] | 70 [64–73] |
| Age group, years | ||||||
| <65 | 2 [3%] | 6 [3%] | 3 [7%] | 9 [8%] | 4 [25%] | 12 [27%] |
| 65–69 | 7 [12%] | 20 [11%] | 0 [0%] | 0 [0%] | 2 [13%] | 6 [13%] |
| 70–74 | 25 [42%] | 75 [43%] | 18 [44%] | 54 [46%] | 7 [44%] | 21 [47%] |
| 75–79 | 13 [22%] | 39 [22%] | 8 [20%] | 18 [15%] | 3 [19%] | 6 [13%] |
| 80 | 12 [20%] | 35 [20%] | 12 [29%] | 36 [31%] | 0 [0%] | 0 [0%] |
| Race | ||||||
| White | 44 [75%] | 131 [75%] | 26 [63%] | 75 [64%] | 9 [56%] | 27 [60%] |
| Black | 12 [20%] | 36 [21%] | 12 [29%] | 36 [31%] | 6 [38%] | 15 [33%] |
| Other/Unknown | 3 [5%] | 8 [5%] | 3 [7%] | 6 [5%] | 1 [6%] | 3 [7%] |
| Starting PSA, ng/mL | 6 [0.71–14] | 2 [0.60–9] | 5 [0.39–14] | 1 [0.50–5] | 25 [3–142] | 6 [1–37] |
| Gleason Score | ||||||
| <8 | 22 [37%] | 64 [37%] | 14 [34%] | 42 [36%] | 1 [6%] | 3 [7%] |
| 8 | 33 [56%] | 99 [57%] | 25 [61%] | 72 [62%] | 13 [81%] | 36 [80%] |
| Unknown | 4 [7%] | 12 [7%] | 2 [5%] | 3 [3%] | 2 [13%] | 6 [13%] |
| Comorbidity index | ||||||
| <5 | 49 [83%] | 146 [83%] | 30 [73%] | 90 [77%] | 13 [81%] | 39 [87%] |
| 5 | 10 [17%] | 29 [17%] | 10 [24%] | 27 [23%] | 3 [19%] | 6 [13%] |
| Unknown | 0 [0%] | 0 [0%] | 1 [2%] | 0 [0%] | 0 [0%] | 0 [0%] |
| g-rates/day | 0.001357 | 0.000858 | 0.001889 | 0.000607 | 0.001914 | 0.001161 |
| p-valued | – | 0.34 | – | 0.002e | – | 0.37e |
Matched on 5 year interval age groups, race, PSA (<1, 1–10, >10), Gleason score (<8, 8) and prior lines of therapy (0,1, 2).
59, 41, and 16 of the abiraterone, enzalutamide and docetaxel cohorts had received the cohort-defining drug in the first line and these numbers defined the size of an additional 3:1 cohort. 177 (59 × 3), 123 (41 × 3) 48 (16 × 3) for the abiraterone, enzalutamide and docetaxel cohort, respectively.
No matches were found for one patient, thus only N = 47 was selected from the reference cohort.
p-values in each case are obtained by comparing the g-rates of a reference cohort to the olaparib cohort.
These p-values come from Wilcoxon rank-sum test.
Discussion
We report our efforts examining olaparib efficacy in the therapy of prostate cancer (PC) harboring alterations/mutations in homologous recombination repair (HRR) genes in a real-world setting. This analysis 1) demonstrates the value of the approach we use to assess efficacy, one that we believe is ideal for real-world analyses given its indifference to the timing of data collection; 2) provides an example of a methodology that can use a patient as his/her own control to assess treatment efficacy by leveraging a paradigm that scored a longer PFS duration in a sequential treatment as evidence of efficacy17; and 3) as regards olaparib in the therapy of mPC, an indication approved by regulatory agencies including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), ratifies clinical trial results and especially does so in Black men, an important observation, given only three such men were enrolled in the PROfound registration trial, an under-representation of an important minority group disproportionately impacted by PC.5,6
As a measure of efficacy, we estimated rates of tumor growth, designated g-rate, a value we and the US FDA have shown in numerous cancers treated with chemotherapy, targeted therapies, and immunotherapy inversely correlates with overall survival.7, 8, 9, 10, 11, 12, 13,15 Estimable using clinical data, a g-rate is ideal for real-world analyses because the simple equations used to estimate it include time as a variable, rendering assessment intervals irrelevant. Given the widely disparate intervals of assessments in the real-world, our approach allows for estimates of efficacy using data not obtained on the rigid schedules used in a clinical trial and, importantly, will enable patients to serve as their own control. Note here that it would allow patients followed in the real-world that enroll on a protocol to have their pre-enrollment g-rates estimated as the comparator for their protocol g-rates regardless of prior assessment intervals. Specifically, the previous paradigm that scored a longer PFS duration in a sequential treatment as evidence of efficacy required prior enrollment on a protocol and even then, had to contend with differences in the intervals of assessment—eight weeks in the study compared with six or longer than eight in the prior to trial—a difference that confounded the comparisons.17 Because the approach described here is indifferent to the timing of assessment, patients with rare alterations/mutations, always difficult to find and recruit to trials of novel therapies increasingly being conducted, can serve as their own control without prior trial enrollment. For example, a patient who presents to their real-world oncologist and begins on a standard of care (SOC) regimen before or despite the rare alteration/mutation having been identified, can then enroll on the trial of the novel agent targeting their tumor's alteration/mutation, such as a basket trial, after disease progression and have the novel agent's efficacy compared to their prior therapy, in this case, the accepted SOC. Note that if the results with the novel agent are found to be demonstrably better, a strong argument could even be made that the data shows it is superior to the SOC and it should be considered for earlier administration.
An essential goal of this effort was to evaluate a novel method of analysis as an approach others could use to assess treatment efficacy in patients with tumors considered rare or harboring rare mutations, the latter a challenge increasingly encountered as rare mutations increasingly define rare tumors. A previous publication had argued that because successive therapies are invariably associated with less benefit, one could infer that a therapy administered in a clinical trial that achieves a PFS 1.3-fold longer than the previous therapy is likely beneficial.17 Consistent with this, we found in earlier analyses of Veteran data median g-rates (doubling time, DT) for abiraterone followed by enzalutamide of 0.0032/d (DT, 216 days) and 0.0062/d (DT, 112 days), respectively, and for enzalutamide followed by abiraterone of 0.0030/d (DT, 231 days) and 0.0083/d (DT, 83 days), demonstrating decrements in efficacy with faster rates of growth of two well-established effective therapies administered in succession.8 Given these considerations, we compared olaparib efficacy to that of the prior therapy. We chose to represent efficacy more intuitively as DTs, a value easily calculated by dividing the natural log of 2 (0.693) by the estimated g-rate and, in turn, defined the relative efficacy as the DT ratio by dividing the DT on olaparib by the DT on the drug before olaparib. With a DT ratio cutoff of 1 — indicative of comparable or better efficacy of the sequential therapy — but not DT ratio cutoffs lower than 1, we found statistically significant differences in the overall survivals of the groups with DT ratios <1 and DT ratios 1 and chose DT ratios 1 as a value indicative of meaningful olaparib efficacy.
With DT ratios as the metric, 62/139 patients (45%) had a DT ratio ≥1, including 21/42 (50%) Black Veterans, with DT ratios ≥1 observed in 20–75% of all HRR alterations/mutations most notably in tumors harboring BRCA2 mutations. The latter, a recurring observation indicating the mutations harbored by some tumors, may not confer much, if any, vulnerability, encouraging further studies to better identify those mutations that can result in meaningful benefit (Table 1). Importantly, there was no statistically significant difference in the groups with DT ratios <1 and DT ratios ≥1 in terms of age, race, number of prior lines of therapy, and starting PSA value (Fig. 1). We feel confident this data provides valuable support for its use in Black men and offers a way forward to assess efficacy in minorities repeatedly under-represented in clinical trials even as we continue to strive for their enrollment. We would note that while a DT ratio of 1 emerged as a distinguishing metric in our analysis, different cutoffs may provide greater discrimination in other settings. Finally, Supplemental Table S3 summarizes some of the data regarding PSA responses/declines, a metric often used but that in our analyses has proven less discriminatory.
Clinical trials such as PROpel, TALAPRO, PROfound, and TRITON3 have noted that control arms of abiraterone, enzalutamide, or docetaxel seem to have limited benefit when compared to a combination of PARP inhibitors with these drugs or PARP inhibitors alone.6,18, 19, 20 However, there have only been a few retrospective direct comparisons of the efficacy of first-line use of these drugs in patients with mPC with or without HRR alterations.21, 22, 23 Analyses such as the present study, especially leveraging the enormous data we have amassed in Veterans treated at VAMCs—a real-world cohort of more than 38,000 Veterans with mPC treated with one or more of the established therapies for which we also have invaluable information on treatment efficacy—allow interrogations often not possible in a clinical trial but potentially informative. Specifically, finding a cohort matched for desired variables is very achievable. In the current analyses, we thus created cohorts matched 3:1 and queried the response of tumors harboring alterations/mutations in HRR genes to commonly used treatment options. Such information would support either their use or avoidance in managing tumors harboring alterations/mutations in HRR genes.
Surprisingly, we found enzalutamide efficacy in first-line was inferior in Veterans comprising the olaparib cohort than in the matched reference cohorts. In contrast, g-rates for both abiraterone and docetaxel were comparable in the olaparib and reference cohorts. While others have reported similar results as ours — poorer outcomes with ARPIs in men whose mCRPC tumors harbor alterations/mutations in the genes associated with HRR,21,23 we cautiously see these findings as hypothesis-generating. Although published results have described enzalutamide's impact on DNA damage repair, none has implicated this as critical to its activity.24,25 Were it critical; one might envision reduced enzalutamide activity in cells harboring HRR gene defects that over time could adapt to disrupted repair. As regards docetaxel, we note that while conclusions regarding docetaxel in the therapy of prostate cancers harboring deletions/mutations in HRR genes remain inconclusive, the results in this analysis support docetaxel use against these tumors, recognizing all analyses, including the current, have involved small numbers of patients.26, 27, 28, 29 We also compared g-rates of first line abiraterone vs. enzalutamide vs. docetaxel in our 139 HRR patient cohort and found no statistically significant difference, but small sample size was one of the limiting factors (Supplemental Table S4). Finally, we found minimal impact of prior docetaxel on olaparib administration or tolerability, with only a modest hemoglobin decrement in those with DT ratios ≥1 likely associated with longer olaparib administration.
As with all studies, this analysis has limitations, beginning with its use of collected data, although the data was analyzed prospectively and blindly. Comprised of 139 Veterans its statistical power is limited. This cohort represents a specific group of men whose mutations were tested at Veterans Administration Medical Centers and received their care at these institutions, and this could introduce a selection bias and compromise generalizability. Additionally, the hazard ratio is subject to selection bias as the hazard is calculated conditional on those who have survived and confidence intervals for these hazard ratios are wide due to the sample size. Also, there could be other unmeasured confounding variables. Finally, we assumed the majority of the nearly 38,000 Veterans comprising our real-world data from which the comparator cohorts were drawn do not harbor alterations/mutations in genes encoding HRR proteins, given the studied mutations have been found in only a small percentage of Veterans with prostate cancer,30 and as such the comparator cohorts at worse would only have been minimally “contaminated”.
In summary, we report a real-world analysis of olaparib efficacy with the greatest benefit derived against tumors harboring BRCA2 mutations. Evidence is provided of comparable efficacy in Black men. g-rates have been shown time and again to correlate inversely with overall survival. Its indifference to intervals of assessment makes it ideal for real-world analyses. It demonstrates the enormous value of data such as that available in Veterans with mPC treated at VAMCs if they can be linked to estimates of efficacy.8 Finally, by allowing any individual to serve as their own control, this approach could prove valuable for assessing treatment efficacy in individuals with rare cancers or in cancers with rare alterations/mutations.
Contributors
Harshraj Leuva, Nader Jamaleddine, Mina Meseha and Mengxi Zhou have accessed and verified the data.
Harshraj Leuva and Tito Fojo were responsible for the decision to submit the manuscript.
All authors have read and approved the final version of the manuscript.
Harshraj Leuva, MBBS: Conceptualization, data curation, investigation, validation, methodology, writing, funding acquisition.
Mengxi Zhou, MSc: Methodology, validation, software, formal analysis, visualization, writing.
Nader Jamaleddine, MD: Data curation, investigation, validation.
Mina Meseha, MD: Data curation, investigation.
Izak Faiena, MD: Writing.
Yeun-Hee Anna Park, MD: Methodology, writing, supervision.
Glen McWilliams MD: Resources.
Carol Luhrs, MD: Resources, supervision.
Kara Maxwell, MD, PhD: Writing.
Daniel Von Hoff, MD: Conceptualization, methodology, writing.
Susan E. Bates, MD: Conceptualization, writing, supervision.
Tito Fojo MD, PhD: Conceptualization, methodology, writing, supervision, project administration.
Data sharing statement
The US Veterans Health Administration prohibits sharing data unless there is a signed data access agreement with the VA research team requesting it and it is approved by the IRB.
Declaration of interests
All other authors declare no conflicts of interest.
Acknowledgements
NA.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105288.
Contributor Information
Harshraj Leuva, Email: hleuva@unmc.edu.
Tito Fojo, Email: atf2116@cumc.columbia.edu.
Appendix ASupplementary data
References
- 1.Miller K.D., Nogueira L., Devasia T., et al. Cancer treatment and survivorship statistics, 2022. CA A Cancer J Clin. 2022;72(5):409–436. doi: 10.3322/caac.21731. [DOI] [PubMed] [Google Scholar]
- 2.Elmehrath A.O., Afifi A.M., Al-Husseini M.J., et al. Causes of death among patients with metastatic prostate cancer in the US from 2000 to 2016. JAMA Netw Open. 2021;4(8):e2119568. doi: 10.1001/jamanetworkopen.2021.19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abida W., Armenia J., Gopalan A., et al. Prospective genomic profiling of prostate cancer across disease States reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;1:1–16. doi: 10.1200/PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson D., Van Allen E.M., Wu Y.M., et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateo J., Porta N., Bianchini D., et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162–174. doi: 10.1016/S1470-2045(19)30684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain M., Mateo J., Fizazi K., et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 7.Wilkerson J., Abdallah K., Hugh-Jones C., et al. Estimation of tumour regression and growth rates during treatment in patients with advanced prostate cancer: a retrospective analysis. Lancet Oncol. 2017;18(1):143–154. doi: 10.1016/S1470-2045(16)30633-7. [DOI] [PubMed] [Google Scholar]
- 8.Leuva H., Sigel K., Zhou M., et al. A novel approach to assess real-world efficacy of cancer therapy in metastatic prostate cancer. Analysis of national data on Veterans treated with abiraterone and enzalutamide. Semin Oncol. 2019;46(4-5):351–361. doi: 10.1053/j.seminoncol.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Yeh C., Zhou M., Sigel K., et al. Tumor growth rate informs treatment efficacy in metastatic pancreatic adenocarcinoma: application of a growth and regression model to pivotal trial and real-world data. Oncol. 2023;28(2):139–148. doi: 10.1093/oncolo/oyac217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y., Mason J., Shen Y.-L., et al. An FDA analysis of the association of tumor growth rate and overall and progression-free survival in metastatic non-small cell lung cancer (NSCLC) patients. J Clin Oncol. 2020;38(15_suppl):9541. [Google Scholar]
- 11.Stein W.D., Yang J., Bates S.E., Fojo T. Bevacizumab reduces the growth rate constants of renal carcinomas: a novel algorithm suggests early discontinuation of bevacizumab resulted in a lack of survival advantage. Oncol. 2008;13(10):1055–1062. doi: 10.1634/theoncologist.2008-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein W.D., Figg W.D., Dahut W., et al. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncol. 2008;13(10):1046–1054. doi: 10.1634/theoncologist.2008-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein W.D., Gulley J.L., Schlom J., et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17(4):907–917. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alba P.R., Gao A., Lee K.M., et al. Ascertainment of veterans with metastatic prostate cancer in electronic Health records: demonstrating the case for natural language processing. JCO Clin Cancer Inform. 2021;5:1005–1014. doi: 10.1200/CCI.21.00030. [DOI] [PubMed] [Google Scholar]
- 15.Maitland M.L., Wilkerson J., Karovic S., et al. Enhanced detection of treatment effects on metastatic colorectal cancer with volumetric CT measurements for tumor burden growth rate evaluation. Clin Cancer Res. 2020;26(24):6464–6474. doi: 10.1158/1078-0432.CCR-20-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkerson J. R package version 004; 2016. Tumor growth rate analysis. [Google Scholar]
- 17.Von Hoff D.D., Stephenson J.J., Jr., Rosen P., et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28(33):4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 18.Fizazi K., Piulats J.M., Reaume M.N., et al. Rucaparib or physician's choice in metastatic prostate cancer. N Engl J Med. 2023;388(8):719–732. doi: 10.1056/NEJMoa2214676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal N., Azad A.A., Carles J., et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398):291–303. doi: 10.1016/S0140-6736(23)01055-3. [DOI] [PubMed] [Google Scholar]
- 20.Clarke N.W., Armstrong A.J., Thiery-Vuillemin A., et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022;1(9) doi: 10.1056/EVIDoa2200043. [DOI] [PubMed] [Google Scholar]
- 21.Fettke H., Dai C., Kwan E.M., et al. BRCA-deficient metastatic prostate cancer has an adverse prognosis and distinct genomic phenotype. eBioMedicine. 2023;95 doi: 10.1016/j.ebiom.2023.104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annala M., Struss W.J., Warner E.W., et al. Treatment Outcomes and Tumor Loss of Heterozygosity in Germline DNA Repair–deficient Prostate Cancer. Eur Urol. 2017;72(1):34–42. doi: 10.1016/j.eururo.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Warner E., Herberts C., Fu S., et al. BRCA2, ATM, and CDK12 defects differentially shape prostate tumor driver genomics and clinical aggression. Clin Cancer Res. 2021;27(6):1650–1662. doi: 10.1158/1078-0432.CCR-20-3708. [DOI] [PubMed] [Google Scholar]
- 24.Li L., Karanika S., Yang G., et al. Androgen receptor inhibitor-induced "BRCAness" and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10(480) doi: 10.1126/scisignal.aam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekhar K.R., Wang J., Freeman M.L., Kirschner A.N. Radiosensitization by enzalutamide for human prostate cancer is mediated through the DNA damage repair pathway. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0214670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tutt A., Tovey H., Cheang M.C.U., et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nientiedt C., Heller M., Endris V., et al. Mutations in BRCA2 and taxane resistance in prostate cancer. Sci Rep. 2017;7(1):4574. doi: 10.1038/s41598-017-04897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng H.H., DeJong M., Yu E.Y., et al. A pilot study of docetaxel and carboplatin for treatment of patients with mCRPC containing biallelic inactivation of genes in the BRCA1/2 pathway. J Clin Oncol. 2020;38(6_suppl):127. [Google Scholar]
- 29.Gallagher D.J., Cronin A.M., Milowsky M.I., et al. Germline BRCA mutation does not prevent response to taxane-based therapy for the treatment of castration-resistant prostate cancer. BJU Int. 2012;109(5):713–719. doi: 10.1111/j.1464-410X.2011.10292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung A., Candelieri D., Li Y., et al. Tumor testing and treatment patterns in veterans with metastatic castration-resistant prostate cancer. Semin Oncol. 2023;50(1-2):11–24. doi: 10.1053/j.seminoncol.2023.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.