Summary
Background
The extent to which COVID-19 diagnosis and vaccination during pregnancy are associated with risks of common and rare adverse pregnancy outcomes remains uncertain. We compared the incidence of adverse pregnancy outcomes in women with and without COVID-19 diagnosis and vaccination during pregnancy.
Methods
We studied population-scale linked electronic health records for women with singleton pregnancies in England and Wales from 1 August 2019 to 31 December 2021. This time period was divided at 8th December 2020 into pre-vaccination and vaccination roll-out eras. We calculated adjusted hazard ratios (HRs) for common and rare pregnancy outcomes according to the time since COVID-19 diagnosis and vaccination and by pregnancy trimester and COVID-19 variant.
Findings
Amongst 865,654 pregnant women, we recorded 60,134 (7%) COVID-19 diagnoses and 182,120 (21%) adverse pregnancy outcomes. COVID-19 diagnosis was associated with a higher risk of gestational diabetes (adjusted HR 1.22, 95% CI 1.18–1.26), gestational hypertension (1.16, 1.10–1.22), pre-eclampsia (1.20, 1.12–1.28), preterm birth (1.63, 1.57–1.69, and 1.68, 1.61–1.75 for spontaneous preterm), very preterm birth (2.04, 1.86–2.23), small for gestational age (1.12, 1.07–1.18), thrombotic venous events (1.85, 1.56–2.20) and stillbirth (only within 14-days since COVID-19 diagnosis, 3.39, 2.23–5.15). HRs were more pronounced in the pre-vaccination era, within 14-days since COVID-19 diagnosis, when COVID-19 diagnosis occurred in the 3rd trimester, and in the original variant era. There was no evidence to suggest COVID-19 vaccination during pregnancy was associated with a higher risk of adverse pregnancy outcomes. Instead, dose 1 of COVID-19 vaccine was associated with lower risks of preterm birth (0.90, 0.86–0.95), very preterm birth (0.84, 0.76–0.94), small for gestational age (0.93, 0.88–0.99), and stillbirth (0.67, 0.49–0.92).
Interpretation
Pregnant women with a COVID-19 diagnosis have higher risks of adverse pregnancy outcomes. These findings support recommendations towards high-priority vaccination against COVID-19 in pregnant women.
Funding
BHF, ESRC, Forte, HDR-UK, MRC, NIHR and VR.
Keywords: Adverse pregnancy outcomes, COVID-19, Vaccination, Trimester, COVID-19 variants
Research in context.
Evidence before this study
We searched for prospective epidemiological studies of COVID-19 diagnosis and vaccination investigating adverse pregnancy outcomes published in any language up until Dec 1, 2023 (with no specified earliest date), in MEDLINE, Scientific Citation Index Expanded, and Embase using relevant terms ((‘pregnan’∗ OR ‘gestation’ OR ‘birth’) AND (‘diabetes’ OR ‘hypertens’∗ OR ‘preeclampsia’ OR ‘preterm’ OR ‘premature’∗ OR ‘pregnancy’ ‘complication’ OR (‘adverse’ AND ‘birth’ AND ‘outcome∗’) OR ‘stillbirth’ OR (‘pregnan’∗ AND ‘loss’) OR ‘cardiovascular’ OR (‘venous’ AND ‘thrombo∗’)) AND (‘COVID’ OR ‘SARS-CoV-2’ OR ‘coronavirus’)) (n = 9309). We found many primary reports and literature-based reviews showing COVID-19 diagnosis was associated with higher risks of common adverse pregnancy outcomes (e.g., gestational hypertensive disorders in pregnancy and preterm) and COVID-19 vaccination offered protection against adverse pregnancy outcomes. However, there was conflicting evidence about how COVID-19 diagnosis relates to more rare and serious outcomes including stillbirth and venous thrombosis. Few studies investigated how the risks are affected by the timing of the COVID-19 diagnosis and vaccination during pregnancy.
Added value of this study
Our study used population-scale linked electronic health records from 864,654 pregnant women in England and Wales to reliably examine the relationships between timing of COVID-19 diagnosis and vaccination and adverse pregnancy outcomes. This study afforded several advantages. First, it is the largest study to date, providing enhanced generalisabllity. Second, we studied a wide range of common and rare (including venous thrombosis and stillbirth) adverse outcomes after both COVID-19 infection and vaccination. Third, we conducted our analyses by pregnancy trimester, time since COVID-19 diagnosis and vaccination, before and after the vaccination rollout in England and Wales, and COVID-19 variant. Fourth, we adjusted for a wide-range of potential confounders available in electronic health records.
Implications of all the available evidence
The chief implication of this study for public policy is to support recommendations towards high-priority vaccination against COVID-19 in pregnant women to avoid high risks of adverse pregnancy outcomes from COVID-19 especially in the 3rd trimester. The chief implication for scientific understanding is the novel evidence about the associations between timing of COVID-19 and common and rare adverse pregnancy outcomes, which are stronger within 14-days since COVID-19 infection, when COVID-19 occurs in the 3rd trimester and which remain even in the vaccination roll-out era.
Introduction
The physiological maternal adaptations to pregnancy, such as expansion of blood volume, increase of insulin resistance, and immunological changes, can unmask a latent predisposition in a woman to cardiovascular and cardiometabolic complications, such as pre-eclampsia, gestational hypertension, and gestational diabetes. These complications can, in turn, affect fetal development, leading to growth restriction (as for pre-eclampsia), overgrowth (associated with gestational diabetes), and preterm birth, often requiring medical intervention and increasing the risk of stillbirth.1 Additional viremia and inflammatory phases of respiratory virus-related infections such as H1N1 influenza, and more recently COVID-19 may further hamper maternal adaptation inducing pro-coagulation state, affecting uteroplacental circulation and, consequently, fetal growth.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
Studies investigating COVID-19 in pregnant women have largely focused either on COVID-19 infected women alone or on comparisons between COVID-19 infected and non-infected women at the time of birth.10,15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Few population-wide studies and multi-cohort studies, including a study from Denmark (n = 111,186)13 and Canada (n = 6012),7 and a multi-national cohort study involving 18 countries,9 examined COVID-19 during pregnancy. These studies showed that pregnant women diagnosed with COVID-19 are more likely to experience pre-eclampsia,8,12,13,25 gestational hypertension9 and preterm birth.7,8,12, 13, 14,26 Whilst a meta-analysis of 117,552 vaccinated pregnant women underscores COVID-19 vaccine efficacy in preventing infection and hospitalization,27,28 few studies have addressed the safety and effectiveness of COVID-19 vaccination during pregnancy.22,23,28, 29, 30, 31, 32 Current studies also overlook, with few exceptions,8,12,13,33 the relationship between COVID-19 diagnosis and vaccination during pregnancy, including the risks of rare outcomes (such as stillbirth and venous thrombotic events), and how risks differ by time since infection or vaccination, pregnancy trimester or secular time period (e.g., accounting for changes in COVID-19 variants and vaccination rollouts).
To address these gaps, we leveraged population-wide linked electronic health record (EHR) data sources from England and Wales to build the largest population-wide pregnancy cohort of women with a record of birth during the COVID-19 pandemic period. In our study we compared the incidence of adverse pregnancy outcomes in women with and without COVID-19 diagnosis and vaccination during pregnancy stages, accounting for a wide range of potential confounding factors. Our aim was to provide quantitative evidence for recommendations concerning antenatal care of women during their pregnancy and their vaccination against current and future COVID-19 outbreaks.
Methods
Study setting and population
A population-wide pregnancy cohort was defined using Hospital Episode Statistics (HES) maternity information in England and the Maternity Indicator Dataset (MIDS) in Wales. We included 865,654 women with (i) a singleton birth episode, (ii) estimated pregnancy start date for the 1st record of birth after 1st August 2019 and with a delivery date before 31st December 2021, and (iii) registered with a primary care general practice in England or Wales at the estimated pregnancy start date. The pregnancy cohort was linked with primary care events, emergency events, hospital admissions, critical care admissions, outpatient visits, COVID-19 test results, community dispensing records, and deaths. For women without a recorded estimated gestational age at delivery (n = 200,020, 23.1%), we considered a standard pregnancy duration of 280 days. See Supplementary Methods for more detail in the definition of the pregnancy cohort.
The pregnancy cohort was divided at 8th December 2020 (the start of the vaccination programme in the UK) into two subcohorts of pregnancies during pre-vaccination and vaccination roll-out eras. The subcohort for the pre-vaccination era included women with an estimated pregnancy start date from 1st August 2019, and delivery date up to 8th December 2020. The subcohort for the vaccination era included women with an estimated pregnancy start date after 8th December 2020 and delivery date up to 31st December 2021. Pregnancies that spanned across both periods were excluded from the subcohorts.
We categorized the periods of the COVID-19 pandemic into original, alpha, and delta eras, based on the prevalent strain: original variant: 1st July 2020 to 7th December 2020; alpha variant: 8th December 2020 to 17th May 2021; delta variant: 18th May 2021 to 13th December 2021.34
Data were accessed in National Health Service (NHS) England's Secure Data Environment (SDE) service for England35 and in the Secure Anonymised Information Linkage (SAIL) Databank for Wales, via the British Heart Foundation Data Science Centre's CVD-COVID-UK/COVID-IMPACT Consortium. The analysis was performed according to a prespecified protocol. The phenotypes and associated code is available at https://github.com/BHFDSC/CCU018_01. The study is reported in agreement with the RECORD and STROBE statements for observational studies using routinely collected health data.
COVID-19 diagnosis
COVID-19 diagnosis was defined at the earliest recorded date of a positive COVID-19 polymerase chain reaction (PCR) or antigen test or a confirmed COVID-19 diagnosis found in either primary care event records or secondary care hospital admission records, as defined in previous analyses.36 Hospitalisation related to COVID-19 was defined as a hospital admission record with a COVID-19 diagnosis code in the primary position within 4 weeks of from COVID-19 diagnosis as defined above.
COVID-19 vaccination
COVID-19 vaccination during pregnancy was defined as having received dose 1 or dose 1 and dose 2 of ChAdOx1-S vaccine, BNT162b2 vaccine or a mix of these vaccines during pregnancy.
Adverse pregnancy outcomes
We examined the following adverse pregnancy outcomes: gestational diabetes, gestational hypertension, pre-eclampsia, preterm birth (<37 weeks of pregnancy), very preterm birth (<32 weeks of pregnancy), small-for-gestational-age (<5th percentile), stillbirth and venous thrombotic events. The definition of each condition was based on rule-based phenotyping algorithms using SNOMED-CT (Systematised Nomenclature of Medicine–Clinical Terms), Read V2 (Read Coded Clinical Terms) and ICD-10 (International Classification of Diseases 10th Revision) (more details on the description of outcomes and data-sources in Table S1 and https://github.com/BHFDSC/CCU018_01).
Confounders
We pre-specified the following as confounders based on them being known or plausibly associated with COVID-19 diagnosis/vaccination and adverse pregnancy outcomes: age (years), deprivation at residential area level (quintiles), calendar week, region (England only), ethnic group (categories of White, other ethnic groups, unknown ethnic group), previous pregnancy (yes/no), smoking status (categories of smoker, former smoker, non-smoker), history of hypertension (yes/no), history of diabetes (yes/no), history of haematological and cardiovascular diseases (yes/no), overweight/obesity (yes/no), history of depression (yes/no) and other conditions (yes/no, including at least one of chronic obstructive pulmonary disease, liver disease, chronic kidney disease, cancer and surgical intervention). All confounders were defined using primary and secondary care records and occured before the estimated pregnancy start date. Individuals with missing values for deprivation levels (0.4%) and smoking status (6.1%) were excluded from the analysis.
Statistical analysis
We estimated nation-specific hazard ratios (HRs) in England and Wales, comparing the incidence of adverse pregnancy outcomes after a diagnosis of COVID-19 during pregnancy with the incidence of these outcomes in women before or without a diagnosis of COVID-19 during pregnancy. We estimated HRs in separate time periods (in days: [0,14), [14+,]) after diagnosis of COVID-19 and, separately, by pregnancy trimester of diagnosis (1st trimester ≤84 estimated pregnancy duration in days, 2nd trimester >84 < days ≤182, 3rd trimester >182) and by COVID-19 variants (original, alpha and delta era). Analyses used Cox regression models with estimated gestational age in days as the time scale and the estimated pregnancy start date as the time origin, fitted separately by nation. Censoring was at the earliest of the date of the outcome, delivery date or end of cohort follow-up date (i.e., 8th December 2020 or 31st December 2021). Nation-specific HRs for England and Wales were combined using inverse-variance weighted meta-analyses with fixed effect models. We visually assessed the proportional hazards assumption within each of these time periods for the main outcomes using log–log plots (see Supplementary Methods for more detail); there was no evidence of strong violation. To quantify the effectiveness of dose 1 of COVID-19 vaccine during pregnancy, we estimated nation-specific HRs, comparing the incidence of COVID-19 diagnosis in pregnant women after dose 1 of COVID-19 vaccine during pregnancy with the incidence of COVID-19 diagnosis in pregnant women before or without dose 1 of COVID-19 vaccine during pregnancy. Similarly, amongst pregnant women who had received dose 1 of COVID-19 vaccine during pregnancy, we estimated nation-specific HRs, comparing the incidence of COVID-19 diagnosis in women after dose 2 of COVID-19 vaccine during pregnancy with the incidence of COVID-19 diagnosis in women before or without dose 2 of COVID-19 vaccine. To quantify the association of dose 1 of COVID-19 vaccine during pregnancy and adverse pregnancy outcomes we estimated nation-specific HRs, comparing the incidence of adverse pregnancy outcomes after dose 1 of COVID-19 vaccine during pregnancy with the incidence of these outcomes in pregnant women before or without dose 1 of COVID-19 vaccine during pregnancy. Similarly, amongst pregnant women who had received dose 1 of COVID-19 vaccine during pregnancy, we estimated HRs comparing the incidence of adverse pregnancy outcomes after dose 2 of COVID-19 vaccine during pregnancy with the incidence of these outcomes in pregnant women before or without dose 2 of COVID-19 vaccine. Analyses used Cox regression models as described above, and were restricted to women with a pregnancy start date from 9th December 2020 and a delivery date up to 31st December 2021 and without a COVID-19 vaccination before pregnancy. In analyses of dose 2 of COVID-19 vaccine, the time origin was defined as the date of receiving the dose 1 of COVID-19 vaccine.
HRs were adjusted for (i) age and deprivation and (ii) age, deprivation, calendar week, region (England only) and a propensity score (spline term with 3 knots, with knots placed at the 25th, 50th, and 75th percentiles) incorporating all other available confounders. In analyses of dose 2 of COVID-19 vaccine, we further adjusted for the time between the estimated pregnancy start date and date of receiving dose 1 of COVID-19 vaccine. The propensity scores were estimated using logistic regression models; further details are provided in the Supplementary Methods.
We conducted subgroup analyses by COVID-19 vaccination status, (receiving two or more vaccine doses vs receiving fewer than 2 vaccine doses) age group (18–29/30–39/40–55 years), ethnic group (six categories: Black or Black British, Asian or Asian British, White, Mixed, Ethnic minorities and Unknown ethnic group), deprivation index, previous pregnancy, history of COVID-19 diagnosis before pregnancy and existing health conditions (i.e., at least one condition of haematological diseases, cardiovascular disease, hypertensive disorders, diabetes disorders, chronic obstructive pulmonary disease, liver disease, chronic kidney disease, cancer and surgical intervention during the last year before pregnancy). We also conducted a series of sensitivity analyses as specified in detail in Table S2. These included analyses restricted to women with known gestation age; with an estimated pregnancy start date <11th March 2020; with an estimated pregnancy start date ≥11th March 2020; who had not received any doses of COVID-19 vaccine up to 31st December 2021; who had received at least one dose of COVID-19 vaccine up to 31st December 2021 and who had received at least one dose of COVID-19 vaccine from 18th June 2021 to 31st December 2021 and in analyses which included outcomes occurring from day 1 (instead of day 0) of follow-up, when follow-up was censored at the maximal outcome week for preterm birth and very preterm birth and for spontaneous preterm births (rather than planned and spontaneous).
Results from analyses involving subgroups with less than ten incident cases are not reported, adhering to policies and processes of the NHS England SDE and the SAIL Databank.
Analyses used SQL, Python, and RStudio Version 1.3.1093.1 driven by R Version 4.0.3. Codelists used to define phenotypes (for eligibility, outcome and confounder information) are available at https://github.com/BHFDSC/CCU018_01.
The North East–Newcastle and North Tyneside 2 research ethics committee provided ethical approval for the CVD-COVID-UK research program (REC no. 20/NE/0161) to access, within secure trusted research environments, unconsented, whole-population, de-identified data from EHRs collected as part of patients’ routine healthcare.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
COVID-19 diagnosis analysis
Of the 865,654 pregnant women eligible for analysis (829,180 in England and 36,474 in Wales, Figure S1), 60,134 (6.9%) women had a diagnosis of COVID-19 during pregnancy (Table 1). Median age was 30 years and 551,787 (63.7%) had a previous pregnancy, 84,740 (9.8%) had a history of hypertension, 9590 (1.1%) had a history of cardiovascular or haematological diseases and 44,549 (5.1%) had a history of diabetes. Characteristics of pregnant women were similar in the pre-vaccination and vaccination eras, and in those with and without COVID-19 diagnosis during pregnancy with few exceptions (Table 1 and Table S3): in the vaccination era there was a higher proportion of COVID-19 diagnosis than in the pre-vaccination era. Characteristics were comparable between Wales and England, with a few exceptions (Table S4): pregnant women were generally younger, more likely to be current smokers, have a history of depression and less ethnically diverse in Wales than England.
Table 1.
Characteristics of the pregnancy cohort in the overall period, pre-vaccination era and vaccination era in England and Wales.
| Characteristic | Overall, N = 865,654 n (%) | Pre-vaccination era, N = 318,192 n (%) | Vaccination era, N = 162,733 n (%) |
|---|---|---|---|
| Age (years) | |||
| Median (interquartile range) | 30 (26–34) | 30 (26–33) | 30 (26–34) |
| <30 | 403,318 (46.6) | 152,268 (47.9) | 73,590 (45.2) |
| 30–39 | 434,849 (50.2) | 156,033 (49) | 83,577 (51.4) |
| ≥40 | 27,487 (3.2) | 9891 (3.1) | 5571 (3.4) |
| Deprivation | |||
| 1 (least) | 214,763 (24.8) | 79,462 (25.5) | 39,026 (24) |
| 2 | 190,090 (22) | 69,368 (22.3) | 35,473 (21.8) |
| 3 | 167,387 (19.3) | 60,019 (19.3) | 31,411 (19.3) |
| 4 | 152,663 (17.6) | 53,482 (17.2) | 29,464 (18.1) |
| 5 (most) | 136,868 (15.8) | 47,612 (15.3) | 26,557 (16.3) |
| Unknown | 3883 (0.4) | 1428 (0.5) | 807 (0.5) |
| Ethnic group | |||
| Black or Black British | 40,784 (4.7) | 15,018 (4.7) | 7745 (4.8) |
| Asian or Asian British | 105,156 (12.1) | 38,979 (12.3) | 19,624 (12.1) |
| White | 647,493 (74.8) | 236,290 (74.3) | 122,260 (75.1) |
| Mixed | 19,219 (2.2) | 6885 (2.2) | 3752 (2.3) |
| Ethnic minorities | 39,968 (4.6) | 15,593 (4.9) | 6953 (4.3) |
| Unknown ethnic group | 13,029 (1.5) | 5427 (1.7) | 2399 (1.5) |
| Previous pregnancy | 551,787 (63.7) | 201,725 (63.4) | 102,611 (63.1) |
| COVID-19 diagnosis during pregnancy | 60,134 (6.9) | 6821 (2.1) | 17,260 (10.6) |
| COVID-19 Hospitalization | 3626 (0.4) | 451 (0.1) | 1292 (0.8) |
| Trimester of exposure | |||
| 1st | 11,250 (1.3) | 175 (0.1) | 3197 (2) |
| 2nd | 18,532 (2.1) | 673 (0.2) | 5558 (3.4) |
| 3rd | 30,352 (3.5) | 5973 (1.9) | 8505 (5.2) |
| Dose 1 of COVID-19 vaccine during pregnancy | 62,478 (7.2) | 70,614 (43.4) | |
| Dose 2 of COVID-19 vaccine during pregnancy | 98,977 (11.4) | 50,133 (30.8) | |
| Smoking status | |||
| Current | 152,078 (17.6) | 57,780 (18.2) | 27,702 (17) |
| Ex | 118,843 (13.7) | 43,708 (13.7) | 22,070 (13.6) |
| Never | 542,178 (62.6) | 196,999 (61.9) | 102,486 (63) |
| History of cardiovascular and haematological diseasesa | 9590 (1.1) | 3500 (1.1) | 1822 (1.1) |
| History of hypertensiona | 84,740 (9.8) | 28,661 (9) | 17,558 (10.8) |
| History of diabetesa | 44,549 (5.1) | 15,966 (5) | 8721 (5.4) |
| History of depressiona | 178,352 (20.6) | 64,645 (20.3) | 34,429 (21.2) |
| History of other conditionsa | 131,338 (15.2) | 54,043 (17) | 17,848 (11) |
In the year before pregnancy.
During follow-up, we identified 182,120 adverse pregnancy outcomes as follows: gestational diabetes (n = 66,595), gestational hypertension (n = 24,440), pre-eclampsia (n = 13,655), preterm births (n = 42,850, including n = 7985 very preterm), infants born small for gestational age (n = 32,170), stillbirths (n = 1065) and venous thrombotic events (n = 1345) (Table 2).
Table 2.
Incidence of adverse pregnancy outcomes by pandemic period, follow-up, total number of events and incidence rate (IR) per 1000 person-years.
| Adverse pregnancy outcomes | Overall |
Pre-vaccination era |
Vaccination era |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up (person-years) | N events | IR | Follow-up (person-years) | N events | IR | Follow-up (person-years) | N events | IR | |
| Gestational diabetes | 638,641.2 | 66,595 | 104.28 (103.49, 105.07) | 234,839.1 | 23,030 | 98.07 (96.80, 99.34) | 119,367.0 | 13,385 | 112.13 (110.24, 114.03) |
| Gestational hypertension | 648,841.1 | 24,440 | 37.67 (37.20, 38.14) | 238,266.5 | 8705 | 36.53 (35.77, 37.30) | 121,438.3 | 4935 | 40.64 (39.51, 41.77) |
| Pre-eclampsia | 649,334.8 | 13,655 | 21.03 (20.68, 21.38) | 238,435.0 | 4990 | 20.93 (20.35, 21.51) | 121,538.9 | 2850 | 23.45 (22.59, 24.31) |
| Preterm birtha | 496,260.9 | 42,850 | 86.35 (85.53, 87.16) | 184,342.8 | 17,255 | 93.6 (92.21, 95.00) | 94,892.1 | 10,090 | 106.33 (104.27, 108.41) |
| Very preterm birtha | 496,261.0 | 7985 | 16.09 (15.74, 16.44) | 184,342.8 | 3470 | 18.82 (18.20, 19.45) | 94,892.1 | 2305 | 24.29 (23.30, 25.29) |
| Small for gestational agea | 496,261.0 | 32,170 | 64.82 (64.12, 65.53) | 184,342.8 | 12,215 | 66.26 (65.09, 67.44) | 94,892.1 | 6360 | 67.02 (65.38, 68.68) |
| Stillbirtha | 469,647.7 | 1065 | 2.27 (2.13, 2.40) | 174,644.7 | 395 | 2.26 (2.04, 2.49) | 89,090.0 | 235 | 2.64 (2.30, 2.99) |
| Thrombotic venous event | 621,958.8 | 1345 | 2.16 (2.05, 2.28) | 228,495.2 | 455 | 1.99 (1.81, 2.18) | 115,535.9 | 285 | 2.47 (2.18, 2.76) |
Excluding women without a recorded estimated gestational age at delivery.
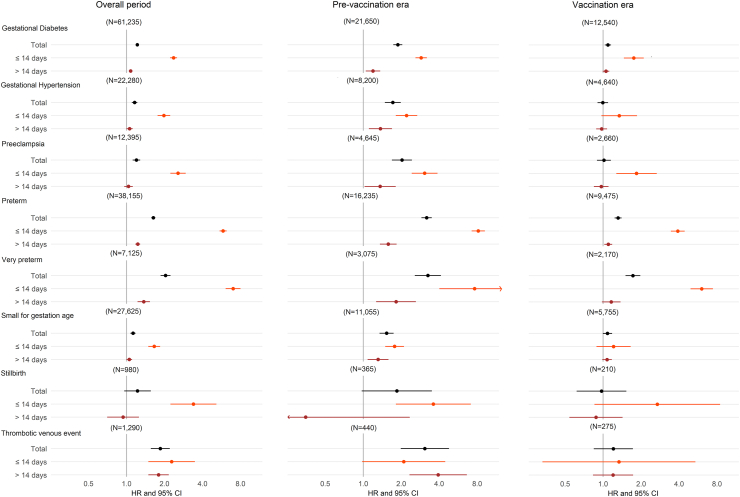
In the overall pregnancy cohort, COVID-19 diagnosis during pregnancy was associated with a higher risk of gestational diabetes (HR 1.22, 95% CI 1.18–1.26), gestational hypertension (HR 1.16, 1.10–1.22), pre-eclampsia (HR 1.20, 1.12–1.28), preterm birth (HR 1.63, 1.57–1.69; and HR 1.68, 1.61–1.75 for spontaneous preterm), very preterm birth (HR 2.04, 1.86–2.23), small for gestational age (HR 1.12, 1.07–1.18), and thrombotic venous events (HR 1.85, 1.56–2.20) (Fig. 1). Risks were higher within 14 days following COVID-19 diagnosis and remained elevated, although weaker, after 14 days with the exception of pre-eclampsia and small for gestational age. Association with stillbirth was observed only within 14 days following COVID-19 diagnosis (HR 3.39, 2.23–5.15). All associations were stronger in the pre-vaccination era, and remained elevated but weaker during the vaccination era for gestational diabetes (HR 1.09, 1.03–1.16), preterm birth (HR 1.31, 1.23–1.40), very preterm birth (HR 1.72, 1.50–1.97). Associations with hospitalised COVID-19 were generally stronger than associations with non-hospitalized COVID-19 (Figure S2).
Fig. 1.
Fully adjusted hazard ratios (log scale) for adverse pregnancy outcomes after COVID-19 diagnosis by pandemic period (overall n = 865,654, prevaccination era n = 318,192, vaccination era n = 162,733).
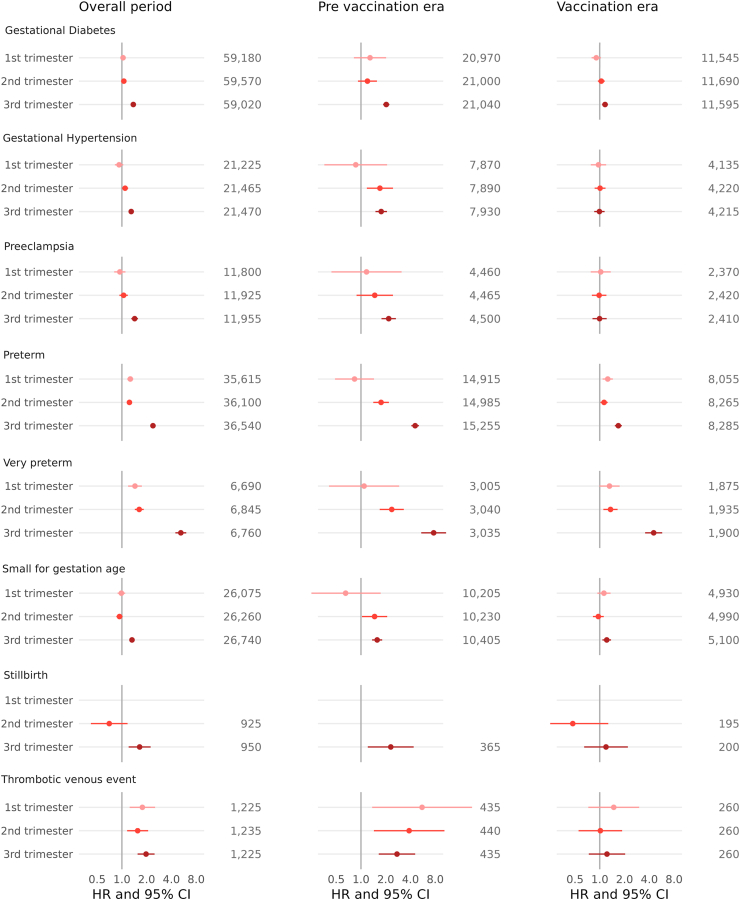
All associations between COVID-19 diagnosis and adverse pregnancy outcomes were confirmed in the 3rd trimester and remained elevated in the 1st and 2nd trimesters for preterm, very preterm, and thrombotic venous events (Fig. 2 and Figure S3). Hazard ratios for associations between COVID-19 diagnosis in the 3rd trimester and adverse pregnancy outcomes were generally stronger in magnitude during the pre-vaccination era, and remained elevated for gestational diabetes, preterm, very preterm birth and small for gestational age during the vaccination era. All associations were stronger in the original variant period, and remained elevated but weaker during the alpha and delta eras for gestational diabetes (Figure S4).
Fig. 2.
Fully adjusted hazard ratios (log scale) for adverse pregnancy outcomes after COVID-19 diagnosis by pandemic period and trimester of infection (n = 829,180, including only England).
Associations between COVID-19 diagnosis and adverse pregnancy outcomes were weaker in pregnant women who were fully vaccinated compared those who were not fully vaccinated for gestational diabetes, preterm and very preterm birth (Figure S5).
In subgroup analyses, having a previous COVID-19 diagnosis before pregnancy modified the association between COVID-19 diagnosis during pregnancy with gestational hypertension (HRs for women with vs without a previous COVID-19 diagnosis: 1.57 (1.13, 2.20) vs 1.15 (1.09–1.21), p-valueinteraction = 0.0129) and pre-eclampsia (1.82 (1.19–2.80) vs 1.19 (1.11–1.27), p-valueinteraction = 0.0029). No significant effect modifiers were observed on the multiplicative scale (Figures S6–S11).
Results remained consistent in sensitivity analyses (Figures S12 and S13).
COVID-19 vaccination
Among 148,841 pregnant women unvaccinated before pregnancy, 60,875 (40.9%) women were vaccinated with dose 1 of COVID-19 vaccine during pregnancy and 15,680 (10.5%) women were diagnosed with COVID-19 (Table S5). Compared to those vaccinated with dose 1, unvaccinated women were generally younger, more likely to be current smokers, from non-white ethnic groups and resided in areas with higher deprivation level.
COVID-19 vaccination during pregnancy was associated with a lower risk of COVID-19 diagnosis during pregnancy (Figure S14). After dose 1 of COVID-19 vaccine, HRs were 0.70 (0.60–0.81) in the first 4 weeks, 0.66 (0.59–0.73) during week 5–8, and 0.60 (0.58–0.62) from 9 weeks. After dose 2, HRs declined from 0.75 (0.68–0.84) in the first 4 weeks to 0.67 (0.60–0.73) and 0.55 (0.51–0.59) in subsequent periods. We found similar patterns of associations in subgroup and sensitivity analyses (Figures S15 and S16), with the notable exception that there was not a clear benefit of vaccination in pregnant women with a history of COVID-19 diagnosis.
During follow-up, there were 11,920 records of gestational diabetes, 4355 gestational hypertension, 2470 pre-eclampsia, 250 venous thrombotic, 8605 preterm birth (including 1860 very preterm birth), 5830 small for gestational age and 215 stillbirth events (Table S6).
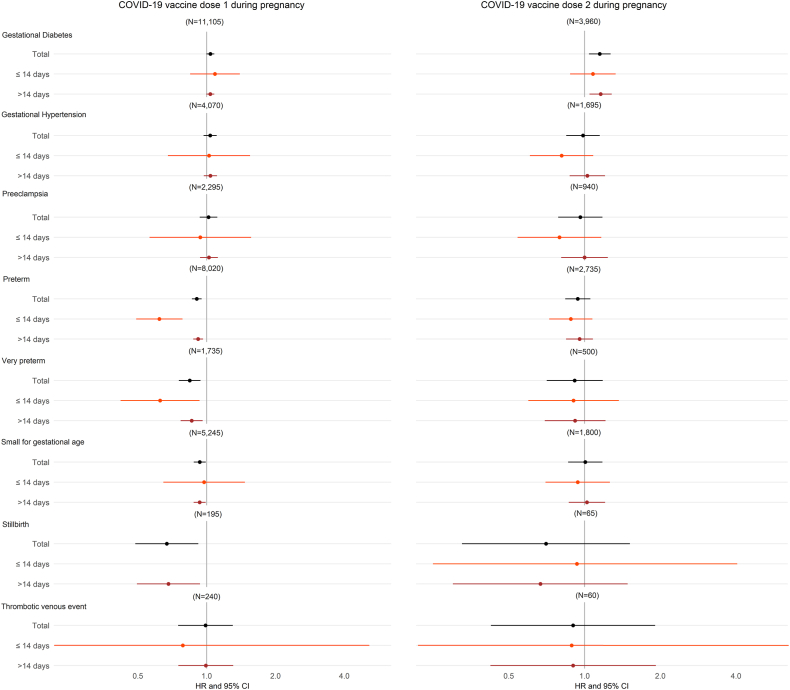
Dose 1 of COVID-19 vaccine during pregnancy was associated with lower risks of preterm birth (HR: 0.90, 0.86–0.95), very preterm birth (HR: 0.84, 0.76–0.94), small for gestational age (HR: 0.93, 0.88–0.99), and stillbirth (HR: 0.67, 0.49–0.92) (Fig. 3). However, we did observe a marginal higher incidence of gestational diabetes in women after dose 1 of COVID-19 vaccine during pregnancy in comparison to women without dose 1 of COVID-19 vaccine during pregnancy (HR: 1.04, 1.00–1.08). This association appeared to be restricted to women under 30 years of age or those in the 3rd trimester of pregnancy (Figures S17–S19). Similarly, we observed a slightly higher incidence of gestational diabetes in women after dose 2 of COVID-19 vaccine during pregnancy in comparison to women receiving only dose 1 of COVID-19 vaccine during pregnancy (Fig. 3 and Table S7). Notably, in sensitivity analyses, these associations attenuated to the null upon censoring follow-up at incident COVID-19 diagnosis (Figure S20). There was no evidence to suggest a higher incidence of other adverse pregnancy outcomes after COVID-19 vaccination during pregnancy (Fig. 3), which was consistent across subgroups and in sensitivity analyses (Figures S17–S21).
Fig. 3.
Fully adjusted hazard ratios (log scale) for adverse pregnancy outcomes, after dose 1 and 2 of COVID-19 vaccine during pregnancy (dose 1 analysis n = 148,841, dose 2 analysis n = 57,885, dose 2 analysis includes only England).
Discussion
The main finding of this analysis was that COVID-19 diagnosis during pregnancy was associated with higher risk of common adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders, preterm birth and small for gestational age, as well as rare outcomes including very preterm, preeclampsia, venous thrombosis and stillbirth (only within 14 days after COVID-19 diagnosis). These risks were more pronounced within 14 days of COVID-19 diagnosis and when COVID-19 diagnosis occurred in the 3rd trimester of pregnancy. These risks were less pronounced after the vaccination rollout in England and Wales. There was no evidence to suggest COVID-19 vaccination during pregnancy was associated with higher risks of adverse pregnancy outcomes. Our study's findings are consistent with previous research that indicate associations of COVID-19 diagnosis during pregnancy with subsequent risk of adverse pregnancy outcomes for pre-eclampsia (HR 1.22 vs RRs in prior studies ranging from 1.31 to 1.769,12,13,20), gestational hypertension (HR 1.16 vs RR 1.46),9 and preterm birth (HR 1.63 vs RRs in prior studies ranging from 1.15 to 2.177,8,12, 13, 14). This population-wide study is the largest to date and adds novel findings because we were able to quantify how the risks of adverse pregnancy outcomes vary by time since COVID-19, pregnancy trimester, variant era and before and after vaccination roll-outs. Risks of adverse pregnancy outcomes were lower during the vaccination era compared to the pre-vaccination era, as well as during the alpha and delta eras compared to the original variant period. Disentangling the potential role of the vaccination rollout from the emergence of new variants is challenging due to overlapping time periods and also changes in testing strategies between 2020 and 2021, which may influence the severity of reported COVID-19 diagnoses during these periods. Elevated risk of stillbirth was only observed within 14 days of a COVID-19 diagnosis and not beyond, whilst elevated risks for other adverse pregnancy outcomes were highest within 14 days. This may reflect ascertainment bias, whereby women presenting with a pregnancy complication are more likely to have a COVID-19 test, or may reflect an acute effect of COVID-19, which requires further investigation.
Our study contributes towards the understanding of the risk-benefit ratio of COVID-19 vaccination during pregnancy. Specifically, we find that pregnant women who received the vaccine had a lower risk of COVID-19 diagnosis after receiving a COVID-19 vaccine during pregnancy as shown in studies in the general population.37,38 We also observed a lower risk of gestational diabetes, preterm birth, and very preterm birth following COVID-19 in pregnant women who were fully vaccinated compared to those who were not fully vaccinated. Furthermore, we confirm the safety of the vaccine even for rare and severe events such as stillbirth and venous thrombotic events.39, 40, 41 Vaccination was also associated with a lower risk of preterm births, small for gestational age and stillbirth, likely as a result of fewer and less severe COVID-19. Whilst we observed an association between COVID-19 vaccination and gestational diabetes, this attenuated to the null on censoring for COVID-19 and on excluding pregnant women with comorbidities and in priority groups for COVID-19 vaccination, suggesting this may be due to confounding. Specifically, COVID-19 or other comorbidities may increase the likelihood of gestational diabetes and being vaccinated. Alternatively, the positive association may reflect a type 1 error arising from multiple testing or may have been influenced by differences in healthcare utilisation and outcome recording between vaccinated and unvaccinated women. For example, women with better access to care are more likely to receive both the oral glucose tolerance test for gestational diabetes and the COVID-19 vaccination.
There are several key strengths to our study. To limit reverse causality, we focused on COVID-19 diagnosis during pregnancy and compared the magnitude of the associations over the pregnancy and split the follow-up time into 0–14 and 14+ days since COVID-19 diagnosis or vaccination. To limit confounding, we adjusted for several potential confounders using non-linear propensity scores. To examine possible misclassification of COVID-19 diagnosis during the 1st wave of COVID-19 pandemic, we performed analyses separately for the pandemic period and the vaccination era. To minimise possible misclassification of the outcomes, we integrated primary care, secondary care, and maternity data. Our results were robust to a variety of sensitivity analyses, and the national nature of the pregnancy cohorts supported the generalizability of the findings.
Our study has some potential limitations. First, gestational week at birth was imputed for about 25% of women in England. It is likely that missing gestational week is independent of outcomes and does not affect the time between exposure and the outcomes. Missing data could have resulted in non-differential misclassification of exposure early in pregnancy. However, sensitivity analyses among women with known estimated gestational week confirmed the findings. Second, pregnant women diagnosed with COVID-19 may have received more intensive monitoring than those non-diagnosed. This increased surveillance could lead to a more rigorous case ascertainment, potentially biasing associations away from the null. Third, our analyses exclude home deliveries (about 2.5%) in England.42 Fourth, we used a stringent definition for small for gestational age, employing the 5th percentile. This helps in identifying the smallest babies, thereby increasing specificity (i.e., the babies we pinpoint are more likely to face health risks). However, this approach might decrease sensitivity (i.e., we could overlook some babies who have health risks due to their size). Fifth, this study cannot exclude residual confounding, particularly since we lacked data on body mass index and instead adjusted for a history of overweight and obesity and measurement errors in smoking behavior may have biased the estimates toward the null, as smoking behavior is negatively associated with the risk of COVID-19 infection.43 Sixth, the potential for reverse causation remains due to relative short pregnancy time periods. Seventh, the results from this study are based on the whole populations in England and Wales with singleton birth and include, original, alpha, and delta variant periods (up to 31st December 2021), but may not be generalisable to other countries, non-singleton birth pregnancies or other pandemic periods. Eighth, this study cannot exclude the possibility of effect modification on scales other than multiplicative ones.
In conclusion, our study provides evidence that COVID-19 diagnosis during pregnancy was associated with a higher risk of common and rare adverse pregnancy outcomes. These risks were more pronounced within 14 days of COVID-19 diagnosis and when COVID-19 diagnosis occurred in the 3rd trimester of pregnancy, and before the vaccination rollout. Notably, there was no evidence to suggest higher risks of adverse pregnancy outcomes following vaccination. These findings support recommendations towards high-priority vaccination against COVID-19 in pregnant women to avoid high risks of adverse pregnancy outcomes from COVID-19 especially in the 3rd trimester.
Contributors
ER, TB, GS, AA, KH, GC, DAL, MC, AMW contributed to Conceptualization. ER, TB, JN, LZ, RD, EA, AA, DAL, AMW contributed to Methodology. ER contributed to Formal analysis. ER, AMW contributed to Investigation. ER, TB, JN, HA contributed to Data Curation. ER, AMW contributed to Writing–Original Draft. All authors contributed to Writing–Review & Editing. ER contributed to Visualization. AMW contributed to Supervision.
Data sharing statement
The data used in this study are available in NHS England's Secure Data Environment (SDE) service for England, but as restrictions apply they are not publicly available (https://digital.nhs.uk/services/secure-data-environment-service). The CVD-COVID-UK/COVID-IMPACT programme led by the BHF Data Science Centre (https://bhfdatasciencecentre.org/) received approval to access data in NHS England's SDE service for England from the Independent Group Advising on the Release of Data (IGARD) (https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/independent-group-advising-on-the-release-of-data) via an application made in the Data Access Request Service (DARS) Online system (ref. DARS-NIC-381078-Y9C5K) (https://digital.nhs.uk/services/data-access-request-service-dars/dars-products-and-services). The CVD-COVID-UK/COVID-IMPACT Approvals & Oversight Board (https://bhfdatasciencecentre.org/areas/cvd-covid-uk-covid-impact/) subsequently granted approval to this project to access the data within NHS England's SDE service for England. The de-identified data used in this study were made available to accredited researchers only. Those wishing to gain access to the data should contact bhfdsc@hdruk.ac.uk in the first instance.
Declaration of interests
G Smith: GSK. Consultant and member of expert panel for RSV vaccination in pregnancy. GSK. Member of Data Safety Monitoring Committee for trials of RSV vaccination in pregnancy Moderna. Member of Data Safety Monitoring Committee for trials of RSV vaccination in pregnancy. No other conflicts of interest to be disclosed. D A Lawlor: European Research Council, Research grant administered by University of Bristol. No conflict with this paper; and Diabetes UK, Research grant administered by University of Bristol. No conflict with this paper.
Acknowledgements
This work is carried out with the support of the BHF Data Science Centre led by HDR UK. This study makes use of de-identified data held in NHS England's Secure Data Environment service for England and the SAIL Databank, and made available via the BHF Data Science Centre's CVD-COVID-UK/COVID-IMPACT consortium. This work uses data provided by patients and collected by the NHS as part of their care and support. We would also like to acknowledge all data providers who make health relevant data available for research.
This study makes use of anonymised data held in the Secure Anonymised Information Linkage (SAIL) Databank. This work uses data provided by patients and collected by the NHS as part of their care and support. We would also like to acknowledge all data providers who make anonymised data available for research. We wish to acknowledge the collaborative partnership that enabled acquisition and access to the de-identified data, which led to this output. The collaboration was led by the Swansea University Health Data Research UK team under the direction of the Welsh Government Technical Advisory Cell (TAC) and includes the following groups and organisations: the SAIL Databank, Administrative Data Research (ADR) Wales, Digital Health and Care Wales (DHCW), Public Health Wales, NHS Shared Services Partnership (NWSSP) and the Welsh Ambulance Service Trust (WAST). All research conducted has been completed under the permission and approval of the SAIL independent Information Governance Review Panel (IGRP) project number 0911.
The British Heart Foundation Data Science Centre (grant No SP/19/3/34678, awarded to Health Data Research (HDR) UK) funded co-development (with NHS England) of the Secure Data Environment service for England, provision of linked datasets, data access, user software licences, computational usage, and data management and wrangling support, with additional contributions from the HDR UK Data and Connectivity component of the UK Government Chief Scientific Adviser's National Core Studies programme to coordinate national COVID-19 priority research. This work was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council (MC_PC_20059) and by the CONVALSCENCE study, which is funded by NIHR (COV-LT-0009). This work was supported by the Con-COV team funded by the Medical Research Council (grant number: MR/V028367/1). This work was supported by Health Data Research UK, which receives its funding from HDR UK Ltd (HDR-9006) funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation (BHF) and the Wellcome Trust. This research has been supported by the ADR Wales programme of work. ADR Wales, part of the ADR UK investment, unites research expertise from Swansea University Medical School and WISERD (Wales Institute of Social and Economic Research and Data) at Cardiff University with analysts from Welsh Government. ADR UK is funded by the Economic and Social Research Council (ESRC), part of UK Research and Innovation. This research was supported by ESRC funding, including Administrative Data Research Wales (ES/W012227/1). This research was supported by the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre (BRC-1215-20014; NIHR203312) and the British Heart Foundation (RG/18/13/33946: RG/F/23/110103).
Consortium partner organisations funded the time of contributing data analysts, biostatisticians, epidemiologists, and clinicians. This research was supported by the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre (BRC-1215-20014; NIHR203312). ER was supported by HDR-UK2022.0173; ‘Developing capacity and capability to undertake UK-wide studies on >65 M people using COVID-19 as an exemplar (COALESCE)’ and by The Swedish Research Council for Health, Working Life and Welfare (Forte 2022-00882) and The Swedish Research Council (VR 2023-01982). LZ's contribution to this study is supported by Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (grant ref MC_PC_20058), with additional support by The Alan Turing Institute via ‘Towards Turing 2.0’ EPSRC Grant Funding and by the Health Data Science Centre, Human Technopole, Milan (Italy). RD's contribution to this study is supported by NIHR Bristol Biomedical Research and Health Data Research UK South-West. DAL's contribution to this study is supported by the British Heart Foundation (CH/F/20/90003 and AA/18/1/34219) and the UK Medical Research Council (MC_UU_00032/05). AMW is supported by the BHF Data Science Centre (HDRUK2023.0239) and as an NIHR Research Professor (NIHR303137). Cambridge BHF Centre of Research Excellence (RE/18/1/34212), BHF Chair Award (CH/12/2/29428) and by Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome. AMW conducted this research whilst part of the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement No 116074 and whilst supported by the BHF-Turing Cardiovascular Data Science Award (BCDSA∖100005).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101037.
Contributor Information
Elena Raffetti, Email: er578@cam.ac.uk.
Angela M. Wood, Email: amw79@cam.ac.uk.
Appendix A. Supplementary data
References
- 1.Cunningham F.G., Leveno K.J., Bloom S.L., et al. McGraw-Hill Medical; New York: 2014. Williams obstetrics. [Google Scholar]
- 2.Siston A.M., Rasmussen S.A., Honein M.A., et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rad H.S., Röhl J., Stylianou N., et al. The effects of COVID-19 on the placenta during pregnancy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.743022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aabakke A.J.M., Krebs L., Petersen T.G., et al. SARS-CoV-2 infection in pregnancy in Denmark-characteristics and outcomes after confirmed infection in pregnancy: a nationwide, prospective, population-based cohort study. Acta Obstet Gynecol Scand. 2021;100(11):2097–2110. doi: 10.1111/aogs.14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ (Clinical research ed) 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linehan L., O'Donoghue K., Dineen S., White J., Higgins J.R., Fitzgerald B. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–266. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClymont E., Albert A.Y., Alton G.D., et al. Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. JAMA. 2022;327(20):1983–1991. doi: 10.1001/jama.2022.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan A.K., Arah O.A., Fell D.B., Sullivan S.G. SARS-CoV-2 infection during pregnancy and associated perinatal health outcomes: a national US cohort study. J Infect Dis. 2022;225(5):759–767. doi: 10.1093/infdis/jiab626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villar J., Ariff S., Gunier R.B., et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman M., Navér L., Söderling J., et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA. 2021;325(20):2076–2086. doi: 10.1001/jama.2021.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chmielewska B., Barratt I., Townsend R., et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Global Health. 2021;9(6):e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metz T.D., Clifton R.G., Hughes B.L., et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327(8):748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aabakke A.J.M., Petersen T.G., Wøjdemann K., et al. Risk factors for and pregnancy outcomes after SARS-CoV-2 in pregnancy according to disease severity: a nationwide cohort study with validation of the SARS-CoV-2 diagnosis. Acta Obstet Gynecol Scand. 2023;102(3):282–293. doi: 10.1111/aogs.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith L.H., Dollinger C.Y., VanderWeele T.J., Wyszynski D.F., Hernández-Díaz S. Timing and severity of COVID-19 during pregnancy and risk of preterm birth in the international registry of coronavirus exposure in pregnancy. BMC Pregnancy Childbirth. 2022;22(1):775. doi: 10.1186/s12884-022-05101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sentilhes L., De Marcillac F., Jouffrieau C., et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020;223(6):914.e1–914.e15. doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neelam V., Reeves E.L., Woodworth K.R., et al. Pregnancy and infant outcomes by trimester of SARS-CoV-2 infection in pregnancy-SET-NET, 22 jurisdictions, January 25, 2020-December 31, 2020. Birth Defects Res. 2023;115(2):145–159. doi: 10.1002/bdr2.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvert C., Carruthers J., Denny C., et al. A population-based matched cohort study of early pregnancy outcomes following COVID-19 vaccination and SARS-CoV-2 infection. Nat Commun. 2022;13(1):6124. doi: 10.1038/s41467-022-33937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favre G., Maisonneuve E., Pomar L., et al. Maternal and perinatal outcomes following pre-Delta, Delta, and Omicron SARS-CoV-2 variants infection among unvaccinated pregnant women in France and Switzerland: a prospective cohort study using the COVI-PREG registry. Lancet Reg Health Eur. 2023;26 doi: 10.1016/j.lanepe.2022.100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jering K.S., Claggett B.L., Cunningham J.W., et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181(5):714–717. doi: 10.1001/jamainternmed.2020.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurol-Urganci I., Jardine J.E., Carroll F., et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225(5):522.e1–522.e11. doi: 10.1016/j.ajog.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karasek D., Baer R.J., McLemore M.R., et al. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Health Am. 2021;2 doi: 10.1016/j.lana.2021.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsay L., Calvert C., Shi T., et al. Neonatal and maternal outcomes following SARS-CoV-2 infection and COVID-19 vaccination: a population-based matched cohort study. Nat Commun. 2023;14(1):5275. doi: 10.1038/s41467-023-40965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torche F., Nobles J. Vaccination, immunity, and the changing impact of COVID-19 on infant health. Proc Natl Acad Sci U S A. 2023;120(49) doi: 10.1073/pnas.2311573120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskenazi B., Rauch S., Iurlaro E., et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study. Am J Obstet Gynecol. 2022;227(1):74.e1–74.e16. doi: 10.1016/j.ajog.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papageorghiou A.T., Deruelle P., Gunier R.B., et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225(3):289.e1–289.e17. doi: 10.1016/j.ajog.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliani F., Oros D., Gunier R.B., et al. Effects of prenatal exposure to maternal COVID-19 and perinatal care on neonatal outcome: results from the INTERCOVID Multinational Cohort Study. Am J Obstet Gynecol. 2022;227(3):488.e1–488.e17. doi: 10.1016/j.ajog.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosworth M.L., Schofield R., Ayoubkhani D., et al. Vaccine effectiveness for prevention of covid-19 related hospital admission during pregnancy in England during the alpha and delta variant dominant periods of the SARS-CoV-2 pandemic: population based cohort study. BMJ Med. 2023;2(1) doi: 10.1136/bmjmed-2022-000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piekos S.N., Hwang Y.M., Roper R.T., et al. Effect of COVID-19 vaccination and booster on maternal-fetal outcomes: a retrospective cohort study. Lancet Digit Health. 2023;5(9):e594–e606. doi: 10.1016/S2589-7500(23)00093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnus M.C., Örtqvist A.K., Dahlqwist E., et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327(15):1469–1477. doi: 10.1001/jama.2022.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavares Veras Florentino P., Cerqueira-Silva T., Freire De Carvalho L., et al. Safety of BNT162b2 and CoronaVac during pregnancy on birth outcomes and neonatal mortality: a cohort study from Brazil. Int J Epidemiol. 2023;52:1708. doi: 10.1093/ije/dyad120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stock S.J., Carruthers J., Calvert C., et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28(3):504–512. doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villar J., Soto Conti C.P., Gunier R.B., et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet (London, England) 2023;401(10375):447–457. doi: 10.1016/S0140-6736(22)02467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stock S.J., Moore E., Calvert C., et al. Pregnancy outcomes after SARS-CoV-2 infection in periods dominated by delta and omicron variants in Scotland: a population-based cohort study. Lancet Respir Med. 2022;10(12):1129–1136. doi: 10.1016/S2213-2600(22)00360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.COG-UK mutation explorer. https://sars2.cvr.gla.ac.uk/cog-uk/
- 35.Wood A., Denholm R., Hollings S., et al. Linked electronic health records for research on a nationwide cohort of more than 54 million people in England: data resource. BMJ. 2021;373:n826. doi: 10.1136/bmj.n826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight R., Walker V., Ip S., et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2022;146(12):892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 38.Lau J.J., Cheng S.M.S., Leung K., et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat Med. 2023;29(2):348–357. doi: 10.1038/s41591-023-02219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fell D.B., Dhinsa T., Alton G.D., et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478–1487. doi: 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe A., Yasuhara J., Iwagami M., et al. Peripartum outcomes associated with COVID-19 vaccination during pregnancy: a systematic review and meta-analysis. JAMA Pediatr. 2022;176(11):1098–1106. doi: 10.1001/jamapediatrics.2022.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piekos S.N., Roper R.T., Hwang Y.M., et al. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit Health. 2022;4(2):e95–e104. doi: 10.1016/S2589-7500(21)00250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Office for National Statistics . 2020. Birth characteristics in England and Wales.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2020 [Google Scholar]
- 43.Galanti M.R., Andersson F., Caspersen I.H., et al. Current tobacco use and COVID-19 diagnoses in a cohort of adult clients of public dental clinics in Sweden. Sci Rep. 2023;13(1):1204. doi: 10.1038/s41598-023-28091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.