Abstract
Background
A pulmonary artery-to-aorta (PA/A) ratio of ≥1 is a reliable indicator of pulmonary hypertension and is associated with an increased risk of acute exacerbation of chronic obstructive pulmonary disease (COPD) and long-term mortality in patients with stable COPD. However, it is unclear whether a PA/A ratio of ≥1 is associated with mortality in patients hospitalized with acute exacerbation of COPD. The purpose of this study was to evaluate the clinical course and mortality of patients with PA/A ratios of ≥1 who were hospitalized with acute exacerbation of COPD.
Methods
We retrospectively reviewed the medical charts of patients admitted to a tertiary referral hospital and a secondary hospital with acute exacerbation of COPD between 2016 and 2021. Chest computed tomography was used to measure the pulmonary artery (PA), aorta (A) diameter, and the PA/A ratio. The study involved 324 and 111 patients with PA/A ratios <1 and ≥1, respectively.
Results
The average age in the two groups was 74.1 and 74.5 years, which was not significantly different. When compared with the group with PA/A ratios of <1, the group with PA/A ratios of ≥1 had a lower proportion of males (71.2% vs. 89.5%, P<0.001), more patients with type 2 respiratory failure (35.1% vs. 18.8%), higher high-flow nasal cannula use (10.8% vs. 4.6%), higher use of non-invasive ventilation (NIV) (21.6% vs. 7.7%), and longer hospital stay (10.9 vs. 9.5 days). In-hospital mortality was not significantly different between the two groups. A PA/A ratio of ≥1 was identified as an independent predictor of the need for high-flow nasal cannula, NIV, and intubation in COPD patients.
Conclusions
Patients with PA/A ratios of ≥1 had a high incidence of type 2 acute respiratory failure and required advanced treatment, including high-flow nasal cannula, NIV, and intubation. Therefore, hospitalized patients with acute exacerbation of COPD and PA/A ratios of ≥1 require more aggressive treatment.
Keywords: Chronic obstructive pulmonary disease (COPD), acute exacerbation, pulmonary artery-to-aorta ratio (PA/A ratio)
Highlight box.
Key findings
• In patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease (COPD), a pulmonary artery-to-aorta (PA/A) ratio of ≥1 was identified as an independent predictor of the need for high-flow nasal cannula, non-invasive ventilation, and intubation.
What is known and what is new?
• PA/A ratios of ≥1 are associated with an increased risk of acute exacerbation of COPD and long-term mortality in patients with stable COPD.
• It is unclear whether PA/A ratios of ≥1 are associated with mortality in patients hospitalized with acute exacerbation of COPD. In this study, in-hospital mortality did not differ significantly between patients with PA/A ratios of ≥1 vs. <1. However, PA/A ratios of ≥1 were identified as a good predictor of needing high-flow nasal cannula [odds ratio (OR) =2.382; 95% confidence interval (CI): 1.051–5.395], non-invasive ventilation (OR =4.801; 95% CI: 1.977–11.658), and intubation (OR =2.095; 95% CI: 1.043–4.209).
What is the implication, and what should change now?
• When hospitalized with acute exacerbation of COPD, patients with PA/A ratios of ≥1 should be treated more aggressively.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms caused by airway and/or alveoli abnormalities that result in persistent, often progressive, airflow obstruction (1). COPD is one of the top three causes of death worldwide (2,3). In patients with COPD, acute exacerbations are associated with increased morbidity and mortality (1,4-6). Patients hospitalized with acute exacerbation of COPD have an in-hospital mortality rate of 3–9%, but this rate increases to 11% if non-invasive ventilation (NIV) is required (7-10). The mortality rate one year after acute COPD exacerbation is 28% (7-10). Acute exacerbation of COPD is associated with several mortality risk factors, including advanced age, being male, prior hospitalization, an arterial carbon dioxide partial pressure (PaCO2) of >45 mmHg, and a blood urea nitrogen level of >22 mg/dL (11).
Pulmonary hypertension is associated with poor prognosis in patients with COPD (4,12). A pulmonary artery-to-aorta (PA/A) ratio of ≥1 is a reliable indicator of pulmonary hypertension (13,14). PA/A ratios of ≥1 are associated with an increased risk of acute exacerbation of COPD and long-term mortality in patients with COPD (15,16). Few studies have investigated the association between PA/A ratios and clinical outcomes in patients hospitalized with acute exacerbation of COPD and previous results have varied across studies. Several studies have associated increased PA/A ratios with higher in-hospital mortality among patients hospitalized with acute exacerbation of COPD (17,18), although there is also evidence of no correlation between the two (19,20).
In this study, we evaluated the clinical course and mortality of patients with PA/A ratios of ≥1 who were hospitalized with acute exacerbation of COPD. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-62/rc).
Methods
Study design and population
We retrospectively reviewed the medical charts of patients with COPD who were admitted to Chonnam National University Hospital, a tertiary referral hospital and Chonnam National University Bitgoeul Hospital, a secondary hospital between January 2016 and August 2021. COPD diagnosis was based on the global initiative for chronic obstructive lung disease (GOLD) guidelines, whereby COPD is indicated by a forced expiratory volume in 1 second-to-forced vital capacity ratio of <70% of the predicted value (21). The diagnosis of acute exacerbation of COPD was also based on GOLD guidelines and is indicated by an acute worsening of respiratory symptoms that results in additional therapy (21). Patients without recent pulmonary function tests or with pneumothorax, pleural effusion, pulmonary embolism, pulmonary edema, or arrhythmia were excluded from the study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (approval No. 2024-011). Informed consent was waived by Chonnam National University Hospital’s Institutional Review Board because of the study’s minimal risk and retrospective design. Patient data were anonymized and deidentified before the analyses. All participating institutions were also informed and agreed the study.
Definitions
The results of arterial blood gas analysis within 24 hours of admission were used to determine the type of acute respiratory failure (ARF). Type 1 ARF was indicated by an arterial oxygen partial pressure of <60 mmHg in room air, or an arterial oxygen partial pressure-to-fractional inspired oxygen ratio of <300. Type 2 ARF was indicated by respiratory acidosis (a PaCO2 value of >45 mmHg and an arterial pH of <7.35). Chest computed tomography was used to measure the pulmonary artery (PA), aorta (A) diameter, and the PA/A ratio, as previously described (15). Hypoventilation and ventilation-perfusion mismatch were assessed and defined as follows: PaCO2 >45 mmHg, increased alveolar-arterial oxygen gradient, and correction of hypoxemia with oxygen (22).
Data collection
Data on the patients’ age, sex, body mass index, smoking status, underlying diseases (hypertension and diabetes, tuberculosis, bronchiectasis, ischemic heart disease, atrial fibrillation, congestive heart failure, chronic kidney disease, and previous cerebrovascular accidents) combined with pneumonia, pulmonary function tests, arterial blood gas levels, initial laboratory findings (white blood cell counts and the levels of hemoglobin, platelet, blood urea nitrogen, creatinine, C-reactive protein, procalcitonin, fibrinogen, fibrinogen degradation products, and D-dimer), GOLD stage and group (21), previous treatments, treatment with conventional oxygen therapy, high-flow nasal cannula (HFNC) and NIV use, intensive care unit admission, intubation, and antibiotic and systemic steroid use, were analyzed. Data on in-hospital mortality, readmission within one year, and one-year mortality were also analyzed.
Statistical analyses
All data are presented as mean (standard deviation) or number (percentage). Differences between two groups of continuous variables were compared using the Student’s t-test. Differences between categorical variables were compared using the Chi-square test. Kaplan-Meier analysis was used to evaluate one-year mortality after admission with acute COPD exacerbation and readmission within one year after discharge. Univariate logistic regression analysis was used to identify factors associated with in-hospital mortality, followed by multivariate logistic regression analyses of the univariate analysis variables with P<0.25. We used logistic regression analysis to evaluate whether PA/A ≥1 was an independent risk factor for advanced treatments such as NIV, HFNC, and intubation. Additionally, we adjusted for the following clinical indicators: age, sex, pack-years, body mass index, hypertension, diabetes, pulmonary tuberculosis, bronchiectasis, ischemic heart disease, atrial fibrillation, chronic kidney disease, previous cerebrovascular disease, forced vital capacity (% pred), forced expiratory volume in 1 second (% pred), diffusion lung capacity of carbon monoxide, GOLD group, white blood cells, hemoglobin, platelets, blood urea nitrogen, creatinine, C-reactive protein, ventilation-perfusion mismatch, and hypoventilation. All statistical analyses were done on SPSS version 25.0 (IBM, Armonk, NY, USA). P<0.05 indicates statistically significant differences.
Results
We reviewed data on 871 patients with COPD who were hospitalized during the study period (Figure 1). After excluding 213 patients who were hospitalized for reasons other than acute exacerbation, 219 patients who did not undergo pulmonary function tests, and four patients with asthma, 435 patients were included in the analysis. Of these, 324 and 111 had PA/A ratios of <1 and ≥1, respectively (Figure 1).
Figure 1.
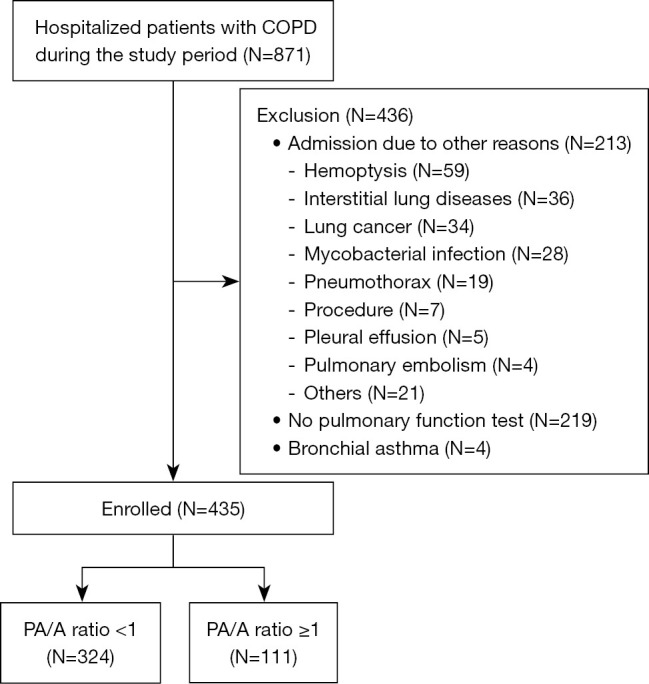
Study flowchart. COPD, chronic obstructive pulmonary disease; PA/A ratio, pulmonary artery-to-aorta ratio.
Baseline characteristics of the groups with high and low PA/A ratios
The average ages in the two groups were 74.1 and 74.5 years, which was not significantly different (Table 1). When compared with the group with PA/A ratios of <1, the group with ratios of ≥1 had a significantly lower proportion of men (71.2% vs. 89.5%, P<0.001), significantly more never smokers and fewer current smokers (37.8% vs. 19.8% and 49.5% vs. 63.6%, P=0.001), significantly lower forced vital capacity (2.12 vs. 2.63 L, P<0.001) and forced expiratory volume in 1 second (1.07 vs. 1.23 L, P=0.002), and significantly lower pH levels (7.36 vs. 7.39, P=0.006) and higher PaCO2 levels (54.0 vs. 46.0 mmHg, P<0.001). However, underlying diseases, GOLD stage, and GOLD group did not differ significantly between the two groups.
Table 1. Basal characteristics of patients with acute exacerbation of COPD based on PA/A ratios.
| Variables | PA/A ratio ≥1 (n=111) | PA/A ratio <1 (n=324) | P value |
|---|---|---|---|
| Age (years) | 74.1 (0.8) | 74.5 (0.4) | 0.72 |
| Males | 79 (71.2) | 290 (89.5) | <0.001 |
| Smoking status | 0.001 | ||
| Never | 42 (37.8) | 64 (19.8) | |
| Ex | 14 (12.6) | 54 (16.7) | |
| Current | 55 (49.5) | 206 (63.6) | |
| Pack-years | 22.0 (2.4) | 30.2 (1.1) | 0.001 |
| Body mass index (kg/m2) | 22.1 (0.3) | 21.7 (0.2) | 0.38 |
| Underlying disease | |||
| Hypertension | 50 (45.0) | 151 (46.6) | 0.82 |
| Diabetes | 32 (28.8) | 84 (25.9) | 0.61 |
| Pulmonary TB | 36 (32.4) | 89 (27.5) | 0.33 |
| Bronchiectasis | 16 (14.4) | 35 (10.8) | 0.30 |
| Ischemic heart disease | 18 (16.2) | 50 (15.4) | 0.88 |
| Atrial fibrillation | 12 (10.8) | 52 (16.0) | 0.21 |
| Chronic kidney disease | 5 (4.5) | 20 (6.2) | 0.64 |
| Previous cerebrovascular accident | 10 (9.0) | 25 (7.7) | 0.68 |
| Pulmonary function test | |||
| FVC (L) | 2.12 (0.08) | 2.63 (0.04) | <0.001 |
| FVC (% pred) | 58.0 (1.9) | 68.0 (1.1) | <0.001 |
| FEV1 (L) | 1.07 (0.04) | 1.23 (0.02) | 0.002 |
| FEV1 (% pred) | 43.9 (1.5) | 48.0 (1.0) | 0.041 |
| FEV1/FVC | 53.1 (1.2) | 48.2 (0.7) | 0.001 |
| DLco | 46.6 (1.9) | 50.5 (1.2) | 0.11 |
| GOLD stage | 0.19 | ||
| 1 | 2 (1.8) | 17 (5.2) | |
| 2 | 31 (27.9) | 111 (34.3) | |
| 3 | 55 (49.5) | 143 (44.1) | |
| 4 | 23 (20.7) | 53 (16.4) | |
| GOLD group | 0.52 | ||
| A | 17 (15.3) | 52 (16.0) | |
| B | 80 (72.1) | 240 (74.1) | |
| C | 0 (0.0) | 3 (0.9) | |
| D | 14 (12.6) | 29 (9.0) |
Data are presented as mean (standard deviation) or number (%). COPD, chronic obstructive pulmonary disease; PA/A ratio, pulmonary artery-to-aorta ratio; TB, tuberculosis; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLco, diffusion lung capacity of carbon monoxide; GOLD, global initiative for chronic obstructive lung disease.
The clinical variables of patients with acute exacerbation of COPD based on PA/A ratios
When compared with the group with PA/A ratios of <1, the group with ratios of ≥1 had more patients with type 2 ARF (35.1% vs. 18.8%), a higher rate of HFNC (10.8% vs. 4.6%) and NIV (21.6% vs. 7.7%) use, and a longer hospital stay (average 10.9 vs. 9.5 days) (Table 2). The rates of in-hospital mortality, readmission, and mortality within one year were not significantly different between the two groups (Figures 2,3).
Table 2. Clinical variables of patients with acute exacerbation of COPD based on PA/A ratio.
| Variables | PA/A ratio ≥1 (n=111) | PA/A ratio <1 (n=324) | P value |
|---|---|---|---|
| Arterial blood gas analysis | |||
| pH | 7.36 (0.01) | 7.39 (0.09) | 0.006 |
| PaCO2 (mmHg) | 54.0 (1.9) | 46.0 (1.0) | <0.001 |
| P/F ratio | 259.2 (11.7) | 284.2 (7.3) | 0.07 |
| V/Q mismatch + hypoventilation | 20 (18.0) | 31 (9.6) | 0.02 |
| ARF type | 0.001 | ||
| No ARF | 32 (28.8) | 138 (42.6) | |
| Type 1 ARF | 40 (36.0) | 125 (38.6) | |
| Type 2 ARF | 39 (35.1) | 61 (18.8) | |
| Laboratory findings | |||
| White blood cells (×103/μL) | 10,274 (423) | 11,251 (301) | 0.08 |
| Neutrophil (%) | 77.0 (1.1) | 75.6 (0.7) | 0.29 |
| Eosinophil (%) | 1.2 (0.2) | 2.1 (0.2) | 0.01 |
| Hemoglobin (g/dL) | 12.7 (0.1) | 13.1 (0.1) | 0.08 |
| Platelet count (×103/μL) | 229.6 (0.9) | 231.9 (4.6) | 0.81 |
| Blood urea nitrogen (mg/dL) | 19.8 (1.1) | 19.2 (0.5) | 0.59 |
| Creatinine (mg/dL) | 0.94 (0.05) | 0.92 (0.02) | 0.65 |
| C-reactive protein (mg/dL) | 7.87 (0.76) | 7.33 (0.46) | 0.55 |
| Treatment | |||
| Conventional oxygen therapy | 102 (91.9) | 292 (90.1) | 0.70 |
| High-flow nasal cannula | 12 (10.8) | 15 (4.6) | 0.03 |
| Non-invasive ventilation | 24 (21.6) | 25 (7.7) | <0.001 |
| ICU admission | 13 (11.7) | 28 (8.6) | 0.34 |
| Intubation | 15 (13.5) | 25 (7.7) | 0.08 |
| Antibiotics | 109 (98.2) | 314 (96.9) | 0.73 |
| Steroids | 74 (66.7) | 218 (67.3) | 0.90 |
| Hospital stay (days) | 10.9 (0.6) | 9.5 (0.3) | 0.050 |
| In-hospital mortality | 1 (0.9) | 9 (2.8) | 0.46 |
| Readmission within 12 months | 31 | 88 | 0.39 |
| Death within 12 months | 5 | 24 | 0.42 |
Data are presented as mean (standard deviation), number (%), or number. COPD, chronic obstructive pulmonary disease; PA/A ratio, pulmonary artery-to-aorta ratio; PaCO2, arterial carbon dioxide partial pressure; P/F ratio, arterial oxygen partial pressure to fractional inspired oxygen; V/Q, ventilation-perfusion; ARF, acute respiratory failure; ICU, intensive care unit.
Figure 2.
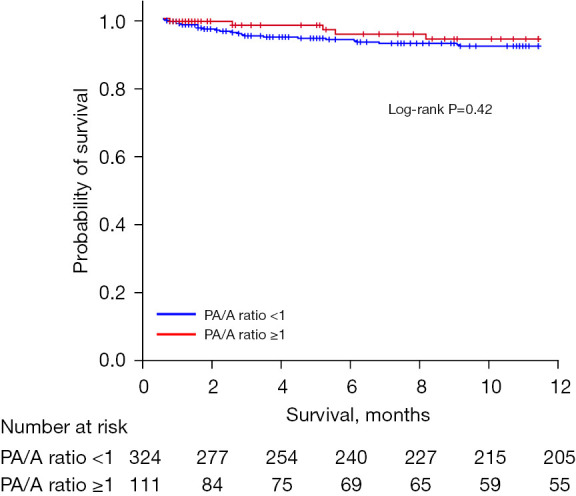
One-year survival of patients with acute exacerbation of COPD based on PA/A ratios. PA/A ratio, pulmonary artery-to-aorta ratio; COPD, chronic obstructive pulmonary disease.
Figure 3.
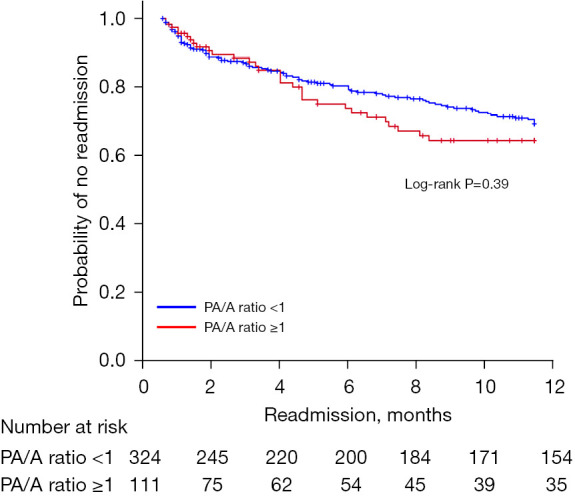
Readmission within one year after discharge based on PA/A ratios. PA/A ratio, pulmonary artery-to-aorta ratio.
Risk factors for in-hospital mortality in patients with acute exacerbation of COPD
Multivariate analysis (Table 3) revealed that older age [odds ratio (OR) =1.157; 95% confidence interval (CI): 1.025–1.306; P=0.01], lower eosinophil levels (OR =0.042; 95% CI: 0.002–0.987; P=0.049), HFNC (OR =6.061; 95% CI: 1.122–32.756; P=0.03) or NIV use (OR =5.463; 95% CI: 1.257–23.750; P=0.02), and intubation (OR =6.192; 95% CI: 1.244–30.819; P=0.02) were significantly associated with in-hospital mortality. However, PA/A ratios of ≥1 were not associated with in-hospital mortality (OR =0.318; 95% CI: 0.040–2.540; P=0.28).
Table 3. Risk factors for in-hospital mortality in patients with acute exacerbation of COPD.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age | 1.112 | 1.007–1.228 | 0.03 | 1.157 | 1.025–1.306 | 0.01 | |
| Sex | 0.709 | 0.14–3.416 | 0.66 | – | – | – | |
| Smoking (pack-years) | 1.024 | 1.001–1.048 | 0.040 | – | – | – | |
| Body mass index | 0.874 | 0.728–1.048 | 0.14 | – | – | – | |
| Hypertension | 2.778 | 0.709–10.889 | 0.14 | – | – | – | |
| Diabetes mellitus | 0.682 | 0.143–3.260 | 0.63 | – | – | – | |
| Atrial fibrillation | 2.557 | 0.644–10.159 | 0.18 | – | – | – | |
| FVC (% pred) | 1.000 | 0.971–1.031 | 0.98 | – | – | – | |
| FEV1 (% pred) | 1.015 | 0.984–1.046 | 0.34 | – | – | – | |
| DLco | 0.970 | 0.934–1.007 | 0.11 | – | – | – | |
| White blood cells | 1.000 | 1.000–1.000 | 0.02 | – | – | – | |
| Neutrophil | 1.124 | 1.030–1.228 | 0.009 | – | – | – | |
| Eosinophil | 0.036 | 0.001–1.020 | 0.051 | 0.042 | 0.002–0.987 | 0.049 | |
| Hemoglobin | 0.905 | 0.666–1.228 | 0.52 | – | – | – | |
| Platelet count | 1.000 | 0.993–1.008 | 0.89 | – | – | – | |
| Creatinine | 0.981 | 0.235–4.102 | 0.97 | – | – | – | |
| C-reactive protein | 0.943 | 0.849–1.048 | 0.27 | – | – | – | |
| High-flow nasal cannula | 7.161 | 1.742–29.442 | 0.006 | 6.061 | 1.122–32.756 | 0.03 | |
| Non-invasive ventilation | 8.659 | 2.412–31.090 | 0.001 | 5.463 | 1.257–23.750 | 0.02 | |
| Intubation | 7.204 | 1.943–26.709 | 0.003 | 6.192 | 1.244–30.819 | 0.02 | |
| Systemic steroids | 1.986 | 0.416–9.475 | 0.38 | – | – | – | |
| PA/A ratio ≥1 | 0.318 | 0.040–2.540 | 0.28 | – | – | – | |
COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLco, diffusion lung capacity of carbon monoxide; PA/A ratio, pulmonary artery-to-aorta ratio.
PA/A ratios of ≥1 predict the need for advanced treatment in patients hospitalized with acute exacerbation of COPD
Having a PA/A ratio of ≥1 was identified as an independent predictor of the need for HFNC, NIV, and intubation in COPD patients (Table 4). A PA/A ratio of ≥1 remained a strong predictor of the need for NIV even after adjusting for several variables (OR =4.801; 95% CI: 1.977–11.658; P=0.001).
Table 4. PA/A ratios of ≥1 predict the requirement for advanced treatment in patients hospitalized with acute exacerbation of COPD.
| Advanced treatment | OR | 95% CI | P value |
|---|---|---|---|
| High-flow nasal cannula | |||
| Crude | 2.497 | 1.131–5.513 | 0.02 |
| Adjustment† | 2.382 | 1.051–5.395 | 0.03 |
| Adjustment‡ | 2.359 | 0.814–6.834 | 0.11 |
| Non-invasive ventilation | |||
| Crude | 3.299 | 1.795–6.065 | <0.001 |
| Adjustment† | 3.247 | 1.736–6.074 | <0.001 |
| Adjustment‡ | 4.801 | 1.977–11.658 | 0.001 |
| Intubation | |||
| Crude | 1.869 | 0.947–3.689 | 0.07 |
| Adjustment† | 2.095 | 1.043–4.209 | 0.03 |
| Adjustment‡ | 2.041 | 0.757–5.502 | 0.15 |
†, adjusted for age and sex; ‡, adjusted for age, sex, pack-years, body mass index, hypertension, diabetes, pulmonary tuberculosis, bronchiectasis, ischemic heart disease, atrial fibrillation, chronic kidney disease, previous cerebrovascular disease, forced vital capacity (% pred), forced expiratory volume in 1 second (% pred), diffusion lung capacity of carbon monoxide, global initiative for chronic obstructive lung disease group, white blood cells, hemoglobin, platelets, blood urea nitrogen, creatinine, C-reactive protein, and hypoventilation and ventilation-perfusion mismatch. PA/A ratio, pulmonary artery-to-aorta ratio; COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval.
Discussion
This retrospective study evaluated the PA/A ratios and clinical characteristics of patients hospitalized with acute exacerbation of COPD. When compared with PA/A ratios of <1 in patients with COPD, ratios of ≥1 were associated with lower lung function, higher rates of type 2 ARF, and more HFNC and NIV use. Having a PA/A ratio of ≥1 was identified as an independent risk factor associated with NIV use in patients hospitalized with acute COPD exacerbation. However, having a PA/A ratio of ≥1 was not associated with the rates of in-hospital mortality, 12-month mortality, and readmission within one year.
Having a high PA/A ratio is a reliable indicator of pulmonary hypertension in patients with COPD (13-15,23,24), and a decrease in right ventricular function and pulmonary blood vessel changes are thought to cause an increase in the PA/A ratio (25). Various studies have set the cut-off PA/A ratio for predicting pulmonary hypertension at between 0.8 and 1.0 (13-15,23,24,26,27). However, several studies that have used a PA/A ratio of ≥1 as the cut-off value have demonstrated correlation between PA/A ratios of ≥1 and various clinical prognostic factors in patients with COPD (13-15,23,24). It is reported that 37–42.5% of patients hospitalized with acute exacerbation of COPD have PA/A ratios of ≥1 (17-19). However, in this study, 25% of the patients hospitalized with acute exacerbation of COPD had PA/A ratios of ≥1.
In this study, the proportion of women with PA/A ratios of ≥1 was relatively high. Although some previous studies have reported that the proportion of women with COPD with PA/A ratios of ≥1 is relatively high, others have reported no difference in the proportions of men vs. women, or a higher proportion of men (15,17,19). In patients with COPD, smoking inflames blood vessel walls, which is associated with vascular remodeling and pulmonary hypertension (28). Hypercapnic pulmonary vasoconstriction and increased mean pulmonary arterial pressure are also associated with elevated PaCO2 (29-31). As in the previous study, the group with PA/A ratios of ≥1 had a lower smoking rate and significantly higher PaCO2 levels (15). These results indicate that in patients with COPD, hypercapnia may contribute more to the development of pulmonary hypertension than smoking.
Our analyses show that in patients hospitalized with acute exacerbation of COPD, PA/A ratios of ≥1 were associated with a higher rate of type 2 ARF, which was identified as an independent risk factor for needing advanced treatment with HFNC, NIV, and intubation. Previous findings indicate that in patients admitted with acute exacerbation of COPD, those with PA/A ratios of ≥1 have a high risk of treatment failure and admission into intensive care unit (17,18). In patients hospitalized with acute exacerbation of COPD, for those with PA/A ratios of ≥1, the possibility of type 2 ARF and the need for advanced treatment should be considered. The PA/A ratio may, however, increase during acute exacerbations of COPD that require advanced treatment. Therefore, a multicenter prospective study is needed to compare changes in the PA/A ratio and prognosis before and after an acute exacerbation of COPD.
However, in this study, PA/A ratios of ≥1 were not associated with in-hospital mortality or one-year mortality in patients hospitalized with acute exacerbation of COPD. Previous studies have reported reduced in-hospital mortality and long-term survival in hospitalized patients with acute exacerbation of COPD and PA/A ratios of ≥1 (17,18). The lower mortality rate in this study is attributable to the lower severity of patients hospitalized with acute exacerbation of COPD or appropriate advanced treatment. It may also result from the relatively short follow-up since we evaluated the one-year survival rate. Considering the finding of a population-based study that long-term mortality is increased in patients with moderate to severe COPD and PA/A ratios of ≥1, it is likely that the long-term mortality of patients hospitalized with acute exacerbation of COPD is also high (16).
This study had some limitations. First, as a retrospective study conducted at two centers, the generalizability of our findings is limited, and there may be potential biases in data collection and analysis. Second, this study may not have included patients with acute exacerbation of COPD who were not screened for lung function because of premature death or deterioration. Therefore, relatively severe acute exacerbation of COPD cases may not have been included in the study. Third, the non-ARF group probably had less severe acute exacerbation of COPD than the ARF group, despite following the GOLD guidelines for treating acute exacerbation of COPD. Fourth, this study found no significant association between the PA/A ratio and in-hospital or one-year mortality, which may limit its utility as a prognostic tool. Therefore, further prospective studies are required to confirm the usefulness of PA/A ratios in hospitalized COPD patients with actual exacerbations.
Conclusions
In this study, PA/A ratios of ≥1 were not associated with in-hospital mortality and one-year mortality in patients hospitalized with acute exacerbation of COPD. However, patients with PA/A ratios of ≥1 had a high incidence of type 2 ARF and required advanced treatment, including HFNC, NIV, and intubation. Therefore, patients with PA/A ratios of ≥1 should be treated more aggressively when hospitalized for acute exacerbation of COPD.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (approval No. 2024-011). Informed consent was waived by Chonnam National University Hospital’s Institutional Review Board because of the study’s minimal risk and retrospective design. Patient data were anonymized and deidentified before the analyses. All participating institutions were also informed and agreed the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-62/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-62/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-62/dss
References
- 1.Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am J Respir Crit Care Med 2023;207:819-37. 10.1164/rccm.202301-0106PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpin DMG, Celli BR, Criner GJ, et al. The GOLD Summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int J Tuberc Lung Dis 2019;23:1131-41. 10.5588/ijtld.19.0397 [DOI] [PubMed] [Google Scholar]
- 3.Meghji J, Mortimer K, Agusti A, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet 2021;397:928-40. 10.1016/S0140-6736(21)00458-X [DOI] [PubMed] [Google Scholar]
- 4.McGhan R, Radcliff T, Fish R, et al. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007;132:1748-55. 10.1378/chest.06-3018 [DOI] [PubMed] [Google Scholar]
- 5.Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, et al. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J 2011;37:508-15. 10.1183/09031936.00043710 [DOI] [PubMed] [Google Scholar]
- 6.Çolak Y, Afzal S, Marott JL, et al. Prognosis of COPD depends on severity of exacerbation history: A population-based analysis. Respir Med 2019;155:141-7. 10.1016/j.rmed.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 7.Gunen H, Hacievliyagil SS, Kosar F, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J 2005;26:234-41. 10.1183/09031936.05.00024804 [DOI] [PubMed] [Google Scholar]
- 8.Matkovic Z, Huerta A, Soler N, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary disease. Respiration 2012;84:17-26. 10.1159/000335467 [DOI] [PubMed] [Google Scholar]
- 9.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013;10:81-9. 10.1513/AnnalsATS.201208-043OC [DOI] [PubMed] [Google Scholar]
- 10.Steriade AT, Johari S, Sargarovschi N, et al. Predictors of outcome of noninvasive ventilation in severe COPD exacerbation. BMC Pulm Med 2019;19:131. 10.1186/s12890-019-0892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slenter RH, Sprooten RT, Kotz D, et al. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respiration 2013;85:15-26. 10.1159/000342036 [DOI] [PubMed] [Google Scholar]
- 12.Blanco I, Tura-Ceide O, Peinado VI, et al. Updated Perspectives on Pulmonary Hypertension in COPD. Int J Chron Obstruct Pulmon Dis 2020;15:1315-24. 10.2147/COPD.S211841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin S, King CS, Brown AW, et al. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med 2014;108:1626-32. 10.1016/j.rmed.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 14.Wu XG, Shi YJ, Wang XH, et al. Diagnostic value of computed tomography-based pulmonary artery to aorta ratio measurement in chronic obstructive pulmonary disease with pulmonary hypertension: A systematic review and meta-analysis. Clin Respir J 2022;16:276-83. 10.1111/crj.13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367:913-21. 10.1056/NEJMoa1203830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terzikhan N, Bos D, Lahousse L, et al. Pulmonary artery to aorta ratio and risk of all-cause mortality in the general population: the Rotterdam Study. Eur Respir J 2017;49:1602168. 10.1183/13993003.02168-2016 [DOI] [PubMed] [Google Scholar]
- 17.Wells JM, Morrison JB, Bhatt SP, et al. Pulmonary Artery Enlargement Is Associated With Cardiac Injury During Severe Exacerbations of COPD. Chest 2016;149:1197-204. 10.1378/chest.15-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Li L, Tu X, et al. The Main Pulmonary Artery to the Ascending Aorta Diameter Ratio (PA/A) as a Predictor of Worse Outcomes in Hospitalized Patients with AECOPD. Int J Chron Obstruct Pulmon Dis 2022;17:1157-65. 10.2147/COPD.S357696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortaç Ersoy E, Durusu Tanriover M, Öcal S, et al. Measurement of pulmonary artery to aorta ratio in computed tomography is correlated with pulmonary artery pressure in critically ill chronic obstructive pulmonary disease patients. J Crit Care 2016;33:42-6. 10.1016/j.jcrc.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 20.Iliaz S, Tanriverdio E, Chousein EGU, et al. Importance of pulmonary artery to ascending aorta ratio in chronic obstructive pulmonary disease. Clin Respir J 2018;12:961-5. 10.1111/crj.12612 [DOI] [PubMed] [Google Scholar]
- 21.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 22.Bach JF. Hypoxemia: a quick reference. Vet Clin North Am Small Anim Pract 2008;38:423-6, vii. 10.1016/j.cvsm.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 23.Mohamed Hoesein FA, Besselink T, Pompe E, et al. Accuracy of CT Pulmonary Artery Diameter for Pulmonary Hypertension in End-Stage COPD. Lung 2016;194:813-9. 10.1007/s00408-016-9926-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gašparović K, Pavliša G, Hrabak Paar M, et al. Diagnostic accuracy, sensitivity, and specificity of CT pulmonary artery to aorta diameter ratio in screening for pulmonary hypertension in end-stage COPD patients. Croat Med J 2021;62:446-5. 10.3325/cmj.2021.62.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuttica MJ, Bhatt SP, Rosenberg SR, et al. Pulmonary artery to aorta ratio is associated with cardiac structure and functional changes in mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis 2017;12:1439-46. 10.2147/COPD.S131413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging 2012;5:147-54. 10.1161/CIRCIMAGING.111.968610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung KS, Kim YS, Kim SK, et al. Functional and Prognostic Implications of the Main Pulmonary Artery Diameter to Aorta Diameter Ratio from Chest Computed Tomography in Korean COPD Patients. PLoS One 2016;11:e0154584. 10.1371/journal.pone.0154584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gredic M, Blanco I, Kovacs G, et al. Pulmonary hypertension in chronic obstructive pulmonary disease. Br J Pharmacol 2021;178:132-51. 10.1111/bph.14979 [DOI] [PubMed] [Google Scholar]
- 29.Youssef HH, Edeen HE, Elgammal MY. Hypercapnic pulmonary hypertension. (A preliminary report). Dis Chest 1968;53:328-31. 10.1378/chest.53.3.328 [DOI] [PubMed] [Google Scholar]
- 30.Swenson ER. Hypercapnic Pulmonary Vasoconstriction as a Mechanism for Regional Perfusion Redistribution in Asthma. Am J Respir Crit Care Med 2018;197:683-4. 10.1164/rccm.201708-1774LE [DOI] [PubMed] [Google Scholar]
- 31.Zuoyou L, Shiota S, Morio Y, et al. Borderline pulmonary hypertension associated with chronic hypercapnia in chronic pulmonary disease. Respir Physiol Neurobiol 2019;262:20-5. 10.1016/j.resp.2019.01.003 [DOI] [PubMed] [Google Scholar]


