Summary
Increasing evidence indicates that immunotherapy is hindered by a hostile tumor microenvironment (TME) featured with deprivation of critical nutrients and pooling of immunosuppressive metabolites. Tumor cells and immunosuppressive cells outcompete immune effector cells for essential nutrients. Meanwhile, a wide range of tumor cell-derived toxic metabolites exerts negative impacts on anti-tumor immune response, diminishing the efficacy of immunotherapy. Nanomedicine with excellent targetability offers a novel approach to improving cancer immunotherapy via metabolically reprogramming the immunosuppressive TME. Herein, we review recent strategies of enhancing immunotherapeutic effects through rewiring tumor metabolism via nanomedicine. Attention is drawn on immunometabolic tactics for immune cells and stromal cells in the TME via nanomedicine. Additionally, we discuss future directions of developing metabolism-regulating nanomedicine for precise and efficacious cancer immunotherapy.
Keywords: Metabolic reprogramming, Immunometabolism, Anti-tumor immunity, Tumor immune microenvironment, Nanomedicine
Search strategy and selection criteria.
We searched the databases of PubMed for relevant articles published only in English, using the search terms “immune evasion”, “immunotherapy”, “metabolism”, “tumor microenvironment”, “nanomedicine”, “glucose” or “lactate” or “glutamine” or “arginine” or “tryptophan” or “methionine” or “FAs” or “cholesterol” or “PGE2” or “adenosine” from 2005 to 2024, primarily within the past 5 years.
Introduction
In recent decades, immunotherapy has achieved remarkable clinical breakthroughs and gained popularity in treating cancer.1 However, the overall response to immunotherapy is usually impeded by an immune-resistant tumor microenvironment (TME). Specifically, tumor cells rewrite their metabolism and create a TME featured with critical nutrient deficiency and immunoinhibitory metabolite accumulation, inhibiting antigen presentation by dendritic cells (DCs) and deactivating the tumor-killing function of cytotoxic T lymphocytes (CTLs) and natural killer cells (NK cells). Moreover, immunosuppressive cells such as tumor associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and cancer-associated fibroblasts (CAFs) can readily adapt themselves to the nutritionally deprived TME due to their metabolic flexibility, and after adaptation, they often become accomplices in tumor immune evasion. In this context, metabolic reprogramming of the TME could be a promising strategy to enhance anti-tumor immunotherapeutic effects,2 and fully elucidating the intertwined metabolic networks among various cells in the TME is essential for performing targeted immunometabolic therapy.
To metabolically reprogram the immune-resistant TME, the use of nanomedicine has emerged as a promising approach. On the one hand, nanomaterials improve targetability, pharmacokinetics and bioavailability of delivered small molecular metabolic regulators, thereby enhancing the efficacy of immunometabolic therapy while reducing adverse effects.3 On the other hand, nanomaterials in synergy with other cancer treatment modalities like radiotherapy, which often induces tumor immunogenic cell death (ICD) to facilitate release of disease-associated molecular patterns (DAMPs) and activate immune cells, bolstering immunotherapy outcomes.4,5 Encouragingly, nanomedicine-mediated tumor cell-targeting immunotherapies have been progressively explored, while metabolic manipulation of immune cells and stromal cells via nanomedicine to rebuild an immunocompetent TME remains to be discovered. Leveraging engineered nanomaterials to deliver metabolic modulators to non-tumor cells in the TME has the potential to offer more precise and potent therapeutic methods.
Herein, we initially elaborate the impact of metabolic reprogramming of tumor cells and stromal cells on immunocompetent cells from the aspects of nutrient deprivation and suppressive metabolite accumulation. Next, we survey the current landscape of therapies by harnessing nanomedicine to manipulate tumor metabolism to overcome the barriers in the TME. In addition, the potential of nanomedicine to revitalize the immunosuppressive TME by modulating metabolism of immune cells as well as stromal cells is elaborated. Finally, forward-looking strategies for enhancing nanomedicine-assisted immunometabolic therapies are offered. Overall, we discuss nanomedicine-assisted metabolic reprogramming strategies to regulate an immunosuppressive TME, paving a way for efficacious cancer immunotherapy.
Metabolic profiles in the immunosuppressive TME
Tumor cells are capable of rewriting their metabolic profiles to consume more nutrients to promote macromolecule biosynthesis and maintain redox homeostasis, thus facilitating rapid cell proliferation and tumor metastasis. Interestingly, tumor cells prefer aerobic glycolysis, an inefficient way of generating energy, over oxidative phosphorylation (OXPHOS) to generate intermediate metabolites for biosynthesis, known as the “Warburg effect”. Besides, tumor cells adaptively consume certain non-essential amino acids including glutamine and arginine that are also indispensable for activation of the tumor-lysis function of immune effector cells. For example, metabolically active tumor cells overexpress nitric oxide synthase 2 (NOS2) to consume excessive arginine, which negatively impacts the survival of T cells.6 Lipids not only provide tumor cells with building blocks for cell membranes, but also provide ATP during energy stress. It is recently discovered that oxysterols and prostaglandin E2 (PGE2), the lipid derivatives, display immunosuppressive properties and promote tumor immune evasion.7,8 In addition, abundant adenosine leads to unsatisfactory immunotherapeutic effects.9,10
Altered tumor metabolism impairs immune cells in various ways (Fig. 1). Notably, increasing attention has been drawn to stromal cell metabolism, especially CAFs, the most abundant and heterogeneous stromal cells within the TME. The metabolism of CAFs favors tumor immune evasion via supplying metabolites to tumor cells and deteriorating nutrient deprivation. For glucose metabolism, tumor compressive and oxidative stress upregulate glycolysis and promote lactate production in CAFs, and CAF-derived lactate can be recycled by tumor cells to generate energy and nutrients.11, 12, 13 For amino acid metabolism, CAFs can rewrite their metabolic product profiles and supply glutamine, arginine and aspartate to tumor cells.14, 15, 16 Additionally, CAFs can consume SAM to downregulate histone methylation in ovarian cancer cells, promoting tumor metastasis.17 For lipid metabolism, colorectal cancer cells can uptake CAF-synthesized fatty acids via CD36, and CAFs induce castration resistance in prostate cancer cells through elevating cholesterol and steroid biosynthesis.18,19
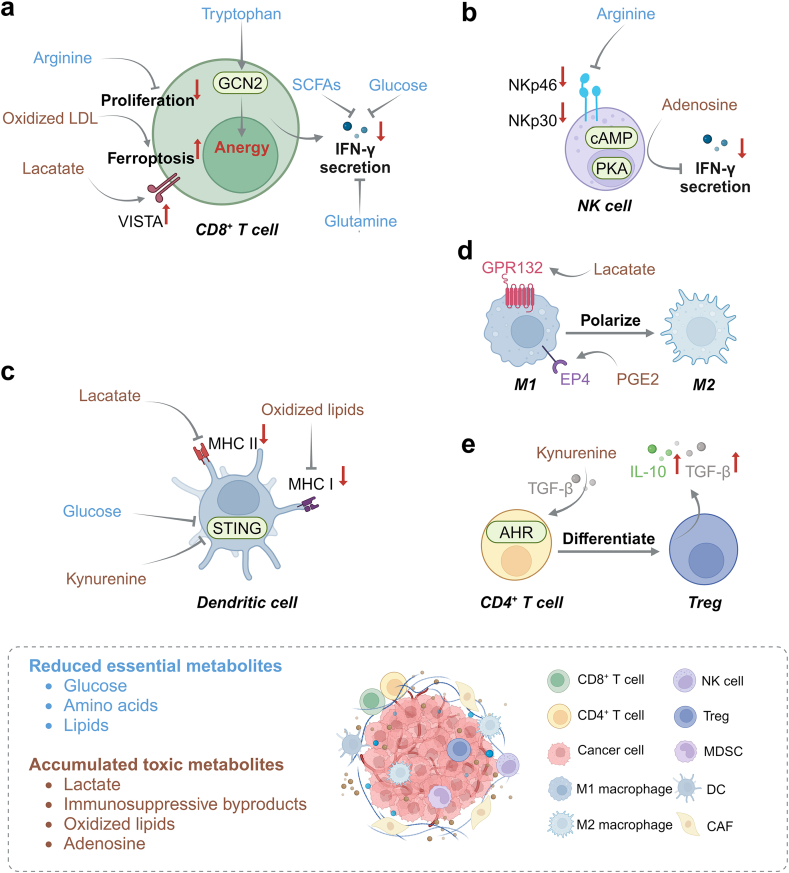
Fig. 1.
A hostile TME is characterized by nutrient deprivation and toxic metabolite accumulation. (a) Deprivation of nutrients impairs the anti-tumor immune response of CD8+ T cells: inadequate supply of glucose, glutamine and short-chain fatty acids (SCFAs) reduce IFN-γ secretion; inadequate supply of arginine leads to impaired proliferation; tryptophan deficiency induces anergy in CD8+ T cells via activating general control nondepressible 2 (GCN2); accumulation of oxidized LDL induces ferroptosis in CD8+ T cells; and accumulated lactate activates co-inhibitory receptor VISTA on CD8+ T cells. (b) Arginine deficiency reduces NK cell viability via downregulating the expression of activating receptors including NK cell protein 30 (NKp30) and NKp46, and the accumulation of adenosine inhibits IFN-γ secretion of NK cells via upregulating the cAMP-PKA signaling pathway. (c) Excessive kynurenine and insufficient glucose inhibit STING pathway-mediated innate immunity in DCs; and the accumulated lactate and oxidized lipids impair antigen presentation via inhibiting the function of MHC II and MHC I, respectively. (d) A high level of lactate and PGE2 in the TME induces M1-to-M2 polarization of TAMs via GPR132 and EP4, respectively. (e) Kynurenine binds to the aryl hydrocarbon receptor (AHR) in CD4+ T cells to induce their differentiation into Tregs in the presence of TGF-β, and Tregs excrete a large amount of immunosuppressive TGF-β and IL-10.
In this section, we dive into the mechanisms of restoring anti-tumor immune cell functions through tumor metabolic reprogramming in a hostile TME.
Deprivation of nutrients
Glucose
In the metabolic “tug-of-war” for key nutrients between tumor cells and immune cells, glucose is one of the most extensively studied nutrients (Fig. 2a). Glucose deprivation impairs the interferon-γ (IFN-γ) production of effector T cells (Teffs) and NK cells.20,21 Besides, DCs need hyperactive glycolysis to initiate the stimulator of interferon genes (STING) pathway for type I interferon (IFN-I) secretion,22 and it can be inferred that their anti-tumor immunity is attenuated when glucose is constrained. Glucose deprivation also hampers the anti-tumor immunity through altering an epigenetic landscape of T cells. Insufficient glucose promotes the expression of miRNA26a and miRNA101 in CD8+ T cells to impair lysine 27 trimethylation on histone H3 (H3K27me3) via downregulating the enhancer of zeste homolog 2 (EZH2), ultimately hampering the production of IFN-γ and tumor necrosis factor (TNF).23 This finding reveals the intricate link between metabolic profiles and epigenetic changes in reshaping immune response against tumors, and targeting EZH2 in CTLs could provide a novel insight into preventing tumor immune evasion.
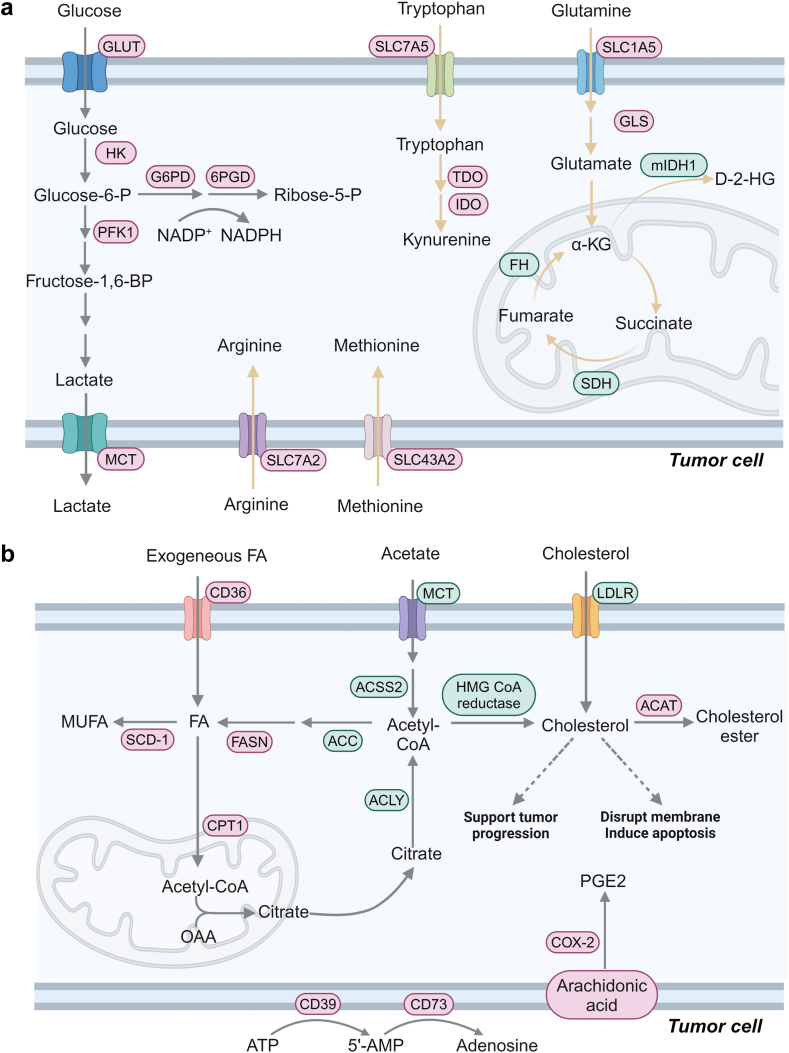
Fig. 2.
Precisely reprogramming metabolism in tumor cells via nanomedicine. (a) Cancer-promoting factors, such as MYC and hypoxia-inducible factor (HIF-1α), induce tumor cells to overexpress glucose transporter 1 (GLUT1), hexokinase (HK) and lactate dehydrogenase-A (LDH-A), leading to enhanced glucose uptake and lactate production. Besides, the pentose phosphate pathway (PPP) coordinates the modulation of tumor growth, and tumor cells upregulate the expression of glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (6PGD) to maintain a high flux of PPP, which provides tumor cells with ribose-5-phosphate and reducing equivalent NADPH to support the biosynthesis of nucleotides and lipids. Glutamine can enter cells via SLC1A5, and it is catabolized to glutamate with the help of glutaminase (GLS) to enter the tricarboxylic acid (TCA) cycle for biosynthesis. Tumor cells usually express hypofunctional SDH and hyperactive mIDH1, leading to the accumulation of succinate and immunosuppressive D-2-HG, respectively. Tryptophan enters tumor cells via SLC7A5 and it is catabolized to immunosuppressive kynurenine by IDO and TDO. Substantial arginine and methionine predominantly enter tumor cells via SLC7A2 and SLC43A2, respectively. (b) The building block for fatty acids is acetyl coenzyme A, which can be transformed from acetate and citrate by acetyl-coenzyme A synthetase 2 (ACSS2) and ATP-citrate lyase (ACLY), respectively. Catalyzed by Acetyl-CoA Carboxylase (ACC), acetyl coenzyme A is converted to malonyl coenzyme A, which is prolonged by fatty acid synthase (FASN) to form saturated fatty acids. Finally, saturated fatty acids are transformed to unsaturated ones with the help of stearoyl-CoA desaturase-1 (SCD-1). To maintain rapid proliferation, tumor cells uptake exogenous cholesterol via LDLR or synthesize ingenuous cholesterol, while an excessive level of cholesterol may induce tumor cell apoptosis. PGE2 is derived from arachidonic acid and it is predominately mediated by cyclooxygenase-2 (COX-2). Besides, adenosine is converted from ATP via CD39 and CD73. Targets in red texts are discussed in this review, while those in green texts remain to be elucidated. Solid arrows are used to indicate the process of metabolites from uptake to metabolic conversion by tumor cells; dashed arrows to introduce distinct roles of different cholesterol levels in tumor cells.
Amino acids
Tumors consume a great amount of amino acids, and immune cells fail to perform their normal functions without adequate supply of these amino acids (Fig. 2a). Among these amino acids, glutamine, arginine, tryptophan and methionine are the most widely studied. Upon entry into cells, glutamine is involved in nucleotide biosynthesis, alternatively, it can be catabolized into glutamate and glutathione (GSH), which are responsible for energy production and redox balance, respectively. Thus, a deficiency in glutamine in the TME weakens CD8+ T cell activation24 and induces Treg differentiation, a major type of immune cells promoting immune evasion.25 Inadequate glutamine also impairs T cell proliferation via inhibiting fatty acid synthesis (FAS) under hypoxic conditions.26
Due to downregulated expression of the enzymes for de novo synthesis of arginine including argininosuccinate synthase 1 (ASS1) and argininosuccinate lyase (ASL),27 tumor cells predominately uptake exogenous arginine. It has been reported that arginine is critical for survival of T cells in vitro,6 and a deficiency in arginine impairs their proliferation due to loss of cyclin D3 and cyclin-dependent kinase 4.28 Besides, arginine is a substrate for the synthesis of NO, a pro-inflammatory cytokine, by M1 macrophages via NOS2, which facilitates tumor regression.29 An arginine-deficient environment impairs the viability and proliferation of NK cells via inhibiting NKp30 and NKp46.30 Therefore, inhibiting the arginine transporter31 or directly depleting arginine32 in arginine-dependent tumors could serve as a promising anti-cancer therapeutic approach.
Tryptophan deficiency induces T cell exhaustion through activating the general control non-depressible 2 (GCN2).33 The upregulation of IDO in DCs can increase kynurenine production and induce Treg differentiation via the activation of AHR.34 IDO-mediated tryptophan depletion can be rescued by tryptophan supplementation or IDO inhibition. Targeting IDO and/or TDO is able to increase the availability of tryptophan in the TME and revitalize CTLs to become functional against tumors.
Methionine is an important raw material for S-adenosyl-methionine (SAM), which is involved in the methylation of various nucleic acids and proteins. Deprivation of methionine induces apoptosis in CD8+ T cells via inhibiting dimethylation of histone H3 at lysine 79 (H3K79me2), weakening the anti-tumor immunity.35 Besides, a reduction in H3K79me2 increases PD-1 expression on CD4+ T cells, which promotes tumor immune evasion.36 The supplementation of SAM enhances the anti-PD-1 antibody efficacy via promoting the proliferation of CD8+ T cells, and their production of IFN-γ and TNF-α.37 These results suggest that blocking the methionine transporter SLC43A2 of tumors or specifically supplying methionine to T cells can enhance anti-tumor immunity and prevent metastasis.
Lipids
In addition to glucose and amino acid metabolism, lipid metabolic reprogramming in tumors attenuates anti-tumor immune response (Fig. 2b). Proliferating tumor cells usually upregulate FAS for cell membrane construction and energy production.38 Tissue-resident memory T (Trm) cells rely on fatty acid oxidation (FAO) rather than glucose metabolism for their survival and biological functions. Trm cells are induced to death when they are co-cultured with gastric adenocarcinoma cells, which suggests that tumor cells outcompete Trm cells for securing fatty acids.39 FAO can also increase the spare respiration capacity in CD8+ memory T cells in response to stress, thus their long-term survival may be impaired under a lipid-deficiency environment.40 Besides, gut microbiota-derived SCFAs are involved in modulating immune cell functions. Among them, pentanoate and butyrate have been shown to improve TNF-α and IFN-γ secretion of CTLs via inhibiting histone deacetylation of tumor suppressor genes41 and promote antigen re-encounter response and long-term survival of CD8+ T cells via fueling OXPHOS.42 A comprehensive elucidation of the influence of microbiota ecology on immune cell metabolism in tumors could be harnessed to improve the anti-tumor immune effect.
Cancer cells are often in great demand of cholesterol. Tumor cells can synthesize a large quantity of cholesterol through the mevalonate pathway (MVA pathway) by up-regulation of sterol regulatory element binding proteins-2 (SREBP-2).43 Apart from constituting cell membranes, cholesterol facilitates cancer stem cell renewal, promotes proliferation and induces drug resistance.44 Therefore, tumors often have an insatiable appetite for cholesterol, while access to cholesterol by immune effector cells is restricted, which impairs anti-tumor immunity. For example, membrane cholesterol is essential for T cell receptor (TCR) clustering and immune synapse formation.45 Besides, NK cells with abundant cholesterol-containing lipid rafts on the membrane exhibit enhanced anti-tumor activity.46 Moreover, DCs that are deficient in cholesterol efflux promote pro-inflammatory T cell differentiation.47 Interestingly, subcellular location of cholesterol in immune cells has a great impact on their functions, but its mechanistic details remain to be elucidated.
Immunosuppressive metabolites
Lactate
Lactate accumulation in the TME has been shown to be related to poor prognosis. The majority of lactate is exported by monocarboxylate transporter protein 4 (MCT4) to prevent acidosis. It is well accepted that lactate in an acidic TME impairs the function of T cells, specifically, an influx of a high concentration of lactate into T cells via MCT inhibits cellular glycolysis, preventing their activation and IFN-γ secretion.48 In addition, an acidic environment can inhibit CTLs proliferation through promoting the binding of P-selectin glycoprotein ligand-1 (PSGL-1) to VISTA.49 G-protein coupled receptor 81 (GPR81) is overexpressed on tumor cells, which can be activated upon binding to lactate, leading to angiogenesis, drug efflux and PD-L1 expression.50 Additionally, GPR81 expression is upregulated in DCs and lactate-GPR81 interaction inhibits the transfer of MHC Ⅱ to the cell surface, ultimately impairing activation of T cells and promoting tumor immune evasion.51 Lactate also induces the polarization of TAMs towards M2 via binding to GPR132 and promotes tumor metastasis.52 Alleviating immunosuppressive effects of lactate in the TME can be achieved by breakdown of lactate or prevention of lactate production or efflux.
Intermediates in the TCA cycle
It has been demonstrated that intermediates in the TCA cycle also facilitate tumor immune evasion (Fig. 2a). The IFN-γ secretion of T cells is reduced in patients with pheochromocytoma and paraganglioma because a high extracellular succinate concentration disturbs their TCA metabolism.53 In addition, D-2-HG alters glycolysis in CD8+ T cells via an LDH inhibition-mediated decrease in the NAD+/NADH ratio, ultimately leading to diminished IFN-γ production.54 Encouragingly, L-2-HG, a D-2-HG isomer, boosts the tumor-killing ability of CD8+ T cells when they are cultured in vitro, which may be attributed to the epigenetic modification effect of L-2-HG. To be specific, L-2-HG can inhibit H3K27me3 demethylation and promote CD62L transcription, and CD62L transcription is essential for central memory CD8+ T cell differentiation.55 Therefore, future efforts should be made into exploring the role of the two enantiomers of 2-HG in tumor immunity.
Kynurenine
Tumor cells usually overexpress IDO and TDO to consume tryptophan and produce immunosuppressive kynurenine, which inhibits anti-tumor immunity in an AHR-dependent manner. It has been demonstrated that CAFs can educate normal fibroblasts to upregulate their expression of IDO-1, which is similar to that in CAFs.56 Through interaction with AHR, kynurenine can induce the differentiation of Tregs in the presence of transforming growth factor-β (TGF-β).57 In addition, kynurenine can promote the production of IL-10 in NK cell and downregulate IFN-I response in DCs, thus supporting tumor immune evasion.58,59 Blocking kynurenine-AHR engagement can reverse the suppressive activity of Tregs and TAM and enhance tumor-killing of CD8+ T cells.60 Accordingly, IDO inhibitors61 and kynureninase62 have been explored to reduce immunosuppressive kynurenine in the IDO-kynurenine-AHR signaling pathway.
Lipids and their derivatives
The accumulation of oxidized lipids in DCs has been shown to undermine their ability of antigen cross-presentation, in turn affecting CTLs activation, which may be due to the accumulation of peptide-MHC I complexes in lysosomes.63 Besides, TAMs increase the uptake of lipids via CD36 from the TME for fatty acid oxidation (FAO) to initiate the JAK1-STAT6 signaling to achieve M2 polarization and promote tumor growth.64 Therefore, targeting CD36 and/or FAO may become a new strategy of reprogramming TAMs for anti-tumor therapy. Oxidized lipids can stimulate the exhaustion of tumor-infiltrating T cells (TILs) since the uptake of oxidized low-density lipoproteins (oxLDLs) via CD36 leads to lipid peroxidation and a reduction in the production of IFN-γ and TNF.65 Therefore, inhibition of CD36 and overexpression of glutathione peroxidase 4 (GPX4) can mitigate lipid peroxidation and restore anti-tumor immunity in TILs.
Overexpressed PD-L1 on tumor cells exhausts CTLs, leading to poor overall survival. Cholesterol can stabilize PD-L1 by binding to the transmembrane domain of PD-L1, suggesting that modulation of cholesterol metabolism may increase the efficacy of anti-PD-L1 antibodies.66 27-hydroxycholesterol has been found to inhibit SREBP-1 activation and interfere with glucose metabolism and secretion of IFN-γ by NK cells.67 In addition, 22-hydroxycholesterol can recruit tumor-promoting neutrophils in a CXCR2-dependent manner for tumor angiogenesis, thus favoring tumor growth.7 Overall, both oxidized lipids show a wide range of immunosuppressive properties.
Cyclooxygenase-2 (COX-2) is commonly overexpressed in a wide range of tumor tissues, producing abundant immunosuppressive PGE2 to promote tumor progression via MDSCs and Tregs (Fig. 2b). PGE2 potentiates MDSCs to induce immunosuppressive IL-10 production by Tregs.8 Meanwhile, PGE2 can induce arginase 1 (ARG1) expression via E prostanoid 4 receptor (EP4) in MDSCs to deplete arginine for T cells, thus resulting in a less potent anti-tumor effect in the TME.68 Through binding to EP2 and EP4, PGE2 not only diminishes the cytotoxicity of NK cells and γδ T cells via the cAMP-PKA signaling pathway,69 but also enhances the recruitment of Tregs by mature regulatory DCs via secreting CCL17 and CCL22.70 In addition, PGE2 is involved in the regulation of macrophage polarization from M1 phenotype to pro-tumor M2 phenotype, reducing the release of TNF-α and IL-12.71 Considering a high expression level of COX-2 in tumor tissues, a reduction in immunosuppressive PGE2 production could be realized by inhibiting COX-2 or blocking PGE2 receptors via nanomedicine.
Adenosine
ATP can be released to the extracellular matrix in response to cellular stress and it is sequentially converted to 5′-AMP and adenosine predominately by extracellular nucleotidases including CD39 and CD73 (Fig. 2b). Excessive extracellular 5′-AMP blocks the differentiation of MDSCs into DCs.72 Interestingly, the interaction between CAFs and TILs accelerates adenosine production: TILs upregulate the CD73 expression on CAFs, and in turn CAFs promote CD39 expression on TILs.73 The overexpression of CD39 and CD73 on tumor cells, tumor-derived exosomes, Tregs,74 CAFs and TILs synchronizes to produce excessive adenosine, which promotes tumor immune evasion through interacting mainly with adenosine receptors A2AR and A2BR. A great amount of adenosine inhibits the IFN-γ secretion of CTLs via A2AR.9 Notably, hypoxia and HIF-1α upregulate the expression of the A2BR in DCs as well as inhibit their antigen uptake and migration, ultimately weakening the activation of CTLs. It is noted that adenosine directs the differentiation of monocytes into M2 macrophages upon binding to A2BR.75 These results suggest that antagonists of CD39, CD73, and adenosine receptors can remodel the TME to alleviate tumor immune evasion.
Nanomedicine improves the TME via metabolic reprogramming
Since tumor cells uptake and catabolize abundant nutrients through overexpressing their transporters and enzymes, as well as excrete a myriad of immunosuppressive metabolites, modulation of essential nodes in the metabolism of tumor cells could ameliorate the hostile TME to some extent. Besides, modulation of the metabolism in immune cells and CAFs can further enhance anti-tumor immunity. However, the use of metabolism-modulating agents faces challenges because of their severe toxicity due to their off-target effects and poor bioavailability. The application of nanomedicine can realize the delivery of metabolic regulators to the target cell types. On the one hand, nanoparticles can passively accumulate in tumor sites through the enhanced permeability and retention (EPR) effect. On the other hand, specific ligand-modified nanoparticles can actively conjugate to the targeted cells through ligand–receptor interactions. For example, 2-Deoxyglucose (2-DG), a glucose mimetic drug, inhibits glycolysis in tumor cells while induces inevitable cardiotoxicity,76 but tumor cell-targeting 2-DG nanovesicles could eliminate lung cancer cells and reveals no significant damage to major organs 14 days posttreatment.77 Overall, nanomedicine-aided metabolic reprogramming of the TME has great potential to augment the efficacy and safety of immunometabolism-modulating agents.
The mainstream flow: targeting tumor cells
The progress in the field of immunometabolism has revolutionized immunotherapy, and the vast majority of immunometabolic strategies are devoted to modulating the metabolism of tumor cells. Nanomedicine has been applied to reprogram the metabolism of tumor cells through various approaches: (1) blocking glycolysis and preventing lactate production via inhibiting HK2, phosphofructokinase (PFK), LDHA, GLUT1 and MCT478, 79, 80, 81, 82, 83; (2) decreasing NADPH production via inhibiting G6PD and 6PGD84,85; (3) restricting glutamine metabolism via GLS inhibitors, 6-Diazo-5-oxo-l-norleucine (DON) and SLC1A5 blockade86, 87, 88; (4) decreasing arginine uptake via SLC7A2 blockade and depleting arginine via arginine deiminase31,32,89; (5) interfering with tryptophan metabolism via IDO inhibitors and kynureninase61,90; (6) inhibiting methionine uptake via genetic knockout of SLC43A291; (7) interfering with fatty acid and cholesterol metabolism via downregulating the expression of FASN, SCD-1 and CD3692, 93, 94, 95, 96; (8) decreasing immunosuppressive PGE2 production via downregulating HIF-1α97,98; (9) reducing the production of adenosine via inhibiting CD39 and CD7399 (Supplementary Tables S1–S5). Details on reprograming the metabolism of tumor cells via nanomedicine can be seen in Supplementary Material.
A breath of fresh air: targeting tumor-suppressing immune cells
We have discussed advances in reprogramming tumor metabolic pathways to ameliorate the immunosuppressive TME, but the immune-modulatory effect is often indirectly induced through the ICD effect. Since immune cells are one of the critical components in the TME, the modulation of an immunosuppressive TME could be realized via targeting immune cells in the TME.100,101 There are very few reports on nanomedicine-mediated metabolic reprogramming of immune cells. Herein, we will cover current advances in using nanomedicine for metabolic reprogramming in immune cells in the TME.
A tumor–immunity cycle typically begins with the release of antigens after triggering tumor cell death from therapeutic treatment. Immature DCs can uptake and process these antigens, and they become mature. Mature DCs activate T cells via antigen presentation in tumor-draining lymph nodes (TDLNs). The activated T cells infiltrate to tumor sites via the vascular system. CTLs are the most effective anti-tumor immune cells via secreting IFN-γ, granzyme B and the Fas/FasL pathway. The release of antigens from fresh dead tumor cells allows the tumor–immunity cycle to continue sustainably102 (Fig. 3a). However, there remain various metabolic barriers to this cycle in the TME. For example, glucose shortage affects the infiltration of CTLs and their anti-tumor effects48; excessive lipid accumulation in the TME impairs antigen processing and presentation of DCs.103 Strategies have been developed for metabolic rewiring in DCs and T cells via nanomedicine, especially surface-engineering of T cells is very promising in promoting anti-tumor immunity (Supplementary Table S6).
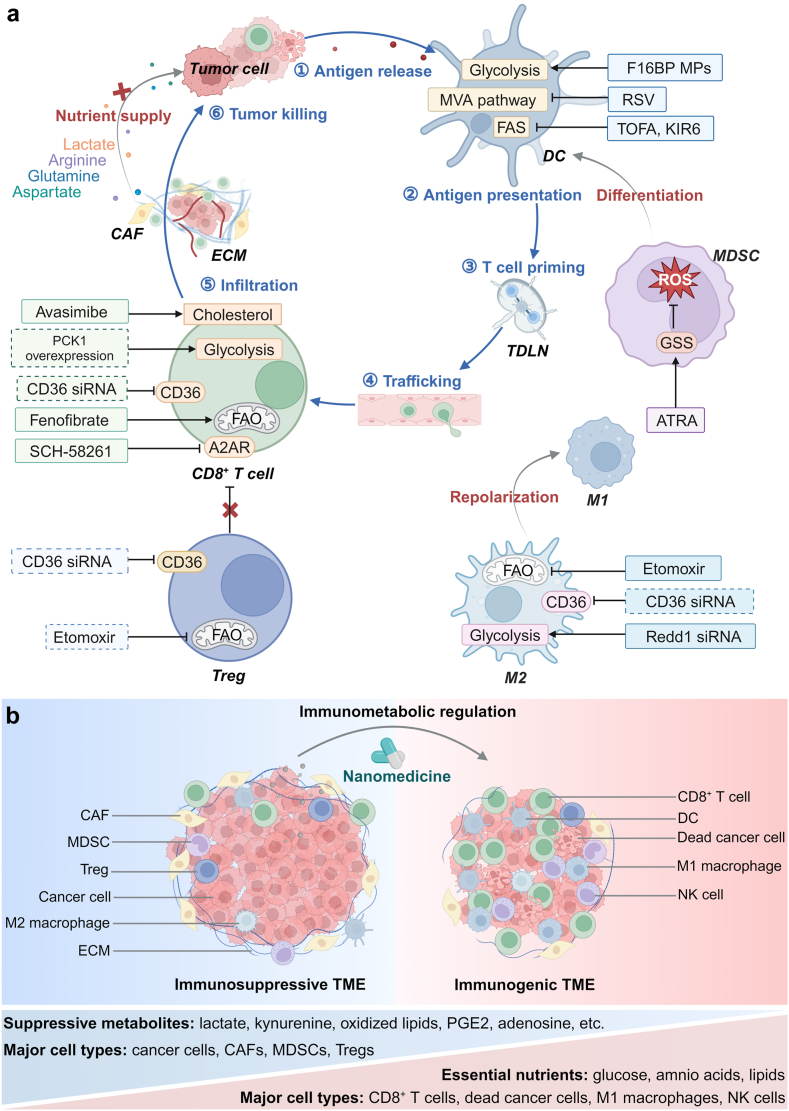
Fig. 3.
Overview of metabolic reprogramming of immune cells and CAFs via nanomedicine. (a) Schematic illustration of a tumor–immunity cycle including the following steps: antigen release, antigen presentation, T cell priming, CD8+ T cell trafficking through the vascular system, infiltration into deep tumor tissues, and tumor killing. Several targets have been identified to remodel an immunosuppressive TME via modulating metabolic pathways in cancer cells, CAFs and immune cells: (1) nanomedicine-based metabolic reprogramming strategies help restoring the proliferation of CD8+ T cells and their secretion of effector cytokines, and facilitating antigen-presentation by DCs; (2) MDSCs and M2 macrophages can be transformed to mature DCs and M1 macrophages via nanomedicine-assisted metabolic modulation, respectively; (3) metabolic reprogramming of Tregs via nanomedicine can attenuate their suppressive activity; and (4) regulating glycolysis and amino acid metabolism in CAFs can block their nutrient supply to tumor cells. Well-established targets are shown in solid boxes, while targets that remain to be explored are in dashed boxes. (b) Nanomedicine-aided immunotherapy can reverse an immunosuppressive TME to an immunogenic one via immunometabolic regulation.
Enhancing antigen presentation by DCs
Glycolysis is essential for both activation of DCs, which mediate T cell activation. Instead of complex genetic upregulation of glucose transporters and glycolysis enzymes in DCs, directly replenishing glycolytic ingredients to DCs may be an effective and feasible approach. Inamdar et al. formulated F-1,6-BP microparticles (F16BP MPs), which contained an immune adjuvant and melanoma cell antigens. These microparticles were phagocytosed by DCs, rescuing glycolysis and activation of DCs after systemic administration of PFK15, a glycolysis inhibitor. Encouragingly, treatment with F16BP MPs elevated CTL/Treg ratio, ultimately promoting immune response against melanoma.104 The strategy realized specific inhibition of glycolysis in tumor cells and replenished substrates for DC-targeting glycolysis, thus efficiently activating anti-tumor immunity. Through the same approach, exhausted T cells in the TME can be rescued via targeted delivery of critical substrates.
Furthermore, abnormal lipid metabolism has a negative impact on antigen cross-presentation in DCs and priming of T cells. 5-tetradecyloxy-2-furoic acid (TOFA) is often used to block FAS via inhibiting ACC, but its poor aqueous solubility and bioavailability diminish its efficacy via an oral administration route. Recently, Qin et al. constructed a lipid metabolism-manipulating nanovaccine, TPOP, which was comprised of TOFA-loaded PLGA nanoparticles coated with bacteria outer membranes. Pathogen-associated molecular patterns on the bacteria outer membrane can be recognized by pattern recognition receptors on DCs, thus TPOP was successfully phagocytosed by DCs. Therefore, the TPOP nanovaccine could specifically target DCs. After administration of doxorubicin (DOX) and TPOP, DOX induced the ICD effect to release tumor-associated antigens, which were captured by the TPOP nanovaccine via the Michael addition reaction. Uptake of the TPOP nanovaccine by DCs decreased in the intracellular level of triglyceride and cholesterol ester, and promoted maturation and antigen presentation of DCs. Consequently, the TPOP nanovaccine helped increasing the level of anti-tumor cytokines and the population of effector memory T cells, thus effectively suppressing the primary tumor and distant lesions in B16F10 tumor-bearing mice103 (Supplementary Figure S1). This suggests that normalization of FAS is essential for antigen cross-presentation of DCs, which facilitates restoring the weakened anti-tumor immunity.
In line with excessive fatty acids accumulation, abnormal cholesterol metabolism also exerts a negative impact on antigen cross-presentation by DCs. Specifically, the activity in the MVA pathway of tumor-infiltrating DCs is upregulated, and its downstream metabolite, geranylgeranyl diphosphate (GGPP), can activate GTPase Rab5 to induce antigen degradation by lysosomes, thus impairing the ability of antigen cross-presentation of DCs.105 Rosuvastatin (RSV), an inhibitor for the MVA pathway, has been widely used to decrease the plasma cholesterol level in clinical practices. To realize precise and long-term modulation of cholesterol metabolism in DCs, Yang et al. developed a mannose-decorated hydrogel delivery system to load RSV to construct Gel@NPs. After binding to overexpressed mannose receptors on DCs, Gel@NPs were specifically internalized by DCs. RSV was released sustainably to reactivate antigen cross-presentation of DCs and strengthen tumor-killing of CTLs, thus forming an immunocompetent TME106 (Supplementary Figure S1). This result implies that nanomedicine-assisted metabolic modulation could enhance activation and antigen presentation of DCs in the TME, thus activating the anti-tumor immunity.
Revitalizing tumor-suppressive effects of T cells
A hypoglycemic TME often exhausts T cells through interfering with aerobic glycolysis which is essential for IFN-γ secretion and proliferation.48 It has been found that phosphoenolpyruvate (PEP) can promote activation of T cells and enhance production of cytotoxic effector molecules.107 Therefore, overexpression of phosphoenolpyruvate carboxykinase 1 (PCK1), which produces PEP from oxaloacetate (OAA), can enhance anti-tumor effects of T cells. However, the challenging issue is to achieve specific overexpression of PCK1 in TILs, but not in tumor cells. T cells-targeting nanomedicine might be employed to specifically upregulate PCK1 in TILs.
In addition, CD8+ T cells maintain their anti-tumor ability under a glucose-deprived condition via upregulating peroxisome proliferators-activated receptor-α (PPAR-α) and FAO.108 To selectively deliver fenofibrate, an agonist of PPAR-α, to T cells, Kim et al. encapsulated fenofibrate into anti-CD3e f (ab')2 fragment-modified amphiphilic polygamma glutamic acid-based nanoparticles (aCD3/F/ANs). It was demonstrated that aCD3/F/ANs were specifically uptaken by CD3+ T cells. Through activating PPAR-α, aCD3/F/ANs promoted FAO in T cell mitochondria via upregulating expression of the related enzymes and transporters. This nanomedicine increased tumor-infiltrating CD8+ T cell proliferation and enhanced their cytokine production, effectively inhibiting B16F10 tumor growth109 (Fig. 4). The result indicates that mitochondria are essential for augmenting the T cell function and reprogramming lipid metabolism in adoptive T cells may enhance their survival and anti-tumor effects.
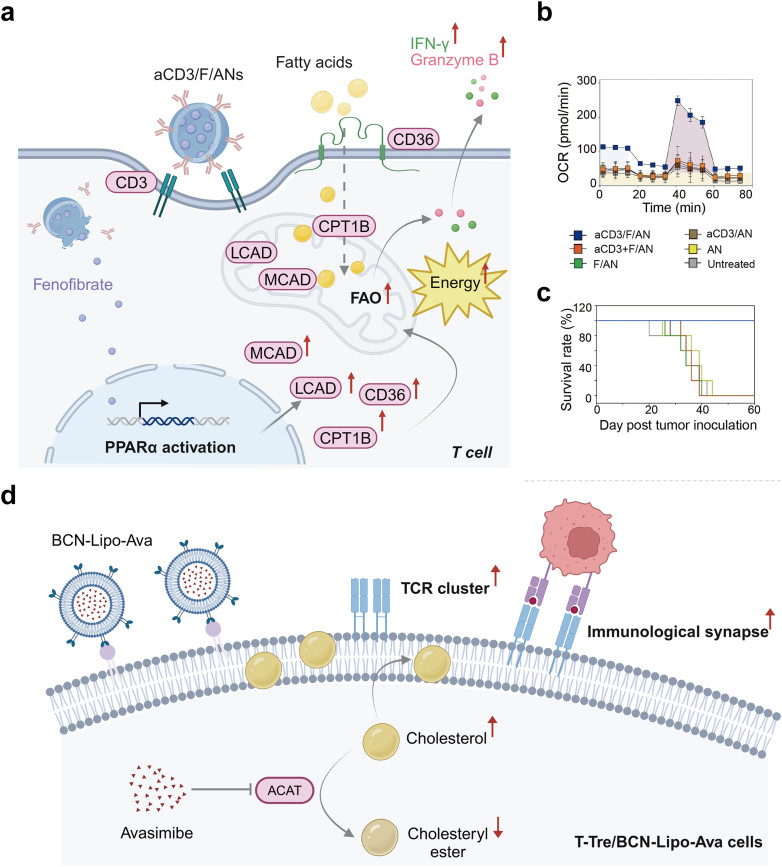
Fig. 4.
Precisely reprogramming FAO and cholesterol metabolism in T cells via nanomedicine. (a) Scheme for aCD3/F/ANs to reprogram FAO in T cells via upregulating the expression of CD36, CPT1B, long chain acyl-CoA dehydrogenase (LCAD) and medium chain acyl-CoA dehydrogenase (MCAD), leading to an elevation in the secretion level of IFN-γ and granzyme β. OCR (b) and survival rate (c) of the aCD3/F/ANs-treated group compared to those from other treatment groups. Reprinted with permission from ref.109 Copyright 2021 Springer Nature. (d) Schematic illustration of the mechanism for an enhancement in anti-tumor response of T-Tre/BCN-Lipo-Ava cells.
A high level of cholesterol in the plasma membrane is positively correlated with the tumor-killing ability of CD8+ T cells.45 Compared with free avasimibe, Hao et al. innovatively anchored liposomes that contained avasimibe onto the membrane of T cells, then constructed T-Tre/BCN-Lipo-Ava cells without any impact on the physiology of T cells. The liposomal avasimibe was stably attached to the cell surface for up to 4 days and avasimibe was sustainably released to increase the membrane cholesterol concentration. The surface-engineered T cells exhibited enhanced TCR clustering and immunological synapse maturation, which promoted anti-tumor cytokines secretion and boosted the tumor-killing capacity both in B16F10 and LN-299 tumor-bearing mice110 (Fig. 4). This elegant method circumvents targeted delivery of metabolic modulators to T cells via nanoparticles, since vasculature extravasation of these nanoparticles is often hindered by a high interstitial fluid pressure in the hostile TME. Enhancing anti-tumor activity of adoptive T cells by anchoring essential nutrients onto the immune cell surface via nanotechnology is a feasible approach, but side effects like the cytokine storm must be thoroughly examined before clinical translation.
Accumulation of adenosine in the TME suppresses various immune effector cells through ligation with A2AR. Masjedi et al. fabricated A2AR siRNA-loaded nanoparticles to downregulate the expression of A2AR in T cells, which relieved adenosine-mediated immunosuppression, enhanced the DCs vaccination efficacy, and increased CD8+ T cell infiltration.111 However, the delivery of nanomedicine into immune cells is often hindered by a hostile TME. A2AR antagonist SCH-58261 (SCH)-loading multilamellar liposomes, cMLVs, were constructed by Siriwon et al. and cMLVs were coupled onto the membrane of CAR-T cells, without affecting CAR-T cell function. The engineered CAR-T cells can effectively infiltrate into tumor sites via responding to chemoattractant gradients. Encouragingly, due to blockade of A2AR, the surface-engineered CAR-T cells recovered their tumor-killing potency and inhibited SKOV3 tumor growth. Unfortunately, IFN-γ secretion by the surface-engineered CAR-T cells was less than that of counterparts from healthy mice, indicating there were other immunosuppressive mechanisms.112 Conjugation of multiple metabolic modulators to the membrane of engineered T cells has the potential to improve the anti-tumor immunity, while such a complex procedure for surface engineering of T cells may be very challenging for translation into clinical practice.
Notably, CD4+ T cells can promote CD8+ T cell anti-tumor immunity. Th1 cell infiltration is a positive prognostic indicator in esophageal squamous cell carcinoma patients, since Th1 cells can secrete IFN-γ, which improves antigen presentation via upregulating MHC II expression on DCs.113 Th1 cells are involved in CD8+ T cell proliferation, recruitment and memory response.114 Besides, Th1 cells exhibit cytotoxicity independently of CTLs. Tumor cells often downregulate MHC I expression to evade CTL recognition while Th1 cell produce IFN-γ to induce MHC II expression on tumor cells for recognition and secrete granzyme B to kill them.115 Therefore, Th1 cell may become a novel target for metabolic-reprogramming nanomedicine to enhance the anti-tumor response.
These results have suggested that nanomedicine-aided metabolic reprogramming of T cells could resolve the issues associated with direct administration of small molecular agents, particularly, nanomedicine can enhance their T cell-targeting ability, thus revitalizing exhausted T cells in the TME.
Make a friend out of an enemy: targeting tumor-promoting immune cells
Low immune response of immunotherapy may be stemmed from the presence of a substantial number of immunosuppressive cells (MDSCs and Tregs) and other immune cells (Th17 cells and TAMs) which exhibit paradoxical roles in promotion of tumor progression or inhibition of tumor growth. For example, Th17 cells are shown to promote anti-tumor immunity via inducing infiltration of DCs and CTLs into tumor sites, while Th17 cells can overexpress CD39 and CD73 to produce immunosuppressive adenosine in the presence of TGF-β.116 In this section, we will elaborate the metabolic plasticity of MDSCs, TAMs and Th17 cells, and summarize recent advances in metabolic regulation of these cells via nanomedicine to amplify anti-tumor immunity.
Repolarizing TAMs to immunocompetent M1 phenotype
Tumors usually recruit circulating monocytes via secreting chemokine CCL2, and these monocytes are then differentiated into macrophages in the TME. The macrophages are referred to TAMs.117 TAMs can be classified into M1 and M2 phenotypes, depending on their anti-tumor or pro-tumor role, respectively. Anti-tumor M1 macrophages primarily perform glycolysis for energy supply, while M2 macrophages, the predominant type of TAMs, prefer to mitochondria-mediated OXPHOS and FAO for energy production. Pro-tumor M2 macrophages weaken the antigen presentation by DCs via secreting IL-10 and TGF-β, and upregulate ARG1 expression to exhaust T cells.118
Repolarizing macrophages (M2 to M1) can significantly activate anti-tumor immune response (Supplementary Table S6). Since OXPHOS heavily depends on intact mitochondria, disruption of the mitochondrial activity could shift the metabolism from OXPHOS to glycolysis, promoting M1 repolarization. RSL3 can sensitize lipid peroxidation and ferroptosis via inhibiting GPX4. Thus, Gu et al. constructed an iron-based metal organic framework with the Fenton activity to realize metabolic conversion in TAMs. Fe-metal organic framework nanoparticles (MIL88B) were employed to load hydrophobic RSL3 to yield MIL88B/RSL3. The nanomedicine promoted lipid peroxidation on mitochondrial membranes, resulting in a shift in the energy production from OXPHOS to glycolysis, and ultimately M1 repolarization of TAMs. M1 macrophages generated via induction of MIL88B/RSL3 exhibited an enhancement in phagocytic killing, thus inhibiting 4T1 tumor growth.119 However, the nanosystem was not equipped with the M2 macrophage-targeting ability.
It is desirable to increase the glycolysis level in M2 microphages instead of tumor cells. Knockdown of Redd1 can upregulate the glycolysis level through activating the mTOR pathway, thus promoting repolarization of M2 macrophages towards M1. Guo et al. loaded Redd1 siRNA into bacterial outer membrane vesicles (OMVs), and the nanovesicles were then modified with mannose and paclitaxel (PTX) to formulate siRNA@M-/PTX-CA-OMVs. Through the EPR effect, siRNA@M-/PTX-CA-OMVs accumulated in acidic tumor tissues and released PTX to induce the ICD effect. Specific binding of the nanovesicles onto M2 macrophages was realized through interaction between CD206 on M2 macrophages and mannose on the nanovesicles. The knockdown of Redd1 via Redd1 siRNA in nanovesicles selectively promoted glycolysis in M2 macrophages and repolarized them towards M1. Besides, the nanovesicles helped increasing the number of TILs and effector cytokines levels, ameliorating the TME and inhibiting 4T1 tumor progression120 (Supplementary Figure S2).
Interestingly, it has been found that tumor-derived long-chain fatty acids (LCFAs) are preferentially internalized by metastasis-associated macrophages via CD36, which drives their polarization towards M2, leading to a metastasis-promoting TME.121 Therefore, nanomedicine-aided CD36 inhibition in TAMs may provide a prospective approach of repolarization of M2 macrophages towards their M1 phenotype. To conclude, nanomedicine-mediated metabolic reprogramming in TAMs has been demonstrated to remodel an immunosuppressive TME. The abundant TAMs assist in tumor progression; thus efforts should be made into exploring unveiled metabolic regulators for re-educating TAMs to the M1 phenotype for enhancing anti-tumor immunity.
Promoting the differentiation of MDSCs into DCs
MDSCs are a major type of immunosuppressive cells originated from hematopoietic stem cells and they can be differentiated into macrophages, DCs, and granulocytes. However, the TME prevents MDSCs differentiation, and immature heterogeneous MDSCs exhibit potent immunosuppressive properties. For example, MDSCs can overexpress ARG1 and NOS2. ARG1 could deplete arginine and impair T cell activation, and NOS2 produces NO to promote tumor angiogenesis through upregulating VEGF expression. In addition, PD-L1 expression and TGF-β secretion are enhanced in MDSCs, and a high level of ROS is produced, thus exhausting CD8+ T cells.122
Notably, MDSCs have metabolic plasticity, which provides a foundation for differentiating immature MDSCs into mature cells via metabolic reprogramming. ATRA has been reported to induce the differentiation of MDSCs into mature macrophages and DCs through upregulating the expression of glutathione synthase (GSS) to synthesize GSH for scavenging ROS.5,123 Zheng et al. constructed ATRA-encapsulated liposomes (L-ATRA) to significantly improve the solubility and the encapsulation efficiency of ARTA. L-ATRA effectively promoted MDSC differentiation into mature DCs and enhanced their expression of MHC II, thus improving the viability of CD8+ T cells.124 Therefore, nanoplatforms for ROS-scavenging and antioxidation may be used to promote differentiation of MDSCs into mature DCs and ameliorate an immunosuppressive TME.125 Additionally, it has been reported that proliferation and suppressive activities of MDSCs are predominantly prompted by HIF-1α-driven glycolysis,126 and tumor-infiltrating MDSCs exhibit a higher FAO level than their splenic counterparts in tumor-bearing mice.127 Therefore, inhibition of glycolysis and FAO in MDSCs may alter their immunosuppressive properties. It is noted that these strategies have been reported to be feasible for tumor cells. Since different subsets of MDSCs are featured with distinct metabolic pathways, the interference with the metabolism in MDSCs to ameliorate an immunosuppressive TME remains to be elucidated.
Modulating the Th17/Treg axis to augment anti-tumor immunity
CD8+ CTLs are the major type of effector cells in immunotherapy, whereas CD4+ T cells including Th17, Th1, Th2, and Tregs are involved in immunomodulation predominantly through the secretion of cytokines. Notably, Th17 cells exhibit environmentally-dependent plasticity,128 indicating that they can be transformed into other types of CD4+ T cells, primarily including anti-tumor Th1 cells and pro-tumor Tregs, which lays the groundwork for the following discussion.
Th17 cells are featured with the production of IL-17, and Th17 cells have been shown to have a contradictory influence on tumor development. For example, Th17 cells can express CD39 and CD73 to produce immunosuppressive adenosine by decomposing the extracellular ATP116 but produces IL-17 to recruit DCs to TDLNs for CD8+ T cell activation.129 IL-17 can facilitate tumor cell renewal,130 and upregulate the expression of VEGF and CD31 to promote tumor angiogenesis,131 but IL-17 level is positively associated with the prognosis of cervical adenocarcinoma.132
Tregs can express an array of immune checkpoints such as CTLA4, which can downregulate CD80 and CD86 on DCs to weaken their immunostimulatory ability.133 In addition, Tregs induce the production of immunosuppressive adenosine to promote tumor immune evasion.74 Therefore, we suggest that an elevation in the Th17/Treg ratio may be beneficial for anti-tumor immunity.
It has been found that activation and proliferation of Th17 cells are dependent on glycolysis and FAS,134 but Tregs are predominantly driven by OXPHOS and FAO from tumor-derived lactate and free fatty acids.135 It is noteworthy that the trans-differentiation of Th17 cells to Tregs is promoted under the suppression of glycolysis in Th17 cells and the presence of PGE2.136 Therefore, inhibition of COX-2 could reduce trans-differentiation of Th17 cells into pro-tumor Tregs, which could be realized through nanomedicine with precise targeting and efficient drug delivery capabilities.
Blocking metabolic crosstalk between tumor cells and CAFs
CAF-derived critical nutrients can be utilized by tumor cells, which is one of the latest pro-tumor mechanisms. Therefore, nanomedicine-aided metabolic regulation of CAFs could help reducing the production of these nutrients, starving tumor cells to inhibit tumor growth (Supplementary Table S7).
Cancer cells and CAFs outcompete immune effector cells in gaining glucose via hyperactive glycolysis to produce lactate, which also promotes tumor immune evasion. To kill two birds with one stone, Zang et al. constructed homologous targeting nanoparticles, PTX/PFK15-SLN@[4T1-3T3] NPs, by co-loading PTX and PFK15 (a glycolysis inhibitor) onto solid lipid nanoparticles camouflaged with hybrid membranes from tumor cells and fibroblasts. Through the EPR effect, PTX/PFK15-SLN@[4T1-3T3] NPs were readily internalized by tumor cells and CAFs, inhibiting glycolysis and reducing lactate production in both cells. It is noted that glycolysis inhibition and reduced lactate production can starve tumor cells and attenuate lactate-mediated immunosuppression. Therefore, treatment with these nanoparticles increased CD8+ T cell infiltration, enhanced effector cytokine secretion, and repolarized M2 macrophages towards M1, thus reshaping the immunosuppressive TME.137 This strategy of targeting both tumor cells and CAFs to inhibit their glycolysis and attenuate lactate-mediated immunosuppression could be very effective in revitalize anti-tumor immunity.
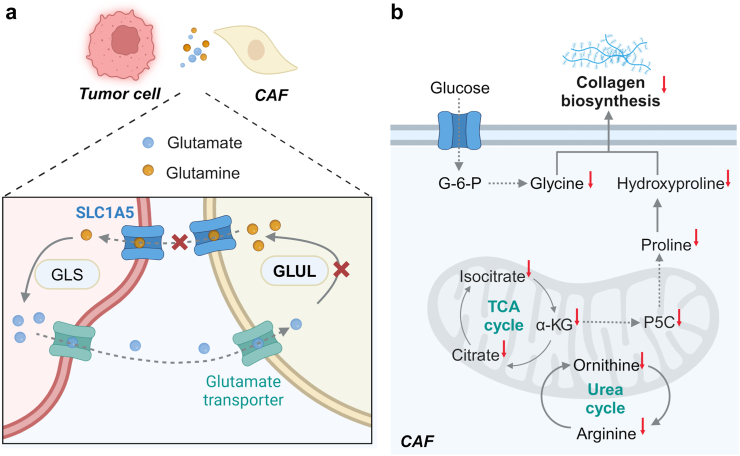
CAFs are often stimulated to synthesize glutamine from tumor cell-derived glutamate via glutamate ammonia ligase (GLUL) to meet a high demand for glutamine by tumor cells.14 To block the glutamine–glutamate cycle, Ai et al. constructed FH peptide-conjugated nanodroplets to co-load V9302 and siGLUL, resulting in FH-V9302-siGLUL-NDs. The nanodroplets exhibited a negligible hemolysis rate compared to free V9302. Since FH peptides have high affinity to overexpressed tenascin C on CAFs, FH-V9302-siGLUL-NDs could precisely target CAFs (Fig. 5). Both V9302 and siGLUL were rapidly released in a CAF-enriched TME under ultrasound irradiation to simultaneously downregulate the GLUL expression in CAFs and the SLC1A5 expression on tumor cells, inhibiting melanoma tumor growth and ECM deposition.138
Fig. 5.
Nanomedicine-aided precise metabolic reprogramming of CAFs. (a) Schematic illustration of FH-V9302-siGLUL-NDs to interfere with glutamine metabolic crosstalk between CAFs and tumor cells via blocking SLC1A5 and GLUL. (b) The mechanism of reducing collagen biosynthesis in CAFs by P-DAS via sequentially delivering dasatinib and epirubicin into CAFS to interfere with glycolysis and amino acid metabolism.
Considering that CAFs are the main contributors to a dense ECM, which blocks the intratumoral penetration of nanodrugs and the infiltration of T cells, leading to unsatisfactory nanomedicine-mediated immunotherapy outcomes. It was reported that dendritic polymer-based sequential delivery of dasatinib and epirubicin exhibited great inhibition of collagen biosynthesis in CAFs by downregulating amino acid metabolism and energy production, resulting in an increased level of CTL infiltration139 (Fig. 5). Although rewriting the metabolism of CAFs to ameliorate an immunosuppressive TME is still at an early stage, the findings are very encouraging in enhancing anti-tumor immunity and curbing tumor growth.
Conclusions and future directions
Adapted metabolic profiles in tumor cells and CAFs create a TME that contains abundant immunosuppressive metabolites while inadequate essential nutrients, which suppress anti-tumor activities of both intrinsic and adaptive immune cells, ultimately promoting tumor immune evasion. Immunometabolic strategies targeting the critical metabolic pathways in tumor cells have been demonstrated to ameliorate the immunosuppressive TME and boost immune response. More recently, immune cells and CAFs in the TME serve as new targets for immunometabolic therapy. Nanomedicine can improve biodistribution, biocompatibility and bioavailability of metabolic modulators through stimuli-responsive drug release and targeting specific cell types, thus enhancing the efficiency in metabolic reprogramming and minimizing their systemic toxicity. Eventually, nanomedicine-assisted metabolic interventions can effectively and precisely reverse an immunologically cold milieu to an immunologically hot TME (Fig. 3b).
Nanomedicine-aided metabolic reprogramming of the TME for cancer immunotherapy has made great progress in fundamental studies, but clinical translation of this approach is still challenging. Collaborative efforts should be made into the following directions: (1) Unveiling metabolic crosstalk between various cell types in a heterogenous TME; (2) Improving selective targetability of nanomedicine on different cell types within the TME to promote anti-tumor immunity and reduce systemic toxicity; (3) Effectively reactivating anti-tumor immune response of T cells; (4) Reducing nonspecific clearance of nanomedicine by the reticuloendothelial system; (5) Reducing physical and chemical barriers of ECM for the intra-tumoral infiltration of nanomedicine and immune cells; (6) Developing precise and personalized metabolic reprogramming nanomedicine for different patients and/or tumor subtypes; (7) Developing advanced in vitro and in vivo models for human tumors.
Outstanding questions
Rewriting metabolism via nanomedicine to ameliorate a suppressive TME represents a fascinating approach for tumor immunotherapy, however, clinical trials of this approach remain scarce. To bridge the gap between fundamental research and preclinical/clinical trials: (1) Nanomaterials as vehicles for a broad spectrum of metabolic regulators should be developed with better targetability and higher delivery efficiency to reduce systemic toxicity of metabolic regulators; (2) Immunometabolic nanomedicine should target not only tumor cells but also immune cells and CAFs; (3) Holistically regulating the TME via nanomedicine should be considered, rather than targeting a single cell type, because of the presence of complex metabolic interplay among tumor cells, immune cells and CAFs in the TME, which could effectively potentiate immunometabolic therapy.
Contributors
Jieyu Liu, Xiaoling Li and Kui Luo conceived the outline of this review. Jieyu Liu and Yinan Bai drafted the manuscript and performed the creation of figures, tables and text boxes. Xiaoling Li and Yinggang Li critically revised the review. Kui Luo finalized this manuscript. Jieyu Liu and Yinan Bai contributed equally to this draft. All authors have read and approved the final version of the manuscript.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (32271445, 52073193, 52303198), National Science and Technology Major Project of China (2023YFB3810004), Sichuan Science and Technology Program (2024NSFJQ0050, 2024NSFSC1019) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYGD23026). All diagrams were created with BioRender.com. The funders play no role in paper design, data collection, data analysis, interpretation, and writing of the paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105301.
Contributor Information
Xiaoling Li, Email: xiaoling@scu.edu.cn.
Kui Luo, Email: luokui@scu.edu.cn.
Appendix A. Supplementary data
References
- 1.Weiss S.A., Wolchok J.D., Sznol M. Immunotherapy of melanoma: facts and hopes. Clin Cancer Res. 2019;25(17):5191–5201. doi: 10.1158/1078-0432.CCR-18-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17(4):251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z., Zhang Q., Li Z., et al. Branched glycopolymer prodrug-derived nanoassembly combined with a STING agonist activates an immuno-supportive status to boost anti-PD-L1 antibody therapy. Acta Pharm Sin B. 2024;14(5):2194–2209. doi: 10.1016/j.apsb.2024.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Gong Q., Luo K. Biomarker-driven molecular imaging probes in radiotherapy. Theranostics. 2024;14(10):4127–4146. doi: 10.7150/thno.97768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiger R., Rieckmann J.C., Wolf T., et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829–842. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raccosta L., Fontana R., Maggioni D., et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210(9):1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomić S., Joksimović B., Bekić M., et al. Prostaglanin-E2 potentiates the suppressive functions of human mononuclear myeloid-derived suppressor cells and increases their capacity to expand IL-10-producing regulatory T cell subsets. Front Immunol. 2019;10:475. doi: 10.3389/fimmu.2019.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang R., Elsaadi S., Misund K., et al. Conversion of ATP to adenosine by CD39 and CD73 in multiple myeloma can be successfully targeted together with adenosine receptor A2A blockade. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lokshin A., Raskovalova T., Huang X., et al. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66(15):7758–7765. doi: 10.1158/0008-5472.CAN-06-0478. [DOI] [PubMed] [Google Scholar]
- 11.Fiaschi T., Marini A., Giannoni E., et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72(19):5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 12.Kim B.G., Sung J.S., Jang Y., et al. Compression-induced expression of glycolysis genes in CAFs correlates with EMT and angiogenesis gene expression in breast cancer. Commun Biol. 2019;2(1):313. doi: 10.1038/s42003-019-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faubert B., Li K.Y., Cai L., et al. Lactate metabolism in human lung tumors. Cell. 2017;171(2):358–371. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L., Achreja A., Yeung T.L., et al. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 2016;24(5):685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao H., Li Y., Zheng Y., Lin J.-M. Stability of the arginine–ornithine–citrulline cycle maintained by tumor–stroma interactions in cell coculture hydrogel microspheres. Anal Chem. 2023;95(29):10999–11006. doi: 10.1021/acs.analchem.3c01134. [DOI] [PubMed] [Google Scholar]
- 16.Bertero T., Oldham W.M., Grasset E.M., et al. Tumor-Stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 2019;29(1):124–140. doi: 10.1016/j.cmet.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert M.A., Coscia F., Chryplewicz A., et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature. 2019;569(7758):723–728. doi: 10.1038/s41586-019-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong J., Lin Y., Zhang H., et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020;11(4):267. doi: 10.1038/s41419-020-2434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuwirt H., Bouchal J., Kharaishvili G., et al. Cancer-associated fibroblasts promote prostate tumor growth and progression through upregulation of cholesterol and steroid biosynthesis. Cell Commun Signal. 2020;18(1):11. doi: 10.1186/s12964-019-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blagih J., Coulombe F., Vincent E.E., et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42(1):41–54. doi: 10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly R.P., Loftus R.M., Keating S.E., et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193(9):4477–4484. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z., Yu X., Ding R., et al. Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J Clin Invest. 2023;133(7) doi: 10.1172/JCI166031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao E., Maj T., Kryczek I., et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol. 2016;17(1):95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best S.A., Gubser P.M., Sethumadhavan S., et al. Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab. 2022;34(6):874–887. doi: 10.1016/j.cmet.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Matias M.I., Yong C.S., Foroushani A., et al. Regulatory T cell differentiation is controlled by αKG-induced alterations in mitochondrial metabolism and lipid homeostasis. Cell Rep. 2021;37(5) doi: 10.1016/j.celrep.2021.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce E.L., Poffenberger M.C., Chang C.H., Jones R.G. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342(6155) doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scalise M., Console L., Rovella F., et al. Membrane transporters for amino acids as players of cancer metabolic rewiring. Cells. 2020;9(9):2028. doi: 10.3390/cells9092028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez P.C., Quiceno D.G., Ochoa A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmieri E.M., Gonzalez-Cotto M., Baseler W.A., et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun. 2020;11(1):698. doi: 10.1038/s41467-020-14433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamas B., Vergnaud-Gauduchon J., Goncalves-Mendes N., et al. Altered functions of natural killer cells in response to L-Arginine availability. Cell Immunol. 2012;280(2):182–190. doi: 10.1016/j.cellimm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Wang S., Guo X., et al. Arginine supplementation targeting tumor-killing immune cells reconstructs the tumor microenvironment and enhances the antitumor immune response. ACS Nano. 2022;16(8):12964–12978. doi: 10.1021/acsnano.2c05408. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Alfa G.K., Qin S., Ryoo B.Y., et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol. 2018;29(6):1402–1408. doi: 10.1093/annonc/mdy101. [DOI] [PubMed] [Google Scholar]
- 33.Rashidi A., Miska J., Lee-Chang C., et al. GCN2 is essential for CD8(+) T cell survival and function in murine models of malignant glioma. Cancer Immunol Immunother. 2020;69(1):81–94. doi: 10.1007/s00262-019-02441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Cui L., Lu S., Xu S. Amino acid metabolism in tumor biology and therapy. Cell Death Dis. 2024;15(1):42. doi: 10.1038/s41419-024-06435-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bian Y., Li W., Kremer D.M., et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 2020;585(7824):277–282. doi: 10.1038/s41586-020-2682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandit M., Kil Y.S., Ahn J.H., et al. Methionine consumption by cancer cells drives a progressive upregulation of PD-1 expression in CD4 T cells. Nat Commun. 2023;14(1):2593. doi: 10.1038/s41467-023-38316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehdi A., Attias M., Mahmood N., et al. Enhanced anticancer effect of a combination of S-adenosylmethionine (SAM) and immune checkpoint inhibitor (ICPi) in a syngeneic mouse model of advanced melanoma. Front Oncol. 2020;10:1361. doi: 10.3389/fonc.2020.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Röhrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16(11):732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 39.Lin R., Zhang H., Yuan Y., et al. Fatty acid oxidation controls CD8(+) tissue-resident memory T-cell survival in gastric adenocarcinoma. Cancer Immunol Res. 2020;8(4):479–492. doi: 10.1158/2326-6066.CIR-19-0702. [DOI] [PubMed] [Google Scholar]
- 40.van der Windt G.J., Everts B., Chang C.H., et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luu M., Riester Z., Baldrich A., et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12(1):4077. doi: 10.1038/s41467-021-24331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachem A., Makhlouf C., Binger K.J., et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity. 2019;51(2):285–297. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Huang B., Song B-l, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2(2):132–141. doi: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 44.Mok E.H.K., Leung C.O.N., Zhou L., et al. Caspase-3-Induced activation of SREBP2 drives drug resistance via promotion of cholesterol biosynthesis in hepatocellular carcinoma. Cancer Res. 2022;82(17):3102–3115. doi: 10.1158/0008-5472.CAN-21-2934. [DOI] [PubMed] [Google Scholar]
- 45.Yang W., Bai Y., Xiong Y., et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin W.H., Yang Z.S., Li M., et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. 2020;158(6):1713–1727. doi: 10.1053/j.gastro.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 47.Westerterp M., Gautier E.L., Ganda A., et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 2017;25(6):1294–1304. doi: 10.1016/j.cmet.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang C.H., Curtis J.D., Maggi L.B., Jr., et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston R.J., Su L.J., Pinckney J., et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature. 2019;574(7779):565–570. doi: 10.1038/s41586-019-1674-5. [DOI] [PubMed] [Google Scholar]
- 50.Lundø K., Trauelsen M., Pedersen S.F., Schwartz T.W. Why warburg works: lactate controls immune evasion through GPR81. Cell Metab. 2020;31(4):666–668. doi: 10.1016/j.cmet.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Brown T.P., Bhattacharjee P., Ramachandran S., et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene. 2020;39(16):3292–3304. doi: 10.1038/s41388-020-1216-5. [DOI] [PubMed] [Google Scholar]
- 52.Chen P., Zuo H., Xiong H., et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;114(3):580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gudgeon N., Munford H., Bishop E.L., et al. Succinate uptake by T cells suppresses their effector function via inhibition of mitochondrial glucose oxidation. Cell Rep. 2022;40(7) doi: 10.1016/j.celrep.2022.111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Notarangelo G., Spinelli J.B., Perez E.M., et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8(+) T cell function. Science. 2022;377(6614):1519–1529. doi: 10.1126/science.abj5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tyrakis P.A., Palazon A., Macias D., et al. S-2-hydroxyglutarate regulates CD8(+) T-lymphocyte fate. Nature. 2016;540(7632):236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh G., Takagane K., Fukushi Y., et al. Cancer-associated fibroblasts educate normal fibroblasts to facilitate cancer cell spreading and T-cell suppression. 2022;16(1):166–187. doi: 10.1002/1878-0261.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gandhi R., Kumar D., Burns E.J., et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11(9):846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagage S., John B., Krock B.L., et al. The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J Immunol. 2014;192(4):1661–1670. doi: 10.4049/jimmunol.1300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada T., Horimoto H., Kameyama T., et al. Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat Immunol. 2016;17(6):687–694. doi: 10.1038/ni.3422. [DOI] [PubMed] [Google Scholar]
- 60.Campesato L.F., Budhu S., Tchaicha J., et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun. 2020;11(1):4011. doi: 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu D., Chen B., Mo Y., et al. Redox-activated porphyrin-based liposome remote-loaded with indoleamine 2,3-dioxygenase (IDO) inhibitor for synergistic photoimmunotherapy through induction of immunogenic cell death and blockage of IDO pathway. Nano Lett. 2019;19(10):6964–6976. doi: 10.1021/acs.nanolett.9b02306. [DOI] [PubMed] [Google Scholar]
- 62.Zeng Z., Zhang C., Li J., et al. Activatable polymer nanoenzymes for photodynamic immunometabolic cancer therapy. Adv Mater. 2021;33(4) doi: 10.1002/adma.202007247. [DOI] [PubMed] [Google Scholar]
- 63.Veglia F., Tyurin V.A., Mohammadyani D., et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun. 2017;8(1):2122. doi: 10.1038/s41467-017-02186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su P., Wang Q., Bi E., et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020;80(7):1438–1450. doi: 10.1158/0008-5472.CAN-19-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu S., Chaudhary O., Rodríguez-Morales P., et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54(7):1561–1577. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q., Cao Y., Shen L., et al. Regulation of PD-L1 through direct binding of cholesterol to CRAC motifs. Sci Adv. 2022;8(34) doi: 10.1126/sciadv.abq4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kedia-Mehta N., Tobin L., Zaiatz-Bittencourt V., et al. Cytokine-induced natural killer cell training is dependent on cellular metabolism and is defective in obesity. Blood Adv. 2021;5(21):4447–4455. doi: 10.1182/bloodadvances.2021005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez P.C., Hernandez C.P., Quiceno D., et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinet L., Jean C., Dietrich G., et al. PGE2 inhibits natural killer and γδ T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol. 2010;80(6):838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Thumkeo D., Punyawatthananukool S., Prasongtanakij S., et al. PGE2-EP2/EP4 signaling elicits immunosuppression by driving the mregDC-Treg axis in inflammatory tumor microenvironment. Cell Rep. 2022;39(10) doi: 10.1016/j.celrep.2022.110914. [DOI] [PubMed] [Google Scholar]
- 71.Albu D.I., Wang Z., Huang K.C., et al. EP4 Antagonism by E7046 diminishes Myeloid immunosuppression and synergizes with Treg-reducing IL-2-Diphtheria toxin fusion protein in restoring anti-tumor immunity. OncoImmunology. 2017;6(8) doi: 10.1080/2162402X.2017.1338239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiu D.K.-C., Tse A.P.-W., Xu I.M.-J., et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8(1):517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Connor R.A., Chauhan V., Mathieson L., et al. T cells drive negative feedback mechanisms in cancer associated fibroblasts, promoting expression of co-inhibitory ligands, CD73 and IL-27 in non-small cell lung cancer. OncoImmunology. 2021;10(1) doi: 10.1080/2162402X.2021.1940675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deaglio S., Dwyer K.M., Gao W., et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teo K.Y.W., Zhang S., Loh J.T., et al. Mesenchymal stromal cell exosomes mediate M2-like macrophage polarization through CD73/ecto-5’-nucleotidase activity. Pharmaceutics. 2023;15(5):1489. doi: 10.3390/pharmaceutics15051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minor R.K., Smith D.L., Jr., Sossong A.M., et al. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243(3):332–339. doi: 10.1016/j.taap.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li B., Yang T., Liu J., et al. Genetically engineered PD-1 displaying nanovesicles for synergistic checkpoint blockades and chemo-metabolic therapy against non-small cell lung cancer. Acta Biomater. 2023;161:184–200. doi: 10.1016/j.actbio.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Meng Z., Zhang X., Tan H., Lian H. Zinc-enriched nanosystem for dual glycolysis regulation and photothermal therapy to synergistically inhibit primary melanoma and lung metastasis. Chem Eng J. 2022;435 [Google Scholar]
- 79.Chen Z., Chen L., Ma Y., et al. Peptide-appended nanosonosensitizers targeting tumor glycolysis for synergistic sonodynamic-immunometabolic therapy of spinal-metastasized tumors. Adv Mater. 2023;35(42) doi: 10.1002/adma.202304246. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen Cao T.G., Truong Hoang Q., Kang J.H., et al. Bioreducible exosomes encapsulating glycolysis inhibitors potentiate mitochondria-targeted sonodynamic cancer therapy via cancer-targeted drug release and cellular energy depletion. Biomaterials. 2023;301 doi: 10.1016/j.biomaterials.2023.122242. [DOI] [PubMed] [Google Scholar]
- 81.Wu S., Zhang K., Liang Y., et al. Nano-enabled tumor Systematic energy exhaustion via zinc (II) interference mediated glycolysis inhibition and specific GLUT1 depletion. Adv Sci. 2022;9(7) doi: 10.1002/advs.202103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Z.X., Liu M.D., Guo D.K., et al. A MSN-based tumor-targeted nanoplatform to interfere with lactate metabolism to induce tumor cell acidosis for tumor suppression and anti-metastasis. Nanoscale. 2020;12(5):2966–2972. doi: 10.1039/c9nr10344a. [DOI] [PubMed] [Google Scholar]
- 83.Tang J., Meka A.K., Theivendran S., et al. Openwork@Dendritic mesoporous silica nanoparticles for lactate depletion and tumor microenvironment regulation. Angew Chem Int Ed Engl. 2020;59(49):22054–22062. doi: 10.1002/anie.202001469. [DOI] [PubMed] [Google Scholar]
- 84.Vijayalakshmi S., Mariadoss A.V.A., Ramachandran V., et al. Polydatin encapsulated poly [Lactic-co-glycolic acid] nanoformulation counteract the 7,12-Dimethylbenz[a] anthracene mediated experimental carcinogenesis through the inhibition of cell proliferation. Antioxidants. 2019;8(9):375. doi: 10.3390/antiox8090375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Chen J., Duan R., et al. High-Z-sensitized radiotherapy synergizes with the intervention of the pentose phosphate pathway for in situ tumor vaccination. Adv Mater. 2022;34(13) doi: 10.1002/adma.202109726. [DOI] [PubMed] [Google Scholar]
- 86.Tang Y., Wang S., Li Y., et al. Simultaneous glutamine metabolism and PD-L1 inhibition to enhance suppression of triple-negative breast cancer. J Nanobiotechnology. 2022;20(1):216. doi: 10.1186/s12951-022-01424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu C., Wang N., Chen X., et al. A photodynamic-mediated glutamine metabolic intervention nanodrug for triple negative breast cancer therapy. Mater Today Bio. 2023;19 doi: 10.1016/j.mtbio.2023.100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen G., Xu Q., Feng Z., et al. Glutamine antagonist synergizes with electrodynamic therapy to induce tumor regression and systemic antitumor immunity. ACS Nano. 2022;16(1):951–962. doi: 10.1021/acsnano.1c08544. [DOI] [PubMed] [Google Scholar]
- 89.Lam S.K., Li Y.Y., Xu S., et al. Growth suppressive effect of pegylated arginase in malignant pleural mesothelioma xenografts. Respir Res. 2017;18(1):80. doi: 10.1186/s12931-017-0564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Triplett T.A., Garrison K.C., Marshall N., et al. Reversal of indoleamine 2,3-dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol. 2018;36(8):758–764. doi: 10.1038/nbt.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang Y., Qin G., Cui T., et al. A bimetallic nanoplatform for STING activation and CRISPR/Cas mediated depletion of the methionine transporter in cancer cells restores anti-tumor immune responses. Nat Commun. 2023;14(1):4647. doi: 10.1038/s41467-023-40345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khan A., Aljarbou A.N., Aldebasi Y.H., et al. Fatty acid synthase (FASN) siRNA-encapsulated-her-2 targeted fab'-immunoliposomes for gene silencing in breast cancer cells. Int J Nanomedicine. 2020;15:5575–5589. doi: 10.2147/IJN.S256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang M.H., Ye Y., Zhang M., et al. Exosome-mediated delivery of SCD-1 siRNA promoted the death of anaplastic thyroid carcinoma cells via regulating ROS level. Clin Transl Oncol. 2022;24(2):288–296. doi: 10.1007/s12094-021-02682-x. [DOI] [PubMed] [Google Scholar]
- 94.Wang B., Yan N., Wu D., et al. Combination inhibition of triple-negative breast cancer cell growth with CD36 siRNA-loaded DNA nanoprism and genistein. Nanotechnology. 2021;32:395101. doi: 10.1088/1361-6528/ac0d1e. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J., Yin Y., Zhang J., et al. Suppression of energy metabolism in cancer cells with nutrient-sensing nanodrugs. Nano Lett. 2022;22(6):2514–2520. doi: 10.1021/acs.nanolett.2c00356. [DOI] [PubMed] [Google Scholar]
- 96.Lee S.S.-Y., Li J., Tai J.N., et al. Avasimibe encapsulated in human Serum albumin blocks cholesterol esterification for selective cancer treatment. ACS Nano. 2015;9(3):2420–2432. doi: 10.1021/nn504025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karpisheh V., Fakkari Afjadi J., Nabi Afjadi M., et al. Inhibition of HIF-1α/EP4 axis by hyaluronate-trimethyl chitosan-SPION nanoparticles markedly suppresses the growth and development of cancer cells. Int J Biol Macromol. 2021;167:1006–1019. doi: 10.1016/j.ijbiomac.2020.11.056. [DOI] [PubMed] [Google Scholar]
- 98.Wang L., Ding K., Zheng C., et al. Detachable nanoparticle-enhanced chemoimmunotherapy based on precise killing of tumor seeds and normalizing the growing soil strategy. Nano Lett. 2020;20(9):6272–6280. doi: 10.1021/acs.nanolett.0c01415. [DOI] [PubMed] [Google Scholar]
- 99.Mao C., Yeh S., Fu J., et al. Delivery of an ectonucleotidase inhibitor with ROS-responsive nanoparticles overcomes adenosine-mediated cancer immunosuppression. Sci Transl Med. 2022;14(648) doi: 10.1126/scitranslmed.abh1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao Q., Li X., Liu C., et al. Improving cancer immunotherapy via co-delivering checkpoint blockade and thrombospondin-1 downregulator. Acta Pharm Sin B. 2023;13(8):3503–3517. doi: 10.1016/j.apsb.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu Y., Xiong J., Sun X., Gao H. Targeted nanomedicines remodeling immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Acta Pharm Sin B. 2022;12(12):4327–4347. doi: 10.1016/j.apsb.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y.T., Sun Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365–5386. doi: 10.7150/thno.58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qin Y.-T., Liu X.-H., An J.-X., et al. Dendritic cell-based in situ nanovaccine for reprogramming lipid metabolism to boost tumor immunotherapy. ACS Nano. 2023;17(24):24947–24960. doi: 10.1021/acsnano.3c06784. [DOI] [PubMed] [Google Scholar]
- 104.Inamdar S., Suresh A.P., Mangal J.L., et al. Rescue of dendritic cells from glycolysis inhibition improves cancer immunotherapy in mice. Nat Commun. 2023;14(1):5333. doi: 10.1038/s41467-023-41016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y., Song Z., Zhang Z., et al. Preparation and characterization of pickering emulsion stabilized by lovastatin nanoparticles for vaccine adjuvants. Int J Pharm. 2024;653 doi: 10.1016/j.ijpharm.2024.123901. [DOI] [PubMed] [Google Scholar]
- 106.Yang J., Pan X., Zhang J., et al. Reprogramming dysfunctional dendritic cells by a versatile metabolism nano-intervenor for enhancing cancer combinatorial immunotherapy. Nano Today. 2022;46 [Google Scholar]
- 107.Ho P.C., Bihuniak J.D., Macintyre A.N., et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162(6):1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y., Kurupati R., Liu L., et al. Enhancing CD8(+) T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32(3):377–391. doi: 10.1016/j.ccell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim D., Wu Y., Li Q., Oh Y.K. Nanoparticle-mediated lipid metabolic reprogramming of T cells in tumor microenvironments for immunometabolic therapy. Nanomicro Lett. 2021;13(1):31. doi: 10.1007/s40820-020-00555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hao M., Hou S., Li W., et al. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Sci Transl Med. 2020;12(571):eaaz6667. doi: 10.1126/scitranslmed.aaz6667. [DOI] [PubMed] [Google Scholar]
- 111.Masjedi A., Ahmadi A., Ghani S., et al. Silencing adenosine A2a receptor enhances dendritic cell-based cancer immunotherapy. Nanomedicine. 2020;29 doi: 10.1016/j.nano.2020.102240. [DOI] [PubMed] [Google Scholar]
- 112.Siriwon N., Kim Y.J., Siegler E., et al. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol Res. 2018;6(7):812–824. doi: 10.1158/2326-6066.CIR-17-0502. [DOI] [PubMed] [Google Scholar]
- 113.Yuan J., Weng Z., Tan Z., et al. Th1-involved immune infiltrates improve neoadjuvant chemoradiotherapy response of esophageal squamous cell carcinoma. Cancer Lett. 2023;553 doi: 10.1016/j.canlet.2022.215959. [DOI] [PubMed] [Google Scholar]
- 114.Laidlaw B.J., Craft J.E., Kaech S.M. The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat Rev Immunol. 2016;16(2):102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quezada S.A., Simpson T.R., Peggs K.S., et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chalmin F., Mignot G., Bruchard M., et al. Stat 3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36(3):362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 117.Cassetta L., Pollard J.W. Tumor-associated macrophages. Curr Biol. 2020;30(6):R246–R248. doi: 10.1016/j.cub.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 118.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gu Z., Liu T., Liu C., et al. Ferroptosis-strengthened metabolic and inflammatory regulation of tumor-associated macrophages provokes potent tumoricidal activities. Nano Lett. 2021;21(15):6471–6479. doi: 10.1021/acs.nanolett.1c01401. [DOI] [PubMed] [Google Scholar]
- 120.Guo Q., Li X., Zhou W., et al. Sequentially triggered bacterial outer membrane vesicles for macrophage metabolism modulation and tumor metastasis suppression. ACS Nano. 2021;15(8):13826–13838. doi: 10.1021/acsnano.1c05613. [DOI] [PubMed] [Google Scholar]
- 121.Yang P., Qin H., Li Y., et al. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat Commun. 2022;13(1):5782. doi: 10.1038/s41467-022-33349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu Y., Yi M., Niu M., et al. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer. 2022;21(1):184. doi: 10.1186/s12943-022-01657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nefedova Y., Fishman M., Sherman S., et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67(22):11021–11028. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 124.Zheng A., Xie F., Shi S., et al. Sustained drug release from liposomes for the remodeling of systemic immune homeostasis and the tumor microenvironment. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.829391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deng H., Yang W., Zhou Z., et al. Targeted scavenging of extracellular ROS relieves suppressive immunogenic cell death. Nat Commun. 2020;11(1):4951. doi: 10.1038/s41467-020-18745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu Y., Liu H., Bi Y., et al. Glucocorticoid receptor promotes the function of myeloid-derived suppressor cells by suppressing HIF1α-dependent glycolysis. Cell Mol Immunol. 2018;15(6):618–629. doi: 10.1038/cmi.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hossain F., Al-Khami A.A., Wyczechowska D., et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015;3(11):1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muranski P., Restifo N.P. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121(13):2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin-Orozco N., Muranski P., Chung Y., et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xiang T., Long H., He L., et al. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene. 2015;34(2):165–176. doi: 10.1038/onc.2013.537. [DOI] [PubMed] [Google Scholar]
- 131.Liu J., Duan Y., Cheng X., et al. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407(2):348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 132.Punt S., van Vliet M.E., Spaans V.M., et al. FoxP3(+) and IL-17(+) cells are correlated with improved prognosis in cervical adenocarcinoma. Cancer Immunol Immunother. 2015;64(6):745–753. doi: 10.1007/s00262-015-1678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tekguc M., Wing J.B., Osaki M., et al. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. 2021;118(30) doi: 10.1073/pnas.2023739118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cluxton D., Petrasca A., Moran B., Fletcher J.M. Differential regulation of human Treg and Th17 cells by fatty acid synthesis and glycolysis. Front Immunol. 2019;10:115. doi: 10.3389/fimmu.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y., Huang T., Gu J., Lu L. Targeting the metabolism of tumor-infiltrating regulatory T cells. Trends Immunol. 2023;44(8):598–612. doi: 10.1016/j.it.2023.06.001. [DOI] [PubMed] [Google Scholar]
- 136.Downs-Canner S., Berkey S., Delgoffe G.M., et al. Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+) T(reg) cells are a source of tumour-associated T(reg) cells. Nat Commun. 2017;8 doi: 10.1038/ncomms14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zang S., Huang K., Li J., et al. Metabolic reprogramming by dual-targeting biomimetic nanoparticles for enhanced tumor chemo-immunotherapy. Acta Biomater. 2022;148:181–193. doi: 10.1016/j.actbio.2022.05.045. [DOI] [PubMed] [Google Scholar]
- 138.Ai C., Sun X., Xiao S., et al. CAFs targeted ultrasound-responsive nanodroplets loaded V9302 and GLULsiRNA to inhibit melanoma growth via glutamine metabolic reprogramming and tumor microenvironment remodeling. J Nanobiotechnology. 2023;21(1):214. doi: 10.1186/s12951-023-01979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y., Fang Z., Pan D., et al. Dendritic polymer-based nanomedicines remodel the tumor stroma: improve drug penetration and enhance antitumor immune response. Adv Mater. 2024;36(25) doi: 10.1002/adma.202401304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.