Abstract
This study aimed to evaluate the safety and efficacy of pharmacomechanical catheter-directed thrombolysis (PCDT) and stenting for treating acute iliofemoral deep venous thrombosis (DVT) combined with iliac vein compression syndrome (IVCS), and to identify the predictors of stent restenosis. Patients with acute proximal DVT combined with IVCS underwent PCDT and stenting from January 2017 to December 2022 were enrolled. Primary and secondary patency were assessed by duplex ultrasound (DUS). The morbidity of postthrombotic syndrome (PTS) was assessed by the Villalta score. Risk factors for stent restenosis were assessed using univariate and multivariate Cox regression models. Total of 254 patients were included. The mean follow-up time was 36.06 ± 17.66 months. The primary patency rates at 1 year, 3 years, and 5 years were 92.5%±1.7%, 85.4%±2.4%, and 82.4%±2.9%, respectively. The incidence of stent restenosis was 14.2%. Discontinuation of anticoagulants within one year [hazard ratio (HR) = 5.03; P = .048] was the factor associated with acute in-stent thrombosis. Previous DVT history (HR =2.29; P = .037) and stent placement across the inguinal ligament (HR =6.70; P < .001) were identified as independent risk factors significantly associated with stent restenosis. The overall PTS rate was 19.3%. PCDT with stenting is safe and effective for patients with iliofemoral DVT secondary to IVCS, leading to low rates of PTS. Previous DVT history and stents placed across the inguinal ligament may be predictors of stent restenosis. Furthermore, stent restenosis typically occurs within one year and is mainly caused by acute thrombosis due to discontinuation of anticoagulants.
Keywords: pharmacomechanical catheter-directed thrombolysis, iliofemoral deep venous thrombosis, iliac vein compression syndrome, stent restenosis, postthrombotic syndrome
Introduction
Deep vein thrombosis (DVT) is a common disease, with an annual incidence of approximately 1 case per 1000 persons. 1 Iliac vein compression syndrome (IVCS) has been identified as a risk factor associated with an increased occurrence of DVT. Postthrombotic syndrome (PTS), pulmonary embolism (PE), and phlegmasia cerulea dolens (PCD) arise as comorbidities of DVT combined with iliac compression, resulting in heightened rates of mortality and disability, alongside a decline in overall quality of life.2,3 In addition to anticoagulation, early endovascular therapy has progressively emerged as the primary approach for decreasing these comorbidities. 4
As described by the Clinical Practice Guidelines of the Society for Vascular Surgery and the American Venous Forum, pharmacomechanical catheter-directed thrombolysis (PCDT) is commonly employed in patients with acute iliofemoral DVT.5,6 PCDT serves as an early thrombus removal strategy, contributing to the alleviation of symptoms, restoration of venous patency, and mitigation of the occurrence of PTS. Following thrombolysis, balloon angioplasty and stent placement are commonly performed to address remaining venous stenosis or IVCS for long-term efficacy.6–15 However, previous studies have lacked large sample sizes or long follow-up times. To further prove the efficacy of this treatment strategy, we carried out a retrospective study spanning five years with a median follow-up of three years to evaluate the outcomes of PCDT and stenting in the treatment of acute iliofemoral DVT.
Method
Patient Selection
This retrospective study was reviewed and approved by the institutional review board, which waived the requirement for informed consent, and performed in accordance with the principles of the Declaration of Helsinki.
Our study enrolled patients who were objectively diagnosed with DVT combined with IVCS and treated with PCDT and stenting. From January 2017 to December 2022, 1051 patients with swelling and pain in the leg (symptoms within 14 days) were treated in our hospital. Acute DVT was confirmed in 977 patients by duplex ultrasound (DUS) examination. The presence of IVCS and the accurate extent of thrombosis were further confirmed by computed tomography venography (CTV), as shown in Figure 1. A total of 289 patients were diagnosed with iliofemoral DVT combined with IVCS. Patients with bilateral lower limb lesions, contraindications for intervention, and propensity of conservative treatment were not included in our study. Ultimately, 265 patients underwent PCDT with stenting. During the follow-up process, 3 patients were lost to follow-up, and 8 patients died due to diseases other than PE. A detailed study flow diagram is shown in Figure 2.
Figure 1.
Computed tomography venography (CTV) demonstrated DVT combined with compression of the left common iliac vein by the right common iliac artery in the coronal (A), transverse (B), and sagittal (C) plane images. DVT, deep venous thrombosis.
Figure 2.
Consolidated standards of reporting trials diagram of this retrospective study. DUS, duplex ultrasound; CTV, Computed tomography venography; DVT, deep venous thrombosis; IVCS, iliac vein compression syndrome; PCDT, pharmacomechanical catheter-directed thrombolysis.
Procedure
Patients received standard anticoagulation treatment with either low-molecular-weight heparin or an oral anticoagulant on the day of diagnosis. 16 With the patients in the supine position and after local anesthesia, the contralateral common femoral vein was punctured by the Seldinger method with the placement of a 6F sheath (Cordis, Fremont, Calif). According to the Chinese guideline, retrievable inferior vena cava (IVC) filters were placed to prevent PE before pharmacomechanical thrombolysis (PMT). 17 The ipsilateral popliteal vein or tibial vein was punctured, and the extent of the thrombus was visualized under digital subtraction angiography with the injection of contrast medium. Under the direction of the C2 or multi-function catheter, the guidewire was inserted until reaching the IVC. Following the guidewire, an AngioJet catheter (Boston Scientific, Marlborough, Mass) was introduced into the thrombus in the iliac common femoral vein. PMT was performed with a power pulse lytic model and an initial administration of urokinase (150 000 to 200 000 IU) into the thrombus. After waiting for 15 min to allow the pharmacologic thrombolytic effect to take place, the AngioJet catheter was switched to the rheolytic thrombectomy mode. Venography was performed to identify the thrombus burden, and the procedure was repeated if significant residual thrombus remained. After repeated aspiration, 3D imaging with an Allura 3D-RA Xper (Philips) system was used to evaluate the residual stenosis. If stenosis > 50% (residual thrombus or extrinsic compression) was identified by intraoperative venography, the stenotic iliofemoral vein was dilated with a balloon catheter and stented with the S.M.A.R.T. Control (Cordis) or Wallstent (Boston Scientific). (Figure 3) If there was little residual thrombus left, antegrade thrombolysis through the distal popliteal or tibial vein with urokinase (total 600 000 to 1 200 000 U/d) was continuously performed. The intravenous infusion was simultaneously conducted to administer unfractionated heparin, and the unfractionated heparin dose was modified to increase the activated partial thromboplastin time by 1.2 to 1.5 times. Venography was performed daily during lysis to check the results. The concomitant administration of antithrombotic agents other than unfractionated heparin ceased during catheter-directed thrombolysis (CDT). The timing to discontinue thrombolysis is specifically guided by venography findings and changes in coagulation indices. The Venous Registry Index (VRI) was used to assess the occlusion status of each segment of the vascular lumen preoperatively and postoperatively. 6 Subsequently, the IVC filters were removed in 3 to 7 days. Postoperatively, patients were recommended to continue standard anticoagulation therapy with rivaroxaban at a dosage of 15 mg twice daily for the initial 21 days, followed by a daily dosage of 20 mg for more than one year, while necessitating periodic evaluation of the potential for bleeding complications.18,19
Figure 3.
Intraoperative 3D imaging before stent placement: Anteroposterior view (A); Left anterior oblique 90 degrees (LAO) (B); Right anterior oblique 90 degrees (RAO) (C). Intraoperative 3D imaging after stent placement: Anteroposterior view (D); Left anterior oblique 90 degrees (LAO) (E); Right anterior oblique 90 degrees (RAO) (F). X-per CT based on intraoperative 3D imaging showed a small caliber left common iliac vein. (G) Post-stenting imaging (H) showed restoration of lumen of the left common iliac vein.
Follow-up
Patients were followed up at 3 months, 6 months, 12 months, and annually thereafter. The primary endpoints were primary stent patency and risk factors for restenosis. The secondary endpoints were secondary stent patency and rates of PTS. DUS was conducted to evaluate the patency of the stent via color flow imaging. Patency was defined as 50% maximal lumen stenosis with antegrade flow and a spontaneous Doppler signal. 20 All ultrasound follow-ups were performed using Intersocietal Accreditation Commission criteria by our specialized vascular ultrasound team of physicians. 21 The assessment of PTS was conducted using the Villalta scores. 22
Statistical Analysis
Statistical analysis was performed with SPSS version (27.0) software. The results are expressed as medians ± standard deviation or numbers. The primary patency, secondary patency, and freedom from target lesion reintervention rates are presented by Kaplan‒Meier curves. Univariate and multivariate analysis Cox regression models were built to judge the factors related to stent restenosis.
Results
Baseline Characteristics
The study included 254 patients with a mean age of 61.57 ± 13.39 years, of who 160 (63.0%) were women. Of all the patients, 228 patients (89.8%) had symptoms on the left side. The average symptom duration was 6.3 ± 4.3 days, with 23 patients (9.1%) also presenting with PE. In the study, 36 patients (14.2%) were identified as smokers, and 24 patients (9.4%) had a history of DVT before. DVT was most frequently observed in both the iliac and femoral veins, accounting for 62.2% (158/254) of patients. The remaining cases were distributed as follows: 9.1% (23/254) occurred solely in the common iliac or external iliac vein, while 28.7% (73/254) extended from the iliac to the popliteal vein. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline Patient Characteristics.
| Variables | |
|---|---|
| Number of patients | 254 |
| Age, years | 61.57 ± 13.39 |
| Gender | |
| Male | 94 (37.0) |
| Female | 160 (63.0) |
| Limb | |
| Left-side | 228 (89.8) |
| Right-side | 26 (10.2) |
| Symptom duration, days | 6.3 ± 4.3 |
| Symptoms of PE | 23 (9.1) |
| Previous DVT History | 24 (9.4) |
| Smoking | 36 (14.2) |
| DVT extent | |
| Common iliac or external vein only | 23(9.1) |
| Both iliac and femoral veins | 158(62.2) |
| Iliac to popliteal vein | 73(28.7) |
Abbreviations: DVT, Deep venous thrombosis; PE, pulmonary embolism.
Categorical variables are presented as numbers (%). Continuous variables are presented as mean ± standard deviation.
Procedural Characteristics
The average duration from diagnosis to initiation of endovascular treatment was 36-48 h. The mean duration of thrombolysis was 54.80 ± 19.06 h, and the mean amount of urokinase was 2.1 ± 0.7 million IU. The VRI decreased from 6.77 ± 1.65 (preoperative) to 0.57 ± 0.50 (postoperative). There were no cases of mortality within 30 days. A minor bleeding complication occurred in 3.5% (9/254) of patients. The emboli were trapped in the IVC filter in 12.2% (31/254) of cases. The average length of stents was 138.3 ± 68.6 mm. A stent diameter of 16, 14, or 12 mm could be selected. The self-expanding Wallstent was utilized exclusively in 59.4% (151/254) of patients, while the S.M.A.R.T. Control stent was employed exclusively in 19.7% (50/254) of patients. The remaining 20.9% (53/254) were treated with a combination of both types of stents. Stent across the inguinal ligament was observed in 38.6% (98/254) of the cases. Small hematomas at the puncture site occurred in 1.6% (4/254) of patients during IVC filter removal. During postoperative anticoagulation, 10.2% (26/254) of cases discontinued anticoagulants on their own. The procedural characteristics are outlined in Table 2.
Table 2.
Procedural Characteristics.
| Variables | |
|---|---|
| Duration of urokinase, hours | 54.80 ± 19.06 |
| Amount of urokinase, million IU | 2.1 ± 0.7 |
| Venous Registry Index (pre- to post-operative) | 6.77 ± 1.65 to 0.57 ± 0.50 |
| Stent placement | |
| Type of stents | |
| Wallstent | 151(59.4) |
| S.M.A.R.T. | 50(19.7) |
| Both of Wallstent and S.M.A.R.T. | 53(20.9) |
| Stent position | |
| Across inguinal ligament | 98(38.6) |
| Not across inguinal ligament | 156(61.4) |
| Length of stents, mm | 138.3 ± 68.6 |
| Across inguinal ligament | 211.8 ± 47.8 |
| Not across inguinal ligament | 92.2 ± 26.6 |
| Complications | |
| Major bleeding | 0 |
| Minor bleeding | 9(3.5) |
| PE syndrome | 0 |
| 30-Day mortality | 0 |
| Emboli trapped in the IVC filter | 31 (12.4) |
Abbreviations: PE, pulmonary embolism; IVC, retrievable inferior vena cava.
Categorical variables are presented as numbers (%). Continuous variables are presented as mean ± standard deviation.
Follow-up Outcomes
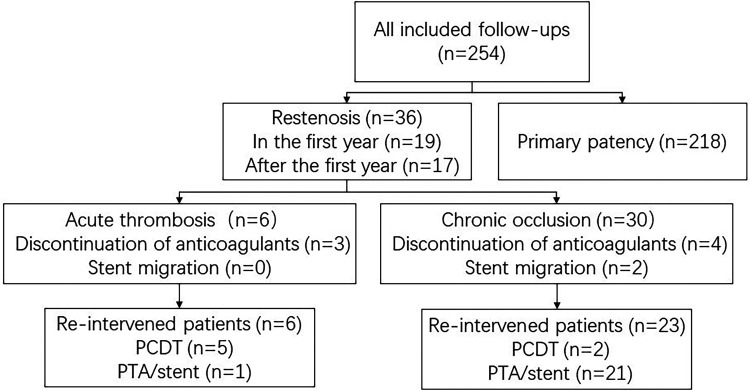
The mean follow-up time was 36.06 ± 17.66 months, and the median follow-up time was 36.00 months (range, 12-72 months). The primary patency rates at 1 year, 2 years, 3 years, 4 years, and 5 years were 92.5%±1.7%, 87.4%±2.2%, 85.4%±2.4%, 82.4%±2.9%, 82.4%±2.9%, respectively. The secondary patency rates at 1 year, 2 years, 3 years, 4 years, and 5 years were 99.6%±0.4%, 98.6%±0.8%, 98.0%±1.0%,97.0%±1.4% and 97.0%±1.4%%, respectively, as shown in Figure 4. The freedom from target lesion reintervention rates at 1 year, 2 years, 3 years, and 4 years, 5 years were 93.3%±1.6%, 90.0%±1.9%, 88.7%±2.1%, 85.5%±2.7%, and 85.5%±2.7%%, respectively, as shown in Figure 5. The incidence of stent restenosis was 14.2% (36/254), with 6 cases of acute thrombosis (2.4%) and 30 cases of chronic occlusion (11.8%). Among the patients, 29 patients with restenosis opted for reintervention; 7 patients were treated with PCDT, while 22 patients were treated with percutaneous transluminal angioplasty (PTA)/stent. Stent migration was observed in two cases with chronic occlusion. (Figure 6) For the remaining 7 patients who chose conservative treatment, most of them exhibited mild symptoms such as lower limb swelling. The overall PTS rate was 19.3% (49/254), while 12.6% (32/254) of patients experienced mild symptoms, and 6.7% (17/254) of patients endured moderate to severe symptoms, including leg swelling, pain, heaviness, pruritus, varicose veins, and ankle ulcers.
Figure 4.
Cumulative Kaplan-Meier estimate of stent patency: primary patency versus secondary patency.
Figure 5.
Plot depicting freedom from target lesion re-intervention (TLR).
Figure 6.
Flowchart restenosis of the included follow-up patients. Pharmacomechanical catheter-directed thrombolysis (PCDT). Percutaneous transluminal angioplasty (PTA).
Univariate and multivariate Cox regression analyses for factors correlated with stent restenosis are presented in Table 3. Discontinuation of anticoagulants [hazard ratio (HR) = 2.49, 95% CI 1.09-5.68, P = .031], previous DVT history (HR =4.05, 95% CI 1.90 −8.63 P < .001), and stent across the inguinal ligament (HR =7.61, 95% CI 3.33-17.39, P < .001) were factors associated with stent restenosis in the univariate analysis. Multivariate Cox regression analysis confirmed that previous DVT history (HR =2.29, 95% CI 1.05-4.99, P = .037) and stent across the inguinal ligament (HR =6.70, 95% CI 2.89-15.54, P < .001) were factors significantly related to stent restenosis. Discontinuation of anticoagulants within one year (HR =5.03, 95% CI 1.01-25.04, P = .048) was the factor associated with acute in-stent thrombosis in the univariate analysis, presented in Table 4.
Table 3.
Univariate and Multivariate Cox Regression Analyses of Factors Associated with Stent Patency.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age(>=65) | 1.38(0.71 −2.65) | .34 | ||
| Male sex | 1.11(0.57-2.17) | .77 | ||
| Previous DVT History | 4.05(1.90 -8.63) | <.001* | 2.29 (1.05-4.99) | .037* |
| Smoking | 1.36(0.56 -3.26) | .50 | ||
| Type of stents | ||||
| Wallstent | 1.0(reference) | |||
| S.M.A.R.T. | 0.74(0.30-1.82) | .52 | ||
| Both of Wallstent and S.M.A.R.T. | 0.64(0.26-1.56) | .64 | ||
| Stent position | ||||
| Across inguinal ligament | 7.61 (3.33-17.39) | <.001* | 6.70 (2.89-15.54) | <.001* |
| Discontinuation of anticoagulants | 2.49 (1.09-5.68) | .031* | 2.24 (0.97-5.19) | 0.060 |
Abbreviations: HR, hazard ratio; CI, confidence interval; DVT, Deep venous thrombosis.
, statistically significant associations.
Table 4.
Univariate Cox Regression Analyses of Factors Associated with Acute in-Stent Thrombosis.
| Variables | Univariate | |
|---|---|---|
| HR (95% CI) | P | |
| Age(≥=65) | 0.83 (0.17-4.10) | .82 |
| Male sex | 0.77 (0.14-4.19) | .76 |
| Previous DVT History | 3.44 (0.70-17.06) | .13 |
| Stent position | ||
| Across inguinal ligament | 1.31 (0.15-11.21) | .81 |
| Discontinuation of anticoagulants | 5.03 (1.01-25.04) | .048* |
Abbreviations: HR, hazard ratio; CI, confidence interval; DVT, Deep venous thrombosis.
, statistically significant associations.
Discussion
Early treatment of acute DVT contributes to mitigating common complications, including PE, PCD, and PTS. Treatment approaches are consistently improved to decrease the incidence of comorbidities. The application of anticoagulants and filters has been suggested for managing potential fatal PE and PCD. The most effective treatment strategies for reducing the incidence of PTS are still under debate. 18 Our previous study indicated that PCDT and stenting are effective for patients with DVT combined with IVCS in the short term. 23 Another study also indicated that the treatment is effective, however, with a sample size of 110 patients. 24 The current study which included 254 patients with a three-year follow-up time further demonstrates the efficacy of this treatment.
Preliminary studies have suggested that iliac vein compression, which is characterized by compression of the left iliac vein by the right common iliac artery and results in the narrowing of the vein in IVCS, leads to stasis and thrombus formation. 25 According to the previous study, approximately one-half to two-thirds of patients with left-sided iliofemoral DVT were combined with IVCS.26,27 Due to the mechanical nature of IVCS, CDT alone is ineffective, as the anatomical stenosis is not relieved. Previous studies have reported that re-thrombosis occurs in up to 40% of patients with a combination of DVT and IVCS who are treated by CDT alone. 28 Without treatment of the underlying obstruction, the rate of acute left iliofemoral DVT recurrence is reportedly 73%. 29 However, it remains a major debate whether PTA and stenting should be performed in the area of outflow from the stenotic region after thrombus clearance. Avgerinos et al underlined the significance of regarding any remaining thrombus as chronic, thereby advocating for stent placement to ensure adequate inflow and outflow. 30 Mewissen et al indicated a positive correlation between enhanced clot lysis and improved vessel patency by quantifying clot lysis levels in patients enrolled in the national venous registry. 31 Additional studies have also demonstrated favorable patency rates associated with the treatment.24,30,32 Furthermore, among patients suffering from chronic venous stasis with iliac vein outflow obstruction, such as those with refractory open ulcers, symptoms improve in 58% of limbs after stent placement. 33 We studied 289 patients combined with IVCS, accounting for 72% of iliofemoral DVT. Of those, 281 patients underwent endovascular therapy (16 in PCDT without stenting, and 265 in PCDT with stenting). The results demonstrated that PCDT with stenting has favorable efficacy in the treatment of IVCS combined with acute DVT.
PTS is regarded as a late complication of lower extremity DVT that is not effectively treated in the acute phase. Once it occurs, the quality of life of patients could be severely affected. In the ATTRACT trial, 34 it was reported that the occurrence of PTS was 47% at 12 months. In parallel, clinical prediction models for PTS have reported a higher incidence of PTS in iliofemoral vein thrombosis.35,36 In our study, PTS was observed in 19.3% of patients, which was far lower than the rate in the ATTRACT trial. This might be attributed to the high rate of stent placement in our research. In the ATTRACT trial, stenting was performed in 28% (82/297) of cases. 37 In our study, stents were implanted in each limb to relieve the obstruction and obtain a better venous outflow tracts.. These results further confirm that PCDT with stenting has an advantage in managing iliofemoral DVT, complementing the results of the ATTRACT trial.
Our study also indicated the highest incidence of stent restenosis within 1 year at 7.5% (19/254), accounting for 52.8% of stent restenosis cases. This suggests that we should appropriately increase the frequency of follow-up within 1 year after stent placement. The main cause of stent restenosis within 1 year may be acute thrombosis associated with discontinuation of anticoagulants. Guidelines recommend continuous anticoagulation for more than 3 months for acute DVT, without providing a specific duration of anticoagulation after stent implantation for IVCS combined with acute DVT. 19 Our study indicated that anticoagulation for more than 1 year after stent placement may be effective in reducing the incidence of acute in-stent thrombosis.
There has been considerable debate as to whether venous stenting across the inguinal ligament affects stent patency rates. The present research revealed a significantly increased incidence of stent restenosis when stents were placed across the inguinal ligament. Stents placed across the inguinal ligament are longer and carry a higher risk of thrombus reformation. Moreover, joint-crossing stents are likely to be compressed, which has been proven to be a risk factor for stent restenosis. 38 Pouncey et al demonstrated a correlation between the necessity for reintervention and stents across the inguinal ligament. 39 However, the validity of this finding has been subject to debate. Several previous studies have indicated no association between stents placed across the inguinal ligament and stent restenosis.40,41 More extended studies will be needed to conclusively demonstrate the correlation between the position and patency of the stent.
Our study also showed that a previous DVT history was associated with an increased risk of stent restenosis. This may be attributed to flow limiting stenoses and incompetent valves. Favorable valve function is an important safeguard for the inflow tract of the stent. According to the “open vein” hypothesis, the obstruction of the venous outflow tract by acute DVT leads to dilation of the venous lumen and disruption of normal venous valve function. These changes can result in inflammation, venous hypertension, and chronic venous insufficiency, which are crucial factors in the development of PTS and contribute to a higher incidence of restenosis. 42
Limitations
Our study has several limitations. First, as a single-center retrospective study, there was inherent bias in the selection of patients. Second, intravascular ultrasound (IVUS), which is considered the most accurate method for diagnosing IVCS, was not available for our study. 39 Preoperatively, we initially assessed patients for IVCS. After completion of thrombus removal, we performed intraoperative 3D imaging with an Allura 3D-RA Xper system to confirm the diagnosis of IVCS and measure the degree of stenosis. The results are generally consistent with those found by CTV. Although CTV and intraoperative 3D imaging are reliable, IVUS is still the most accurate assessment method. Third, our study lacked a control group because the number of patients in our study who chose conservative treatment or PCDT without stenting was too small to set up a control group. Fourth, the S.M.A.R.T. Control stent used in the present study was not a dedicated nitinol venous stent. This may have had an impact on the results, even though such stents have been used by previous investigators to treat IVCS. Finally, according to our guidelines, all patients underwent intraoperative implantation of filters. However, this procedure lacks guidance from global guidelines.
Conclusion
PCDT combined with stenting is a safe and effective treatment strategy for patients with iliofemoral DVT secondary to IVCS within a median 3-year follow-up time and results in a low incidence of PTS. Stent restenosis typically occurs within one year mainly caused by acute thrombosis due to discontinuation of anticoagulants. Previous DVT history and stent across the inguinal ligament may be predictors of stent restenosis.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Research Ethics and Patient Consent: The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (no. K2023-002) on January 6, 2023, with the need for written informed consent waived.
ORCID iD: Fenghe Li https://orcid.org/0009-0007-5548-9404
References
- 1.Monreal M, Agnelli G, Chuang LH, et al. Deep vein thrombosis in Europe—health-related quality of life and mortality. Clin Appl Thromb Hemost. 2019 Jan-Dec;25:1076029619883946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins JMT, Magee TR, Galland RB. Phlegmasia caerulea dolens and venous gangrene. Br J Surg. 1996;83(1):19-23. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SR. The post-thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2016;2016(1):413-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalcante LP, dos Santos Souza JE, Pereira RM, et al. Iliac vein compression syndrome: Literature review. J Vasc Bras. 2015;14(1):78-83. [Google Scholar]
- 5.Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: Clinical practice guidelines of the society for vascular surgery and the American venous forum. J Vasc Surg. 2012;55(5):1449-1462. [DOI] [PubMed] [Google Scholar]
- 6.Vedantham S, Grassi CJ, Ferral H, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2009;20(7):S391-S408. [DOI] [PubMed] [Google Scholar]
- 7.Comerota AJ, Kearon C, Gu C-S, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139(9):1162-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ming Z-B, Li W-D, Yuan R-F, Li X-Q, Ding W-B. Effectiveness of catheter directed thrombolysis and stent implantation on iliofemoral vein thrombosis caused by iliac vein compression. J Thromb Thrombolysis. 2017;44(2):254-260. [DOI] [PubMed] [Google Scholar]
- 9.Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg. 2013;57(4):1163-1169. [DOI] [PubMed] [Google Scholar]
- 10.Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: Systematic review and meta-analysis. Circ Cardiovasc Interv. 2015 Oct;8(10):e002772. [DOI] [PubMed] [Google Scholar]
- 11.Ao A, Me B OA, Cha W HJ. The role of May-Thurner syndrome in recurrent thrombosis after catheter-directed thrombolysis of deep vein thrombosis. Ann Vasc Surg. 2019;54. doi: 10.1016/j.avsg.2018.05.067 [DOI] [PubMed] [Google Scholar]
- 12.Jin W, Yu G, Huang J, Lu K, Huang C. Timing of endovascular interventions for iliac vein compression syndrome with thrombus. Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211026974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sogomonian R, Gonzalez-Lengua CA, Hanumanthu BK, et al. Early and mid-term outcomes of femoro-ilio-caval vein stent implantation. J Invasive Cardiol. 2021 Jul;33(7):E497-E505. [DOI] [PubMed] [Google Scholar]
- 14.Hartung O, Loundou AD, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Endovascular management of chronic disabling ilio-caval obstructive lesions: Long-term results. Eur J Vasc Endovasc Surg. 2009;38(1):118-124. [DOI] [PubMed] [Google Scholar]
- 15.Kahn SR, Julian JA, Kearon C, et al. Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2020 Jan;8(1):8-23.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakkos SK, Gohel M, Baekgaard N, et al. Editor’s choice – European society for vascular surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61(1):9-82. [DOI] [PubMed] [Google Scholar]
- 17.Vascular Surgery Group, Surgery Branch, Chinese Medical Association. Guidelines for the diagnosis and treatment of deep venous thrombosis (3rd edition). Chinese Journal of General Surgery. 2017;32(9):807-812. [Google Scholar]
- 18.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. [DOI] [PubMed] [Google Scholar]
- 19.Twine CP, Kakkos SK, Aboyans V, et al. Editor’s choice – European society for vascular surgery (ESVS) 2023 clinical practice guidelines on antithrombotic therapy for vascular diseases. Eur J Vasc Endovasc Surg. 2023;65(5):627-689. [DOI] [PubMed] [Google Scholar]
- 20.Lim MNHH, Damodharan K, Chan SL, et al. Endovascular deep vein stenting of symptomatic post-thrombotic and non-thrombotic iliac vein stenotic lesions: A multicentre cohort experience from Singapore. Ann Acad Med Singap. 2020;49(8):551-560. [PubMed] [Google Scholar]
- 21.IAC Standards and Guidelines for Vascular Testing Accreditation (2024). 2024.
- 22.Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. Journal of thrombosis and haemostasis : JTH. 2009;7. doi: 10.1111/j.1538-7836.2009.03294.x [DOI] [PubMed] [Google Scholar]
- 23.Jiang C, Zhao Y, Wang X, Liu H, Tan T-W, Li F. Midterm outcome of pharmacomechanical catheter-directed thrombolysis combined with stenting for treatment of iliac vein compression syndrome with acute iliofemoral deep venous thrombosis. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2020. Jan;8(1):24-30. [DOI] [PubMed] [Google Scholar]
- 24.Shi W-Y, Gu J-P, Liu C-J, He X, Lou W-S. Endovascular treatment for iliac vein compression syndrome with or without lower extremity deep vein thrombosis: A retrospective study on mid-term in-stent patency from a single center. Eur J Radiol. 2016;85(1):7-14. [DOI] [PubMed] [Google Scholar]
- 25.Fraser DGW, Moody AR, Martel A, Morgan PS. Re-evaluation of iliac compression syndrome using magnetic resonance imaging in patients with acute deep venous thromboses. J Vasc Surg. 2004;40(4):604-611. [DOI] [PubMed] [Google Scholar]
- 26.Shebel ND, Whalen CC. Diagnosis and management of iliac vein compression syndrome. J Vasc Nurs. 2005;23(1):10-17. [DOI] [PubMed] [Google Scholar]
- 27.Harbin MM, Lutsey PL. May-thurner syndrome: History of understanding and need for defining population prevalence. J Thromb Haemostasis. 2020;18(3):534-542. [DOI] [PubMed] [Google Scholar]
- 28.Murphy EH, Davis CM, Journeycake JM, DeMuth RP, Arko FR. Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May-Thurner syndrome. J Vasc Surg. 2009;49(3):697-703. [DOI] [PubMed] [Google Scholar]
- 29.Oguzkurt L, Ozkan U, Ulusan S, Koc Z, Tercan F. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19(3):366-370. quiz 371. [DOI] [PubMed] [Google Scholar]
- 30.Avgerinos ED, Saadeddin Z, Abou Ali AN, et al. Outcomes and predictors of failure of iliac vein stenting after catheter-directed thrombolysis for acute iliofemoral thrombosis. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2019 Mar;7(2):153-161. [DOI] [PubMed] [Google Scholar]
- 31.Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: Report of a national multicenter registry. Radiology. 1999;211(1):39-49. [DOI] [PubMed] [Google Scholar]
- 32.Pouncey AL, Gwozdz AM, Johnson OW, et al. Angiojet pharmacomechanical thrombectomy and catheter directed thrombolysis vs. Catheter directed thrombolysis alone for the treatment of iliofemoral deep vein thrombosis: A single centre retrospective cohort study. Eur J Vasc Endovasc Surg. 2020;60(4):578-585. [DOI] [PubMed] [Google Scholar]
- 33.Iliac-Femoral venous stenting for lower extremity venous stasis symptoms. Ann Vasc Surg. 2012;26(2):185-189. [DOI] [PubMed] [Google Scholar]
- 34.Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabinovich A, Ducruet T, Kahn SR, et al. Development of a clinical prediction model for the postthrombotic syndrome in a prospective cohort of patients with proximal deep vein thrombosis. J Thromb Haemostasis. 2018;16(2):262-270. [DOI] [PubMed] [Google Scholar]
- 36.Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698-707. [DOI] [PubMed] [Google Scholar]
- 37.Kearon C, Gu C-S, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis in acute femoral-popliteal deep vein thrombosis: Analysis from a stratified randomized trial. Thromb Haemost. 2019;119(04):633-644. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Zhao Y, Chen Z, et al. The effect of stent compression on in-stent restenosis and clinical outcomes in iliac vein compression syndrome. Quant Imaging Med Surg. 2021 Jun;11(6):2245-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pouncey AL, Kahn T, Morris RI, Saha P, Thulasidasan N, Black SA. Risk factors and classification of reintervention following deep venous stenting for acute iliofemoral deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1051-1058.e3. [DOI] [PubMed] [Google Scholar]
- 40.Blanch Alerany M, Izquierdo Lamoca LM, Ramirez Ortega M, Lago Rivas I, Zotta Desboeufs R, Stefanov Kiuri S. Endovascular treatment of iliofemoral chronic post-thrombotic venous flow obstruction. J Vasc Surg Venous Lymphat Disord. 2014;2(1):2-7. [DOI] [PubMed] [Google Scholar]
- 41.Neglén P, Tackett TP, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg. 2008;48(5):1255-1261. [DOI] [PubMed] [Google Scholar]
- 42.Aday AW, Beckman JA. The open vein hypothesis and postthrombotic syndrome: Not dead yet. Circulation. 2021;143(12):1239-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]