Abstract
Background:
Type 2 diabetes, a lifestyle-related disease demanding daily self-management, is a significant health concern. In this context, the use of telemedicine as a management tool is a relatively new and promising approach. This study aims to contribute to the growing body of knowledge by identifying the effectiveness of telemedicine in managing type 2 diabetes through a systematic review approach.
Methods:
Four databases were searched including PubMed, Virtual Health Library, Global Health Library, and Google Scholar on 27 July 2022. Additionally, a manual search was performed to identify any relevant articles that may have been missed. The quality of the included articles was rigorously assessed using the Study Quality Assessment Tools of the National Institute of Health.
Results:
We analyzed data from 134 articles. All 134 studies were published between 2002 and 2022, including 103 controlled intervention trials, 13 cohort studies, 7 before–after (pre–post) studies with no control group, 1 initial trial, 1 case study, 1 pilot study, and 8 two-arm studies that did not report the study design. Accordingly, most studies show positive changes in glycemic index in every group using telemedicine. Overall, although the BMI and weight indices in the studies improved at the end of the course, the improvement values were considered insignificant.
Conclusion:
Telemedicine may be a valuable solution for blood sugar management in patients with type 2 diabetes. However, the effectiveness of telemedicine in improving BMI and quality of life is unclear.
Keywords: Telemedicine, systematic review, type 2 diabetes, effectiveness, management
Introduction
The world is witnessing an increasing number of people having diabetes, in which type 2 diabetes is the predominant case. It is estimated that approximately 1.31 billion people will have diabetes by 2050, with patient groups mainly from North Africa, Middle East, Latin America and Caribbean. 1 WHO defines diabetes as a chronic metabolic disease characterized by elevated levels of blood glucose (or blood sugar), which leads over time to severe damage to the heart, blood vessels, eyes, kidneys, and nerves. 2 Challenges in preventing and controlling type 2 diabetes are still affecting all income levels. The government in the United States spent roughly 237 billion dollars in direct health care costs, and this spending amount of money will become a burden for society. 3
A new technology called Telemedicine—a delivery of health-related services and information via telecommunications technologies—could be a method to address these challenges. This allows clinical services to leverage information technologies, video imaging, and telecommunication linkages to enable doctors to provide healthcare services at a distance by using two-way video, smartphones, wireless tools, and other telecommunications technology. 4 Throughout the decade, telemedicine has been applied in many countries with different approaches. One such approach was installing an integrated app “Mobile Health” on patients’ electronic devices. 5 With this installed application, healthcare professionals or providers can collect data, monitor lifestyle, and evaluate the effectiveness of treatment outcomes. Moreover, patients can immediately contact emergency physicians or nurses or request a health status update.
However, there exist various reported regarding the effectiveness of telemedicine. With the American Diabetes Association (ADA)’s emphasis on person-centered team care combined with a long-term treatment approach for diabetes, applying digital interventions (also known as technology applications in treatment) is generally considered to be fully adaptable to different functions for patient care, especially for type 2 diabetes patients. 3 To be more specific, the ADA guidelines in 2023 recommend that telehealth should be used as a complementary method to optimize glycemic management in people with uncontrolled diabetes. Evidence suggests that various telehealth approaches may improve HbA1c in type 2 diabetes compared with usual care. 3 However, irregular data and applications to different populations suggest a potential gap in outcomes for telehealth intervention. 3 This systematic review aims to evaluate telemedicine’s effectiveness in managing type 2 diabetes comprehensively.
Methods
Protocol and registration
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Checklist 6 (Supplemental Table S1). Our protocol was registered at PROSPERO with ID number CRD42022351941.
Eligibility criteria
We selected original studies published in English that reported the effectiveness of telemedicine in the management of type 2 diabetes. No restrictions were made for publication year or the type of publication. We excluded materials that are not original articles such as thesis, book chapters, editorials, author responses, posters, letters, conference papers, reviews, and patents; abstracts only or studies with limited access; studies unrelated to telemedicine care; studies unrelated to illness such as the introduction of telemedicine techniques; studies that did not report of any glycemic index (HbA1c, FBG, . . .); studies with populations other than patients with type 2 diabetes.
Information sources and search strategies
Four databases, including PubMed, Virtual Health Library (VHL), Global Health Library (GHL), and Google Scholar, were searched on 27 July 2022. The search was completed in 1 day. A manual search was applied to find more relevant articles. Study Quality Assessment Tools (SQAT) of the National Institute of Health assessed the quality of included articles. The research uses a research object filter and a language filter to select research articles with the subject human, and written in English. A manual search using the references of included studies was performed to find more relevant studies. The search terms are provided in Supplemental Table S2.
Study selection
Search results were imported into Endnote X8.1 (Thomson Reuters, CA, USA) to delete duplicates automatically. We selected articles in two phases: 1. title and abstract screening of all searched articles; 2. full-text screening and selecting articles. Two independent reviewers completed these two stages of selection according to our inclusion and exclusion criteria. Any disagreement was discussed among two reviewers to reach a final decision.
Data collection process and data items
We created an extract form in the spreadsheet editor to extract all included articles. The extracted data included basic information (such as author, publication year, country, study design, and follow-up period), population baseline characteristics, descriptions of interventions, and outcomes. For baseline population characteristics, the extracted information should include population description, number of patients, age, sex, HbA1c, BMI, and diabetes duration. We describe telemedicine models based on information such as intervention setting (community-based, primary care-based, or hospital setting-based), medium of communication used (short message service, telephone, web-based, mobile phone app, or video conferencing system), telemedicine strategies (teleconsultation, tele-education, tele-case management, telemonitoring, or telementoring) were collected from the intervention section in the methods section of each article. For example, one article could report patterns in different intervention sites and use multiple telemedicine strategies and communication methods. Our primary outcome is the effectiveness of the patient’s blood sugar control expressed by a glycemic index such as (HbA1c, FBG, . . .). Weight control and patients’ quality of life are also included in the results.
Risk of bias in individual studies
The quality of the selected studies was assessed for risk of bias by two independent reviewers using the Study Quality Assessment Tools (SQAT) 7 of the National Institute of Health. Each item was rated NO for potential flaws or YES for good practice. Additionally, we followed SQAT’s instructions to categorize “NA” (not applicable), “NR” (not reported), or “CD” (cannot be determined). These notations were used for ambiguous fields when our investigators were unsure how scores should be allotted, suggesting caution to others when adopting data from those studies. Each item would receive equal points in the final percentage calculation. The scoring cut-off at 75% or above of the total points places the article as having “good” quality, anything between 75% and 43% is “fair” and articles that are 43% or below are considered “poor” quality.
Results
Systematic search, study selection, and study characteristics
As a result, 1221 articles were identified from 4 databases. After excluding all duplicates by Endnote X8.1, 792 articles had potentially relevant articles. The selection of titles and abstracts resulted in 177 articles, subsequently analyzed as full texts by the reviewers. After excluding studies that did not meet the inclusion criteria and adding 47 articles from the manual search, 134 articles were eligible for systematic review Figure 1. All 134 studies were published between 2002 and 2022, including 103 controlled intervention trials, 13 cohort studies, 7 before–after (pre–post) studies with no control group, 1 initial trial, 1 case study, 1 pilot study, and 8 two-arm studies that did not report the study design.
Figure 1.
PRISMA flow diagram of study selection.
Among the 134 eligible studies, 49 were from the United States, 26 from Korea, 8 from China, 7 from Australia, 6 from United Kingdom, 3 from Canada, 3 from India, 3 from Italy, 3 from Iran, 3 from Malaysia, 2 from Denmark, 2 from Germany, 2 from Ireland, 2 from Poland, 2 from Taiwan, 2 from Spain, 1 from Belgium, 1 from Brazil, 1 from Finland, 1 from France, 1 from Indonesia, 1 from Iraq, 1 from Japan, 1 from Norway, 1 from Singapore, 1 from Slovenia, and 1 from Turkey. All 134 studies were published between 2002 and 2022, with intervention settings including community-based (50 studies), primary care (38 studies), hospital (30 studies), community-based and primary care (7 studies), community-based and hospital (2 studies), primary care and hospital (5 studies), and all three intervention settings (2 studies). A summary of the characteristics of the studies included is depicted in Table 1. Risk of bias and methodological quality of included studies.
Table 1.
Characteristics of study participants in included studies.
| Author, year, country | Study design | Follow-up period | Population description | Intervention | Conjtrol | Baseline characteristics of patients (mean ± SD) | Outcome included in review | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention setting (Community-based = 1, Primary care based = 2, Hospital setting based = 3) | Medium of communication used (Short message service = 1, Telephone = 2, Web-based = 3, Mobile phone app = 4, Video conferencing system = 5) | Telemedicine strategies (Teleconsultation = 1, Tele-education = 2, Telecase-management = 3, Telemonitoring = 4, Telementoring = 5) | N patients | Age (years) | Male sex (n or %) | BMI (kg/m2) | HbA1c | Diabetes duration | ||||||
| Hansel et al., 2017, France 8 | Randomized trial | 4 months | Patients with abdominal obesity and T2DM, HbA1c >5.6% and <8.5% | 3 | 3 | 125 | Usual care | 60c, 60i | 57 ± 9 | 40 (33.3%) | 33 ± 4 | 7.2 ± 1.1 | no data | HbA1c, body weight |
| Kim and Jeong, 2007, South Korea 9 | A control group pretest–posttest design | 6 months | Patients with T2DM | 3 | 13 | 234 | Usual care | 26c, 25i | 47.5 ± 9.1c, 46.8 ± 8.8i | 11c, 11i | 23.4 ± 2.5c, 24.5 ± 2.9i | 7.59 ± 1.09c, 8.09 ± 1.72i | 8.0 ± 4.9c, 5.2 ± 5.9i | HbA1c, FBG, 2HPMG |
| Basudev et al., 2016, United Kingdom 10 | Prospective randomized controlled study | 12 months | Patients with T2DM of >1 year duration and HbA1c > 69 mmol/mol (>8.5%) | 1 | 5 | 134 | Usual care | 115c, 93i | 59.3 ± 12.0c, 60.5 ± 12.3i | 68 (59.6%)c, 51 (54.8%)i | 31.4 ± 7.1c, 30.8 ± 6.7i | 10.4 ± 1.4c, 10.2 ± 1.7i | 9.4 ± 5.2c, 10.7 ± 6.8i | HbA1c, BMI |
| Nicolucci et al., 2015, Italy 11 | A randomized, parallel group (1:1), open-label, multicenter study | 12 months | Patients with T2DM HbA1c between 7.5% and 10% | 1 | 12 | 24 | Usual care | 149c, 153i | 57.8 ± 8.9c, 59.1 ± 10.3i | 61.7c, 61.4i | 29.0 ± 5.0c, 28.7 ± 4.6i | 8.0 ± 0.8c, 7.9 ± 0.7i | 8.7 ± 6.2c, .3 ± 6.2i | HbA1c, weight |
| Oh et al., 2003, South Korea 12 | A randomized comparison experimental design | 3 months | Patients with T2DM, HbA1c ⩾ 7% | 3 | 2 | 124 | Usual care | 25c, 25i | 62.0 ± 5.7c, 59.2 ± 7.2i | 36.0%c, 36.0%i | 24.4 ± 2.6c,24.6 ± 2.6i | 8.3 ± 0.9c, 8.8 ± 1.1i | 162.4 ± 8.35c, 158.1 ± 99.3i (months) | HbA1c, FBG, 2HPMG, BMI |
| Stone et al., 2010, USA 13 | Randomized controlled trial | 6 months | Patients with T2DM, HbA1c ⩾ 7.5% | 12 | 123 | 124 | Usual care | 77c, 73i | One-third of the participants in both groups were aged 65 years | The vast majority were male | No data | 9.4 ± 1.4c, 9.6 ± 1.6i | No data | HbA1c, Weight |
| Kim and Oh, 2003, Korea 14 | Randomized controlled trial | 12 weeks | Patients with T2DM, HbA1c ⩾ 7% | 3 | 2 | 245 | Usual care | 16c, 20i | 60.9 ± 5.8c, 59.7 ± 7.3i | 25%c, 35%i | 24.6 ± 2.8c, 24.6 ± 2.8i | 8.2 ± 0.8c 8.8 ± 1.2i | 13.4 ± 7.7c, 14.0 ± 8.9i | HbA1c |
| Khanna et al., 2014, Spanish 15 | Prospective, randomized, open-label trial with blinded endpoint assessment | 12 weeks | Patients with T2DM, HbA1c >8.5% (not being on insulin) and >8% (being on insulin) | 1 | 2 | 124 | Usual care | 37c, 38i | 53 ± 12c, 51 ± 12i | 68%c, 50%i | 33 ± 7c, 35 ± 10i | 8.9 ± 1.3c, 9.2 ± 1.9i | No data | HbA1c, BMI |
| Cho et al., 2017, Korea 16 | A randomized, prospective open trial | 6 months | Patients with T2DM, HbA1c between 7% and 10% | 3 | 3 | 1234 | Usual care | 240c, 244i | 53.4 ± 8.7c, 52.9 ± 9.2i | 63.3%c, 63.5%i | 25.5 ± 3.2c, 25.6 ± 3.4i | 7.81 ± 0.66c, 7.86 ± 0.69i | No data | HbA1c, FBG, Postprandial glucose, BMI |
| Welch et al., 2011, USA 17 | Randomized controlled trial | 12 months | Patients with T2DM, HbA1c >7.5% within the past 3 months but not >14% | 12 | 3 | 2345 | Usual care | 21c, 25i | 57.5 ± 9.5c, 54.4 ± 10.4i | 38.1%c, 32%i | 35.8 ± 14.0c, 33.8 ± 7.8i | 8.5 ± 1.0c, 9.0 ± 1.2i | 13.8 ± 7.7c, 10.3 ± 8.0i | HbA1c, BMI |
| Fortmann et al., 2017, USA 18 | A randomized, nonblinded, parallel groups clinical trial | 6 months | Patients with T2DM, HbA1c ⩾ 7.5% | 2 | 1235 | 245 | Usual care | 63c, 63i | 49.1 ± 10.6c, 47.8 ± 9.0i | 15 (24%)c, 17 (27%)i | 32.2 ± 6.6c, 31.5 ± 6.0i | 9.6 ± 1.4c, 9.5 ± 1.2i | No data | HbA1c, FBS, BMI |
| Yang et al., 2020, Korea 19 | Randomized controlled trial | 3 months | Patients with T2DM, HbA1c between 7% and 10% | 2 | 1234 | 145 | Usual care | 97c, 150i | 60.6 ± 10.2c, 54.1 ± 10.1i | 45 (46%)c, 80 (53.3%)i | 25.7 ± 3.9c, 26.3 ± 3.7i | 7.9 ± 0.8c, 8 ± 0.8i | No data | HbA1C, BMI |
| Wakefield et al., 2014, Missouri 20 | A single-center randomized controlled clinical trial | 3 months | Patients with T2DM, HbA1c ⩾8% | 2 | 3 | 45 | Usual care | 55c, 53i | 62.5 ± 10.9c, 57.7 ± 10.8i | 41%c, 7%i | No data | 7.4 ± 0.18 (n = 53)c, 7.2 ± 0.21 (n = 41)i | No data | HbA1c |
| Egede et al., 2018, USA 21 | A randomized controlled trial | 12 months | Patients with T2DM | 1 | 25 | 124 | Usual care | 47c, 43i | 62.7 ± 3.4c, 63.5 ± 4.9i | 97.9%c, 97.7%i | No data | 7.3 ± 2.0c, 6.9 ± 1.1i | No data | HbA1c |
| Steventon et al., 2014, United Kingdom 22 | A large cluster randomized trial | 12 months | Patients with T2DM | 1 | 3 | 245 | Usual care | 213c, 300i | 66.2 ± 11.9c, 63.9 ± 13.0i | 64.3%c, 3.3%i | 30.3 ± 5.9 (n = 189)c, 31.8 ± 6.6 (n = 245)i | 8.3 ± 1.7c, 8.5 ± 1.8i | No data | HbA1c |
| Duruturk and Özköslü, 2019, Turkey 23 | A double-blind randomized controlled trial | 6 weeks | Patients with T2DM at least 6 months | 3 | 5 | 15 | Usual care | 21c, 23i | 53.04 ± 10.45c, 52.82 ± 11.86i | 14c, 12i | 29.90 ± 4.63c, 32.07 ± 6.51i | 7.57 ± 0.99c, 7.14 ± 0.91i | 5.23 ± 3.36c, 4.89 ± 3.86i | HbA1c |
| Cho et al., 2011, Korea 24 | A randomized controlled trial | 24 weeks | Patients with T2DM, HbA1c between 6% and 10% | 3 | 13 | 4 | Usual care | 41c, 38i | 51 ± 11c, 48 ± 13i | 68%c, 63%i | 24.2 ± 2.1c, 22.8 ± 2.8i | 6.7 ± 0.8c, 6.9 ± 0.9i | 3.3 ± 0.8c, 3.7 ± 1.0i | HbA1c |
| Wakefield et al., 2011, USA 25 | Randomized controlled trial | 12 months | Patients with T2DM | 2 | 123 | 2345 | Usual care | 107c, 93i (High-intensity group) 102i (Low-intensity group) | 67.9 ± 9.9c, 67.8 ± 10i (High-intensity group) 68.4 ± 9.5i (Low-intensity group) | 96c, 99i (High-intensity group) 99i (Low-intensity group) | 33.8 ± 6.9c, 33.1 ± 6.6i (High-intensity group), 33.1 ± 7.3i (Low-intensity group) | 7.2c, 7.1i (High-intensity group) 7.2i (Low-intensity group) | No data | HbA1c |
| Kim et al., 2008, South Korea 26 | Quasi-experimental design | 12 months | Patients with T2DM and obese | 3 | 123 | 2345 | Usual care | 16c, 18i | 48.5 ± 8.0c, 45.5 ± 9.1i | 7 (43.8%)c, 9 (50.0%)i | 25.0 ± 1.7c, 25.6 ± 2.4i | 7.6 ± 0.7c, 8.1 ± 1.9i | 7.8 ± 5.0c, 4.6i ± 6.3i | HbA1c, FBG, 2HPMG |
| Katula et al., 2022, USA 27 | Single-blind RCT | 12 months | Patients with BMI ⩾ 25 kg/m2 (⩾22 kg/m2 if participant self-identified as Asian), and had baseline HbA1c in the prediabetic range (5.7%−6.4% (39−46 mmol/mol)) | 12 | 3 | 2345 | Usual care | 300c, 299i | 55.6 ± 12.6c, 55.3 ± 12.9i | 116 (38.7%)c, 115 (38.5%)i | 36.1 ± 6.6c, 35.8 ± 6.1i | 5.8 ± 0.2c, 5.8 ± 0.3i | No data | BMI, HbA1c |
| Hu et al., 2021, China 28 | A randomized controlled trial | 6 months | Patients with T2DM more than 3 months | 3 | 4 | 14 | Usual care | 70c, 72i | 52.21 ± 8.38c, 50.04 ± 5.76i | 43c, 51i | 24.05 ± 3.98c, 24.69 ± 3.39i | 8.63 ± 1.62c, 8.96 ± 1.78i | 6.09 ± 1.66c, 6.24 ± 1.95i | HbA1c |
| Warren et al., 2018, Australia 29 | A prospective randomized controlled trial | 6 months | Patients with T2DM, HbA1c level measured at ⩾58 mmol/mol (7.5%) at least once in the previous 12 months | 1 | 35 | 134 | Usual care | 63c, 63i | 61.3 ± 11.4c, 61.3 ± 10.8i | 48%c, 60%i | 34.1 (30.3–40.6)c, 34.2 (29.6–39.8)i | 8.1 (7.1–8.9)c, 8.4 (7.8–9.0)i | No data | HbA1c, BMI |
| Cho et al., 2011, Korea 30 | A randomized controlled design | 3 months | Patients with T2DM, HbA1c 7.0%–11.0% | 2 | 3 | 124 | Usual care | 35c, 36i | 63.1 ± 10.3c, 5.3 ± 9.3i | 34%c, 44%i | 24.7 ± 3.1c, 25.2 ± 3.4i | 8.0 ± 1.0c, 8.0 ± 0.8i | 9.9 ± 9.6c, 7.9 ± 6.8i | HbA1c |
| Jia et al., 2021, China 31 | A cluster randomized trial | 12 months | Patients with T2DM | 123 | 34 | 145 | Usual care | 6509c, 13037i | 60.8 ± 8.4c, 60.4 ± 8.4i | 2613 (40.1%)c, 5447 (41.8%)i | 25.6 ± 3.6c, 25.7 ± 3.5i | 7.83 ± 1.91c, 7.89 ± 1.93i | 6 (3, 11)c median (Q1, Q3), 6 (3, 10)i median (Q1, Q3) | HbA1c < 7.0%, BMI |
| Trief et al., 2016, USA 32 | Randomized clinical trial | 12 months | Patients with T2DM, HbA1c ⩾ 7.5% | 1 | 2 | 2 | — | Diabetes education: 78 individual calls: 93 couples calls: 97 | Diabetes education: 56.9 ± 10.4, individual calls: 55.6 ± 11.4, couples calls: 57.8 ± 10.8 | Diabetes education: 59.0%, individual calls: 62.4%, couples calls: 62.9% | Diabetes education: 36 ± 8.1, individual calls: 36 ± 8.2, couples calls: 35.7 ± 6.3 | Diabetes education: 9.1 ± 1.6, individual calls: 9.3 ± 1.7, couples calls: 8.9 ± 1.3 | Diabetes education: 12.6 ± 8.3, individual calls: 11.9 ± 6.9, couples calls: 12.8 ± 8.5 | HbA1c, BMI |
| Wayne et al., 2015, Canada 33 | Pragmatic randomized controlled trial | 6 months | Patients with T2DM, HbA1c ⩾ 7.3% | 1 | 124 | 345 | Usual care | 49c, 48i | 53.3 ± 11.9c, 53.1 ± 10.9i | 10 (20%)c, 17 (35%)i | 37.00 ± 7.92c, 33.74 ± 6.70i | 8.89 ± 1.30c, 8.69 ± 1.32i | No data | HbA1c, BMI |
| Benson et al., 2019, USA 34 | Randomized controlled trial | 12 months | Patients with T2DM | 23 | 12 | 125 | Usual care | 58c, 60i | 60.0 ± 8.66c, 59.8 ± 10.20i | 56.9%c, 53.3%i | 36.2 ± 6.21c, 37.8 ± 9.80i | 8.3 ± 1.66c, 8.1 ± 1.55i | <1 (10.3%), 1–3 (19.0%), 3–5 (15.5%), 5–10 (29.3%), >10 (25.9%)c, <1 (8.3%), 1–3 (13.3%), 3–5 (10.0%), 5–10 (25.0%), >10 (40.0%)i | HbA1c, BMI |
| Hee-Sung, 2007, Korea 35 | A control group pretest–posttest design | 12 weeks | Patients with T2DM | 3 | 123 | 2345 | Usual care | HbA1c <7.0%, 11c, 13i, HbA1c ⩾ 7.0%, 15c, 12i | 49.2 ± 9.2c, 50.0 ± 8.6i, 46.2 ± 9.1c, 43.4 ± 7.9i | 5c, 5i, 6c, 6i | 23.1 ± 2.9c, 24.6 ± 2.2i, 23.6 ± 2.3c, 24.5 ± 3.6i | 6.71 ± 0.39c, 6.92 ± 0.35i, 8.24 ± 0.98c, 9.35 ± 1.72i | 7.8 ± 5.2c, 5.5 ± 4.8i, 8.1 ± 4.7c, 4.8 ± 7.0i | HbA1c |
| Xu et al., 2020, Missouri 36 | A randomized controlled trial | 6 months | Patients with T2DM, HbA1c > 7% | 2 | 12 | 34 | Usual care | 32c, 33i | 55.34 ± 1.94c, 54.6 ± 1.82i | 25%c, 37.5%i | No data | 9.23 ± 0.32c, 9.8 ± 0.45i | No data | HbA1c, FBG |
| Lu et al., 2021, China 37 | A randomized controlled trial | 6 months | Patients with T2DM, HbA1c 7%–10% | 3 | 3 | 34 | Usual care | 59c, 60i | 53.17 ± 11.44c, 56.75 ± 12.05i | 55.93%c, 53.33%i | No data | 9.20 ± 1.92c, 9.27 ± 2.26i | No data | HbA1c, FBG |
| Anderson et al., 2010, USA 38 | A randomized controlled trial | 12 months | Patients with T2DM | 1 | 2 | 134 | Usual care | 149c, 146i | No data | 64 (43.0%)c, 60 (41.1%)i | 33.7 ± 6.64c, 35.4 ± 8.63i | 8.4 ± 2.33c, 7.6 ± 1.75i | No data | HbA1c, BMI |
| Agarwal et al., 2019, Canada 39 | Multicenter pragmatic randomized controlled trial | 6 months | Patients with T2DM, HbA1c > 8.0% | 23 | 14 | 234 | Usual care | 113c, 110i | 52.1 ± 10.7c, 51.5 ± 10.6i | 55 (49.0%)c, 61 (55.0%)i | no data | 9.03 ± 1.53c, 8.89 ± 1.82i | No data | HbA1c |
| Cho et al., 2009, Korea 40 | Randomized controlled trial | 3 months | Patients with T2DM | 1 | 123 | 12345 | — | Internet group: 34, Phone group: 35 | Internet group: 45.2 ± 11.3, Phone group: 51.1 ± 13.2 | Internet group: 26 (76%), Phone group: 28 (80%) | Internet group: 23.6 ± 3.0, Phone group: 25.3 ± 4.7 | Internet group: 7.6 ± 1.9, Phone group: 8.3 ± 2.3 | Internet group: 5.3 ± 4.8, Phone group: 8.2 ± 7.8 | HbA1c, FBG, 2HPMG |
| Quinn et al., 2016, USA 41 | Randomized controlled trial | 12 months | Patients with T2DM at least 6 months, HbA1c level ⩾7.5% within the past 3 months | 1 | 1234 | 234 | Usual care | Age < 55 years, 29c, 37i, age > 55 years, 27c, 25i | 47.4 ± 7.5c, 47.4 ± 6.8i, 59.5 ± 2.8c, 59.0 ± 2.9i | 62.1%c,37.8%i, 37%c, 68%i | 33.9 ± 5.4c, 36.5 ± 8.3i, 34.7 ± 7.2c, 34.8 ± 4.8i | 9.9 ± 1.8c, 9.9 ± 2.0i, 8.4 ± 1.2c, 9.8 ± 2.3i | 8.9 ± 7.5c, 6.8 ± 4.5i, 9.2 ± 6c, 10.3 ± 5.8i | HbA1c |
| Sun et al., 2019, China 42 | Randomized controlled trial | 6 months | Patients with T2DM, HbA1c 7.0%–10.0% | 3 | 14 | 2345 | Usual care | 47c, 44i | 68.04c, 67.9i | 18 (38%)c, 19 (43%)i | 23.30c, 23.60i | 7.88 ± 0.64c, .84 ± 0.73i | 11.52 ± 7.73c, 11.19 ± 6.39i | HbA1c, BMI |
| Lim et al., 2016, Korea 43 | Randomized, controlled clinical trial | 6 months | Patients with T2DM, HbA1c 7.0%–10.5% | 3 | 13 | 2345 | Usual care | 50c, 50i | 65.8 ± 4.7c, 64.3 ± 5.2i | 35c, 40i | 25.4 ± 3.3c, 25.9 ± 3.6i | 7.9 ± 0.8c, 8.1 ± 0.9i | 14.6 ± 8.4c, 14.4 ± 9.5i | HbA1C, BMI |
| Tang et al., 2013, USA 44 | Randomized clinical trial | 12 months | Patients with T2DM ⩾18 y.o, HbA1c ⩾ 7.5% more than 1 year resulted within 30 days | 123 | 1245 | 345 | Usual care | 213c, 202i | 53.5 ± 10.2, 54.0 ± 10.7 | 61%, 58.9% | No data | 9.28, 9.24 | No data | HbA1c |
| Greenwood et al., 2015, USA 45 | Randomized clinical trial | 6 months | Patients with T2DM | 1 | 3 | 1234 | Usual care | 45c, 45i | 57.5 ± 10.6c, 53.9 ± 10.4i | 79%c, 75%i | 34.1 ± 6.6c, 34.1 ± 6.8i | 8.2 ± 1.1c, 8.5 ± 1.1i | 8.1 ± 5.3c, 8.3 ± 5.5i | HbA1c |
| Williams et al., 2012, Australia 46 | Randomized controlled trial | 6 months | Adults with type 2 diagnosis of ⩾3 months and HbA1c ⩾ 7.5%, | 1 | 2 | 1234 | Usual care | 60c, 60i | 56.4 ± 8.3c, 58.4 ± 8.2i | 63.3%c, 61.7%i | No data | 8.9c, 8.7i | No data | HbA1c, HRQL |
| Ramadas et al., 2018, Malaysia 47 | Randomized clinical trial | 12 months | Patients with T2DM, HbA1c ⩾ 7% | 23 | 13 | 235 | Usual care | 62c, 66i | 51.5 ± 10.3, 49.6 ± 10.7 | 75.8%, 62.1% | no data | 8.9 ± 1.9, 9.1 ± 2.0 | 6.8, 9.3 | HbA1c |
| Egede et al., 2017, United States 48 | Randomized clinical trial | 6 months | Patients with T2DM aged ⩾ 18 years from the southeastern United States, HbA1c ⩾ 8% | 1 | 23 | 234 | Usual care | 59c, 54i | 53.4 ± 10.5, 55.1 ± 11.4 | 18.6%, 18.5% | 36.9 ± 9.4, 34.2 ± 7.8 | 10.1 ± 2.1, 10.1 ± 1.8 | 11.5 ± 7.2, 13.0 ± 8.1 | HbA1c |
| Kim et al., 2016, China 49 | Randomized open-label, parallel group design | 6 months | T2DM Chinese patients were diagnosed ⩾ 1 year, HbA1c level of 7.0%–10.0% | 3 | 123 | 1234 | Usual care | 90c, 92i | 55.6 ± 10.0c, 52.5 ± 9.1i | 43.3%c, 53.3%i | 25.2 ± 3.5c, 25.8 ± 2.7i | 8.0 ± 0.8c, 7.9 ± 0.7i | No data | HbA1c |
| Goode et al., 2015, Australia 50 | A randomized trial | 18 months | Patients with T2DM | 1 | 2 | 14 | Usual care | 151c, 151i | Usual group: no data. Intervention group: Low: 57.1 ± 7.3, Medium: 59.4 ± 7.4, High: 56.8 ± 9.3 | Usual group: no data. Intervention group: - Low: 29 (58.0%), Medium: 23 (50.0%), High: 32 (58.2%) | Usual group: no data. Intervention group: Low: 32.4 ± 6.3, Medium: 33.7 ± 7.1, High: 33.2 ± 5.5 | Usual group: no data. Intervention group: Low: 6.9 ± 7.93. Medium: 7.3 ± 8.33, High: 7.1 ± 7.9 | Usual group: no data. Intervention group: Low: 4 ± 6.3, Medium: 4.5 ± 2, 7.3. High: 4 ± 10 | HbA1c |
| Jeong et al., 2018, Korea 51 | Randomized clinical trial | 24 weeks | Patients with T2DM, HbA1c from 7% to 11% | 3 | 1235 | 12345 | Usual care | 113c, 113i, 112i | 53.16 ± 9.06, 53.65 ± 9.10, 52.46 ± 8.48 | 67.26%, 66.37%, 68.75% | 25.39 ± 3.07, 25.22 ± 3.64, 25.21 ± 3.27 | 8.39 ± 1.10, 8.21 ± 0.93, 8.39 ± 1.10 | No data | HbA1c |
| Nagrebetsky et al., 2013, United Kingdom 52 | Feasibility trial | 12 months | Patients with T2DM, HbA1c 8%–11% | 2 | 123 | 45 | Usual care | 9c, 8i | 60 ± 13, 56 ± 8 | 71%, 71% | 32.4 ± 6.2, 33.4 ± 7.1 | 66 ± 13 mmol/mol, 64 ± 11 mmol/mol | 2.3, 3.0 | HbA1c |
| Wild et al., 2016, United Kingdom 53 | Randomized clinical trial | 9 months | Patients with T2DM aged > 17 years, HbA1c > 58 mmol/mol. | 2 | 23 | 134 | Usual care | 161c, 160i | 61.4 ± 9.8, 60.5 ± 9.8 | 66.2%, 67.1% | 31.9 ± 6.3, 33.8 ± 7.0 | 8.8 ± 1.1, 8.9 ± 1.3 | 7.4 ± 5.8, 7.4 ± 5.7 | HbA1c |
| de Vasconcelos et al., 2018, Brazil 54 | Randomized clinical trial | 24 weeks | Patients with T2DM for at least 1 year | 2 | 2 | 1235 | Usual care | 15c, 16i | 59.6, 60.9 | 5, 2 | 29.87 ± 5.25, 29.99 ± 5.82 | 6.9 ± 1.31, 8.0 ± 2.14 | 8.67 ± 6.39, 10 ± 8.48 | HbA1c, BMI |
| Rasmussen et al., 2016, Denmark 55 | Randomized controlled trial | 6 months | Patients with T2DM | 3 | 5 | 1 | Usual care | 22c, 18i | 64.6c, 60.7i | 14 (63.6%)c, 13 (72.2%)i | 30.4c, 32.6i | 8.1c, 9.0i | 8.4c, 10.7i | HbA1c, Blood glucose level, Weight |
| Rodríguez-Idígoras et al., 2009, Spain 56 | Randomized controlled parallel-group trial | 1 year | Patients with T2DM | 2 | 234 | 234 | Usual care | 167c, 161i | 64.52, 63.32 | 49.10%, 54.04% | No data | 7.41, 7.62 | 10.18, 11.32 | HbA1c |
| von Storch et al., 2019, Germany 57 | Prospective study | 3 months | Patients with T2DM | 2 | 2 | 1345 | Usual care | 55c, 60i | 58.4 ± 7.3, 59.4 ± 6.3 | 85% 78% | 29.3 ± 4.43, 31.9 ± 7.06 | 6.89 ± 1.01, 7 ± 0.96 | 7± 4.1, 7± 4 | HbA1c, BMI |
| Lee et al., 2020, Malaysia 58 | Cluster-randomized controlled trial | 52 weeks | Patients with T2DM | 2 | 2 | 234 | Usual care | 120c, 120i | 56.3 ± 8.6, 56.1 ± 9.2 | 45.8%, 44.2% | No data | 9.00, 9.00 | 6.6 ± 7.0, 6.7 ± 5.3 | HbA1c |
| Lee et al., 2017, Malaysia 59 | Cluster-randomized controlled trial | 12 weeks | Patients with T2DM, HbA1c between 7.5% and 11.0% | 12 | 134 | 234 | Usual care | 40c, 45i | 53.77 ± 8.03c, 53.24 ± 7.29i | 16 (40.00%)c, 24 (60.00%)i | 30.28 ± 5.05c, 29.20 ± 5.98i | 8.79 ± 1.15c, 8.69 ± 1.12i | 10.04 ± 7.64c, 7.91 ± 4.81i | HbA1c, BMI |
| Dario et al., 2017, Italy 60 | Randomized controlled trial | 12 months | Patients with T2DM, HbA1c > 7.0% | 1 | 4 | 4 | Usual care | 91c, 208i | 73.04 ± 5.28c, 73.05 ± 5.79i | 49 (53%)c, 119 (57%)i | No data | 7.93 ± 1.10c, 7.94 ± 0.98i | 16.01 ± 9.84, 15.01 ± 10.24 | HbA1c |
| Egede et al., 2017, USA 61 | Randomized controlled trial | 4 years | Patients with T2DM, HbA1c ⩾ 9% | 23 | 2 | 12345 | Usual care | 64c, 63i (knowledge), 65i (skills) 63i (combination) | 56.1 ± 10.3c, 56.5 ± 11.5i, 58.3 ± 9.5i, 58.2 ± 10.0i | 51.6%c, 55.6%i, 61.5%i, 52.4%i | No data | 9.5 ± 2.5c, 9.3 ± 1.8i, 9.2 ± 2.1i, 9.2 ± 1.9i | 13.5 ± 9.3c, 12.5 ± 8.3i, 13.5 ± 8.8i, 13.7 ± 9.7i | HbA1c |
| Bujnowska-Fedak et al., 2011, Poland 62 | Randomized clinical trial | 6 months | Patients with T2DM | 2 | 124 | 4 | Usual care | 48c, 47i | 57.5 ± 27.4, 53.1 ± 25.2 | 25, 26 | 26.2 ± 6.6, 25.4 ± 7.2 | 7.61 ± 1.65, 7.63 ± 1.53 | 7.7 ± 6.8, 8.1 ± 7.6 | HbA1c |
| Arora et al., 2014, United States 63 | Randomized controlled trial | 6 months | Patients with T2DM, HbA1c ⩾ 8% | 3 | 1 | 23 | Usual care | 64c, 64i | 51.0 ± 10.2, 50.5 ± 10.3 | 20, 26 | No data | 10.0 ± 1.7, 10.2 ± 1.7 | 10.1 ± 6.5 10.9 ± 10.4 | HbA1c |
| Kardas et al., 2016, Poland 64 | A feasibility prospective parallelarm randomized controlled trial | 6 weeks | Patients with T2DM | 2 | 45 | 34 | Usual care | 30c, 30i | 59.0 ± 8.09c, 59.9 ± 5.31i | 19 (63.3%)c, 17 (56.7%)i | 30.3 ± 3.35c, 31.6 ± 5.27i | 6.84 ± 0.98c, 6.78 ± 1.10i | No data | FBG, HbA1c |
| McFarland et al., 2012, USA 65 | Nonrandomized, parallel, control group study | 6 months | Patients with T2DM, HbA1c ⩾ 7% | 12 | 12 | 34 | Usual care | 67i, 36c | 63 ± 10c, 66 ± 9i | 64 (96%)c, 36 (100%)i | No data | 9.1 ± 1.6c, 9 ± 1.5i | No data | HbA1c |
| Hansen et al., 2017, Denmark 66 | Cross-sectional randomized controlled trial | 8 months | Patients with T2DM, HbA1c > 7.5% | 13 | 5 | 134 | Usual care | 82c, 83i | 58.3 ± 9.3c, 57.8 ± 9.4i | 53 (65%)c, 53 (64%)i | 33.6 ± 5.6c, 33.9 ± 6.2i | 9.36 ± 1.3c, 9.25 ± 1.2i | 12.5 ± 7.3c, 12.1 ± 6.6i | HbA1c |
| Zhou et al., 2014, China 67 | Prospective randomized study | 3 months | Patients with T2DM | 3 | 123 | 234 | Usual care | 55c, 53i | No data | No data | 23.64 ± 3.01, 24.72 ± 3.38 | 8.22 ± 1.58, 8.44 ± 1.58 | No data | FBG, HbA1c |
| Luley et al., 2011, Germany 68 | Randomized clinical trial | 6 months | Patients with T2DM with BMI > 25 kg/m2 | 1 | 2 | 45 | Usual care | 35c, 35i | 58 ± 7, 57 ± 9 | 54%, 43% | 34.8 ± 5.9, 35.3 ± 5.7 | 7.6 ± 1.1, 7.5 ± 1.1 | No data | HbA1c, BMI |
| Hsu et al., 2016, USA 69 | A randomized controlled study | 12 ± 2 weeks | Patients with T2DM, HbA1c levels of 9%–14% | 1 | 3 | 234 | Usual care | 20c, 20i | 53.8c, 53.3i | No data | 31.7c, 30.8i | 10.9c, 10.8i | 9c, 9.6i | HbA1c |
| Kleinman et al., 2017, India 70 | A randomized clinical trial | 6 months | Patients with T2DM, HbA1c levels between 7.5% and 12.5% | 3 | 34 | 4 | Usual care | 46c, 44i | 48.0 ± 9.5c, 48.8 ± 9.0i | 58.7%c, 81.8%i | 28.0 ± 4.2c, 29.7 ± 6.0i | 9.1 ± 1.1c, 9.4 ± 1.2i | 8.5c, 10.0i | HbA1c, FBG, BMI |
| Orsama et al., 2013, Finland 71 | A randomized controlled trial | 10 months | Patients with T2DM, HbA1c levels 6.5% and 11% | 1 | 124 | 234 | Usual care | 24c, 24i | 61.5 ± 9.1c, 62.3 ± 6.5i | 54%c, 54%i | 33.5 ± 8.0c, 30.7 ± 4.5i | 7.09 ± 1.51c, 6.86 ± 1.56i | No data | HbA1c, weight |
| Kim et al., 2007, Korea 72 | A randomized controlled trial | 12 weeks | Patients with T2DM | 3 | 123 | 123 | Usual care | 26c, 25i | 47.5 ± 9.1c, 46.8 ± 8.8i | 11c, 11i | 23.4 ± 2.5c, 24.5 ± 2.9i | 7.59 ± 1.09c, 8.09 ± 1.72i | 8.0 ± 4.9c, 5.2 ± 5.9i | HbA1c, 2HPMG |
| Bender et al., 2017, USA 73 | A randomized controlled trial | 6 months | Patients with T2DM and BMI > 23 kg/m2 | 1 | 34 | 245 | Usual care | 23c, 22i | 57.7 ± 10.0c, 57.4 ± 0.8i | 40%c, 37%i | 31.5 ± 5.1c, 28.6 ± 3.6i | 7.44 ± 0.93c, 7.39 ± 0.82i | No data | HbA1c, Fasting glucose, BMI, weight |
| Blackberry et al., 2013, Australia 74 | Prospective, cluster randomized controlled trial | 18 months | Patients with T2DM, HbA1c > 7.5% in the past 12 months | 1 | 2 | 25 | Usual care | 237c, 236i | 61.9 ± 10.5c, 63.6 ± 10.4i | 142 (60%)c, 127 (54%)i | No data | 8.13 ± 1.34c, 7.98 ± 1.22i | 9c,10i | HbA1c, weight |
| Borhani et al., 2013, Kerman 75 | A quasi-experimental study | 3 months | Patients with T2DM, HbA1c > 7% | 1 | 2 | 35 | Usual care | 25c, 25i | No data | No data | 30.69 ± 6.67c, 27.93 ± 4.84i | 9.38 ± 1.53c, 9.98 ± 1.34i | No data | HbA1c, FBS, postprandial glucose, BMI |
| Faridi et al., 2008, USA 76 | A pilot controlled trial | 3 months | Patients with T2DM, BMI > 25, HbA1c < 8% | 2 | 1 | 35 | Usual care | 15c, 15i | 56.7 ± 10.6c, 55.3 ± 8.7i | 33.3%c, 40%i | 36.9 ± 12.5c, 34.3 ± 7.4i | 6.5 ± 0.7c, 6.4 ± 0.6i | No data | HbA1c, BMI, weight |
| Hallberg et al., 2018, USA 77 | An open-label, nonrandomized, controlled, before-and-after 1-year study | 1 year | Patients with T2DM | 1 | 34 | 235 | Usual care | 87c, 262i | 52.33 ± 9.52c, 53.75 ± 8.35i | No data | 36.72 ± 7.26c, 40.43 ± 8.81i | 7.64 ± 1.76c, 7.60 ± 1.50i | 7.85 ± 7.32c, 8.44 ± 7.22i | HbA1c, weight |
| Holmen et al., 2014, Norway 78 | A 3-arm prospective randomized controlled trial | 12 months | Patients with T2DM, HbA1c level ⩾ 7.1% | 1 | 124 | 345 | Usual care | 50c, few touch application: 51i FTA-health counseling: 50i | 55.9 ± 12.2c, 58.6 ± 11.8i, 57.4 ± 12.1i | 60%c, 50%i, 67%i | 32.0 ± 6.0c, 32.4 ± 6.5i, 30.7 ± 5.6i | 8.3 ± 1.2c, 8.1 ± 1.1i, 8.2 ± 1.1i | 9.4 ± 5.5c, 11.2 ± 7.3i, 9.6 ± 8.4i | HbA1c, weight |
| Lim et al., 2011, Korea 79 | A randomized controlled trial | 6 months | Patients with T2DM, A1C level was 6.5%–10.5% | 2 | 123 | 12345 | Usual care | 52c, clinical decision support system (CDSS)-based ubiquitous healthcare: 51i self-monitored blood glucose: 51i | 68.1 ± 5.5c, 67.2 ± 4.1i, 67.2 ± 4.4i | 19c, 23i, 22i | 25.4 ± 3.3c, 24.7 ± 2.3i, 24.9 ± 3.0i | 7.9 ± 0.8c, 7.8 ± 1.0i, 7.9 ± 0.9i | 15.8 ± 10.7c, 14.1 ± 10.1i, 15.4 ± 8.3i | HbA1c |
| Odnoletkova et al., 2016, Belgium 80 | A parallel-group, randomized controlled trial | 18 months | Patients with T2DM | 1 | 2 | 12345 | Usual care | 287c, 287i | 62.4 ± 8.9c, 63.8 ± 8.7i | 63%c, 60%i | 30.6 ± 5.2c, 30.2 ± 4.9i | 7.0 ± 1.0c, 7.0 ± 1.1i | No data | HbA1c, BMI |
| Quinn et al., 2011, USA 81 | A cluster-randomized clinical trial | 12 months | Patients with T2DM, HbA1c level ⩾ 7.5% (within 3 months) | 2 | 3 | 45 | Usual care | 56c, coach-only: 23i coach PCP portal: 22i coach PCP portal with decision support: 62i | 53.2 ± 8.4, 52.8 ± 8.0i, 53.7 ± 8.2i, 52 ± 8.0i | 50%c, 52.2%i, 45.5%i, 50%i | 34.3 ± 6.3c, 36.9 ± 7.5i, 35.5 ± 10.3i, 35.8 ± 7.1i | 9.2 ± 1.7c, 9.3 ± 1.8i, 9.0 ± 1.8i, 9.9 ± 2.1i | 9.0 ± 7.0c, 7.7 ± 5.6i, 6.8 ± 4.9i, 8.2 ± 5.3i | HbA1c |
| Rothman et al., 2005, USA 82 | A randomized controlled trial | 12 months | Patients with T2DM, HbA1c level ⩾ 8.0% | 2 | 2 | 245 | Usual care | 105c, 112i | 57 ± 11c, 54 ± 13i | 44%c, 44%i | 34 ± 8c, 35 ± 9i | 11 ± 3c, 11 ± 2i | 9 ± 9c, 8 ± 9i | HbA1c |
| Varney et al., 2014, Australia 83 | A random controlled trial | 12 months | Patient with T2DM and HbA1c level >7% | 2 | 2 | 245 | Usual care | 47c, 47i | 64c, 59i | 64%c, 72%i | 30.9c, 32.1i | 8.5c, 8.2i | 13.1c, 12.6i | HbA1c, BMI |
| Waki et al., 2014, Japan 84 | A nonblinded randomized controlled study | 3 months | Patient with T2DM | 1 | 13 | 4 | Usual care | 27c, 27i | 57.4 ± 9.4c, 57.1 ± 10.2i | 21c, 20i | 27.1 ± 7.6c, 26.2 ± 6.1i | 7.0 ± 0.9c, 7.1 ± 1.0i | No data | HbA1c, FBS, BMI |
| Wang et al., 2019, China 85 | A random controlled trial | 6 months | Patient with T2DM and HbA1c level >7% | 3 | 4 | 1234 | Usual care | 60c, 60i | 45.8 ± 8.38c, 45.13 ± 7.83i | 31c, 33i | No data | 8.68 ± 2.26c, 8.62 ± 2.33i | No data | HbA1c, FBG, 2HPMG |
| Kusnanto et al., 2019, Indonesia 86 | A randomized experimental study | 3 months | Patient with T2DM and HbA1c level >7% | 1 | 4 | 45 | Usual care | 15c, 15i | No data | 40%c, 46.7%i | No data | 8.18 ± 1.02c, 8.74 ± 1.34i | No data | HbA1c |
| Yoo et al., 2009, Korea 87 | A randomized, controlled clinical trial | 3 months | Patient with T2DM, HbA1c 6.5%–10.0% and BMI ⩾ 23.0 kg/m2 | 13 | 13 | 4 | Usual care | 54c, 57i | 59.4 ± 8.4c, 57.0 ± 9.1i | 64.8c, 52.6i | 25.5 ± 3.3c, 25.6 ± 3.5i | 7.4 ± 0.9c, 7.6 ± 0.9i | 7.2 ± 6.0c, 6.0 ± 5.4i | HbA1c |
| Meigs et al., 2003, USA 88 | A group randomized controlled trial | 12 months | Patient with T2DM | 3 | 3 | 23 | Usual care | 291c, 307i | 67 ± 12c, 68 ± 12i | 50.5%c, 44.9%i | No data | No data | 9.7 ± 5.6c, 9.9 ± 5.5i | HbA1c |
| Tutino et al., 2017, China 89 | A multicenter randomized nonblinded study | 12 months | Patient with T2DM | 3 | 3 | 23 | — | DIAMOND: 1728i, JADE: 1858i | 56.8 ± 11.7i, 56.1 ± 11.6i | 54.5%i, 54.4i% | 25.32 ± 3.62i, 25.18 ± 3.58i | 7.91 ± 2.08i, 7.78 ± 1.95i | 5i, 5i | HbA1c |
| Graziano et al., 2009, USA 90 | A randomized controlled trial | 3 months | Patient with T2DM and HbA1c level ⩾ 7% | 2 | 12 | 24 | Usual care | 58c, 61i | 63.0 ± 9.3c, 60.1 ± 7.4i | 33c, 33i | no data | 8.59 ± 1.96c, 8.71 ± 1.74i | 12.2 ± 8.2c, 13.5 ± 8.4i | HbA1c |
| Middleton et al., 2021, Australisa 91 | A randomized controlled trial | 12 months | Patient with T2DM | 3 | 1 | 123 | Usual care | 19c, 21i | 32.4 ± 4.4c, 33.0 ± 5.8i | 53%c, 48%i | 31.6 ± 5.1c, 31.8 ± 8.6i | 7.3 ± 2.1c, 7.2 ± 1.6i | 5.0 ± 5.9c, 7.6 ± 6.2i | HbA1c, BMI |
| Smith et al., 2008, USA 92 | A randomized controlled trial | 12 months | Patient with T2DM | 2 | 12 | 12 | Usual care | 227c, 358i | 60c, 62i | 50%c, 45%i | 34c, 33i | 7.3c, 7.3i | 4c, 4i | HbA1c |
| Farmer et al., 2021, UK 93 | Two parallel-arm, individually randomized controlled trial | 12 months | Patient with T2DM | 12 | 1 | 2 | Usual care | 561c, 558i | no average | 30.1%c, 30.1%i | 30.8 ± 7.4c, 30.6 ± 6.5i | 10.2 ± 3.6c, 10.1 ± 3.4i | 5.2c, 5.0i | HbA1c |
| Vinitha et al., 2019, India 94 | A multicentric, randomized controlled trial | 24 months | Patient with T2DM | 1 | 1 | 25 | Usual care | 122c, 126i | 44.1 ± 8.9c, 42.4 ± 8.5i | 82c, 86i | 27.3 ± 4.7c, 27.2 ± 4.5i | 9.5 ± 1.9c, 9.5 ± 2.1i | no data | HbA1c, FBG |
| Peimani et al., 2016, Iran 95 | A three-arm randomized controlled trial | 3 months | Patient with T2DM | 2 | 1 | 25 | Usual care | 50c, Tailored-SMS group: 50i, Non-tailored-SMS group: 50i | 54.56 ± 9.88c, 49.78 ± 9.76i, 53.26 ± 10.49i | 26c, 27i, 28i | 27.92 ± 4.97c, 27.71 ± 5.29i, 27.40 ± 4.73i | 7.52 ± 1.49c, 7.29 ± 1.33i, 7.53 ± 1.47i | 9.98 ± 7.51c, 8.09 ± 6.95i, 8.9 ± 6.63 | HbA1c, FBS, BMI |
| Schillinger et al., 2009, USA 96 | A three-arm practical clinical trial | 12 months | Patient with T2DM and HbA1C ⩾ 8.0% | 12 | 12 | 2 | Usual care | 114c, ATSM: 112i, GMV: 113i | 55.8 ± 11.8c, 55.9 ± 12.7i, 56.5 ± 11.4i | 44.7%c, 42%i, 36.3%i | 32.3 ± 13.5c, 30.3 ± 6.7i, 31.9 ± 8.2i | 9.8 ± 2.0c, 9.3 ± 1.8i, 9.4 ± 2.0i | 10.4 ± 8.1c, 9.1 ± 7.3i, 9.2 ± 6.8i | HbA1c |
| Kim et al., 2014, Korea 97 | Clinical trial | 3 months | Patient with T2DM and HbA1C was 7.0%–10.0% | 3 | 4 | 13 | Usual care | 35c, 35i | 53.8 ± 9.0c, 51.8 ± 10.3i | 20c, 20i | 24.9 ± 3.4c, 25.0 ± 3.3i | 7.7 ± 0.5c, 7.7 ± 0.7i | , 11.8 ± 7.3i | HbA1c, BMI |
| Iljaž et al., 2017, Slovenia 98 | A randomized controlled trial | 6 months | Patient with T2DM | 2 | 4 | 124 | Usual care | 62c, 58i | 54.7c, 56.3i | 36c, 37i | no data | 6.8 ± 1.2c, 7.1 ± 1.5i | 5.7 ± 4.8c, 5.1 ± 5.7i | HbA1c |
| Kwon et al., 2004, Korea 99 | A randomized controlled trial | 3 months | Patient with T2DM | 2 | 13 | 1234 | Usual care | 55c, 55i | 54.7 ± 9.4c, 53.5 ± 8.8i | 32c, 35i | 23.9 ± 3.1c, 24.4 ± 3.4i | 7.19 ± 1.17c, 7.59 ± 1.43i | 6.6 ± 5.7c, 7.0 ± 6.3i | HbA1c |
| Lee et al., 2017, Korea 100 | A subanalysis of clinical trial | 6 months | Patient with T2DM and HbA1c ⩾7.5% | 1 | 2 | 14 | Usual care | 91c, infrequent users: 54i, frequent users: 53i | 56.4 ± 8.7c, 53.5 ± 9.6i, 55.8 ± 9.i9 | 55c, 32i, 35i | 35.5 ± 6c, 35.5 ± 6.5i, 34.1 ± 6.4i | 9.2 ± 1.5c, 9.4 ± 1.4i, 9.2 ± 1.4i | No data | HbA1c, BMI |
| Kim et al., 2010, Korea 101 | Clinical trial | 12 weeks | Patient with T2DM and HbA1c was >7.0% and <12.0%, body mass index values <35 kg/m2 | 1 | 13 | 2 | Usual care | 45c, 47i | 49.0 ± 10.7c, 47.8 ± 9.6i | 22c, 24i | 24.4 ± 3.5c, 23.6 ± 2.5i | 9.8 ± 1.2c, 9.8 ± 1.3i | 8.4 ± 6.2c, 8.5 ± 6.4i | HbA1c |
| Song et al., 2009, Korea 102 | A randomized two-group pretest/posttest experimental study | 12 weeks | Patient with T2DM | 1 | 2 | 124 | Usual care | 25c, 24i | 49.5 ± 10.6c, 51.0 ± 11.3i | 50%c, 36%i | 25.5 ± 3.7c, 24.2 ± 3.9i | 9.0 ± 1.2c, 9.4 ± 1.8i | 5.0 ± 5.7c, 4.9 ± 5.3i | HbA1c |
| McKay et al., 2002, USA 103 | A randomized design study | 3 months | Patient with T2DM | 2 | 23 | 125 | Usual care | Information-only condition: 40c, peer support condition: 40i, personal self-management coach condition: 40i, combined condition: 40i | 60.8 ± 9.1c, 57.6 ± 9.2i, 57.6 ± 9.0i, 62.1 ± 9.5i | 47.5%c, 52.5%i, 42.5%i, 45.0%i | No data | 7.2 ± 1.36c, 7.64 ± 1.71i, 7.75 ± 1.33i, 7.46 ± 1.35i | 11.85 ± 6.8c, 11.72 ± 8.71i, 10.00 ± 6.39i, 11.60 ± 9.23i | HbA1c |
| Cho et al., 2006, Korea 104 | A randomized controlled trial | 30 months | Patient with T2DM | 2 | 3 | 24 | Usual care | 40c, 40i | 54.6 ± 8.6c, 51.3 ± 9.1i | 57.5%c, 65%i | 23.8 ± 2.8c, 22.8 ± 2.6i | 7.5 ± 1.3c, 7.7 ± 1.5i | 6.9 ± 5.7c, 6.7 ± 5.3i | HbA1c |
| Eakin et al., 2013, Australia 105 | A two-arm randomized controlled trial | 6 months | Patient with T2DM and BMI ⩾ 25.0 kg/m2 | 1 | 2 | 14 | Usual care | 151c, 151i | 58.3 ± 9.0c, 57.7 ± 8.1i | 57%c, 55.6%i | 33.2 ± 6.0c, 33.1 ± 6.3i | 7.5 ± 1.7c, 7.4 ± 1.5i | 5c, 4i | HbA1c, weight |
| Agboola et al., 2016, USA 106 | A randomized controlled trial | 6 months | Patient with T2DM and HbA1c > 7% | 1 | 12 | 245 | Usual care | 62c, 64i | 52.6 ± 12.6c, 50.3 ± 10.5c | 40%c, 56%i | No data | 8.38 ± 1.37c, 9.02 ± 1.63i | No data | HbA1c |
| Glasgow et al., 2012, USA 107 | A patient-randomized practical effectiveness trial | 12 months | Patient with T2DM and BMI ⩾ 25.0 kg/m2 | 2 | 23 | 12 | Usual care | EUC: 132c, CASM: 169i, CASM+: 162i | 58.7 ± 9.1c, 58.7 ± 9.3i, 57.8 ± 9.3i | 48.5%c, 55.4%i, 46.3%i | 34.8 ± 0.6c, 34.9 ± 0.4i, 34.9 ± 0.4i | 8.16 ± 0.16c, 8.14 ± 0.10i, 8.14 ± 0.10i | No data | HbA1c, BMI |
| Ralston et al., 2009, USA 108 | A pilot randomized trial | 12 months | Patient with T2DM and HbA1c ⩾ 7% | 2 | 3 | 35 | Usual care | 41c, 42i | 57.6c, 57.0i | 48.8%c, 52.4%i | No data | 7.9c, 8.2i | No data | HbA1c |
| Noh et al., 2010, Korea 109 | A randomized controlled trial | 6 months | Patient with T2DM and HbA1c was ⩾7.0% and ⩽10.0% | 3 | 3 | 234 | Usual care | 20c, 20i | 42.3 ± 7.6c, 42.5 ± 10.6i | 75%c, 80%i | 24.7 ± 2.8c, 25.7 ± 3.1i | 8.6 ± 1.2c, 9.0 ± 2.3i | 8.4 ± 5.9c, 4.6 ± 6.9i | HbA1c |
| Murray et al., 2017, England 110 | A multicenter, two-arm individually randomized controlled trial | 12 months | Patient with T2DM | 1 | 123 | 1245 | Usual care | 189c, 185i | 64.7 ± 9.1c, 64.7 ± 9.1i | 69%c, 69%i | 29.6 ± 5.2c, 30.1 ± 5.3i | 7.35 ± 1.37c, 7.26 ± 1.25i | No average | HbA1c |
| Bingham et al., 2021, USA 111 | A retrospective study | 3 months | Patients with T2DM | 1 | 2 | 234 | No control | 444 | 70 [40-75] | 180 (40%) | No data | 7.4 [4.5-13.9] | No data | HbA1c |
| Michaud et al., 2020, Nebraska 112 | Retrospective observational study | 3 months | Patients with T2DM | 3 | 2 | 234 | No control | 1103 | 60.5 ± 11.4 | 0.45 | No data | 7.6 ± 1.9 | No data | HbA1c |
| Kesavadev et al., 2012, India 113 | A retrospective cohort study | 6 months | Patients with T2DM, HbA1c ⩾ 6.5% | 1 | 23 | 134 | No control | 1000 | 53.2 ± 9.8 | 0.64 | 25.4 ± 3.8 | 8.5 ± 1.4 | 10.9 ± 7.1 | HbA1c, FBS, BMI |
| Su et al., 2019, USA 114 | Cohort study | 3 months | Patients with T2DM | 3 | 2 | 14 | No control | 1354 | 59.6 ± 11.8 | 45.1% | BMI ⩾ 30 kg/m2: 74.2% | 7.7 ± 2.0 | No data | HbA1c |
| Musacchio et al., 2011, Italy 115 | Cohort study | 12 months | Patients with T2DM | 2 | 13 | 234 | No control | 1004 | 66.6 ± 9.5 | 54.1% | 29.5 ± 4.8 | 6.9 ± 0.9 | 10.8 ± 7.7 | HbA1c |
| Turner et al., 2009, USA 116 | Exploratory study | 3 months | HbA1c >7.5% commencing treatment with a basal insulin regimen during the past 12 months | 2 | 124 | 35 | No control | 23 | 57.6 ± 12.0 | 18 (78%) | 33.2 ± 6.3 | 9.5 ± 2.2 | 6.4 ± 4.5 | HbA1c |
| Bergenstal et al., 2021, USA 117 | Cohort study | 17 months | Patients with T2DM | 1 | 45 | 1245 | No control | 594 | 53.0 ± 8.4 | 224 | 35.4 ± 7.7 (n = 550) | 7.7 ± 1.6 (n = 563) | No data | HbA1c |
| Michaud et al., 2018, USA 118 | Retrospective, observational study | 3 months | Patients with type 2 diabetes | 1 | 2 | 2345 | No control | 955 | No data | 432 (45%) | 35.59 ± 7.79 | 7.91 ± 2.07 | No data | HbA1c, BMI |
| Cheng et al., 2021119 | Cross-sectional study | 1-month | Patients with T2DM | 1 | 12 | 1234 | Usual care | 207c, 168i | 64.9 ± 13.1c, 66.9 ± 12.0i | 99 (47.8%)c, 76 (45.2%)i | 25.8 ± 4.8c, 25.9 ± 4.2i | 9.3 ± 2.3c, 9.1 ± 2.2i | No data | FBS, 2HPMG, Glucose variability |
| Shane-McWhorter et al., 2014, USA 120 | A nonrandomized prospective observational preintervention–postintervention study | 6 months | Patients with T2DM, HbA1c level >7% | 2 | 12 | 245 | No control | 95 | No data | 40 | No data | 9.73 | No data | HbA1c |
| Yu et al., 2014, Canada 121 | A single-arm pre–post cohort study | 9 months | Patients with T2DM, HbA1c > 7.0% | 2 | 123 | 25 | — | Observational cohort: 81. Qualitative study: 21 | No average | Observational cohort: 54%, Qualitative study: 43% | No data | Observational cohort: 7.64 ± 1.29, Qualitative study: 7.17 ± 0.98 | No average | HbA1c |
| Berman et al., 2018, USA 122 | Cohort study | 12 weeks | Patients with T2DM, HbA1c > 6.5% | 1 | 24 | 125 | No control | 118 | 50.7 ± 9.4 | 18.6% | 38.1 ± 8.8 | 8.1 ± 1.6 | 1.4 ± 0.9 | HbA1c |
| Shane-McWhorter et al., 2015, USA 123 | Cohort study | 6 months | Patients with T2DM | 1 | 123 | 1245 | Usual care | 75c, 75i | 50.57 ± 11.01c, 48.28 ± 10.62i | 33.3%c, 34.7%i | 33.29 ± 6.95c, 33.13 ± 6.79i | 9.44 ± 1.72c, 9.87 ± 2.06i | No data | HbA1c |
| Dixon et al., 2020, United States 124 | Technology report | 6 months | Patients with T2DM | 1 | 1245 | 12345 | No control | 740 | 53.8 ± 8.8 | 0.37 | 35.6 ± 8.5 | 7.7 ± 1.7 | No data | HbA1c |
| Majithia et al., 2020, USA 125 | Prospective single-arm study | 4 months | Patients with T2DM, HbA1c from 8% to 12% | 1 | 4 | 12345 | No control | 55 | 57.3 ± 11.6 | 33 (60%) | 33.7 ± 7.2 | 8.9 ± 1.0 | No data | HbA1c |
| Kim et al., 2006, Korea 126 | Pre–posttest | 12 weeks | Patients with T2DM | 3 | 13 | 234 | No control | 33 | 43.5 ± 12.6 | 42.4% | 24.3 ± 3.7 | 8.1 ± 2.1 | 5.6 ± 5.7 | HbA1c |
| Mayes et al., 2010, USA 127 | Pre–posttest | 3.5 years | T2DM Hispanic patients | 2 | 245 | 123 | No control | 16 | 51 ± 2.5 | 0.19 | no data | 9.6 ± 0.6 | No data | HbA1c |
| McGloin et al., 2020, Ireland 128 | An observational, pre–post, multimethod, and triangulation design | 12 weeks | Patients with T2DM and commencing with insulin therapy | 1 | 2 | 4 | No control | 39 | 62.4 | 0.59 | 30.16 ± 7.32 | 9.62 | No data | HbA1c, BMI |
| Bollyky et al., 2018, USA 129 | Pre–post test | 12 months | T2DM, HbA1c > 7.5%, BMI ⩾ 25 | 1 | 12 | 125 | No control | 330 | 50.3 ± 9.6 | 146 (44.2%) | No data | 7.5 ± 1.9 % | No data | HbA1c |
| McGloin et al., 2015, Ireland 130 | A longitudinal mixed-method case study | 12 months | Patient with T2DM | 1 | 2 | 5 | No control | 10 | 54.5 ± 6.9 | 0.5 | 34.5 ± 6.9 | 7.85 ± 1.98 | 6.5 ± 6.3 | HbA1c |
| Carter et al., 2011, USA 131 | Not reported | 9 months | Patients with T2DM | 2 | 35 | 24 | Usual care | 21c, 26i | 49c, 52i | 9c, 8i | 36.1c, 35.4i | 8.8c, 9.0i | No data | HbA1c, BMI, weight |
| King et al., 2009, USA 132 | Initial pilot program | 12 months | Patients with T2DM | 2 | 2 | 124 | Usual care | 101c, 34i | 61.0 ± 13.7c, 62.8 ± 14.0i | 46.5%c, 48.5%i | No data | 7.8 ± 1.9c, 7.0 ± 1.1i | No data | HbA1c |
| Carallo et al., 2015, Singapore 133 | Not reported | 1 year | Patients with T2DM | 3 | 2 | 1 | Usual care | 208c, 104i | 61.4 ± 11.2c, 63.9 ± 9.3i | 62c, 63i | 30.6 ± 5.8c, 31.0 ± 4.8i | 61 ± 7 mmol/molc, 58 ± 6 mmol/moli | No data | HbA1c |
| Chen et al., 2011, Taiwan 134 | Not reported | 1 year | Patients with T2DM, HbA1c >7% more than 1 year | 23 | 124 | 2345 | Usual care | 32c, 32i | 55.8 ± 17.5, 51.8 ± 15.8 | 43.8%, 46.9% | No data | 9.6 ± 1.5, 9.5 ± 1.8 | 15.1 ± 9.5, 12.3 ± 7.2 | HbA1c |
| Myers et al., 2021, USA 135 | Pilot study | 3 months | Patients with T2DM, HbA1c ⩾ 9% | 1 | 125 | 124 | — | Telephone: 13, Telehealth: 9 | Telephone: 58.69 ± 11.80, Telehealth: 56.56 ± 7.97 | Telephone: 5, Telehealth: 5 | No data | Telephone: 11.1, Telehealth: 10.3 | No data | HbA1c |
| Istepanian et al., 2014, Iraq 136 | Case study | 6 months | Patients with T2DM first year | 2 | 24 | 4 | Usual care | 6c, 6i | 55.2 ± 10.1, 54.8 ± 12.7 | No data | 26.0 ± 3.5, 26.8 ± 3.1 | 8.95 ± 2.17, 8.95 ± 0.73 | 9.7 ± 9.4, 10.7 ± 11.3 | HbA1c |
| Lim et al., 2009, Korea 137 | Not reported | 3 months | Patients with T2DM | 1 | 2 | 124 | Usual care | 34c, 67i | 58.0 ± 1.0c, 59.0 ± 1.3i | 49.3%c, 44.1%i | 24.8 ± 0.6c, 24.4 ± 0.4i | 8.5 ± 0.3c, 8.0 ± 0.2i | 8.6 ± 1.4c, 7.1 ± 0.7i | HbA1c, BMI, FBS |
| Yoon et al., 2008, Korea 138 | Not reported | 12 months | Patients with T2DM | 2 | 13 | 24 | Usual care | 26c, 25i | 47.5 ± 9.1c, 46.8 ± 8.8i | 42.3%c, 0.44%i | 23.4 ± 2.5c, 24.5 ± 2.9i | 7.59 ± 1.09c, 8.09 ± 1.72i | 8.0 ± 4.9c, 5.2 ± 5.9i | HbA1c |
| Nesari et al., 2010, Iran 139 | Not reported | 3 months | Patients with T2DM, HbA1c >7% | 1 | 2 | 123 | Usual care | 30c, 30i | 51 ± 8.2c, 51.9 ± 7.6i | 20%c, 36.7%i | 28.21 ± 4.70c, 28.23 ± 4.01i | 9.60 ± 1.56c, 8.90 ± 1.44i | No average | HbA1c |
| McIlhenny et al., 2011, USA 140 | Not reported | 6 months | Patients with T2DM | 1 | 3 | 124 | Usual care | 50c, 48i | 61.8 ± 10.88c, 65.8 ± 14.04i | 48%c, 54%i | No data | 7.44 ± 1.65c, 7.12 ± 1.61i | No data | HbA1c, weight |
| Kim et al., 2006, Korea 141 | Not reported | 12 weeks | Patients with T2DM, HbA1c was < 10%, FBS <240 mg/dL | 2 | 3 | 145 | Usual care | 23c, Web-based: 28i, Printed-material: 22i | No data | No data | No data | 7.87 ± 1.52c, Web-based: 7.99 ± 1.22i, Printed-materia: 7.51 ± 1.40i | No data | HbA1c, FBS |
The studies were evaluated using the Study Quality Assessment Tools (SQAT) 7 of the National Institute of Health. A total of 49 studies out of 103 controlled intervention trials received a good rating, while 54 received a fair rating (Supplemental Table S3). Among 13 cohort studies, 2 had a good rating, and 11 had a fair rating (Supplemental Table S4). All seven before–after (pre–post) studies with no control group had a fair rating (Supplemental Table S5). One initial trial, one case study, one pilot study, and eight two-arm studies that did not report the study design were evaluated using Quality Assessment of Controlled Intervention Studies, 2 studies received a fair rating, and 9 received a poor rating (Supplemental Table S3).
Description of telemedicine intervention
The application of telemedicine in type 2 diabetes management has adopted various communication procedures to communicate and perform interventions, including short message service (57/134 studies), telephone (77/134 studies), web-based (59/134 studies), mobile phone app (30/134 studies), and video conferencing system (15/134 studies) (Figure 2).
Figure 2.
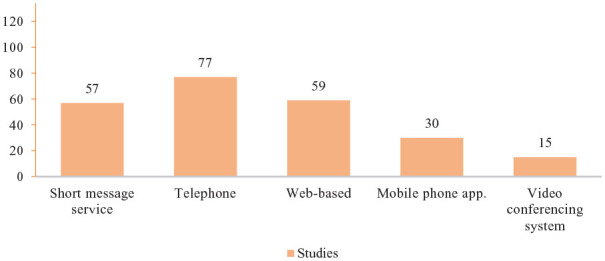
The medium of communication used.
Telemedicine strategies used in the studies included tele-consultation (59/134 studies), tele-education (90/134 studies), tele-case management (67/134 studies), tele-monitoring (100/134 studies) studies, and tele-mentoring (57/134 studies) (Figure 3).
Figure 3.
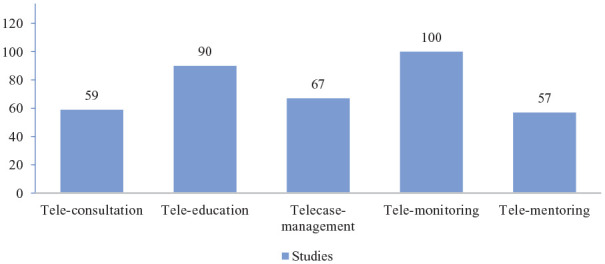
Telemedicine strategies.
The effectiveness of telemedicine in improved glycemic control
The change in glycemic index assessed the effectiveness of telemedicine in glycemic control before and after innovation. In 11 noncontrolled cohort studies and 7 pretest and posttest studies, 2 studies showed no impact of telemedicine use on glycemic control in patients,121,130 3 studies showed telemedicine application helps improve blood sugar index but not statistically significant,116,126,127 13 studies showed that telemedicine significantly improved clinical outcomes in patients.111–115,117,118,120,122,124,125,128,129 There were significant differences in HbA1c at baseline and HbA1c at the end of the follow-up period (Table 2).111–115,117,118,120,122,124,125,128,129 Telemedicine has proved effective in providing glycemic control results that are comparable to therapies that are widely recognized. 113 Better glycemic control results were linked to higher patient activation and engagement levels with telemedicine technology.114,117 Telemedicine solutions might help improve illness management. 120
Table 2.
The effectiveness of telemedicine in improved glycemic control, obesity control, and quality of life.
| Author, Year, Country | Study design | Follow-up period and n patients last visit | Primary Outcome | Secondary Outcome | Conclusion | ||
|---|---|---|---|---|---|---|---|
| Control | Telemedicine | Control | Telemedicine | ||||
| Hansel et al., 2017, France 8 | Randomized trial | 4 months, control group (n = 55), telemedicine group (n = 49) | Average change in HbA1c value was 0.23% (95% CI: 0.73) | Average change in HbA1c value was −0.37% (95% CI: 1.04) (p<0.001 vs. control) (n = 48) | Average change in body weight was 0.2 (kg) (95% CI: 2.6) | Average change in body weight was −2.9 (kg) (95% CI: 3.1) (p<0.001 vs. control) (n = 47) | Body weight and HbA1c changes improved significantly in the intervention |
| Kim and Jeong, 2007, South Korea 9 | A control group pretest–posttest design | 6 months, control group (n = 26), telemedicine group (n = 25) | HbA1c value was 7.70 ± 0.90%, FBG was 149.5 ± 39.3 mg/dl, 2 h post−meal glucose was 218.0 ± 82.0 mg/dl | HbA1c value was 7.04 ± 1.39% (p<0.05 vs. baseline) FBG was 145.7 ± 39.7 mg/dl, 2 h post−meal glucose was 192.6 ± 55.2 mg/dl (p<0.05 vs. baseline) | No data | No data | Web-based intervention using SMS of cellular phone improved HbA1c and 2HPMG for 6 months in type-2 diabetic patients |
| Basudev et al., 2016, United Kingdom 10 | Prospective randomized controlled study | 12 months, control group (n = 88), telemedicine group (n = 79) | HbA1c value was 9.4 ± 1.7%, change in HbA1c value was −0.8 ± 1.9 | HbA1c value was 9.6 ± 1.7%, change in HbA1c value was −0.6 ± 1.7 (p = 0.4 vs. control) | Change in body weight was 0.2 ± 5.4 (kg). Change in BMI was 0.20 ± 1.9 kg/m2 | Change in body value was 0.2 ± 5.4 (kg) (p = 0.99 vs. control), Change in BMI was 0.13 ± 2.0 kg/m2 (p = 0.84 vs. control) | The virtual clinic model showed improvement in metabolic control, HbA1c within 12 months, however it was not significantly superior to the control group |
| Nicolucci et al., 2015, Italy 11 | A randomized, parallel-group (1:1), open-label, multicenter study | 12 months, control group (n = 135), telemedicine group (n = 114) | HbA1c value was 7.78 ± 1.1% | HbA1c value was 7.44 ± 1.0% (p = 0.001 vs. control) | Body weight was 81.3 ± 14.3 kg | Body weight was 82.2 ± 15.4 kg (p = 0.66 vs. control), All SF−36 QoL scores improved in the telemedicine group but not in the control group | Use of the HT system was associated with better metabolic control and quality of life |
| Oh et al., 2003, South Korea 12 | A randomized comparison experimental design | 3 months, control group (n = 18), telemedicine group (n = 20) | Average change in HbA1c value was 0.6 ± 0.9% (p = 0.005 vs. baseline). . BG decreased of 6.9 ± 68.5 mg/dl (p = 0.675 vs. baseline). Two hours post−meal glucose was increased of 19.6 ± 75.3 mg/dl (p = 0.315 vs. baseline) | Average change in HbA1c value was −1.2 ± 1.5% (p = 0.002 vs. baseline, p = 0.000 vs. control). FBG decreased of 15.7 ± 52.0 mg/dl (p = 0.193 vs. baseline, p = 0.245 vs. control). Two hours postmeal glucose was decreased of 42.6 ± 114.8 mg/dl (p = 0.114 vs. baseline, p = 0.071 vs. control) | BMI increased of 0.2 ± 0.6 kg/m2 (p = 0.278 vs. baseline) | BMI increased of 0.3 ± 0.6 kg/m2 (p = 0.068 vs. baseline, p = 0.607 vs. control) | A near-normal glycemic control delivered by telephone would imrprove HbA1c, but would not significantly affect BMI |
| Stone et al., 2010, USA 13 | Randomized controlled trial | 6 months, home telemonitoring (n = 64), monthly care coordination telephone call (n = 73) | HbA1c value was 8.6 ± 1.3% | HbA1c value was 7.9 ± 1.2%, HbA1c was 0.7% lower than monthly care coordination telephone call (p < 0.001) | Body weight was 223.9 ± 48.6 (lb) | Body weight was 229.5 ± 47.6 (lb) (p = 0.49 vs. monthly care coordination telephone call) | Compared with the monthly care coordination telephone call group, the home telemonitoring group demonstrated significantly greater reductions in HbA1c |
| Kim and Oh, 2003, Korea 14 | Randomized controlled trial | 12 weeks, control group (n = 16), telemedicine group (n = 20) | HbA1c value was 8.8 ± 0.9% (p < 0.05 vs. baseline) | HbA1c value was 7.6 ± 1.0% (p < 0.05 vs. baseline) | No data | No data | The nurse telephone intervention can improve HbA1c |
| Khanna et al., 2014, Spanish 15 | Prospective, randomized, open-label trial with blinded endpoint assessment | 12 weeks, control group (n = 26), telemedicine group (n = 23) | Average change in HbA1c value was −0.3% | Average change in HbA1c value was −0.1% (p = 0.41 vs. control) | Average change in BMI value was −0.1 kg/m2 | Average change in BMI value was 0.4 kg/m2 (p = 0.21 vs. control) | There were no statistically or clinically significant differences between these 2 groups in changes in HbA1c |
| Cho et al., 2017, Korea 16 | A randomized, prospective open trial | 6 months, control group (n = 240), telemedicine group (n = 244) | Average change in HbA1c value was −0.11 ± 0.76%, Average change in Fasting blood glucose value was −6.86 ± 33.8 mg/dl, Average change in Postprandial glucose value was −1.65 ± 74.9 mg/dl | Average change in HbA1c value was −0.31 ± 0.7% (p < 0.05 vs. control), Average change in Fasting blood glucose value was −14 ± 40.2 mg/dl, Average change in Postprandial glucose value was −18.6 ± 71.4 (p < 0.05 vs. control) | Average change in BMI value was −0.2 ± 1.28 kg/m2, DTSQ scores were 26.7 ± 5.8 | Average change in BMI value was −0.33 ± 0.77 kg/m2, DTSQ scores were 27.9 ± 6.48 (p < 0.05 vs. control) | Internet-based health gateway device was effective in glucose control, including HbA1c reduction and postprandial glucose level. The intervention did not decrease, patient quality of life. |
| Welch et al., 2011, USA 17 | Randomized controlled trial | 12 months, control group (n = 18), telemedicine group (n = 21) | HbA1c value was 7.9 ± 1.4%, Average change in HbA1c value was −0.6 ± 1.1% | HbA1c value was 7.4 ± 1.4% (p = 0.26 vs. control), Average change in HbA1c value was −1.6 ± 1.4% (p = 0.01 vs. control) | BMI value was 33.8 ± 6.9 g/cm2 | BMI value was 32.6 ± 6.3 g/cm2 | The Comprehensive, Diabetes Management Program intervention was more effective than an attention control condition in helping patients meet evidence-based guidelines for diabetes care |
| Fortmann et al., 2017, USA 18 | A randomized, nonblinded, parallel-groups clinical trial | 6 months, control group (n = 59), telemedicine group (n = 50) | HbA1c value was 9.4 ± 2.0%, FBG value was 186.5 ± 68.5 mg/dl | HbA1c value was 8.5 ± 1.2% (p = 0.03 vs. control), FBG value was 161.3 ± 49.7 mg/dl | BMI value was 32.1 ± 6.6 kg/m2 (n = 58) | BMI value was 31.9 ± 5.4 kg/m2 | Dulce Digital group achieved a significantly greater reduction in HbA1c over time compared with usual care |
| Yang et al., 2020, Korea 19 | Randomized controlled trial | 3 months, control group (n = 94), telemedicine group (n = 145) | Average change in HbA1c value was −0.28% (95% CI: −0.42 to −0.13), Average change in FBG value was −2.41 mg/dl (95% CI: −13.64 to 8.82) | Average change in HbA1c value was −0.63% (95% CI: −0.77 to −0.50), Adjusted mean HbA1c difference to control was −0.30 (95% CI: −0.50 to −0.11) (p = 0.003), Average change in FBG value was −19.11 mg/dl (95% CI: −29.80 to −8.43), Adjusted mean FBG difference to control was −17.29 mg/dl (95% CI: −29.33 to −5.26) (p = 0.005) | BMI changed −0.41 (95% CI: −1.21 to 0.40) (kg/m2) from baseline | BMI changed −0.26 (95% CI: −0.40 to −0.11) (kg/m2) from baseline | The mobile phone–based glucose-monitoring and feedback system was effective in glycemic control when applied in primary care clinic settings. This system could be utilized effectively with diverse institutions and patients. |
| Wakefield et al., 2014, Missouri 20 | A single-center randomized controlled clinical trial | 3 months, control group (n = 53), telemedicine group (n = 41) | HbA1c value was 7.4 ± 0.18% (mean ± SE) | HbA1c value was 7.2 ± 0.2% (mean ± SE) | No data | No data | There were no statistically significant differences in HbA1c between the intervention and control participants |
| Egede et al., 2018, USA 21 | A randomized controlled trial | 12 months, control group (n = 47), telemedicine group (n = 43) | HbA1c value was 7.698% | HbA1c value was 6.875%, Difference between telemedicine and same room was −0.82 (p = 0.0061, 95% CI: −1.405, 0.241) | No data | No data | There was a significant main effect of the treatment group on the mean HbA1c value at the study end |
| Steventon et al., 2014, United Kingdom 22 | A large cluster randomized trial | 12 months, control group (n = 213), telemedicine group (n = 300) | The HbA1c value in the telemedicine group was lower than 0.21% control group (95% CI: 0.04% to 0.38%, p = 0.013) | No data | No data | Telehealth modestly improved glycaemic control in patients with type 2 diabetes over 12 months | |
| Duruturk and Özköslü, 2019, Turkey 23 | A double blind randomized controlled trial | 6 weeks, control group (n = 21), telemedicine group (n = 23) | HbA1c value was 7.92 ± 2.82% (p = 0.23 vs. baseline) | HbA1c value was 5.93 ± 1.46% (p < 0.05 vs. baseline) | No data | No data | Tele-rehabilitation intervention in Patients with T2DM could lead to improvements in glucose control |
| Cho et al., 2011, Korea 24 | A randomized controlled trial | 24 weeks, control group (n = 39), telemedicine group (n = 36) | HbA1c ⩽ 6.5% in both groups maintained their HbA1c at <6.5% (6.0 to 6.4% for the SAVE, group; 6.1 to 6.4% for the control group; p < 0.01 for both), HbA1c was also maintained in patients with baseline, HbA1c >6.5% (7.3 to 7.7% in the SAVE group, p = 0.062; 7.4 to 7.7% in the control group, p = 0.074) | No data | No data | The study showed the efficacy, and safety of the software for online communication in diabetes management | |
| Wakefield et al., 2011, USA 25 | Randomized controlled trial | 12 months, control group (n = 94), High−intensity group (n = 73), Low−intensity group (n = 79) | There was no significant difference between the change scores for the three groups [F(2, 1027) = 0.43, p = 0.65] | No data | No data | The intervention groups were comparable with the control group | |
| Kim et al., 2008, South Korea 26 | Quasi-experimental design | 12 months, control group (n = 16), telemedicine group (n = 18) | HbA1c value was 8.19 ± 0.54%, FBG was 175.8 ± 53.9 mg/dl, Two hours post−meal glucose was 264.7 ± 89.2 mg/dl | HbA1c value was 6.67 ± 0.77% (p < 0.05 vs. baseline), FBG was 149.6 ± 50.0 mg/dl, Two hours post−meal glucose was 169.7 ± 44.7 mg/dl (p < 0.05 vs. baseline) | No data | No data | This web-based intervention using SMS of personal cellular phone improved HbA1c in obese type 2 diabetic patients |
| Katula et al., 2022, USA 27 | Single-blind RCT | 12 months, control group (n = 300), telemedicine group (n = 299) | Average change in HbA1c value was −0.16% (95% CI: − 0.19 to − 0.12) | Average change in HbA1c value was −0.23% (95% CI: − 0.26 to −0.20), The between−group, difference in change in HbA1c was −0.08 (95% CI: −0.12 to −0.03, p < 0.025) | Body weight changed from baseline was −2.18 kg (95% CI: −2.97 to −1.39) | Body weight changed from baseline was −5.52 kg (95% CI: −6.30 to −4.75), The between−group, difference in change in BMI was −3.34 kg/m2 (95% CI: −4.39 to −2.29, p < 0.001) | Digital Diabetes Prevention Programs demonstrated clinical effectiveness and has significant potential for widespread dissemination and impact |
| Hu et al., 2021, China 28 | A randomized controlled trial | 6 months, control group (n = 70), telemedicine group (n = 72) | HbA1c value was 8.22 ± 2.04% | HbA1c value was 7.38 ± 1.67% (p = 0.008 vs. control) | No data | No data | After 6 months of follow-up, the telemedicine group, compared with the control group, showed significant decreases in HbA1c |
| Warren et al., 2018, Australia 29 | A prospective randomised controlled trial | 6 months, control group (n = 63), telemedicine group (n = 63) | HbA1c value was 8.1% [7.4−8.9] | HbA1c value was 7.5% [6.9−8.2], The reduction HbA1c values in the intervention group over time was significantly greater than in the control group (p < 0.01) | BMI value was 33.6 kg/m2 [29.5−38.4] | BMI value was 34.5 kg/m2, [30.3−39.6] | The Townsville Broadband Diabetes Telehealth trial showed that a positive effect on glycaemic control resulted from participation in a telemonitoring intervention when compared with usual care |
| Cho et al., 2011, Korea 30 | A randomized controlled design | 3 months, control group (n = 35), telemedicine group (n = 36) | HbA1c value was 7.8 ± 1.1 % (p = 0.11 vs. baseline) | HbA1c value was 7.5 ± 0.9% (p < 0.01 vs. baseline) | No data | No data | Compared with baseline, HbA1c was significantly reduced at three-month follow-up in the intervention group, but not in the control group |
| Jia et al., 2021, China 31 | A cluster randomized trial | 12 months | No data | Compared with usual care, the intervention led to an absolute improvement in the HbA1c control rate of 7.0% (95% confidence interval [CI] 4.0% to 10.0%) | No data | No data | After 1 year of application and follow-up, HbA1c was significantly reduced in primary care. |
| Trief et al., 2016, USA 32 | Randomized clinical trial | 12 months, diabetes education (n = 78), individual calls (n = 93), couples calls (n = 97) | No data | Significant decreased in HbA1c for all (12 months: cc −0.47%, ic −0.52%, de −0.57%), with no differences between arms | No data | CC showed significant improvement | Education alone was beneficial, but additional intervention is needed to achieve glycemic targets |
| Wayne et al., 2015, Canada 33 | Pragmatic randomized controlled trial | 6 months, control group (n = 49), telemedicine group (n = 48) | HbA1c value was 7.88 ± 1.17% | HbA1c value was 8.13 ± 1.27% | BMI value was 37.21 ± 8.22 kg/m2 (n = 36) | BMI value was 33.53 ± 6.80 kg/m2 (n = 39) | There was not statistically significant at 6 months because the control group’s mean HbA1c reduction improved between 3 and 6 months while the intervention group’s HbA1c level remained stable |
| Benson et al., 2019, USA 34 | Randomized controlled trial | 12 months, control group (n = 58), telemedicine group (n = 60) | HbA1c value was 7.7 ± 0.20% (mean ± SE) | HbA1c value was 7.4 ± 0.15 (mean ± SE) | BMI value was 35.7 ± 0.83 kg/m2 (mean ± SE) | BMI value was 37.9 ± 1.32 kg/m2 (mean ± SE) | The magnitude of change for most individual diabetes measures was somewhat similar in both groups |
| Hee-Sung, 2007, Korea 35 | A control group pretest–post-test design | 12 weeks, HbA1c <7.0% at baseline, control group (n = 11), telemedicine group (n = 13), HbA1c >= 7.0% at baseline, control group (n = 15), telemedicine group (n = 12) | HbA1c <7.0% at baseline: Average change in HbA1c value was 0.43 ± 0.53% (p = 0.034), HbA1c ⩾ 7.0% at baseline: Average change in HbA1c value was −0.22 ± 0.88% (p = 0.336) | HbA1c <7.0% at baseline: Average change in HbA1c value was −0.21 ± 0.57% (p = 0.201), HbA1c ⩾7.0% at baseline: Average change in HbA1c value was −2.15 ± 2.25% (p = 0.007) | No data | No data | There was a significant percentage change in a baseline-glycosylated haemoglobin ⩾ 7.0% for the intervention group; however, no significant change for the control group after 12 weeks. |
| Xu et al., 2020, Missouri 36 | A randomized controlled trial | 12 months, control group (n = 32), telemedicine group (n = 33) | Average change in HbA1c value was −0.03 (95% CI: −0.88 to 0.82), FBG increased by 13.0 mg/dL (95% CI: −47.67, to 73.69) | Average change in HbA1c value was −0.69 (95% CI: −1.41 to 0.02), FBG decreased by 21.6 mg/dL (95% CI: −37.56 to −5.639) | No data | No data | EpxDiabetes helps to reduce HbA1c in patients with uncontrolled T2DM |
| Lu et al., 2021, China 37 | A randomized controlled trial | 6 months, control group (n = 59), telemedicine group (n = 60) | HbA1c value was 8.17 ± 1.30% (p = 0.001 vs. baseline), FBG was 7.64 ± 1.13 mmol/L (p = 0.007 vs. baseline) | HbA1c value was 7.50 ± 0.96% (p = 0.001 vs. baseline, p = 0.002 vs. control), FBG was 7.31 ± 0.84 mmol/L (p = 0.001 vs. baseline, p = 0.077 vs. control) | No data | No data | The telemedicine group showed significantly lower HbA1c at 6 months compared with the control group |
| Anderson et al., 2010, USA 38 | A randomized controlled trial | 12months, control group (n = 117), telemedicine group (n = 94) | HbA1c value was 7.74% | HbA1c value was 7.66% | BMI value was 34.69 kg/m2 | BMI value was 34.50 kg/m2 | A clinic-based telephonic disease management support for underserved patients with diabetes did not improve clinical or behavioral outcomes at 1 year as compared to patients receiving usual care alone |
| Agarwal et al., 2019, Canada 39 | Multicenter pragmatic randomized controlled trial | 6 months, control group (n = 67), telemedicine group (n = 72) | HbA1c value was 8.41% (at 3 months) | HbA1c value was 8.22% (at 3 months) | No data | No data | The results showed no difference between intervention and control arms for the primary clinical outcome of glycemic control measured by HbA1c levels |
| Cho et al., 2009, Korea 40 | Randomized controlled trial | 3 months, Internet group (n = 34), Phone group (n = 35) | no control | HbA1c value was 6.9% (p < 0.01) in internet group, and 7.1% (p < 0.01) in phone group, Two−hour postprandial glucose levels also decreased significantly in both groups after three months (p = 0.001), but FBG levels did not change (p = 0.07) | No data | No data | Mobile, bidirectional communication between doctors and patients using the diabetes phone was as effective for glucose control |
| Quinn et al., 2016, USA 41 | Randomized controlled trial | 12 months, control group (n = 56), telemedicine group (n = 62) | HbA1c changed by−0.3% (95% CI = [−0.9, 0.3]) in older patients and −1.0% (95% CI = [−1.6, −0.4]) in younger group | HbA1c changed by−1.8% (95% CI = [−2.4, −1.1]) in older patients and −2.0% (95% CI = [−2.5, −1.5]) in younger group. The difference in 12−month changes (intervention − control) was −1.4% (95% CI = [−2.3, 0.6], p = 0.001) among older patients and −1.0% (95% CI =[−1.6, −0.4], p = 0.02) in younger group | No data | No data | Mobile PCS can be a useful intervention for those older patients with Type 2 diabetes, which contributed to a significant decrease in HbA1c over the 12-month study period, this could become mainstream in the coming years |
| Sun et al., 2019, China 42 | Randomized controlled trial | 6 months, control group (n = 47), telemedicine group (n = 44) | HbA1c value was 7.22 ± 0.87% | HbA1c value was 6.84 ± 0.76% (p < 0.05 vs. baseline, p = 0.02 vs control) | BMI value was 22.62 kg/m2 | BMI value was 23.8 kg/m2 | Mobile phone–based telemedicine apps help improve glycemic control in older Chinese patients with T2DM |
| Lim et al., 2016, Korea 43 | Randomized, controlled clinical trial | 6 months, control group − SMBG (n = 43), U−heathcare group (n = 42) | HbA1c value was 7.9 ± 1.2 % (p = 0.936 vs. baseline) | HbA1c value was 7.3 ± 0.9 % (p < 0.001 vs.baseline) | BMI value was 26.5 ± 3.7 kg/m2 (p = 0.110 vs. baseline) | BMI value was 25.7 ± 3.6 kg/m2 (p = 0.002 vs. baseline) | the patients using the multidisciplinary u-healthcare service showed better glycemic control with less hypoglycemia than those in the SMBG group |
| Tang et al., 2013, USA 44 | Randomized clinical trial | 12 months, control group (n = 193), telemedicine group (n = 186) | HbA1c value was 8.33 ± 1.81 % | HbA1c value was 8.1 ± 1.68 % (p = 0.133 vs.control) | No data | No data | INT patients achieved greater decreases in A1C at 6 months than UC patients, but the differences were not sustained at 12 months |
| Greenwood et al., 2015, USA 45 | Randomized clinical trial | 6 months, control group (n = 41), telemedicine group (n = 40) | HbA1c value was 7.46% | HbA1c value was 7.35% (p = 0.55 vs. control) | No data | No data | An eHealth model incorporating a complete feedback loop with telehealth remote monitoring and paired glucose testing with asynchronous data analysis significantly improved A1c levels compared to usual care. |
| Williams et al., 2012, Australia 46 | Randomised controlled trial | 6 month, control group (n = 60), telemedicine group (n = 60) | HbA1c value was 8.7% | HbA1c value was 7.9% | HRQ−mental was significantly different between the, two arms at six months (difference = 3.0, p = 0.007), No differences, were observed in HRQL−physical (p = 0.7) | TLC Diabetes program with clinically significant postintervention improvements in both glycaemic control and mental HRQL | |
| Ramadas et al., 2018, Malaysia 47 | Randomized clinical trial | 12 months, control group (n = 55), telemedicine group (n = 63) | HbA1c value was 8.4 ± 2.2% (p = 0.001 vs. baseline), FBG value was 7.7 ± 2.6 mmol/L (p = 0.117 vs. baseline) | HbA1c value was 8.5 ± 1.8% (p = 0.004 vs. baseline), FBG value was 7.9 ± 2.5 mmol/L (p = 0.015 vs. baseline) | No data | No data | E-intervention can be a feasible method for implementing chronic disease management in developing countries. |
| Egede et al., 2017, United States 48 | Randomized clinical trial | 6 months, control group (n = 44), telemedicine group (n = 41) | No data | The levels of HbA1c, in the TACM group were 0.99 points significantly lower compared to the usual care group (p = 0.024) | No data | No data | Participants in the techtechnology-assisted case management intervention group had significantly lower HbA1c levels at 6 months post randomization compared to participants in the usual care group. |
| Kim et al., 2016, China 49 | Randomized open-label, parallel group design | 6 months, control group (n = 90), telemedicine group (n = 92) | HbA1c value was 7.40 ± 1.30% (p < 0.001 vs. baseline), FBG was 7.8 ± 2.4 mmol/L (p = 0.058 vs. baseline), Post−prandial blood glucose was 12.0 ± 3.0 mmol/L (p = 0.088 vs. baseline), | HbA1c value was 6.70 ± 0.70% (p < 0.001 vs. baseline, p < 0.01 vs. control), FBG was 7.1 ± 1.6 mmol/L (p = 0.005 vs. baseline), Post−prandial blood glucose was 10.7 ± 2.0 mmol/L (p < 0.001 vs. baseline), | BMI value was 25.2 ± 3.6 kg/m2 (p = 0.564 vs. baseline) | BMI value was 25.7 ± 2.6 kg/m2 (p = 0.089 vs. baseline) | The Internet-based glucose monitoring system was effective in improving blood sugar levels among patients with diabetes |
| Goode et al., 2015, Australia 50 | A randomized trial | 24 months, control group (n = 131), telemedicine group (n = 181) | no data | Average back transformed from natural log HbA1c was:, − Low: 1.01% (95% CI: 0.96, 1.06), p = 0.69, − Medium: 0.98% (95% CI: 0.94, 1.03), p = 0.44, − High: 0.99 % (95% CI: 0.96, 1.03), p = 0.69 | no data | no data | There was no significant difference in the associations of call completion with any outcome |
| Jeong et al., 2018, Korea 51 | Randomized clinical trial | 24 weeks, control group (n = 101), telemonitoring group (n = 99), telemedicine group (n = 99) | HbA1c reduced 0.66 ± 1.03%. (p < 0.001 vs. baseline) | HbA1c reduced 0.66% ± 1.09% in telemonitoring group, 0.81%± 1.05% in the telemedicine group (p < 0.001 vs. baseline) | No data | No data | Telehealthcare was as effective as conventional care at improving glycemia in patients with type 2 diabetes without serious adverse effects. |
| Nagrebetsky et al., 2013, United Kingdom 52 | Feasibility trial | 6 months, control group (n = 7), telemedicine group (n = 7) | The median (IOR) change in HbA1c was −0.5% [−1.2% to 0.6%] | The median (IOR) change in HbA1c was −0.9% [−1.9% to 0%] | No data | No data | Self-titration of oral glucose-lowering medication in type 2 diabetes with self-monitoring and remote monitoring of blood glucose levels by clinical staff was feasible in primary care and may improve clinical outcomes |
| Wild et al., 2016, United Kingdom 53 | Randomized clinical trial | 9 months, control group (n = 139), telemedicine group (n = 146) | HbA1c value was 8.4 ± 1.3% | HbA1c value was 7.9 ± 1.4%., The absolute mean difference in HbA1c between groups was −0.51% (p = 0.007) | No data | No data | Supported telemonitoring resulted in clinically important improvements in control of glycaemia in patients with type 2 diabetes in family practice |
| de Vasconcelos et al., 2018, Brazil 54 | Randomized clinical trial | 24 weeks, control group (n = 15), telemedicine group (n = 16) | HbA1c value increased from 6.9 ± 1.31% to 7.33 ± 1.73% | HbA1c value decreased from 8.0 ± 2.14% to 7.21 ± 1.19% | BMI value was 30.23 ± 5.29 kg/m2 | BMI value was 29.96 ± 6.04 kg/m2 (p = 0.92 vs. control) | Telecoaching is an effective tool for diabetes management |
| Rasmussen et al., 2016, Denmark 55 | Randomised controlled trial | 6 months, control group (n = 22), telemonitoring group (n = 18) | Average change in HbA1c value was −10.6% (65 to 55 mmol/mol), Average change in blood glucose levels value was −13.1% (10.3 to 8.7 mmol/l) | Average change in HbA1c value was −14.6% (76 to 61 mmol/mol, p=0.016 vs. control), Average change in blood glucose levels value was −17.6% ( 11.7 to 9.7 mmol/l, p=0.015 vs. control) | Average change in Weight value was 1.7 kg (88 to 86.7) | Average change in Weight value was 0.6 kg (99.7 to 99.1, p=0.023 vs. control) | In the direct comparison of home video consultations vs standard outpatient treatment in type 2 diabetes mellitus, telemedicine was a safe and available option with favourable outcomes after six months treatment. |
| Rodríguez-Idígoras et al., 2009, Spain 56 | Randomized controlled parallel-group trial | 12 months, control group (n = 151), telemedicine group (n = 146) | HbA1c value was 7.35 ± 1.38% (p = 0.303 vs. baseline) | HbA1c value was 7.4 ± 1.43% (p = 0.027 vs. baseline) | No data | No data | A teleassistance system using real-time transmission of blood glucose results with an option to make telephone consultations is feasible in the primary care setting as a support tool for family physicians in their follow-up of type 2 diabetes patients. |
| von Storch et al., 2019, Germany 57 | Prospective study | 3 months, control group (n = 55), telemedicine group (n = 60) | HbA1c value was 6.95 ± 1.02 % (p = 0.465 vs. baseline) (n = 54) | HbA1c value was 6.58 ± 0.723 % (p < 0.05 vs. baseline, p < 0.05 vs control) (n = 52) | BMI value was 29.39 ± 4.37 kg/m2 (p < 0.05 vs. baseline) (n =55) | BMI value was 31.8 ± 6.98 kg/m2 (p = 0.569 vs. baseline) (n = 53) | HbA1c values of the intervention group participants were significantly reduced in comparison to those in the control group after 3 months. |
| Lee et al., 2020, Malaysia 58 | Cluster-randomized controlled trial | 52 weeks, control group (n = 104), telemedicine group (n = 104) | HbA1c value was 8.70% | HbA1c value was 8.69%( p = 0.226 vs. control) | No data | No data | The addition of telemedicine in replacement of self-monitoring in diabetes care had limited clinical benefits in improving glycemic control |
| Lee et al., 2017, Malaysia 59 | Cluster-randomised controlled trial | 12 weeks, control group (n = 40), telemedicine group (n = 45) | HbA1c value was 8.55 ± 1.86% (p = 0.33 vs. baseline) | HbA1c value was 7.62 ± 1.61% (p < 0.01 vs. baseline) | BMI value was 30.49 ± 5.11 kg/m2 (p = 0.02 vs. baseline), EuroQoL−5D was 0.81 ± 0.26 | BMI value was 29.42 ± 5.92 kg/m2 (p = 0.01 vs. baseline), EuroQoL−5D was 0.87 ± 0.11 | Mean HbA1c levels in the telemonitoring group improved by 1.07% compared with 0.24% for usual care group at the end of the study. Diabetes education was also found to be able to improve the patients’ quality of life at the end of the study. |
| Dario et al., 2017, Italy 60 | Randomized controlled trial | 12 months, control group (n = 78), telemedicine group (n = 168) | Average change in HbA1c was −0.27 ± 0.99% | Average change in HbA1c was −0.26 ± 0.92% (p = 0.76 vs. control) | No data | No data | There was no statistically, significant difference in HbA1c between the two groups |
| Egede et al., 2017, USA 61 | Randomized controlled trial | 12 months, n = 255 | HbA1c at 12 months for the intervention groups did not differ significantly from that of the control group (knowledge: 0.49, p = 0.123; skills: 0.23, p = 0.456; combined: 0.48, p = 0.105). | Did not show any significant improvement in HRQoL in both groups | Combined education and skills training did not achieve greater reductions in glycemic control at 12 months compared to the control group, education alone, or skills training alone. | ||
| Bujnowska-Fedak et al., 2011, Poland 62 | Randomized clinical trial | 6 months, control group (n = 48), telemedicine group (n = 47) | HbA1c value was 7.43 ± 1.49 % | HbA1c value was 7.37 ± 1.27% (p = 0.72 vs. control) | BMI value was 26.4 ± 6.1 kg/m2 | BMI value was 24.8 ± 6.9 kg/m2 (p = 0.38 vs. control), The difference in QoL between the two groups was not statistically significant | Telehome monitoring is an effective tool in controlling type 2 diabetes in a primary care setting |
| Arora et al., 2014, United States 63 | Randomized controlled trial | 6 months, control group (n = 64), telemedicine group (n = 64) | Hb A1C decreased by 0.60% | Hb A1C decreased by 1.05% (p = 0.230 vs. control) | No data | No data | The TExT-MED program did not result in a statistically significant improvement in HbA1c. |
| Kardas et al., 2016, Poland 64 | A feasibility prospective parallelarm randomized controlled trial | 6 weeks, control group (n = 30), telemedicine group (n = 30) | Average change in FBG (FBG) (mg/dL) was 11.7 ± 36.1 (148.9 ± 43.5 to 137.2 ± 36.6) (p > 0.05), Average change in HbA1c (%) was 0.01 ± 0.36 (6.84 ± 0.98 to 6.78 ± 0.92) (p > 0.05) | Average change in FBG (FBG) (mg/dL) was 9.5 ± 22.5 (145.2 ± 40.7 to 135.7 ± +61.6) (p < 0.05), Average change in HbA1c (%) was 0.04 ± 0.52 (6.78 ± 1.10 to 6.75 ± 0.95) (p > 0.05) | Health related quality of life, as assessed with cumulative utility measure, improved significantly in COMMODITY12 system users (p < 0.05) | mHealth solution was well accepted by type 2 diabetes patients taking part in clinical trial, leading to several clinical benefits, and improved quality of life. | |
| McFarland et al., 2012, USA 65 | Nonrandomized, parallel, control group study | 6 months, control group (n = 67), telemedicine group (n = 36) | Average change in HbA1c (%) was 2.1 ± 1.7 (9.0 ± 1.5 to 6.9 ± 1.0) | Average change in HbA1c (%) was 1.6 ± 1.2 (9.1 ± 1.6 to 7.5 ± 1.1) (p = 0.1987 vs. control) | No data | No data | No statistically significant difference was demonstrated with respect to change in A1C from baseline to 6 months |
| Hansen et al., 2017, Denmark 66 | Cross-sectional randomized controlled trial | 8 months, control group (n = 77), telemedicine group (n = 69) | Average change in HbA1c was 0.18% (p = 0.22 vs. baseline) | Average change in HbA1c was 0.69% (p < 0.000001 vs. baseline) | No data | No data | Video consultations preceded by uploading relevant measurements can lead to clinically and statistically significant improvements in glycemic control among patients who have not responded to standard regimens |
| Zhou et al., 2014, China 67 | Prospective randomized study | 3 months, control group (n = 55), telemedicine group (n = 53) | HbA1c decreased from 8.22±1.58 to 7.60±1.57%.(p = 0.001 vs. baseline), FBG decreased from 8.73±2.60 to 8.02±2.38 mmol/L (p = 0.007 vs. baseline) | HbA1c decreased from 8.44±1.58 to 6.84±1.20% (p < 0.001 vs. baseline), FBG decreased from 8.73±2.61 to 7.06±1.49 mmol/L (p < 0.001 vs. baseline) | BMI value was 23.75±2.93 kg/m2 | BMI value was 24.72±3.36 kg/m2 | Telemedicine system can provide a tighter glycemic control for the treatment of Patients with T2DM |
| Luley et al., 2011, Germany 68 | Randomized clinical trial | 6 months, control group (n = 35), telemedicine group (n = 33) | HbA1c increased by 0.2% (p = 0.053 vs. baseline) | HbA1c decreased by 0.8% (p < 0.0125 vs. basseline) | BMI decreased by 0.1 kg/m2 (no significant) | BMI decreased by 4.1 kg/m2 (p < 0.0125 vs. basseline) | The ABC program effectively lowers body weight, Hb1Ac in patients with type 2 diabetes. |
| Hsu et al., 2016, USA 69 | A randomized controlled study | 12 weeks, control group (n = 16), telemedicine group (n = 19) | Average change in HbA1c was 2.0 ± 2.0% | Average change in HbA1c was 3.2 ± 1.5% (p = 0.048 vs. control) | No data | No data | Mobile health technology could be an effective tool in sharing data, enhancing communication, and improving glycemic control while enabling collaborative decision making in diabetes care. |
| Kleinman et al., 2017, India 70 | A randomized clinical trial | 6 months, control group (n = 46), telemedicine group (n = 44) | Average change in HbA1c was −0.8 ± 1.6%, Average change in FBS was −23.5 ± 70.0 mg/dL | Average change in HbA1c was −1.5 ± 1.1% (p = 0.02 vs. control), Average change in FBS was −32.6 ± 66.4 mg/dL (p = 0.55 vs. control) | Average change in BMI was 0.1 ± 1.1 kg/m2 | Average change in BMI was −0.1 ± 1.0 kg/m2 (p = 0.53 vs. control) | This tool could be an effective way to expand access to quality chronic disease care and improve outcomes |
| Orsama et al., 2013, Finland 71 | A randomized controlled trial | 10 months, control group (n = 24), telemedicine group (n = 24) | Average change in HbA1c was 0.036% | Average change in HbA1c was −0.4% (p = 0.022 vs. control) | Average change in weight was 0.4 kg | Average change in weight was −2.1 kg (p = 0.021 vs. control) | Results showed that the automated feedback intervention had significant effects on HbA1c and on weight, which declined reliably in intervention compared with control participants with type 2 diabetes or type 2 diabetes and hypertension. |
| Kim et al., 2007, Korea 72 | A randomized controlled trial | 12 weeks, control group (n = 26), telemedicine group (n = 25) | Average change in HbA1c was 0.07% (7.59 ± 1.09 to 7.66 ± 0.91), 2HPMG was 13.77 ± 4.2 mmol/l | Average change in HbA1c was −1.15% (8.09 ± 1.72 to 6.94 ± 1.04) (p < 0.05 vs. baseline), 2HPMG was 9.5 ± 4.4 mmol/l (p < 0.05 vs. baseline) | No data | No data | This educational intervention using the Internet and an SMS by cellular phone improved levels of HbA1c and 2HPMG |
| Bender et al., 2017, USA 73 | A randomized controlled trial | 6 months, control group (n = 23), telemedicine group (n = 22) | Average change in HbA1c was −0.3% (7.4 ± 0.93 to 7.1 ± 1.2), Average change in Fasting glucose was −5.4 mg/dL (137.4 ± 30.1 to 132.0 ± 33.0) | Average change in HbA1c was −0.3% (7.4 ± 0.82 to 7.1 ± 0.98), Average change in Fasting glucose was −4.3 mg/dL (133 ± 20.8 to 128.7 ± 30.6) | Average change in BMI was −0.1 kg/m2 (31.5 ± 5.1 to 30.5 ± 5.6), Average change in weight was −2.4 kg (78.8 ± 18.6 to 76.4 ± 19.8) | Average change in BMI was −0.3 kg/m2 (28.5 ± 3.6 to 27.5 ± 3.6), Average change in weight was −1.6 kg (72.6 ± 10.8 to 70.8 ± 11.0) | Improvements in fasting glucose and HbA1c give promise to the efficacy of the PilAm Go4Health mHealth intervention to enhance diabetes self-management. |
| Blackberry et al., 2013, Australia 74 | Prospective, cluster randomised controlled trial | 18 months, control group (n = 222), telemedicine group (n = 220) | Average change in HbA1c was −0.22% (8.13 ± 1.34 to 7.91 ± 1.42) | Average change in HbA1c was −0.13% (7.98 ± 1.22 to 7.85 ± 1.24) (p = 0.84 vs. control) | Average change in weight was 0.5 kg (92.2 ± 20.5 to 92.7 ± 21.0) | Average change in weight was −0.3 kg (91.0 ± 19.5 to 90.7 ± 21.0) (p = 0.89 vs. control) | At 18 months’ follow-up the effect on glycaemic control did not differ significantly between the intervention and control groups |
| Borhani et al., 2013, Kerman 75 | A quasi-experimental study | 3 months, control group (n = 25), telemedicine group (n = 25) | Average change in HbA1c was −0.16% (9.38 ± 1.53 to 9.14 ± 1.59), Average change in FBS was −26.34 mg/dl (188.38 ± 54.20 to 162.04 ± 47.66), Average change in PPG was −16.48 mg/dl (247.43 ± 74.06 to 263.91 ± 69.84) | Average change in HbA1c was −1.83% (9.98 ± 1.34 to 8.15 ± 0.97) (p < 0.001 vs. baseline), Average change in FBS was −38 mg/dl (173.56 ± 54.77 to 135.12 ± 37.54), Average change in PPG was −54.92 mg/dl (257.64 ± 67.48 to 202.72 ± 45.21) (p < 0.001 vs. baseline) | Average change in BMI was −0.77 (30.69 ± 6.67 to 29.92 ± 9.05) | Average change in BMI was −0.2 (27.93 ± 4.84 to 28.13 ± 4.88) | The results showed that phone follow-ups can improve the process of self-care and the control of Glycemic index in patients with type II diabetes |
| Faridi et al., 2008, USA 76 | A pilot controlled trial | 3 months, control group (n = 15), telemedicine group (n = 15) | Average change in HbA1c was 0.3 ± 1.0% (p = 0.3813 vs. baseline) | Average change in HbA1c was −0.1 ± 0.3% (p = 0.1534 vs. baseline) | Average change in BMI was 2.2 ± 7.7 kg/m2, Average change in weight was −3.1 ± 7.5 lbs | Average change in BMI was 0.0 ± 0.9 kg/m2, Average change in weight was −0.1 ± 5.4 lbs | The results indicate the intervention had a positive impact on some clinical outcome and self-efficacy |
| Hallberg et al., 2018, USA 77 | An open-label, nonrandomized, controlled, before-and-after 1-year study | 1 year, control group (n = 72), telemedicine group (n = 204) | Average change in HbA1c was 0.20 ± 1.35)% (p = 0.21 vs. baseline) | Average change in HbA1c was − 1.29 ± 1.32% (p < 0.05 vs. baseline) | Average change in weight was 0.04 ± 5.94 kg (p = 0.95 vs. baseline) | Average change in weight was − 14.24 ± 10.29 (p < 0.05 vs. baseline) | These results demonstrate that a novel metabolic and continuous remote care model can support adults with T2D to safely improve HbA1c, weight |
| Holmen et al., 2014, Norway 78 | A 3-arm prospective randomized controlled trial | 12 months, control group (n = 41), FTA (n = 49), FTA−HC (n = 40) | Average change in HbA1c was −0.16% (95% CI: −0.50, 0.1) | FTA: Average change in HbA1c was −0.31% (95% CI: −0.67, 0.05), FTA−HC: Average change in HbA1c was −0.15% (95% CI: −0.58, 0.29) | Average change in weight was −1.2 kg (95% CI: –2.75, 0.54) | FTA: Average change in weight was −1.3 kg (95% CI: –3.05, 0.43), FTA−HC: Average change in weight was −0.7 kg (95% CI: –2.29, 0.84) | Although HbA1c level declined in all groups, the change did not differ significantly between either of the intervention groups and the control group after 1 year |
| Lim et al., 2011, Korea 79 | A randomized controlled trial | 6 months, control group (n = 48), u−healthcare (n = 49), SMBG (n = 47) | HbA1c decreased from 7.9 ± 0.8% to 7.8 ± 1.0% (p = 0.274) | HbA1c level was significantly decreased from 7.8 ± 1.3% to 7.4 ± 1.0% (p < 0.001) in the u−healthcare group and from 7.9 ± 1.0% to 7.7 ± 1.0% (p = 0.020) in the SMBG group | BMI was 25.8 ± 3.4 kg/m2 (p = 0.005 vs. baseline) | u−healthcare group: BMI was 24.4 ± 2.5 kg/m2 (p = 0.009 vs. baseline), SMBG group: BMI was 25.0 ± 3.2 kg/m2 (p = 0.303 vs. baseline) | The CDSS-based u-healthcare service achieved better glycemic control with less hypoglycemia than SMBG and routine care and may provide effective and safe diabetes management in the elderly diabetic patient |
| Odnoletkova et al., 2016, Belgium 80 | A parallel-group, randomized controlled trial | 18 months, control group (n = 246), telemedicine group (n = 240) | HbA1c level was 7.0 ± 1.1% | HbA1c level was 6.9 ± 1.0% (p = 0.046 vs. control) | BMI was 30.4 ± 5.1 kg/m2 (n = 246) | BMI was 29.9 ± 5.0 kg/m2 (p = 0.602 vs. control) (n = 238) | Twelve months after the intervention completion, there were sustained improvements in glycaemic control |
| Quinn et al., 2011, USA 81 | A cluster-randomized clinical trial | 12 months, control group (n = 51), CO group (n = 21), CPP group (n =21), CPDS group (n = 56) | Average change in HbA1c was −0.7% (95% CI: −1.1, −0.3) | CO: Average change in HbA1c was −1.6 (95% CI: −2.3, −1.0), CPP: Average change in HbA1c was −1.2 (95% CI: −1.8, −0.5), CPDS: Average change in HbA1c was −1.9 (95% CI: −2.3, −1.5) | No data | No data | The mean declines in glycated hemoglobin were 1.9% in the maximal treatment group and 0.7% in the usual care group, a difference of 1.2% (p = 0.001) over 12 months |
| Rothman et al., 2005, USA 82 | A randomized controlled trial | 12 months, control group (n = 95), telemedicine group (n = 99) | Average change in HbA1c was −1.6% | Average change in HbA1c was −2.5% (difference, 0.8%; 95% CI: 0% to 1.7%; p <, 0.05 vs. control) | No data | No data | The comprehensive disease management program reduced HbA1c levels among patients with type 2 diabetes and poor glycemic control. |
| Varney et al., 2014, Australia 83 | A random controlled trial | 12 months, control group (n = 36), telemedicine group (n = 35) | HbA1c level was 8.4% (95% CI: 8.0, 8.7) | HbA1c level was 8.2% (95% CI: 7.9, 8.6) | BMI was 31.7 kg/m2 | BMI was 31.6 kg/m2 | Telephone coaching improved glycaemic control and adherence to complication screening in people with type 2 diabetes, for the duration of its delivery, but these effects were not maintained on withdrawal of the intervention |
| Waki et al., 2014, Japan 84 | A nonblinded randomized controlled study | 3 months, control group (n = 27), telemedicine group (n = 27) | Average change in HbA1c was 0.1 %, Average change in FBS was 16.9 mg/dl | Average change in HbA1c was −0.4% (p = 0.015 vs. control), Average change in FBS was −5.5 (p = 0.019 vs. control) | BMI was 27.1 ± 7.5 kg/m2 | BMI was 25.9 ± 5.9 kg/m2 | HbA1c and FBS values declined significantly in the DialBetics group |
| Wang et al., 2019, China 85 | A random controlled trial | 6 months, control group (n = 60), telemedicine group (n = 60) | HbA1c value was 7.92 ± 2.15% (p < 0.05 vs. baseline), FBG was 7.96 ± 3.63 mmol/l (p < 0.05 vs. baseline), Two hours post−meal glucose was 12.67 ± 3.42 mmol/l (p < 0.05 vs. baseline) | HbA1c value was 7.12 ± 2.01% (p < 0.05 vs. baseline and control), FBG was 6.58 ± 3.02 mmol/l (p < 0.05 vs. baseline and control), Two hours post−meal glucose was 10.43 ± 3.12 mmol/l (p < 0.05 vs. baseline and control) | No data | No data | After the intervention, levels of FPG, 2-hour postprandial blood glucose, and HbA1c were lower in the test group than in the control group; the differences were statistically significant |
| Kusnanto et al., 2019, Indonesia 86 | A randomized experimental study | 3 months, control group (n = 15), telemedicine group (n = 15) | HbA1c value was 7.91 ± 0.88% (p = 0.208 vs. baseline) | HbA1c value was 7.64 ± 1.29% (p = 0.001 vs. baseline, p = 0.005 vs. control) | No data | No data | The HbA1c values in the experimental group was significant and was not significant in the control group. Independent t-tests also showed significant value comparison between two groups |
| Yoo et al., 2009, Korea 87 | A randomized, controlled clinical trial | 12 months, control group (n = 54), telemedicine group (n = 57) | HbA1c value was 7.6 ± 1.0% (p = 0.033 vs. baseline) | HbA1c value was 7.1 ± 0.8% (p < 0.001 vs. baseline) | BMI was 25 ± 3.3 kg/m2 | BMI was 25.1 ± 3.5 kg/m2 | After 12 weeks, there were significant improvements in HbA1c in the intervention group compared with the control group |
| Meigs et al., 2003, USA 88 | A group randomized controlled trial | 12 months, control group (n = 291), telemedicine group (n = 307) | Average change in HbA1c was 0.14% | Average change in HbA1c was −0.23% (p = 0.09 vs. control) | No data | No data | Web-based patient-specific decision support has the potential to improve evidence-based parameters of diabetes care |
| Tutino et al., 2017, China 89 | A multicentre randomized nonblinded study | 12 months, DIAMOND group (n = 1176), JADE group (n = 1383) | No data | DIAMOND: Average change in HbA1c was −0.69% (95% CI: −0.81, −0.57), JADE: Average change in HbA1c was −0.62% (95% CI: −0.73, −0.50) | No data | No data | Integrated care augmented by information technology improved cardiometabolic control, with additional nurse contacts reducing the default rate and enhancing self-care |
| Graziano et al., 2009, USA 90 | A randomized controlled trial | 3 months, control group (n = 58), telemedicine group (n = 61) | Average change in HbA1c was −0.767 ± 1.14% | Average change in HbA1c was −0.834 ± 1.09% (p = 0.84 vs. baseline) | No data | No data | there were no significant differences between the telephone and control groups on mean change HbA1c level |
| Middleton et al., 2021, Australisa 91 | A randomized controlled trial | 12 months, control group (n = 15), telemedicine group (n = 20) | HbA1c value was 6.6 ± 1.7% | HbA1c value was 7.1 ± 1.1% (p = 0.37 vs. control) | BMI was 31.8 ± 5.8 kg/m2 | BMI was 30.4 ± 8.4 kg/m2 (p = 0.57 vs. control) | There was no difference in mean HbA1c between groups |
| Smith et al., 2008, USA 92 | A randomized controlled trial | 12 months, control group (n = 271), telemedicine group (n = 342) | HbA1c value was 6.7% (range: 4.8−13.7) | HbA1c value was 6.7% (range: 4.5−12.8) | No data | No data | Specialty telemedicine did not significantly enhance the value of CCM (the chronic care model) in primary care |
| Farmer et al., 2021, UK 93 | Two parallel-arm, individually randomised controlled trial | 12 months, control group (n = 511), telemedicine group (n = 510) | Average change in HbA1c was −13.0 ± 31.27 mmol/mmol | Average change in HbA1c was −12.5 ± 30.72 mmol/mmol (p = 0.537 vs. control) | No data | No data | Whilst SMS text messages do not lead to improved glycaemia in these low-resource settings |
| Vinitha et al., 2019, India 94 | A multicentric, randomised controlled trial | 24 months, control group (n = 122), telemedicine group (n = 126) | HbA1c value was 7.6 ± 1.3% (p < 0.0001), FBG was 142.4 ± 37.1 mg/dl (p < 0.0001) | HbA1c value was 7.2 ± 1.2% (p < 0.0001), FBG was 128.9 ± 32.2 mg/dl (p < 0.0001) | BMI was 27.7 ± 4.6 kg/m2 (n.s) | BMI was 27.2 ± 4.4 kg/m2 (n.s) | At 24 months, both groups showed significant reduction in blood pressure and glycaemic variables in comparison to the baseline values |
| Peimani et al., 2016, Iran 95 | A three-arm randomized controlled trial | 3 months, control group (n = 50), Tailored−SMS group (n = 50), Non−tailored−SMS group (n =50) | HbA1c value was 7.55 ± 1.44% (p = 0.847), FBS was 165.32 ± 57.85 mg/dl (p = 0.850), | Tailored−SMS group:, HbA1c value was 7.06 ± 1.31% (0.050 vs. baseline), FBS was 152.54 ± 81.09 mg/dl (p = 0.003 vs. baseline), Non−tailored−SMS group:, HbA1c value was 7.26 ± 1.32% (p = 0.075 vs. baseline), FBS was 147.82 ± 47.27 mg/dl (p = 0.026 vs. baseline) | BMI was 28.21 ± 5.15 kg/m2 (p = 0.045) | Tailored−SMS group: BMI was 27.14 ± 5.51 kg/m2 (p < 0.001 vs. baseline), Non−tailored−SMS group: BMI was 26.90 ± 4.57 kg/m2 (p = 0.002 vs. baseline) | Although there were significant differences in the outcomes between the intervention groups and the control one, the differences between intervention groups (tailored and nontailored SMS groups) were not significant |
| Schillinger et al., 2009, USA 96 | A three-arm practical clinical trial | 12 months, control group (n = 103), ATSM group (n = 101), GMV group (n =96) | HbA1c value was 9.0 ± 2.2% | ATSM: HbA1c value was 8.7 ± 1.9%, GMV: HbA1c value was 9.0 ± 2.0% | No data | No data | Glycemic control improved across all three arms, but there were no statistically significant differences in A1C change between three groups |
| Kim et al., 2014, Korea 97 | Clinical trial | 3 months, control group (n = 35), telemedicine group (n = 35) | HbA1c value was 7.7±0.7% (p =0.973 vs. baseline) | HbA1c value was 7.5 ± 0.7% (p = 0.077 vs. baseline) | BMI was 24.3±3.1 kg/m2 (p = 0.066 vs. baseline) | BMI was 25.0±3.4 kg/m2 (p = 0.804 vs. baseline) | Both the smartphone group and the control group showed a tendency towards a decrease in the HbA1c level after 3 months |
| Iljaž et al., 2017, Slovenia 98 | A randomized controlled trial | 6 months, control group (n = 54), telemedicine group (n = 53) | HbA1c value was 6.7 ± 1.5% | HbA1c value was 6.4 ± 0.9% (p < 0.05 vs. baseline) | BMI was 31.8 ± 5.1 kg/m2 | BMI was 32.0 ± 4.7 kg/m2 | The significant reduction of HbA1c values in the interventional group confirmed the application’s potential to improve the regulation of DM type 2 in patients who are not using insulin. |
| Kwon et al., 2004, Korea 99 | A randomized controlled trial | 3 months, control group (n = 50), telemedicine group (n = 51) | Average change in HbA1c was 0.33% | Average change in HbA1c was −0.54% (p < 0.05 vs. baseline) | no data | No data | The intervention group showed a marked decrease in HbA1c levels after 12 weeks of follow-up versus the baseline levels, whereas the control group showed slightly increased HbA1c levels after the same period |
| Lee et al., 2017, Korea 100 | A subanalysis of clinical trial | 6 months, control group (n = 91), Infrequent users (n = 54), Frequent users (n = 53) | Average change in HbA1c was −1.8 ± 1.7% | Infrequent users: Average change in HbA1c was −1.5±1.5%, Frequent users: Average change in HbA1c was −2.4 ± 1.6% (p < 0.05 vs. control and infrequent users) | Average change in BMI was −0.02 ±1.2 kg/m2 | Infrequent users: Average change in BMI was 0.0±1.5 kg/m2, Frequent users: Average change in BMI was −0.1±2.4 kg/m2 | Initial active engagement in self-monitoring with a telemonitoring device could provide incremental improvement of glycemic control over 6 months |
| Kim et al., 2010, Korea 101 | Clinical trial | 12 weeks, control group (n = 45), telemedicine group (n = 47) | HbA1c value was, 7.8 ± 0.8% | HbA1c value was 7.4 ± 0.7% (p = 0.023 vs. control) | Body weight increased 2.2 ± 2.8 kg | Body weight increased 2.4 ± 3.0 kg (p = 0.653 vs. control) | The significant decrease in HbA1C was accomplished with a minimal incidence of hypoglycemia and a small increase in body weight |
| Song et al., 2009, Korea 102 | A randomized two-group pretest/posttest experimental study | 12 weks, control group (n = 24), telemedicine group (n = 25) | HbA1c value was 8.6 ± 1.3% | HbA1c value was 7.1 ± 1.2% (p < 0.05 vs. baseline) | No data | No data | hese findings indicate that the DOIMP can improve HbA1c levels in patients with type 2 diabetes |
| McKay et al., 2002, USA 103 | A randomized design study | 3 months | Information−only condition: HbA1c value was 7.37 ± 1.49% (n= 33) | Peer support condition: HbA1c value was 7.59 ± 1.66% (n = 30), Personal self−management coach condition: HbA1c value was 7.73 ± 1.42% (n = 37), Combined condition: HbA1c value was 7.28 ± 1.28% (n = 33) | No data | No data | There was no significant between-condition differences |
| Cho et al., 2006, Korea 104 | A randomized controlled trial | 30 months, control group (n = 40), telemedicine group (n = 40) | HbA1c value was 7.4 ± 1.3% | HbA1c value was 6.7 ± 0.9% (p < 0.05 vs. baseline) | No data | no data | The mean A1C were significantly lower in the intervention group than in the control group |
| Eakin et al., 2013, Australia 105 | A two-arm randomized controlled trial | 6 months, control group (n = 151), telemedicine group (n = 151) | HbA1c value was 7.5 ± 1.6% | HbA1c value was 7.5 ± 1.7% | Weight value was 95.3 ± 20.9 kg | Weight value was 93.3 ± 19.0 kg | The intervention effects showed, relative to usual care, that the intervention group achieved more weight loss. there was no substantial or statistically significant difference, between groups in HbA1c |
| Agboola et al., 2016, USA 106 | A randomized controlled trial | 6 months, control group (n = 62), telemedicine group (n = 64) | Average change in HbA1c was −0.21% | Average change in HbA1c was −0.43% (p =0.29 vs. control) | No data | No data | Personalized text messaging can be used to improve outcomes in patients with T2DM by employing optimal patient engagement measures |
| Glasgow et al., 2012, USA 107 | A patient-randomized practical effectiveness trial | 12 months | HbA1c value was 8.04 ± 0.14% | HbA1c value was 8.16 ± 0.09% | BMI was 34.8 ± 0.6 kg/m2 | BMI was 34.6 ± 0.4 kg/m2 | The Internet intervention meets the reach and feasibility criteria for a potentially broad public health impact |
| Ralston et al., 2009, USA 108 | A pilot randomized trial | 12 months, control group (n = 35), telemedicine group (n = 39) | Average change in HbA1c was 0.2% | Average change in HbA1c was −0.9% (p < 0.01 vs. control) | No data | No data | GHb declined significantly in the intervention group compared with the usual care group |
| Noh et al., 2010, Korea 109 | A randomized controlled trial | 6 months, control group (n = 20), telemedicine group (n = 20) | Average change in HbA1c was −0.49% (p = 0.257 vs. baseline) | Average change in HbA1c was −1.53% (p = 0.031 vs. baseline) | Average change in BMI was 0 kg/m2 (p = 1 vs. baseline) | Average change in BMI was 0.65 kg/m2 (p = 0.657 vs. baseline) | The improvement in A1C for the intervention group, compared with no difference in the control group after 6 months |
| Murray et al., 2017, England 110 | A multicentre, two-arm individually randomised controlled trial | 12 months, control group (n = 163), telemedicine group (n = 155) | Average change in HbA1c was 0.16 ± 0.07% | Average change in HbA1c was −0.08 ± 0.07% (p = 0.014 vs. control) | Average change in BMI was −0.04 ± 0.2 kg/m2 | Average change in BMI was 0.12 ± 0.2 kg/m2 (p = 0.498 vs. control) | Participants in the intervention group had lower HbA1c than those in the control |
| Bingham et al., 2021, USA 111 | A retrospective study | 3 months, (n = 444) | No data | HbA1c value was 7.1% [4.5−13.6] (p = 0.009 vs. baseline) | No data | No data | There was a significant difference between median HbA1c values pre- and postcomprehensive medication review |
| Michaud et al., 2020, Nebraska 112 | Retrospective observational study | 3 months, (n = 1103) | No data | HbA1c value was 7.1 ± 1.5% (p < 0.001 vs. baseline) | No data | No data | There were significant differences in HbA1C at baseline and HbA1C at the end of remote patient monitoring (RPM) |
| Kesavadev et al., 2012, India 113 | A retrospective cohort study | 6 months, (n = 1000) | No data | HbA1c value was 6.3 ± 0.6%, HbA1c decreased by 2.2% (p < 0.0001 vs. baseline), FBG decreased by 67 mg/dl (p = 0.01 vs. baseline) | No data | BMI decreased by 0.3 (kg/m2) (p < 0.01 vs. baseline) | The Diabetes Tele Management System was successful in achieving glycemic controls at par with internationally accepted treatment |
| Su et al., 2019, USA 114 | Cohort study | 3 months, (n = 1336) | No data | HbA1c value was 7.1 ± 1.5% (p < 0.001 vs. baseline) | No data | No data | Higher levels of patient activation and engagement with remote patient monitoring technology were associated with better glycemic control outcomes |
| Musacchio et al., 2011, Italy 115 | Cohort study | 12 months, telemedicine group (n = 1004) | No data | Patients, HbA1c ⩽ 7.0% increased from 32.7 to 45.8% (p < 0.0001), while those, HbA1c ⩾ 9% decreased from 10.5 to 4.3% (p < 0.0001) | No data | No data | The SINERGIA model is effective in improving metabolic control and major cardiovascular risk factors |
| Turner et al., 2009, USA 116 | Exploratory study | 3 months, telemedicine group (n = 23) | No data | The decrease in HbA1c was 0.52 ± 0.91% | No data | No data | The technology improved the support available for T2D patients commencing insulin treatment. |
| Bergenstal et al., 2021, USA 117 | Cohort study | 10.2 ± 4.0 months, telemedicine group (n = 372) | no data | Significant reductions in HbA1c from baseline (−0.6 ± 1.5%, p < 0.001) | no data | no data | Intermittent use of rtCGM was well-received by adults with T2D and was associated with improvement in HbA1c |
| Michaud et al., 2018, USA 118 | Retrospective, observational study | 3 months, telemedicine group (n = 955) | no data | HbA1c value was 7.09 ± 1.44% (p < 0.001 vs. baseline) | no data | BMI value was 35.23 ± 7.74 kg/m2 (p < 0.001 vs. baseline) | This study found significant differences in clinical outcomes, especially HbA1c, at pre and post the 3-month remote patient monitoring intervention |
| Cheng et al., 2021119 | Cross-sectional study | 1 month, control group (n = 207), telemedicine group (n = 168) | Average change in FPG value was −38.82% (170 ± 6.1 mg/dL to 104 ± 26.1 mg/dL), Average change in 2−h PPG value was −29.29% ( 239 ± 35.5 mg/dL to 169 ± 44.7 mg/dL), Glucose variability value was 65.4 ± 35.1 mg/dL | Average change in FPG value was −41.72% (169 ± 6.3 mg/dL to 98.5 ± 21.1 mg/dL, p=0.027 vs. control), Average change in 2−h PPG value was −54.32% (243 ± 34.6 mg/dL to 111 ± 22.8 mg/dL, p<0.001 vs. control), Glucose variability value was 12.8 ± 7.3 mg/dL (p<0.001 vs. control) | No data | No data | Telemedicine may be a complementary option to assist in the management of glucose variability in diabetes |
| Shane-McWhorter et al., 2014, USA 120 | A nonrandomized prospective observational preintervention–postintervention, Study | 6 months, (n = 95) | No data | Average change in HbA1c was −1.92% (9.73 to 7.81) (p < 0.0001 vs. baseline) | No data | No data | Telemonitoring improved clinical outcomes and may be a useful tool to help enhance disease management |
| Yu et al., 2014, Canada 121 | A single-arm pre–post cohort study | 9 months | No data | Average change in HbA1c was 0.37% in general ( no seperated) | No data | No data | A self-management website for patients with type 2 diabetes did not improve self-efficacy |
| Berman et al., 2018, USA 122 | Cohort study | 12 weeks, (n = 101) | No data | Average change in HbA1c was −0.8 ± 1.3% (p < 0.001 vs. baseline) | No data | No data | Clinically meaningful reductions in HbA1c were observed with use of the FareWell digital therapeutic |
| Shane-McWhorter et al., 2015, USA 123 | Cohort study | 9 months, control group (n = 75), telemedicine group (n = 75) | Average change in HbA1c was −0.66 ± 1.99% (p = 0.009 vs. baseline) | Average change in HbA1c was −2.07 ± 2.36% (p < 0.001 vs. baseline, p < 0.001 vs. control) | Average change in BMI was 0.07 ±1.13 kg/m2 (p = 0.577 vs. baseline) | Average change in BMI was 0.11 ±1.55 kg/m2 (p = 0.535 vs. baseline) | Compared with usual care, a pharmacist-driven telemonitoring program showed a significant improvement in patients’ A1C levels. |
| Dixon et al., 2020, United States 124 | Technology report | The mean follow−up time period was 4.2 months (125.6 ± 22.4 days), (n = 740) | No data | HbA1c decreased by 2.3 ± 1.9%, 0.7 ± 1.0%, and 0.2 ± 0.8% across the baseline categories of > 9.0%, 8.0% to 9.0%, and 7.0% to < 8.0%, respectively (all p < 0.001) | No data | No data | Virtual Diabetes Clinic may be associated with, related to improving HbA1c |
| Majithia et al., 2020, USA 125 | Prospective single-arm study | 4 months, telemedicine group (n = 55) | No data | HbA1c decreased 1.6 ± 1.0% (p < 0.001 vs. baseline) | No data | BMI decreased 1.34 ± 1.5 kg/m2 (p < 0.001 vs. baseline) (n = 54) | After 4 months, there was a decrease in the HbA1C of the participating patients from baseline |
| Kim et al., 2006, Korea 126 | Pre–post test | 12 weeks, telemedicine group (n = 33) | No data | HbA1c value was 7.0 ± 1.1%, average change value was −1.1 ± 2.1 % (p= 0.006) | No data | No data | SMS intervention improved HbA1c level |
| Mayes et al., 2010, USA 127 | Pre–post test | 3.5 years, telemedicine group (n = 16) | no control | The difference between the last and first value for HbA1c (mean 7.2% vs. 9.6%, respectively) was −2.4% (a decrease of 21%) | No data | No data | Video conferencing via the Internet can provide a useful tool to assure that patients who adopt and utilize ADA protocols for diabetes will improve their glucose control |
| McGloin et al., 2020, Ireland 128 | An observational, pre–post, multimethod, and triangulation design | 12 weeks, telemedicine group (n = 39) | No data | HbA1c value was 8.01%, HbA1c (mmol/mol) decreased significantly −17.13 mmol/mol; p < 0.001) | No data | BMI value was 30.15 ± 6.82 kg/m2 | The mean HbA1c (mmol/mol) decreased significantly with no significant impact on weight |
| Bollyky et al., 2018, USA 129 | Pre–posttest | 90 days, telemedicine group (n = 275) | no data | HbA1c value was 7.1 ± 1.4% | no data | no data | Livongo participation significantly improves BG control in people with T2D |
| McGloin et al., 2015, Ireland 130 | A longitudinal mixed method case study | 12 months, (n = 8) | No data | HbA1c level was 7.63 ± 1.5% | No data | BMI was 35.6 ± 6.6 kg/m2 | The change of HbA1c was not significant |
| Carter et al., 2011, USA 131 | Not reported | 9 months, control group (n = 21), telemedicine group (n = 26) | HbA1c value was 7.9 % (p < 0.05 vs. baselime) | HbA1c value was 6.82 % (p < 0.05 vs. baseline) | BMI value was 26.5 (p < 0.05 vs. baselime) | BMI value was 23.8 (p < 0.05 vs. baselime) | Treatment group participants were more likely to achieve positive outcomes in terms of lowered hemoglobin A1c and body mass index measurements than were control group members |
| King et al., 2009, USA 132 | Initial pilot program | 12 months, control group (n = 43), telemedicine group (n = 14) | HbA1c decreased by 0.06% (p = 0.395) | HbA1c decreased by 0.46% (p =0.095) | No data | No data | Reductions in HbA1c did not achieve statistical significance potentially |
| Carallo et al., 2015, Singapore 133 | Not reported | 1 year, control group (n = 208), telemedicine group (n = 104) | The change was not statistically significant | HbA1c value was 54 ± 8 mmol/mol (p = 0.01) | The change was not statistically significant | BMI value was 30.5 ± 4.6 kg/m2 (p = 0.03) | Health care program based on GPs empowerment and taking care plus remote consultation with Consultants is at least as effective as standard outpatient management |
| Chen et al., 2011, Taiwan 134 | Not reported | 1 year, control group (n = 47), telemedicine group (n = 44) | HbA1c changed by −0.6 ± 2.6% (p = 0.202 vs. baseline) | HbA1c changed by −1.4 ± 1.5% (p < 0.001 vs. baseline) | No data | No data | The intensive diabetes management program with the telehealth system is a useful education method to improve blood sugar control in poorly controlled T2D patients receiving insulin injections |
| Myers et al., 2021, USA 135 | Pilot study | 3 months, Telephone (n = 13), Telehealth (n = 9) | The telephone arm had a 0.50% greater reduction in HbA1c (2.07% vs 2.57%, p = 0.70) than the telehealth group | No data | No data | The change in HbA1c was not statistically different across arms | |
| Istepanian et al., 2014, Iraq 136 | Case study | 6 month, control group (n = 6), telemedicine group (n = 6) | HbA1c decreased from 8.95 ± 2.17% to 8.7 ± 1.7% (p = 0.448) | HbA1c decreased from 8.95 ± 0.73% to 8.05 ± 1.31% (p = 0.115) | No data | No data | The key outcome of this study is the effectiveness of the mobile management systems and intervention in lowering the HbA1c level. |
| Lim et al., 2009, Korea 137 | Not reported | 3 months, control group (n = 34), telemedicine group (n = 67) | HbA1c value was 8.6 ± 0.3 %, FBS value was 166.4 ± 7.4 mg/dl | HbA1c value was 7.3 ± 0.2% (p < 0.001 vs. baseline), FBS value was 136.0 ± 4.3 mg/dl (p < 0.001 vs. baseline) | BMI was 24.9 ± 0.5 kg/m2 | BMI was 23.7 ± 0.4 kg/m2 (p < 0.001 vs. baseline) | Subjects in the telephone follow-up group showed a decrease in BMI, FBS, and HbA1c |
| Yoon et al., 2008, Korea 138 | Not reported | 12 months, control group (n = 26), telemedicine group (n = 25) | HbA1c value was 8.40 ± 1.04% (increased 0.81%, p < 0.05 vs. baseline) | HbA1c value was 6.77 ± 0.77% (decrease 1.32%, p < 0.05 vs. baseline) | No data | No data | Participants in the intervention group had lower HbA1c over 12 months when compared with the control group |
| Nesari et al., 2010, Iran 139 | Not reported | 3 months, control group (n = 30), telemedicine group (n = 30) | HbA1c value was 8.60 ± 1.88% (p = 0.150 vs. baseline) | HbA1c value was 7.04 ± 1.18% (p < 0.001 vs. baseline, p < 0.001 vs. control) | No data | No data | A nurse-led telephone follow-up was effective in enhancing the level of adherence to a diabetes therapeutic regimen, such that the HbA1c level decreased |
| McIlhenny et al., 2011, USA 140 | Not reported | 6 months, control group (n = 50), telemedicine group (n = 48) | HbA1c value was 7.49 ± 1.79%, Glucose level was 131.8 ± 45.6 mg/dl | HbA1c value was 6.52 ± 0.99% (p = 0.197 vs control), Glucose level was 102.4 ± 31.9 mg/dl (p = 0.008 vs. control) | Average weight was 98.1 ± 23.2 kg | Average weight was 97.2 ± 20.0 kg (p = 0.378 vs. control) | There was a significant difference in glucose levels between groups at 6 months |
| Kim et al., 2006, Korea 141 | Not reported | 12 weeks, control group (n = 23), WB group (n = 28), PM group (n = 22) | Average change in HbA1c was 0.43 ± 0.81%, Average change in FBS was 4.26 ± 4.48 mg/dl | WB:, Average change in HbA1c was −0.59 ± 0.61% (p = 0.01 vs. control), Average change in FBS was −14.14 ± 14.21 mg/dl (p = 0.01 vs. control), PM:, Average change in HbA1c was −0.51± 1.30 (p = 0.01 vs. control), Average change in FBS was −15.91 ± 13.23 mg/dl (p = 0.01 vs. control) | No data | No data | The findings of this study clearly indicate that both the WB and PM interventions were effective in enhancing the levels of physical activity and better in controlling FBS and HbA1c in Korean adults with type 2 diabetes |
The blood sugar control effectiveness of telemedicine was also evaluated carefully through studies with control groups of patients receiving usual care, with no telemedicine intervention. Two controlled cohort studies showed an improvement affecting the clinical outcomes in the telemedicine group compared to the usual care control group.119,123 Cheng and Kao, 119 showed that managing type 2 diabetes patients with telemedicine for 1 month resulted in a statistically significant difference (p < 0.001) in glucose variability value and 2-h PPG value. 119 Meanwhile, Shane–McWhorter and McAdam–Marx, 123 showed that telemedicine administration for 9 months resulted in a difference in the average change in HbA1c compared to baseline and compared to the control group (p < 0.001). 123 In controlled clinical trials, these results also show that managing type 2 diabetes by using telemedicine can be as effective as or better than conventional care management. Out of a total of 98 clinical trials with usual care control groups, 38 studies showed that telemedicine intervention helped patients improve blood glucose levels with a significant difference compared to others in the control group.8,11–13,17–19,21,22,27–29,37,41,42,46,48,53,55,57,64,69,70,71,79,80,81,82,84,85–88,95,100,101,108,110 The participants in the intervention group had lower HbA1c than the control group.8,11–13,16–18,21,22,27–29,37,42,46,48,55,57,69,70,71,80,82,84,85,86,88,101,108,110 The largest reduction in HbA1c index was 3.2 ± 1.5% after 6 months, compared to the control group received standard care at the clinic (p < 0.05). 69 Additionally, 100 Lee et al. showed that frequent user participation (at least twice daily) in self-monitoring may result in meaningful improvements in glycemic control compared with infrequent user participation. 100 In some studies, patient management by telemedicine has also been shown to be effective in improving FBG, postprandial blood glucose and 2HPMG level,16,64,84,85,95 A total of 19 studies showed that only the group of patients receiving telemedicine care had a significant change in blood glucose index compared to baseline while the change was not significant in the control group.9,23,26,30,35,43,56,59,66,67,68,72,75,77,98,99,102,104,109 Some studies show that telemedicine administration can lead to a significant improvement in HbA1c index (p < 0.05 vs baseline). Hee-Sung et al., 35 showed a significant percentage change in baseline-glycosylated hemoglobin ⩾7.0% for the intervention group. However, there was still no change in the intervention group with baseline HbA1C < 7.0% and the control group. A total of 28 studies showed that managing patients with diabetes via telemedicine improved blood glucose levels comparable to usual care.10,14,24,25,31,34,36,44,45,47,49,51,52,54,60,62,73,74,76,78,83,90,91,94,97,96,106,107 Telemedicine is believed to be a viable method for implementing chronic disease management. Only 14 studies showed that the addition of telemedicine had limited clinical benefit in improving glycemic control.15,20,33,39,38,50,58,61,63,65,92,93,103,105 Pilot studies also show that telemedicine provides equivalent or better diabetes management effectiveness than the control group.126,131–140
The effectiveness of telemedicine in improved obesity control and quality of life
In addition to the effects of telemedicine on glycemic control, the systematic review analyzed other outcomes of telemedicine related to obesity management and quality of patient’s life. The obesity status of patients was studied based on two leading indices: weight and body mass index (BMI). Regarding the effectiveness of improving the quality of life for patients, there are few research articles in this field, and they are only based on the Health-related Quality of Life (HRQoL) rating scale.
There were a total of five studies with a noncontrolled design model that were interested in indicators related to the obesity control status of patients. All the studies found above were of the preinterventional comparative study type. There were no comparative trials between intervention groups (no control groups) and only based on BMI to assess status. Five studies showed telemedicine results that had an improved impact on BMI,113,118,125,131,133 and two studies did not find a difference in BMI before and after the intervention or that difference has no statistical significance.128,130 A total of 54 controlled design articles evaluated BMI or weight values or assessed both as a consequence of the study. Forty-three trials indicated little or no statistically significant improvement10,11–13,15–19,29,33,34,38,42,49,54,57,62,70,73–76,78,79,67,80,83,84,87,91,94,97,98,100,105,107,109,101,110,123,140; five trials showed significant improved outcomes when compared baseline and postintervention, which were observed only in the intervention group; four trials showed that clinical outcomes improved significantly in the intervention compared with control group.8,27,55,71
In addition, seven trials with a model design of a control group and an intervention group assessed the quality of life through the main scale, health-related quality of life (HRQoL), with only three trials performed. Assessing quality of life is the second output next to the glycemic index. In which, three trials showed no improvement in quality of life in groups or statistically significant differences,59,61,62 and four trials showed that telemedicine intervention has positive effects on patients’ lives and activities.11,16,46
Discussion
Type 2 diabetes is a chronic condition characterized by high blood sugar levels resulting from the body’s insufficient response to insulin, a hormone responsible for regulating blood sugar. 141 It is a global health burden that affects millions of people worldwide. 142 Through all studies, we see the benefits of telemedicine when supporting patients with type 2 diabetes. Accordingly, most studies show positive changes in glycemic index in every group using telemedicine. Moreover, when compared with traditional healthcare, these findings suggest that telemedicine can be as effective as, or even superior to, traditional care management.8,11–13,16–19,21,22,27–29,37,41,42,46,48,53,55,57,64,69,70,71,79,80,81,82,84,85–,95,100,101,108,110 Above all, the role of telemedicine is also confirmed to be extremely important in some special cases such as the patient’s residence distance to the center, where there is a hospital with adequate equipment, or the ability for patients to be admitted directly to medical facilities for examination with exception to emergency or urgent cases.
Overall, although the BMI and weight indices in the studies improved at the end of the course, the improvement values were considered insignificant. There were a few cases where the treatment effect changed significantly after the first 3 months of the trial but showed no overall improvement at the end. Quality of life and understanding of diabetes, as well as the level of satisfaction with the treatment course after the study, all tend to increase with statistical significance. Managing type 2 diabetes requires significant lifestyle adjustments, including maintaining a healthy diet, engaging in regular physical activity, monitoring blood sugar levels, and taking medications as prescribed. 143 These ongoing requirements can impact the quality of life, limiting social activities, causing emotional distress, and reducing overall well-being.144,145 The risk of complications associated with uncontrolled diabetes further adds to the burden on individuals living with the condition. 146 Comprehensive efforts, including public health campaigns, health education, increased access to healthcare services, and policies promoting healthy behavior, can play a crucial role in preventing type 2 diabetes and its complications.147,148 Several studies have shown that patients are satisfied with telemedicine services and that managing type 2 diabetes with telemedicine has a positive effect on quality of life scores.46,11,64,104
Type 2 diabetes places a considerable economic burden on healthcare systems. 149 The costs associated with managing diabetes and its related complications, such as cardiovascular disease, kidney disease, and blindness, are substantial.150,151 These costs include medications, hospitalizations, and long-term care, leading to increased healthcare expenditure for individuals, families, and society.152,153 Telemedicine allows healthcare providers to monitor patients’ health conditions remotely; this real-time data can help identify potential issues or trends requiring intervention.31,117,124 Moreover, technology-enabled telemedicine platforms provide tools such as mobile apps or web portals that empower patients to participate in their care management actively.36,39,57 In conclusion, applying technology through telemedicine in outpatient treatment management for patients with type 2 diabetes offers numerous benefits, including remote monitoring, improved access to care, enhanced patient engagement, timely intervention, cost savings, continuity of care, and data-driven decision-making. This integration can significantly improve the overall management and outcomes for individuals with type 2 diabetes.
Our limitation is that the study only focused on evaluating blood sugar index, while comorbidities of hypertension and hyperlipidemia can include many other factors such as cholesterol, triglycerides, HbA1c. These additional factors may provide a more comprehensive view of the intervention’s impact on QoL. In addition, the study examined telemedicine strategies and follow-up duration but did not evaluate intervention frequency. The frequency of intervention can significantly influence the effectiveness of disease management strategies. Future research should consider intervention frequency as an important variable.
Conclusion
This systematic review proved telemedicine in diabetes based-evidence by summarizing 134 studies from 2002 to 2022. Most articles showed that telemedicine, in various ways, could positively impact different aspects of diabetes management. In the end, we strongly agreed that telemedicine could bring benefits to education and the management of diabetes patients. More field research is needed, especially in developing countries, to solidify and prove the effectiveness of telemedicine.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121241271846 for The effectiveness of telemedicine in the management of type 2 diabetes: A systematic review by Truong Van Dat, Van Binh, Thai Minh Hoang, Vo Linh Tu, Pham Dinh Luyen and Le Thi Kim Anh in SAGE Open Medicine
Acknowledgments
None.
Footnotes
Author contributions: TVD provided ideas, organized tasks, and gave instructions. Other authors searched papers and selected relevant ones, extracted data, made tables and figures, and wrote the manuscript under the supervision of TVD, PDL, and LTKA. TVD revised the manuscript and made the final version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Not applicable.
Informed consent: Not applicable.
ORCID iD: Truong Van Dat  https://orcid.org/0000-0002-0018-8336
https://orcid.org/0000-0002-0018-8336
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ong KL, Stafford LK, McLaughlin SA, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023; 402(10397): 203–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roglic G, Varghese C, Thamarangsi T. Diabetes in South-East Asia: burden, gaps, challenges and ways forward. WHO South-East Asia J Public Health 2016; 5(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 3. ElSayed NA, Aleppo G, Aroda VR, et al. 1 Improving care and promoting health in populations: standards of care in diabetes—2023. Diabetes Care 2023; 46(Supplement_1): S10–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verbosky N, Beckey C, Lutfi N. Implementation and evaluation of diabetes management via clinical video telehealth. Diabetes Care 2016; 39(1): e1–e2. [DOI] [PubMed] [Google Scholar]
- 5. Weinstein RS, Lopez AM, Joseph BA, et al. Telemedicine, telehealth, and mobile health applications that work: opportunities and barriers. Am J Med 2014; 127(3): 183–187. [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 7. mNIHN. Study quality assessment tools. National Heart, Lung, and Blood Institute (NHLBI), (2021, accessed 19 September 2022). [Google Scholar]
- 8. Hansel B, Giral P, Gambotti L, et al. A fully automated web-based program improves lifestyle habits and HbA1c in patients with type 2 diabetes and abdominal obesity: randomized trial of patient e-coaching nutritional support (the ANODE study). J Med Intern Res 2017; 19(11): e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim HS, Jeong HS. A nurse short message service by cellular phone in type-2 diabetic patients for six months. J Clin Nurs 2007; 16(6): 1082–1087. [DOI] [PubMed] [Google Scholar]
- 10. Basudev N, Crosby-Nwaobi R, Thomas S, et al. A prospective randomized controlled study of a virtual clinic integrating primary and specialist care for patients with type 2 diabetes mellitus. Diabetic Med 2016; 33(6): 768–776. [DOI] [PubMed] [Google Scholar]
- 11. Nicolucci A, Cercone S, Chiriatti A, et al. A randomized trial on home telemonitoring for the management of metabolic and cardiovascular risk in patients with type 2 diabetes. Diabetes Technol Therap 2015; 17(8): 563–570. [DOI] [PubMed] [Google Scholar]
- 12. Oh JA, Kim HS, Yoon KH, et al. A telephone-delivered intervention to improve glycemic control in type 2 diabetic patients. Yonsei Med J 2003; 44(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 13. Stone RA, Rao RH, Sevick MA, et al. Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care. 2010; 33(3): 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim HS, Oh JA. Adherence to diabetes control recommendations: impact of nurse telephone calls. J Advan Nurs 2003; 44(3): 256–261. [DOI] [PubMed] [Google Scholar]
- 15. Khanna R, Stoddard PJ, Gonzales EN, et al. An automated telephone nutrition support system for Spanish-speaking patients with diabetes. J Diabetes Sci Technol 2014; 8(6): 1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho JH, Kim H-S, Yoo SH, et al. An internet-based health gateway device for interactive communication and automatic data uploading: clinical efficacy for type 2 diabetes in a multi-centre trial. J Telemed Telecare 2017; 23(6): 595–604. [DOI] [PubMed] [Google Scholar]
- 17. Welch G, Allen NA, Zagarins SE, et al. Comprehensive diabetes management program for poorly controlled Hispanic type 2 patients at a community health center. Diabetes Educ 2011; 37(5): 680–688. [DOI] [PubMed] [Google Scholar]
- 18. Fortmann AL, Gallo LC, Garcia MI, et al. Dulce Digital: an mHealth SMS-based intervention improves glycemic control in Hispanics with type 2 diabetes. Diabetes Care 2017; 40(10): 1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Y, Lee EY, Kim HS, et al. Effect of a mobile phone-based glucose-monitoring and feedback system for type 2 diabetes management in multiple primary care clinic settings: cluster randomized controlled trial. JMIR mHealth uHealth 2020; 8(2): e16266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wakefield BJ, Koopman RJ, Keplinger LE, et al. Effect of home telemonitoring on glycemic and blood pressure control in primary care clinic patients with diabetes. Telemed J EHealth 2014; 20(3): 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egede LE, Walker RJ, Payne EH, et al. Effect of psychotherapy for depression via home telehealth on glycemic control in adults with type 2 diabetes: subgroup analysis of a randomized clinical trial. J Telemed Telecare 2018; 24(9): 596–602. [DOI] [PubMed] [Google Scholar]
- 22. Steventon A, Bardsley M, Doll H, et al. Effect of telehealth on glycaemic control: analysis of patients with type 2 diabetes in the whole systems demonstrator cluster randomised trial. BMC Health Serv Res 2014; 14: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duruturk N, Özköslü MA. Effect of tele-rehabilitation on glucose control, exercise capacity, physical fitness, muscle strength and psychosocial status in patients with type 2 diabetes: a double blind randomized controlled trial. Prim Care Diabetes 2019; 13(6): 542–548. [DOI] [PubMed] [Google Scholar]
- 24. Cho JH, Choi YH, Kim HS, et al. Effectiveness and safety of a glucose data-filtering system with automatic response software to reduce the physician workload in managing type 2 diabetes. J Telemed Telecare 2011; 17(5): 257–262. [DOI] [PubMed] [Google Scholar]
- 25. Wakefield BJ, Holman JE, Ray A, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E Health 2011; 17(4): 254–261. [DOI] [PubMed] [Google Scholar]
- 26. Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform 2008; 77(6): 399–404. [DOI] [PubMed] [Google Scholar]
- 27. Katula JA, Dressler EV, Kittel CA, et al. Effects of a digital diabetes prevention program: an RCT. Am J Prevent Med 2022; 62(4): 567–577. [DOI] [PubMed] [Google Scholar]
- 28. Hu Y, Wen X, Ni L, et al. Effects of telemedicine intervention on the management of diabetic complications in type 2 diabetes. Int J Diabetes Dev Ctries 2021; 41(2): 322–328. [Google Scholar]
- 29. Warren R, Carlisle K, Mihala G, et al. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: A randomized controlled trial. J Telemed Telecare 2018; 24(9): 586–595. [DOI] [PubMed] [Google Scholar]
- 30. Cho JH, Kwon HS, Kim HS, et al. Effects on diabetes management of a health-care provider mediated, remote coaching system via a PDA-type glucometer and the Internet. J Telemed Telecare 2011; 17(7): 365–370. [DOI] [PubMed] [Google Scholar]
- 31. Jia W, Zhang P, Zhu D, et al. Evaluation of an mHealth-enabled hierarchical diabetes management intervention in primary care in China (ROADMAP): a cluster randomized trial. PLoS Med 2021; 18(9): e1003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trief PM, Fisher L, Sandberg J, et al. Health and psychosocial outcomes of a telephonic couples behavior change intervention in patients with poorly controlled type 2 diabetes: a randomized clinical trial. Diabetes Care 2016; 39(12): 2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wayne N, Perez DF, Kaplan DM, et al. Health coaching reduces HbA1c in type 2 diabetic patients from a lower-socioeconomic status community: a randomized controlled trial. J Med Inter Res 2015; 17(10): e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benson GA, Sidebottom A, Hayes J, et al. Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) telemedicine randomized controlled trial on diabetes optimal care outcomes in patients with type 2 diabetes. J Acad Nutrit Dietetics 2019; 119(4): 585–598. [DOI] [PubMed] [Google Scholar]
- 35. Hee-Sung K. Impact of web-based nurse’s education on glycosylated haemoglobin in type 2 diabetic patients. J Clin Nurs 2007; 16(7): 1361–1366. [DOI] [PubMed] [Google Scholar]
- 36. Xu R, Xing M, Javaherian K, et al. Improving HbA(1c) with glucose self-monitoring in diabetic patients with EpxDiabetes, a phone call and text message-based telemedicine platform: a randomized controlled trial. Telemed J E Health 2020; 26(6): 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu Z, Li Y, He Y, et al. Internet-based medication management services improve glycated hemoglobin levels in patients with type 2 diabetes. Telemed J E Health 2021; 27(6): 686–693. [DOI] [PubMed] [Google Scholar]
- 38. Anderson DR, Christison-Lagay J, Villagra V, et al. Managing the space between visits: a randomized trial of disease management for diabetes in a community health center. J Gen Intern Med 2010; 25(10): 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agarwal P, Mukerji G, Desveaux L, et al. Mobile app for improved self-management of type 2 diabetes: multicenter pragmatic randomized controlled trial. JMIR mHealth uHealth 2019; 7(1): e10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cho JH, Lee HC, Lim DJ, et al. Mobile communication using a mobile phone with a glucometer for glucose control in type 2 patients with diabetes: as effective as an Internet-based glucose monitoring system. J Telemed Telecare 2009; 15(2): 77–82. [DOI] [PubMed] [Google Scholar]
- 41. Quinn CC, Shardell MD, Terrin ML, et al. Mobile diabetes intervention for glycemic control in 45- to 64-year-old persons with type 2 diabetes. J Appl Gerontol 2016; 35(2): 227–243. [DOI] [PubMed] [Google Scholar]
- 42. Sun C, Sun L, Xi S, et al. Mobile phone-based telemedicine practice in older Chinese patients with type 2 diabetes mellitus: randomized controlled trial. JMIR and uHealth 2019; 7(1): e10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim S, Kang SM, Kim KM, et al. Multifactorial intervention in diabetes care using real-time monitoring and tailored feedback in type 2 diabetes. Acta Diabetol 2016; 53(2): 189–198. [DOI] [PubMed] [Google Scholar]
- 44. Tang PC, Overhage JM, Chan AS, et al. Online disease management of diabetes: engaging and motivating patients online with enhanced resources-diabetes (EMPOWER-D), a randomized controlled trial. J Am Med Inform Assoc 2013; 20(3): 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greenwood DA, Blozis SA, Young HM, et al. Overcoming clinical inertia: a randomized clinical trial of a telehealth remote monitoring intervention using paired glucose testing in adults with type 2 diabetes. J Med Intern Res 2015; 17(7): e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams ED, Bird D, Forbes AW, et al. Randomised controlled trial of an automated, interactive telephone intervention (TLC Diabetes) to improve type 2 diabetes management: baseline findings and six-month outcomes. BMC Public Health 2012; 12: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramadas A, Chan CKY, Oldenburg B, et al. Randomised-controlled trial of a web-based dietary intervention for patients with type 2 diabetes: changes in health cognitions and glycemic control. BMC Public Health 2018; 18(1): 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Egede LE, Williams JS, Voronca DC, et al. Randomized controlled trial of technology-assisted case management in low income adults with type 2 diabetes. Diabetes Technol Therap 2017; 19(8): 476–482. [DOI] [PubMed] [Google Scholar]
- 49. Kim HS, Sun C, Yang SJ, et al. Randomized, open-label, parallel group study to evaluate the effect of internet-based glucose management system on subjects with diabetes in China. Telemed J E Health 2016; 22(8): 666–674. [DOI] [PubMed] [Google Scholar]
- 50. Goode AD, Winkler EA, Reeves MM, et al. Relationship between intervention dose and outcomes in living well with diabetes—a randomized trial of a telephone-delivered lifestyle-based weight loss intervention. Am J Health Promot 2015; 30(2): 120–129. [DOI] [PubMed] [Google Scholar]
- 51. Jeong JY, Jeon JH, Bae KH, et al. Smart care based on telemonitoring and telemedicine for type 2 diabetes care: multi-center randomized controlled trial. Telemed J E Health 2018; 24(8): 604–613. [DOI] [PubMed] [Google Scholar]
- 52. Nagrebetsky A, Larsen M, Craven A, et al. Stepwise self-titration of oral glucose-lowering medication using a mobile telephone-based telehealth platform in type 2 diabetes: a feasibility trial in primary care. J Diabetes Sci Technol 2013; 7(1): 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wild SH, Hanley J, Lewis SC, et al. Supported telemonitoring and glycemic control in people with type 2 diabetes: the telescot diabetes pragmatic multicenter randomized controlled trial. PLoS Med 2016; 13(7): e1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Vasconcelos HCA, Lira Neto JCG, de Araújo MFM, et al. Telecoaching programme for type 2 diabetes control: a randomised clinical trial. Br J Nurs 2018; 27(19): 1115–1120. [DOI] [PubMed] [Google Scholar]
- 55. Rasmussen OW, Lauszus FF, Loekke M. Telemedicine compared with standard care in type 2 diabetes mellitus: a randomized trial in an outpatient clinic. J Telemed Telecare 2016; 22(6): 363–368. [DOI] [PubMed] [Google Scholar]
- 56. Rodríguez-Idígoras MI, Sepúlveda-Muñoz J, Sánchez-Garrido-Escudero R, et al. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol Therap 2009; 11(7): 431–437. [DOI] [PubMed] [Google Scholar]
- 57. von Storch K, Graaf E, Wunderlich M, et al. Telemedicine-assisted self-management program for type 2 diabetes patients. Diabetes Technol Therap 2019; 21(9): 514–521. [DOI] [PubMed] [Google Scholar]
- 58. Lee JY, Chan CKY, Chua SS, et al. Telemonitoring and team-based management of glycemic control on people with type 2 diabetes: a cluster-randomized controlled trial. J Gen Intern Med 2020; 35(1): 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee JY, Wong CP, Tan CSS, et al. Telemonitoring in fasting individuals with type 2 diabetes mellitus during Ramadan: a prospective, randomised controlled study. Scient Rep 2017; 7(1): 10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dario C, Toffanin R, Calcaterra F, et al. Telemonitoring of type 2 diabetes mellitus in Italy. Telemed J E Health 2017; 23(2): 143–152. [DOI] [PubMed] [Google Scholar]
- 61. Egede LE, Williams JS, Voronca DC, et al. Telephone-delivered behavioral skills intervention for African American adults with type 2 diabetes: a randomized controlled trial. J Gen Intern Med 2017; 32(7): 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bujnowska-Fedak MM, Puchała E, Steciwko A. The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland. Telemed J E Health 2011; 17(3): 153–163. [DOI] [PubMed] [Google Scholar]
- 63. Arora S, Peters AL, Burner E, et al. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med 2014; 63(6): 745–754.e746. [DOI] [PubMed] [Google Scholar]
- 64. Kardas P, Lewandowski K, Bromuri S. Type 2 diabetes patients benefit from the COMODITY12 mHealth system: results of a randomised trial. J Med Syst 2016; 40(12): 259. [DOI] [PubMed] [Google Scholar]
- 65. McFarland M, Davis K, Wallace J, et al. Use of home telehealth monitoring with active medication therapy management by clinical pharmacists in veterans with poorly controlled type 2 diabetes mellitus. Pharmacotherapy 2012; 32(5): 420–426. [DOI] [PubMed] [Google Scholar]
- 66. Hansen CR, Perrild H, Koefoed BG, et al. Video consultations as add-on to standard care among patients with type 2 diabetes not responding to standard regimens: a randomized controlled trial. Eur J Endocrinol 2017; 176(6): 727–736. [DOI] [PubMed] [Google Scholar]
- 67. Zhou P, Xu L, Liu X, et al. Web-based telemedicine for management of type 2 diabetes through glucose uploads: a randomized controlled trial. Int J Clin Exp Pathol 2014; 7(12): 8848–8854. [PMC free article] [PubMed] [Google Scholar]
- 68. Luley C, Blaik A, Reschke K, et al. Weight loss in obese patients with type 2 diabetes: effects of telemonitoring plus a diet combination—the Active Body Control (ABC) program. Diabetes Res Clin Pract 2011; 91(3): 286–292. [DOI] [PubMed] [Google Scholar]
- 69. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Therap 2016; 18(2): 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kleinman NJ, Shah A, Shah S, et al. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed J E Health 2017; 23(9): 733–740. [DOI] [PubMed] [Google Scholar]
- 71. Orsama AL, Lähteenmäki J, Harno K, et al. Active assistance technology reduces glycosylated hemoglobin and weight in individuals with type 2 diabetes: results of a theory-based randomized trial. Diabetes Technol Therap 2013; 15(8): 662–669. [DOI] [PubMed] [Google Scholar]
- 72. Kim HS. A randomized controlled trial of a nurse short-message service by cellular phone for people with diabetes. Int J Nurs Stud 2007; 44(5): 687–692. [DOI] [PubMed] [Google Scholar]
- 73. Bender MS, Cooper BA, Park LG, et al. A feasible and efficacious mobile-phone based lifestyle intervention for Filipino Americans with type 2 diabetes: randomized controlled trial. JMIR Diabetes 2017; 2(2): e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Blackberry ID, Furler JS, Best JD, et al. Effectiveness of general practice based, practice nurse led telephone coaching on glycaemic control of type 2 diabetes: the Patient Engagement and Coaching for Health (PEACH) pragmatic cluster randomized controlled trial. BMJ 2013; 347: f5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Borhani F, Lashkari T, Sabzevari S, et al. Effect of telenursing (telephone follow-up) on glycemic control and body mass index (BMI) of type 2 diabetes patients. Iran J Nurs Midwifery Res 2013; 18: 451–456. [PMC free article] [PubMed] [Google Scholar]
- 76. Faridi Z, Liberti L, Shuval K, et al. Evaluating the impact of mobile telephone technology on type 2 diabetic patients’ self-management: the NICHE pilot study. J Eval Clin Pract 2008; 14(3): 465–469. [DOI] [PubMed] [Google Scholar]
- 77. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther 2018; 9(2): 583–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holmen H, Torbjørnsen A, Wahl AK, et al. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR mHealth uHealth 2014; 2(4): e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lim S, Kang SM, Shin H, et al. Improved glycemic control without hypoglycemia in elderly diabetic patients using the ubiquitous healthcare service, a new medical information system. Diabetes Care 2011; 34(2): 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Odnoletkova I, Goderis G, Nobels F, et al. Optimizing diabetes control in people with type 2 diabetes through nurse-led telecoaching. Diabetic Med 2016; 33(6): 777–785. [DOI] [PubMed] [Google Scholar]
- 81. Quinn CC, Shardell MD, Terrin ML, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011; 34(9): 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rothman RL, Malone R, Bryant B, et al. A randomized trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med 2005; 118(3): 276–284. [DOI] [PubMed] [Google Scholar]
- 83. Varney JE, Weiland TJ, Inder WJ, et al. Effect of hospital-based telephone coaching on glycaemic control and adherence to management guidelines in type 2 diabetes, a randomised controlled trial. Intern Med J 2014; 44(9): 890–897. [DOI] [PubMed] [Google Scholar]
- 84. Waki K, Fujita H, Uchimura Y, et al. DialBetics: a novel smartphone-based self-management support system for type 2 diabetes patients. J Diabetes Sci Technol 2014; 8(2): 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Y, Li M, Zhao X, et al. Effects of continuous care for patients with type 2 diabetes using mobile health application: a randomised controlled trial. Int J Health Plan Manag 2019; 34(3): 1025–1035. [DOI] [PubMed] [Google Scholar]
- 86. Kusnanto Widyanata KAJ, Suprajitno, et al. DM-calendar app as a diabetes self-management education on adult type 2 diabetes mellitus: a randomized controlled trial. J Diabetes Metab Disord 2019; 18(2): 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yoo HJ, Park MS, Kim TN, et al. A ubiquitous chronic disease care system using cellular phones and the internet. Diabetic Med 2009; 26(6): 628–635. [DOI] [PubMed] [Google Scholar]
- 88. Meigs JB, Cagliero E, Dubey A, et al. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care 2003; 26(3): 750–757. [DOI] [PubMed] [Google Scholar]
- 89. Tutino GE, Yang WY, Li X, et al. A multicentre demonstration project to evaluate the effectiveness and acceptability of the web-based Joint Asia Diabetes Evaluation (JADE) programme with or without nurse support in Chinese patients with type 2 diabetes. Diabetic Med 2017; 34(3): 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Graziano JA, Gross CR. A randomized controlled trial of an automated telephone intervention to improve glycemic control in type 2 diabetes. ANS Adv Nurs Sci 2009; 32(3): E42–E57. [DOI] [PubMed] [Google Scholar]
- 91. Middleton T, Constantino M, McGill M, et al. An enhanced SMS text message-based support and reminder program for young adults with type 2 diabetes (TEXT2U): randomized controlled trial. J Med Intern Res 2021; 23(10): e27263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Smith SA, Shah ND, Bryant SC, et al. Chronic care model and shared care in diabetes: randomized trial of an electronic decision support system. Mayo Clinic Proc 2008; 83(7): 747–757. [DOI] [PubMed] [Google Scholar]
- 93. Farmer A, Bobrow K, Leon N, et al. Digital messaging to support control for type 2 diabetes (StAR2D): a multicentre randomised controlled trial. BMC Public Health 2021; 21(1): 1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vinitha R, Nanditha A, Snehalatha C, et al. Effectiveness of mobile phone text messaging in improving glycaemic control among persons with newly detected type 2 diabetes. Diabetes Res Clin Pract 2019; 158: 107919. [DOI] [PubMed] [Google Scholar]
- 95. Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes 2016; 10(4): 251–258. [DOI] [PubMed] [Google Scholar]
- 96. Schillinger D, Handley M, Wang F, et al. Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: a three-arm practical clinical trial. Diabetes Care 2009; 32(4): 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim HS, Choi W, Baek EK, et al. Efficacy of the smartphone-based glucose management application stratified by user satisfaction. Diabetes Metab J 2014; 38(3): 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Iljaž R, Brodnik A, Zrimec T, et al. E-healthcare for diabetes mellitus type 2 patients—a randomised controlled trial in Slovenia. Zdravstveno Varstvo 2017; 56(3): 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kwon HS, Cho JH, Kim HS, et al. Establishment of blood glucose monitoring system using the internet. Diabetes Care 2004; 27(2): 478–483. [DOI] [PubMed] [Google Scholar]
- 100. Lee MK, Lee KH, Yoo SH, et al. Impact of initial active engagement in self-monitoring with a telemonitoring device on glycemic control among patients with type 2 diabetes. Scient Rep 2007; 7(1): 3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim CS, Park SY, Kang JG, et al. Insulin dose titration system in diabetes patients using a short messaging service automatically produced by a knowledge matrix. Diabetes Technol Therap 2010; 12(8): 663–669. [DOI] [PubMed] [Google Scholar]
- 102. Song MS, Kim HS. Intensive management program to improve glycosylated hemoglobin levels and adherence to diet in patients with type 2 diabetes. Appl Nurs Res 2009; 22(1): 42–47. [DOI] [PubMed] [Google Scholar]
- 103. McKay HG, Glasgow RE, Feil EG, et al. Internet-based diabetes self-management and support: initial outcomes from the diabetes network project. Rehabil Psychol 2002; 47(1): 31–48. [Google Scholar]
- 104. Cho JH, Chang SA, Kwon HS, et al. Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability: a 30-month follow-up study for diabetes management with a ubiquitous medical care system. Diabetes Care 2006; 29(12): 2625–2631. [DOI] [PubMed] [Google Scholar]
- 105. Eakin EG, Reeves MM, Winkler E, et al. Six-month outcomes from living well with diabetes: a randomized trial of a telephone-delivered weight loss and physical activity intervention to improve glycemic control. Ann Behav Med 2013; 46(2): 193–203. [DOI] [PubMed] [Google Scholar]
- 106. Agboola S, Jethwani K, Lopez L, et al. , Text to move: a randomized controlled trial of a text-messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus. J Med Int Res 201; 18(11): e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Glasgow RE, Kurz D, King D, et al. Twelve-month outcomes of an Internet-based diabetes self-management support program. Patient Educ Counsel 2012; 87(1): 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ralston JD, Hirsch IB, Hoath J, et al. Web-based collaborative care for type 2 diabetes: a pilot randomized trial. Diabetes Care 2009; 32(2): 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noh JH, Cho YJ, Nam HW, et al. Web-based comprehensive information system for self-management of diabetes mellitus. Diabetes Technol Therap 2010; 12(5): 333–337. [DOI] [PubMed] [Google Scholar]
- 110. Murray E, Sweeting M, Dack C, et al. Web-based self-management support for people with type 2 diabetes (HeLP-Diabetes): randomised controlled trial in English primary care. BMJ Open 2017; 7(9): e016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bingham JM, Stanislaw J, Warholak T, et al. Assessment of glycosylated hemoglobin outcomes following an enhanced medication therapy management service via telehealth. Int J Environ Res Public Health 2021; 18(12): 6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Michaud TL, Siahpush M, Estabrooks P, et al. Association between weight loss and glycemic outcomes: a post hoc analysis of a remote patient monitoring program for diabetes management. Telemed E Health 2020; 26(5): 621–628. [DOI] [PubMed] [Google Scholar]
- 113. Kesavadev J, Shankar A, Pillai PBS, et al. Cost-effective use of telemedicine and self-monitoring of blood glucose via Diabetes Tele Management System (DTMS) to achieve target glycosylated hemoglobin values without serious symptomatic hypoglycemia in 1,000 subjects with type 2 diabetes mellitus—a retrospective study. Diabetes Technol Therap 2012; 14(9): 772–776. [DOI] [PubMed] [Google Scholar]
- 114. Su D, Michaud TL, Estabrooks P, et al. Diabetes management through remote patient monitoring: the importance of patient activation and engagement with the technology. Telemed E Health 2019; 25(10): 952–959. [DOI] [PubMed] [Google Scholar]
- 115. Musacchio N, Lovagnini Scher A, Giancaterini A, et al. Impact of a chronic care model based on patient empowerment on the management of Type 2 diabetes: effects of the SINERGIA programme. Diabetic Med 2011; 28(6): 724–730. [DOI] [PubMed] [Google Scholar]
- 116. Turner J, Larsen M, Tarassenko L, et al. Implementation of telehealth support for patients with type 2 diabetes using insulin treatment: an exploratory study. Inform Primary Care 2009; 17(1): 47–53. [DOI] [PubMed] [Google Scholar]
- 117. Bergenstal RM, Layne JE, Zisser H, et al. Remote application and use of real-time continuous glucose monitoring by adults with type 2 diabetes in a virtual diabetes clinic. Diabetes Technol Therap 2021; 23(2): 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Michaud TL, Siahpush M, Schwab RJ, et al. Remote patient monitoring and clinical outcomes for postdischarge patients with type 2 diabetes. Popul Health Manag 2018; 21(5): 387–394. [DOI] [PubMed] [Google Scholar]
- 119. Cheng P-C, Kao C-H. Telemedicine assists in the management of proatherogenic dyslipidemia and postprandial glucose variability in patients with type 2 diabetes mellitus: a cross-sectional study. Endocr Connect 2021; 10(7): 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shane-McWhorter L, Lenert L, Petersen M, et al. The Utah Remote Monitoring Project: improving health care one patient at a time. Diabetes Technol Therap 2014; 16(10): 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yu CH, Parsons JA, Mamdani M, et al. A web-based intervention to support self-management of patients with type 2 diabetes mellitus: effect on self-efficacy, self-care and diabetes distress. BMC Med Inform Decis Making 2014; 14(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Berman MA, Guthrie NL, Edwards KL, et al. Change in glycemic control with use of a digital therapeutic in adults with type 2 diabetes: cohort study. JMIR Diabetes 2018; 3(1): e9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shane-McWhorter L, McAdam-Marx C, Lenert L, et al. Pharmacist-provided diabetes management and education via a telemonitoring program. J Am Pharm Assoc 2015; 55(5): 516–526. [DOI] [PubMed] [Google Scholar]
- 124. Dixon RF, Zisser H, Layne JE, et al. A virtual type 2 diabetes clinic using continuous glucose monitoring and endocrinology visits. J Diabetes Sci Technol 2020; 14(5): 908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Majithia AR, Kusiak CM, Armento Lee A, et al. Glycemic outcomes in adults with type 2 diabetes participating in a continuous glucose monitor-driven virtual diabetes clinic: prospective trial. J Med Intern Res 2020; 22(8): e21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. J Nurs Care Qual 2006; 21(3): 266–271. [DOI] [PubMed] [Google Scholar]
- 127. Mayes PA, Silvers A, Prendergast JJ. New direction for enhancing quality in diabetes care: utilizing telecommunications and paraprofessional outreach workers backed by an expert medical team. Telemed J E Health 2010; 16(3): 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. McGloin H, O’Connell D, Glacken M, et al. Patient empowerment using electronic telemonitoring with telephone support in the transition to insulin therapy in adults with type 2 diabetes: observational, pre-post, mixed methods study. J Med Intern Res 2020; 22(5): e16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bollyky JB, Bravata D, Yang J, et al. Remote lifestyle coaching plus a connected glucose meter with certified diabetes educator support improves glucose and weight loss for people with type 2 diabetes. J Diabetes Res 2018; 2018: 3961730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McGloin H, Timmins F, Coates V, et al. A case study approach to the examination of a telephone-based health coaching intervention in facilitating behaviour change for adults with type 2 diabetes. J Clin Nurs 2015; 24(9–10): 1246–1257. [DOI] [PubMed] [Google Scholar]
- 131. Carter EL, Nunlee-Bland G, Callender C. A patient-centric, provider-assisted diabetes telehealth self-management intervention for urban minorities. Perspect Health Inf Manag 2011; 8(Winter): 1b. [PMC free article] [PubMed] [Google Scholar]
- 132. King AB, Wolfe GS. Evaluation of a diabetes specialist–guided primary care diabetes treatment program. J Am Acad of Nurse Pract 2009; 21(1): 24–30. [DOI] [PubMed] [Google Scholar]
- 133. Carallo C, Scavelli FB, Cipolla M, et al. Management of type 2 diabetes mellitus through telemedicine. PLoS One 2015; 10(5): e0126858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen S-Y, Chang Y-H, Hsu H-C, et al. One-year efficacy and safety of the telehealth system in poorly controlled type 2 diabetic patients receiving insulin therapy. Telemed E Health 2011; 17(9): 683–687. [DOI] [PubMed] [Google Scholar]
- 135. Myers A, Presswala L, Bissoonauth A, et al. Telemedicine for disparity patients with diabetes: the feasibility of utilizing telehealth in the management of uncontrolled type 2 diabetes in black and Hispanic disparity patients; a pilot study. J Diabetes Sci Technol 2021; 15(5): 1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Istepanian RS, Mousa A, Haddad N, et al. The potential of m-health systems for diabetes management in post conflict regions a case study from Iraq. Ann Int Conf IEEE Eng Med Biol Soc 2014; 2014: 3650–3653. [DOI] [PubMed] [Google Scholar]
- 137. Lim H-M, Park J-E, Choi Y-J, et al. Individualized diabetes nutrition education improves compliance with diet prescription. Nutrit Res Pract 2009; 3(4): 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yoon K-H, Kim H-S. A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Research Clin Pract 2008; 79(2): 256–261. [DOI] [PubMed] [Google Scholar]
- 139. Nesari M, Zakerimoghadam M, Rajab A, et al. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Japan J Nurs Sci 2010; 7(2): 121–128. [DOI] [PubMed] [Google Scholar]
- 140. McIlhenny CV, Guzic BL, Knee DR, et al. Using technology to deliver healthcare education to rural patients. Rural Remote Health 2011; 11(4): 72–82. [PubMed] [Google Scholar]
- 141. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32(Suppl 1): S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. The Lancet. Diabetes: a defining disease of the 21st century. Lancet 2023; 401(10394): 2087. [DOI] [PubMed] [Google Scholar]
- 143. American Diabetes Association. 4. Lifestyle management: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(Supplement_1): S38–S50. [DOI] [PubMed] [Google Scholar]
- 144. Sriram S, Chack LE, Ramasamy R, et al. Impact of pharmaceutical care on quality of life in patients with type 2 diabetes mellitus. J Res Med Sci 2011; 16(Suppl1): S412. [PMC free article] [PubMed] [Google Scholar]
- 145. Shalihin SE, Fauzi A, Zulkifli NA, et al. Anti-diabetic medication burden amongst older persons with diabetes and associated quality of life. Med J Malaysia 2020; 75(5): 525. [PubMed] [Google Scholar]
- 146. Cannon A, Handelsman Y, Heile M, et al. Burden of illness in type 2 diabetes mellitus. J Managed Care Special Pharm 2018; 24(9-a Suppl): S5–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chahardah-Cherik S, Gheibizadeh M, Jahani S, et al. The relationship between health literacy and health promoting behaviors in patients with type 2 diabetes. Int J Commun Based Nurs Midwifery 2018; 6(1): 65. [PMC free article] [PubMed] [Google Scholar]
- 148. Silva-Tinoco R, Cuatecontzi-Xochitiotzi T, De la Torre-Saldaña V, et al. Influence of social determinants, diabetes knowledge, health behaviors, and glycemic control in type 2 diabetes: an analysis from real-world evidence. BMC Endocr Disord 2020; 20: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Barbosa A, Whiting S, Ding D, et al. Economic evaluation of physical activity interventions for type 2 diabetes management: a systematic review. Eur J Public Health 2022; 32(Supplement_1): i56–i66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Friel KM, Gillespie P, Coates V, et al. Estimating and examining the costs of inpatient diabetes care in an Irish Public Hospital. Diabetic Med 2022; 39(4): e14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jha V, Al-Ghamdi SMG, Li G, et al. 2023. Global economic burden associated with chronic kidney disease: a pragmatic review of medical costs for the inside CKD research programme. Adv Ther 2023; 40(10): 4405–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. J Am Pharm Assoc 2020; 60(6): e1–e18. [DOI] [PubMed] [Google Scholar]
- 153. Visaria J, Iyer NN, Raval AD, et al. Healthcare costs of diabetes and microvascular and macrovascular disease in individuals with incident type 2 diabetes mellitus: a ten-year longitudinal study. Clinicoecon Outcomes Res 2020; 12: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121241271846 for The effectiveness of telemedicine in the management of type 2 diabetes: A systematic review by Truong Van Dat, Van Binh, Thai Minh Hoang, Vo Linh Tu, Pham Dinh Luyen and Le Thi Kim Anh in SAGE Open Medicine