Abstract
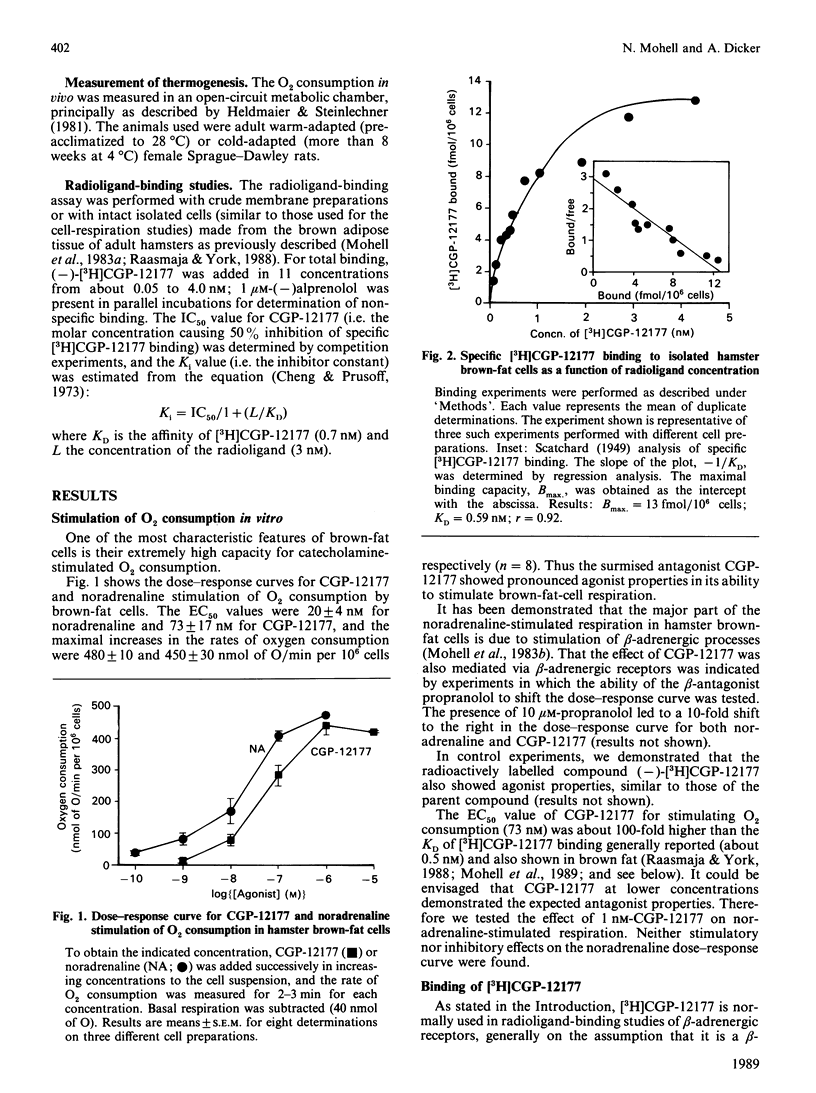
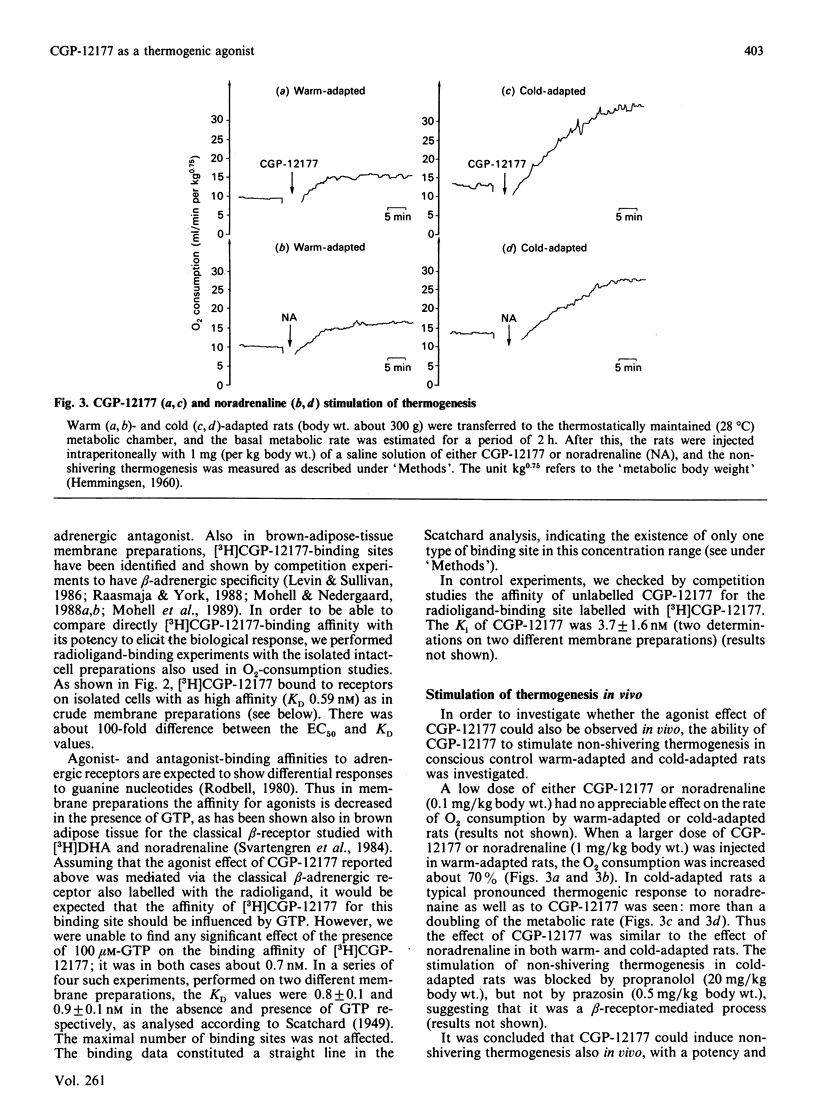
The effect of CGP-12177, originally developed as a radioligand with antagonist properties for binding studies of beta-adrenergic receptors, was investigated in brown adipose tissue. Contrary to expectations, CGP-12177 showed clear agonist properties in experiments with hamster brown-fat cells, with a maximal effect in stimulating oxygen consumption similar to that of the physiological stimulator noradrenaline, and also with a potency similar to that of noradrenaline [EC50 (50% effective concn.) approx. 70 nM]. This value could be contrasted with the very high affinity of CGP-12177 (KD about 1 nM) for ligand-binding sites on the cells. It is therefore suggested that the high-affinity binding site may not be the one that mediates the CGP-12177-stimulated thermogenesis in isolated cells. Also, when injected into cold-adapted rats, CGP-12177 stimulated non-shivering thermogenesis similarly to noradrenaline. This observation, in conjunction with the reported low general sympathomimetic effect of CGP-12177, may indicate that CGP-12177 could be of interest for the development of anti-obesity drugs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Bukowiecki L., Follea N., Vallieres J., Leblanc J. Beta-Adrenergic receptors in brown-adipose tissue. Characterization and alterations during acclimation of rats to cold. Eur J Biochem. 1978 Dec 1;92(1):189–196. doi: 10.1111/j.1432-1033.1978.tb12737.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Foster D. O. Quantitative contribution of brown adipose tissue thermogenesis to overall metabolism. Can J Biochem Cell Biol. 1984 Jul;62(7):618–622. doi: 10.1139/o84-082. [DOI] [PubMed] [Google Scholar]
- Lacasa D., Agli B., Giudicelli Y. Direct assessment of beta-adrenergic receptors in intact rat adipocytes by binding of [3H]CGP 12177. Evidence for agonist high-affinity binding complex and for beta 1 and beta 2 receptor subtypes. Eur J Biochem. 1985 Jan 15;146(2):339–346. doi: 10.1111/j.1432-1033.1985.tb08658.x. [DOI] [PubMed] [Google Scholar]
- Lacasa D., Mauriège P., Lafontan M., Berlan M., Giudicelli Y. A reliable assay for beta-adrenoceptors in intact isolated human fat cells with a hydrophilic radioligand, [3H]CGP-12177. J Lipid Res. 1986 Apr;27(4):368–376. [PubMed] [Google Scholar]
- Lang P. H., Lemmer B. Evidence for two specific affinity states of 3H-antagonist binding to cardiac beta-adrenergic receptors and influence of Gpp(NH)p. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(4):341–360. [PubMed] [Google Scholar]
- Levin B. E., Comai K., O'Brien R. A., Sullivan A. C. Abnormal brown adipose composition and beta-adrenoceptor binding in obese Zucker rats. Am J Physiol. 1982 Sep;243(3):E217–E224. doi: 10.1152/ajpendo.1982.243.3.E217. [DOI] [PubMed] [Google Scholar]
- Levin B. E., Sullivan A. C. Beta-1 receptor is the predominant beta-adrenoreceptor on rat brown adipose tissue. J Pharmacol Exp Ther. 1986 Mar;236(3):681–688. [PubMed] [Google Scholar]
- Mohell N., Connolly E., Nedergaard J. Distinction between mechanisms underlying alpha 1- and beta-adrenergic respiratory stimulation in brown fat cells. Am J Physiol. 1987 Aug;253(2 Pt 1):C301–C308. doi: 10.1152/ajpcell.1987.253.2.C301. [DOI] [PubMed] [Google Scholar]
- Mohell N., Nedergaard J., Cannon B. Quantitative differentiation of alpha- and beta-adrenergic respiratory responses in isolated hamster brown fat cells: evidence for the presence of an alpha 1-adrenergic component. Eur J Pharmacol. 1983 Sep 30;93(3-4):183–193. doi: 10.1016/0014-2999(83)90136-x. [DOI] [PubMed] [Google Scholar]
- Mohell N., Svartengren J., Cannon B. Identification of [3H]prazosin binding sites in crude membranes and isolated cells of brown adipose tissue as alpha 1-adrenergic receptors. Eur J Pharmacol. 1983 Aug 19;92(1-2):15–25. doi: 10.1016/0014-2999(83)90103-6. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Lindberg O. The brown fat cell. Int Rev Cytol. 1982;74:187–286. doi: 10.1016/s0074-7696(08)61173-0. [DOI] [PubMed] [Google Scholar]
- Portenier M., Hertel C., Müller P., Staehelin M. Some unique properties of CGP-12177. J Recept Res. 1984;4(1-6):103–111. doi: 10.3109/10799898409042542. [DOI] [PubMed] [Google Scholar]
- Raasmaja A., York D. A. Alpha 1- and beta-adrenergic receptors in brown adipose tissue of lean (Fa/?) and obese (fa/fa) Zucker rats. Effects of cold-acclimation, sucrose feeding and adrenalectomy. Biochem J. 1988 Feb 1;249(3):831–838. doi: 10.1042/bj2490831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J., Sudera D. K. Beta-adrenoreceptors in rat brown adipose tissue: proportions of beta 1- and beta 2-subtypes. Am J Physiol. 1985 Apr;248(4 Pt 1):E397–E402. doi: 10.1152/ajpendo.1985.248.4.E397. [DOI] [PubMed] [Google Scholar]
- Seydoux J., Giacobino J. P., Girardier L. Impaired metabolic response to nerve stimulation in brown adipose tissue of hypothyroid rats. Mol Cell Endocrinol. 1982 Feb;25(2):213–226. doi: 10.1016/0303-7207(82)90054-5. [DOI] [PubMed] [Google Scholar]
- Sher E., Clementi F. Agonist-induced internalization of the beta adrenergic receptor in a smooth muscle cell line. Biochem Biophys Res Commun. 1984 Nov 14;124(3):863–870. doi: 10.1016/0006-291x(84)91037-4. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Simons P., Jaeggi K., Wigger N. CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem. 1983 Mar 25;258(6):3496–3502. [PubMed] [Google Scholar]
- Svartengren J., Svoboda P., Cannon B. Desensitisation of beta-adrenergic responsiveness in vivo. Decreased coupling between receptors and adenylate cyclase in isolated brown-fat cells. Eur J Biochem. 1982 Nov 15;128(2-3):481–488. [PubMed] [Google Scholar]
- Svartengren J., Svoboda P., Drahota Z., Cannon B. The molecular basis for adrenergic desensitization in hamster brown adipose tissue: uncoupling of adenylate cyclase activation. Comp Biochem Physiol C. 1984;78(1):159–170. doi: 10.1016/0742-8413(84)90064-1. [DOI] [PubMed] [Google Scholar]
- Svoboda P., Svartengren J., Snochowski M., Houstek J., Cannon B. High number of high-affinity binding sites for (-)-[3H]dihydroalprenolol on isolated hamster brown-fat cells. A study of the beta-adrenergic receptors. Eur J Biochem. 1979 Dec;102(1):203–210. doi: 10.1111/j.1432-1033.1979.tb06281.x. [DOI] [PubMed] [Google Scholar]
- Takayanagi I., Koike K., Nakagoshi A. Interactions of some partial agonists with high and low affinity binding sites in beta-adrenoceptors. Can J Physiol Pharmacol. 1987 Jan;65(1):18–22. doi: 10.1139/y87-004. [DOI] [PubMed] [Google Scholar]
- Toews M. L., Waldo G. L., Harden T. K., Perkins J. P. Relationship between an altered membrane form and a low affinity form of the beta-adrenergic receptor occurring during catecholamine-induced desensitization. Evidence for receptor internalization. J Biol Chem. 1984 Oct 10;259(19):11844–11850. [PubMed] [Google Scholar]


