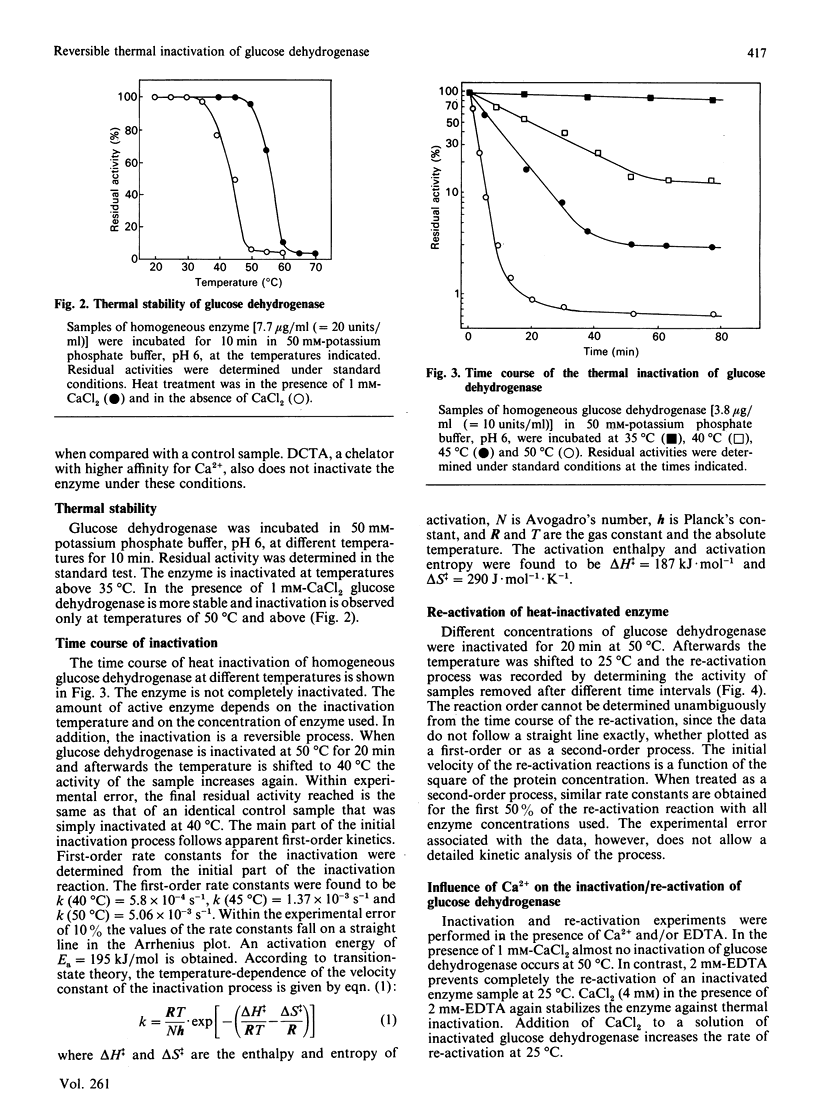
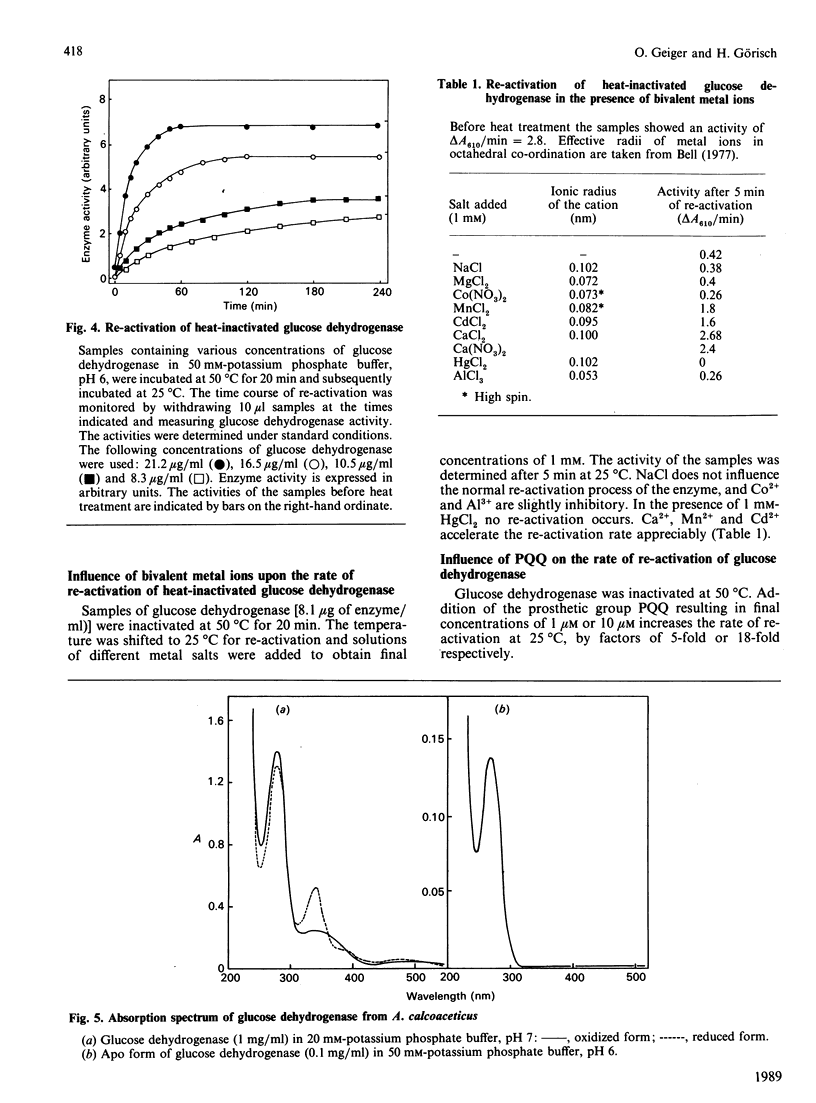
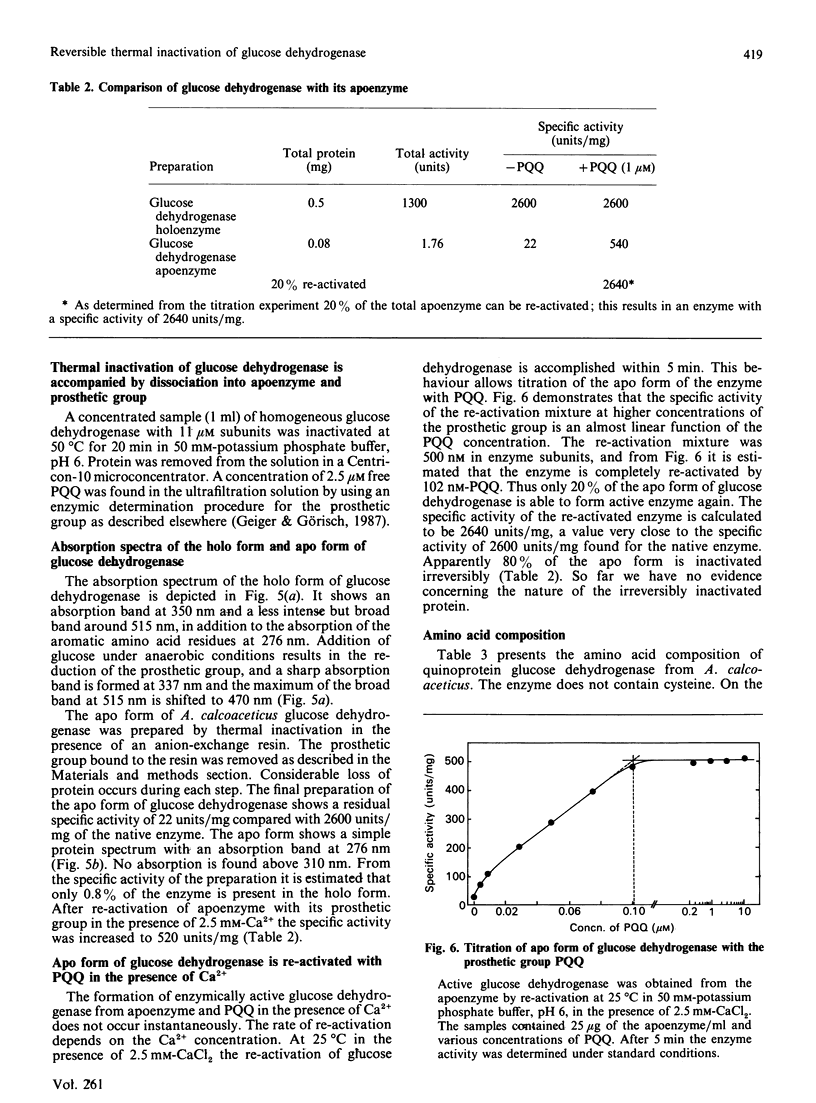
Abstract
The soluble form of the homogeneous quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus is reversibly inactivated at temperatures above 35 degrees C. An equilibrium is established between active and denatured enzyme, this depending on the protein concentration and the inactivation temperature used. Upon thermal inactivation the enzyme dissociates into the prosthetic group pyrroloquinoline quinone and the apo form of glucose dehydrogenase. After inactivation at 50 degrees C active enzyme is re-formed again at 25 degrees C. Ca2+ ions are necessary for the re-activation process. The velocity of re-activation depends on the protein concentration, the concentration of the prosthetic group pyrroloquinoline quinone and the Ca2+ concentration. The apo form of glucose dehydrogenase can be isolated, and in the presence of pyrroloquinoline quinone and Ca2+ active holoenzyme is formed. Even though native glucose dehydrogenase is not inactivated in the presence of EDTA or trans-1,2-diaminocyclohexane-NNN'NH-tetra-acetic acid, Ca2+ stabilizes the enzyme against thermal inactivation. Two Ca2+ ions are found per subunit of glucose dehydrogenase. The data suggest that pyrroloquinoline quinone is bound at the active site via a Ca2+ bridge. Mn2+ and Cd2+ can replace Ca2+ in the re-activation mixture.
Full text
PDF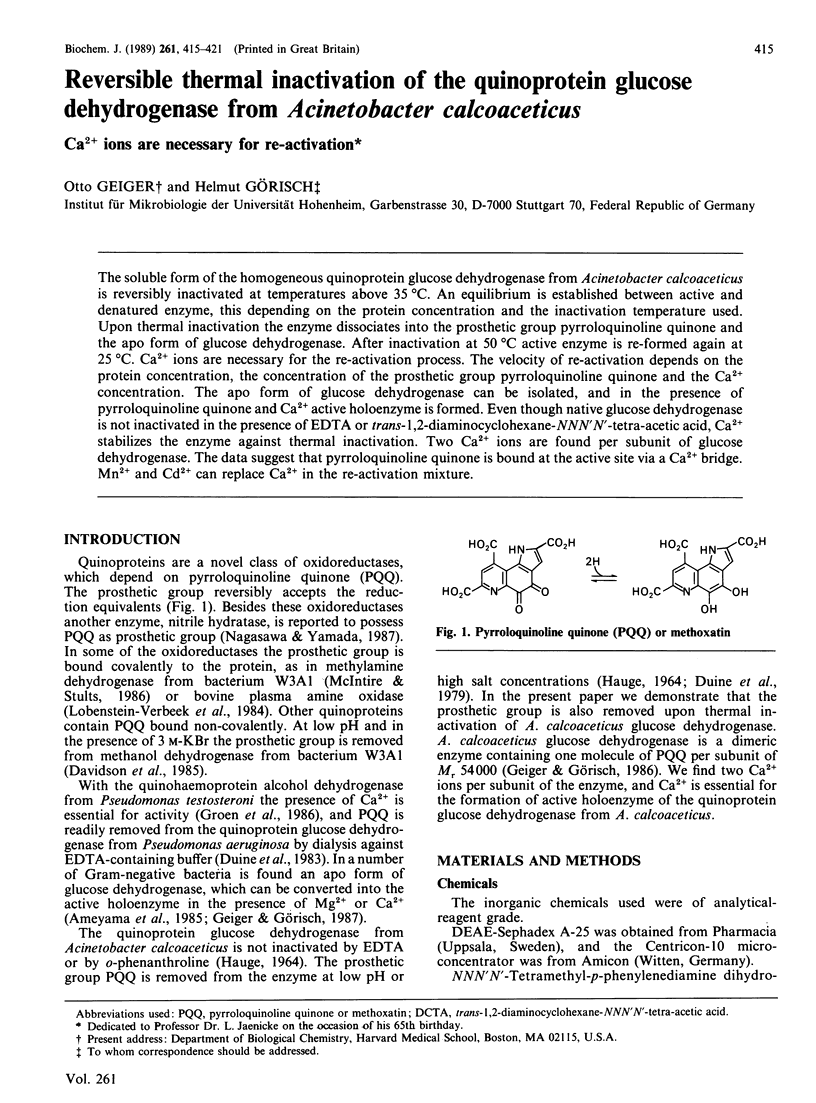


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davidson V. L., Neher J. W., Cecchini G. The biosynthesis and assembly of methanol dehydrogenase in bacterium W3A1. J Biol Chem. 1985 Aug 15;260(17):9642–9647. [PubMed] [Google Scholar]
- Dokter P., Frank J., Duine J. A. Purification and characterization of quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus L.M.D. 79.41. Biochem J. 1986 Oct 1;239(1):163–167. doi: 10.1042/bj2390163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jongejan J. A. Detection and determination of pyrroloquinoline quinone, the coenzyme of quinoproteins. Anal Biochem. 1983 Aug;133(1):239–243. doi: 10.1016/0003-2697(83)90249-x. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Geiger O., Görisch H. Enzymatic determination of pyrroloquinoline quinone using crude membranes from Escherichia coli. Anal Biochem. 1987 Aug 1;164(2):418–423. doi: 10.1016/0003-2697(87)90513-6. [DOI] [PubMed] [Google Scholar]
- Groen B. W., van Kleef M. A., Duine J. A. Quinohaemoprotein alcohol dehydrogenase apoenzyme from Pseudomonas testosteroni. Biochem J. 1986 Mar 15;234(3):611–615. doi: 10.1042/bj2340611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUGE J. G. GLUCOSE DEHYDROGENASE OF BACTERIUM ANITRATUM: AN ENZYME WITH A NOVEL PROSTHETIC GROUP. J Biol Chem. 1964 Nov;239:3630–3639. [PubMed] [Google Scholar]
- HAUGE J. G. Purification and properties of glucose dehydrogenase and cytochrome b from Bacterium anitratum. Biochim Biophys Acta. 1960 Dec 4;45:250–262. doi: 10.1016/0006-3002(60)91449-9. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Ameyama M. D-Glucose dehydrogenase from Pseudomonas fluorescens, membrane-bound. Methods Enzymol. 1982;89(Pt 500):149–154. doi: 10.1016/s0076-6879(82)89026-5. [DOI] [PubMed] [Google Scholar]
- McIntire W. S., Stults J. T. On the structure and linkage of the covalent cofactor of methylamine dehydrogenase from the methylotrophic bacterium W3A1. Biochem Biophys Res Commun. 1986 Dec 15;141(2):562–568. doi: 10.1016/s0006-291x(86)80210-8. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Yamada H. Nitrile hydratase is a quinoprotein. A possible new function of pyrroloquinoline quinone: activation of H2O in an enzymatic hydration reaction. Biochem Biophys Res Commun. 1987 Sep 15;147(2):701–709. doi: 10.1016/0006-291x(87)90987-9. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Verwiel P. E., Frank J., Verwiel E. J. Characterization of the second prosthetic group in methanol dehydrogenase from hyphomicrobium X. Eur J Biochem. 1981 Aug;118(2):395–399. doi: 10.1111/j.1432-1033.1981.tb06415.x. [DOI] [PubMed] [Google Scholar]


