Abstract
1. Subcellular-compartment-specific decreased-activity mutants of phosphoglucose isomerase in Clarkia xantiana were used to analyse the control of sucrose and starch synthesis during photosynthesis. Mutants were available in which the plastid phosphoglucose isomerase complement is decreased to 75% or 50% of the wild-type level, and the cytosol complement to 64%, 36% or 18% of the wild-type level. 2. The effects on the [product]/[substrate] ratio and on fluxes to sucrose or starch and the rate of photosynthesis were studied with the use of saturating or limiting light intensity to impose a high or low flux through these pathways. 3. Removal of a small fraction of either phosphoglucose isomerase leads to a significant shift of the [product]/[substrate] ratio away, from equilibrium. We conclude that there is no 'excess' of enzyme over that needed to maintain its reactants reasonably close to equilibrium. 4. Decreased phosphoglucose isomerase activity can also alter the fluxes to starch or sucrose. However, the effect on flux does not correlate with the extent of disequilibrium, and also varies depending on the subcellular compartment and on the conditions. 5. The results were used to estimate Flux Control Coefficients for the chloroplast and cytosolic phosphoglucose isomerases. The chloroplast isoenzyme exerts control on the rate of starch synthesis and on photosynthesis in saturating light intensity and CO2, but not at low light intensity. The cytosolic enzyme only exerts significant control when its complement is decreased 3-5-fold, and differs from the plastid isoenzyme in exerting more control in low light intensity. It has a positive Control Coefficient for sucrose synthesis, and a negative Control Coefficient for starch synthesis. 6. The Elasticity Coefficients in vivo of the cytosolic phosphoglucose isomerase were estimated to lie between 5 and 8 in the wild-type. They decrease in mutants with a lowered complement of cytosolic phosphoglucose isomerase. 7. The implications of these results for regulation and for evolution are discussed.
Full text
PDF
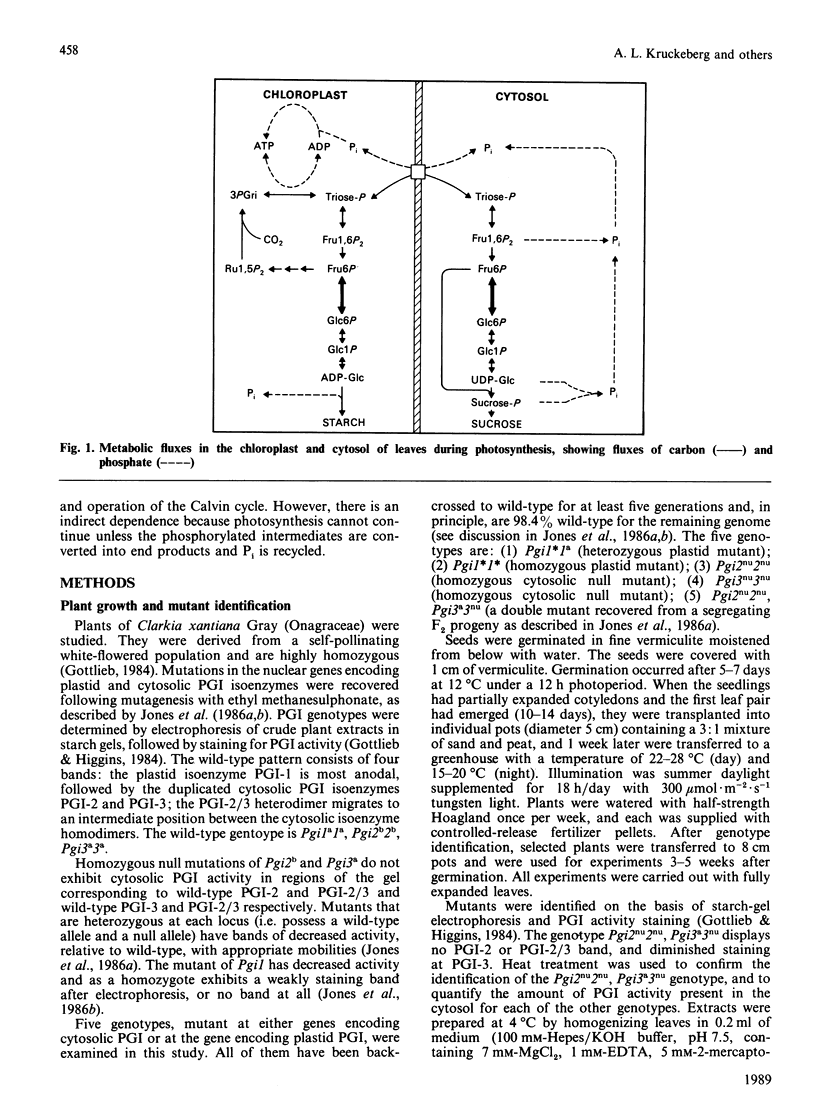

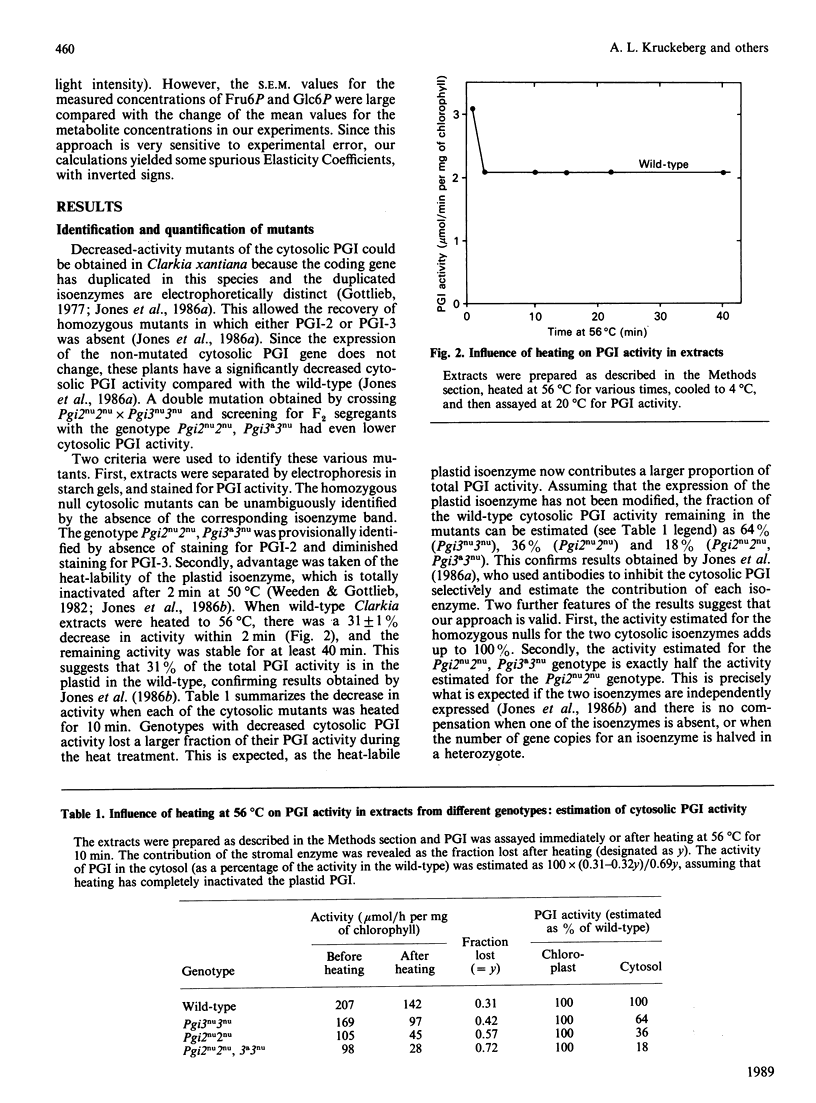
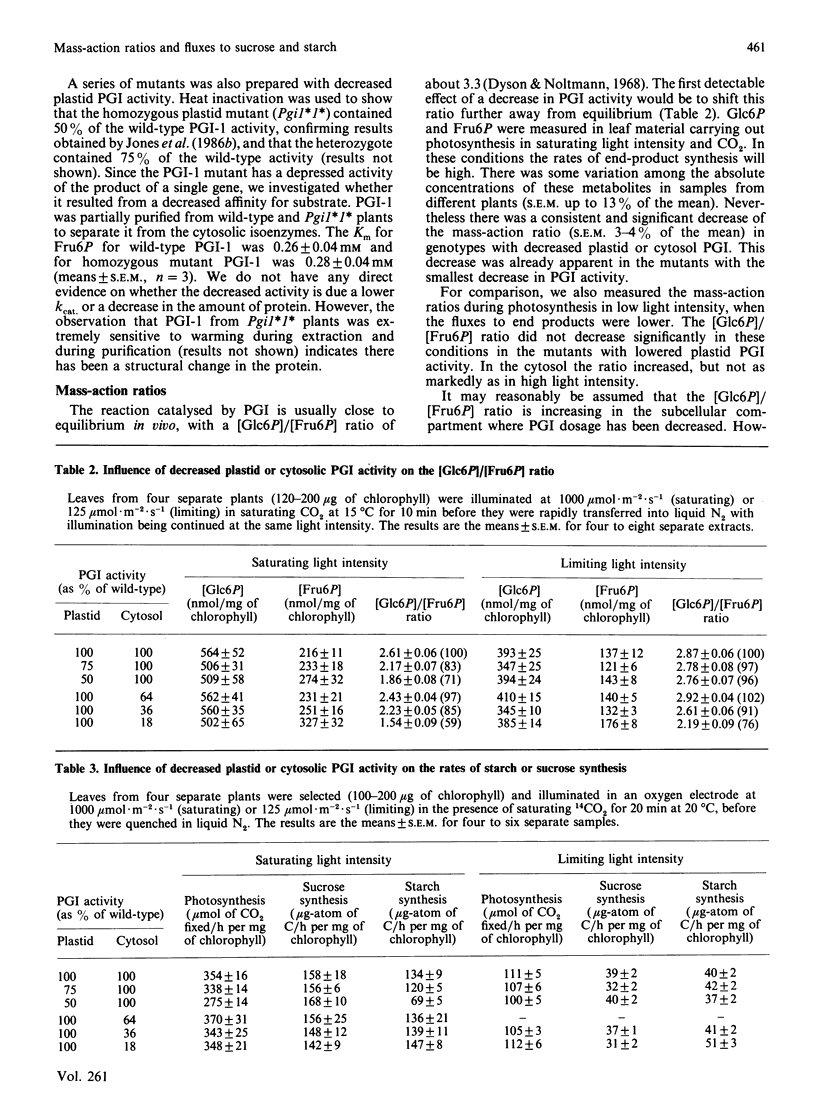
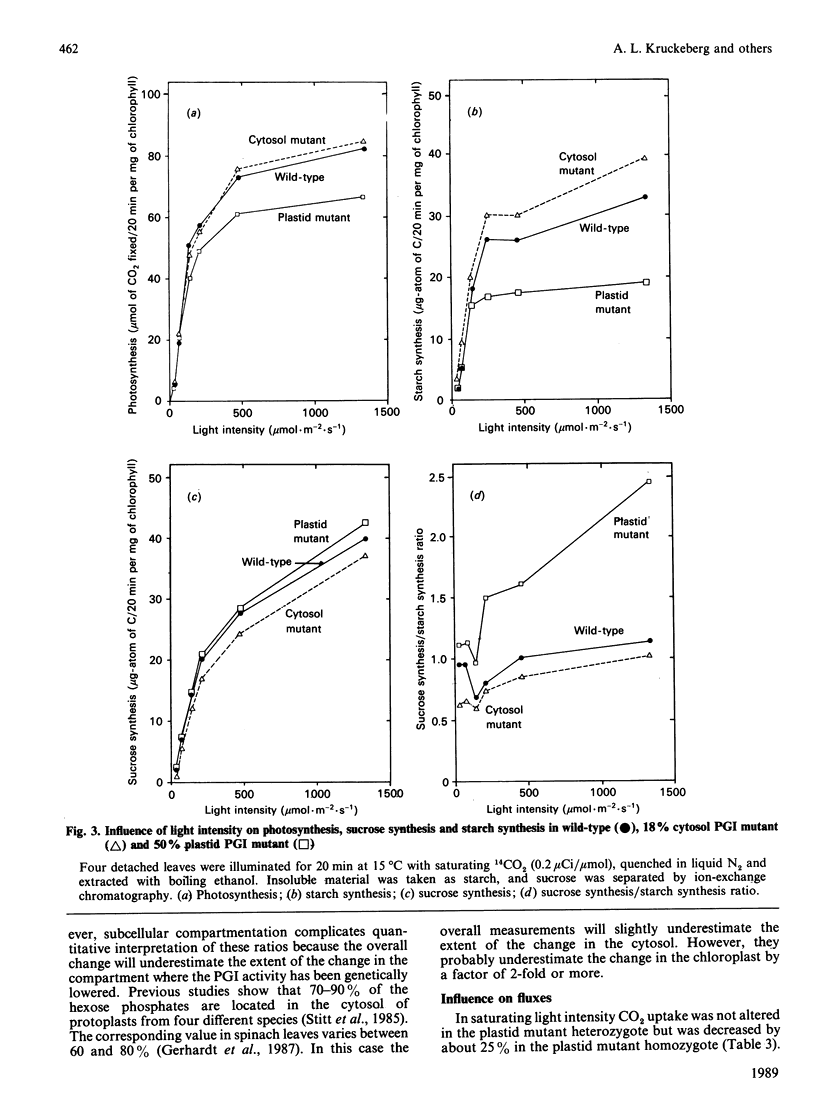

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dyson J. E., Noltmann E. A. The effect of pH and temperature on the kinetic parameters of phosphoglucose isomerase. Participation of histidine and lysine in a proposed dual function mechanism. J Biol Chem. 1968 Apr 10;243(7):1401–1414. [PubMed] [Google Scholar]
- Gerhardt R., Stitt M., Heldt H. W. Subcellular Metabolite Levels in Spinach Leaves : Regulation of Sucrose Synthesis during Diurnal Alterations in Photosynthetic Partitioning. Plant Physiol. 1987 Feb;83(2):399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb L. D. Evidence for duplication and divergence of the structural gene for phosphoglucoisomerase in diploid species of clarkia. Genetics. 1977 Jun;86(2):289–307. doi: 10.1093/genetics/86.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb L. D., Higgins R. C. Phosphoglucose Isomerase Expression in Species of Clarkia with and without a Duplication of the Coding Gene. Genetics. 1984 May;107(1):131–140. doi: 10.1093/genetics/107.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzhütter H. G., Jacobasch G., Bisdorff A. Mathematical modelling of metabolic pathways affected by an enzyme deficiency. A mathematical model of glycolysis in normal and pyruvate-kinase-deficient red blood cells. Eur J Biochem. 1985 May 15;149(1):101–111. doi: 10.1111/j.1432-1033.1985.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Jones T. W., Gottlieb L. D., Pichersky E. Reduced enzyme activity and starch level in an induced mutant of chloroplast phosphoglucose isomerase. Plant Physiol. 1986 Jun;81(2):367–371. doi: 10.1104/pp.81.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. W., Pichersky E., Gottlieb L. D. Enzyme activity in ems-induced null mutations of duplicated genes encoding phosphoglucose isomerases in clarkia. Genetics. 1986 May;113(1):101–114. doi: 10.1093/genetics/113.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. MOlecular democracy: who shares the controls? Biochem Soc Trans. 1979 Oct;7(5):1149–1160. doi: 10.1042/bst0071149. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The molecular basis of dominance. Genetics. 1981 Mar-Apr;97(3-4):639–666. doi: 10.1093/genetics/97.3-4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacser H. The control of enzyme systems in vivo: elasticity analysis of the steady state. Biochem Soc Trans. 1983 Jan;11(1):35–40. doi: 10.1042/bst0110035. [DOI] [PubMed] [Google Scholar]
- Middleton R. J., Kacser H. Enzyme variation, metabolic flux and fitness: alcohol dehydrogenase in Drosophila melanogaster. Genetics. 1983 Nov;105(3):633–650. doi: 10.1093/genetics/105.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres N. V., Mateo F., Meléndez-Hevia E., Kacser H. Kinetics of metabolic pathways. A system in vitro to study the control of flux. Biochem J. 1986 Feb 15;234(1):169–174. doi: 10.1042/bj2340169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden N. F., Gottlieb L. D. Dissociation, reassociation, and purification of plastid and cytosolic phosphoglucose isomerase isozymes. Plant Physiol. 1982 Mar;69(3):717–723. doi: 10.1104/pp.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]


