Abstract
Background
Scutellariae radix (SR) is the dried root of Scutellaria baicalensis Georgi. It has a long history of ethnic medicinal use, traditionally recognized for its efficacy in clearing heat, drying dampness, eliminating fire, removing toxins, stopping bleeding and tranquilizing fetus to prevent miscarriage. Clinically, it is used to treat cold, fever, migraine, hand-foot-and-mouth diseases, liver cancer and inflammatory diseases.
Purpose
The review aims to provide a comprehensive reference on the ethnobotanical uses, processing, phytochemistry, pharmacological effect, quality control and influence factors of biosynthesis for a deeper understanding of SR.
Results and conclusion
A total of 210 isolated components have been reported in the literature, including flavonoids and their glycosides, phenylpropanoids, phenylethanoid glycosides, phenolic acids, volatile components, polysaccharides and others. The extract of SR and its main flavonoids such as baicalin, baicalein, wogonin, wogonoside, and scutellarin showed antioxidant, anti-inflammatory, anti-tumor, antiviral, hepatoprotective, and neuroprotective effects. However, further studies are required to elucidate its mechanisms of action and clinical applications. The pharmacodynamic evaluation based on traditional efficacy should be conducted. Although various analytical methods have been established for the quality control of SR, there are gaps in the research regarding efficacy-related quality markers and the development of quality control standards for its processed products. The regulatory mechanisms of flavonoids biosynthesis remain to be explored while the influence of environmental and transcription factors on the biosynthesis have been studied. In conclusion, SR is a promising herbal medicine with significant potential for future development.
Keywords: Biosynthesis, Flavonoids, Quality control, Scutellariae radix
Graphical abstract
Highlights
-
•
SR is an attractive herb with multiple traditional efficacy and excellent prospects.
-
•
The main components of SR are flavonoids which have various pharmacological effects.
-
•
The historical applications, processing, and biosynthesis of SR are summarized.
-
•
The quality markers related to the biological activity of SR are yet to be explord.
1. Introduction
Scutellariae radix (SR) is the dried root of Scutellaria baicalensis Georgi, belonging to the family of Labiatae, and is typically harvested in autumn [1]. It was first recorded in Shennong Bencao Jing and had a long history of usage in many classical Chinese medical works, such as Shanghan Zabing Lun, Wupu Bencao, Bencao Gangmu [2]. In China, SR mainly originates from Inner Mongolia, Sichuan, Shanxi, Shandong, Hebei, Henan, and other provinces [3]. As a commonly medicinal herb, SR is also cultivated in other countries and regions around the world, particularly in Korea, Japan, and Southeast Asia [4].
According to the theory of traditional Chinese medicines (TCMs), SR acts on the lungs, gall bladder, spleen, stomach, large intestine and small intestine. It is traditionally believed to clear heat and dry dampness, eliminate fire and remove toxins, stop bleeding and tranquilize fetus to prevent miscarriage [5]. In modern clinical practice, SR has been extensively studied. It is used to treat cold, fever, migraine, primary hepatocellular carcinoma, hand-foot-and-mouth diseases (HFMD), gingivitis and other diseases [[6], [7], [8], [9], [10]]. At present, a total of 210 components has been reported in the literatures, including flavonoids and their glycosides, phenylpropanoids, phenylethanoid glycosides, phenolic acids, polysaccharides, volatile components and others. It was reported that the extract of SR, baicalin, baicalein, wogonin, wogonoside, and scutellarin had antioxidant, anti-inflammatory, anti-tumor, antiviral, hepatoprotective, and neuroprotective effects.
Although the botany, chemical components, pharmacological activities, pharmacokinetics and toxicology of SR studies have been reviewed [[11], [12], [13]], there is a gap in summarizing and evaluating its traditional historical applications, processing methods, quality control, and factors influencing biosynthesis. The retrospect of SR historical uses can better analyze its medicinal properties to provide a theoretical basis for modern pharmacological research based on TCM theories. Quality control is the basis for ensuring the effectiveness and safety of SR. The biosynthetic research of SR promotes the development of synthetic biology and metabolic engineering of root-specific flavonoids. Hence, this paper provides an updated review of the ethnobotanical uses, processing, phytochemistry, pharmacological effects, quality control, and influence factors of biosynthesis of SR for scientific study.
2. Methods
The reviewed information was obtained from published literature in various scientific databases. The search was conducted by using the keywords “Scutellaria baicalensis”, “Scutellaria baicalensis Georgi”, or “Scutellariae radix” in PubMed, Springer, Web of Science, ScienceDirect, Wiley Online Library, CNKI, and WanFang Database. The search covered literatures were published from 2010 to 2024. Additionally, information from Chinese medical books and Ch. P were also included to provide a comprehensive overview of SR. The botanical information of SR was described using the Flora of China (http://www.iplant.cn/foc).
3. Botany
SR is a perennial herb that blooms from July to August and bears fruit from August to September (Fig. 1). The SR plants has branched, fleshy rhizomes, and up to 2 cm in diameter. The stems are ascending and have subglabrous or antrorsely to spreading puberulent. The papery leaves are lanceolate to linear-lanceolate in shape with puberulent petioles. The front of the blade is darker in color compared to the back. Racemes grow at the tip of stems and branches, which basal bracts are similar to the leaves while the upper bracts are subglabrous and ovate-lanceolate to lanceolate. Pedicels of 3 mm in length are puberulent. The corollas are about 2.3–3 cm long. Their outer part is densely glandular pubescent and the inner saccate part is pubescent. Nutlets are ovoid and have a fruiting umbilicus on the ventral side near the base (http://www.iplant.cn/foc). Details of the various parts of the SR plant are collated in Table 1.
Fig. 1.
Photograph of Scutellariae radix: inflorescences (A), stems and leaves (B), and medicinal materials (C).
Table 1.
Detailed information on the main parts of the Scutellariae radix plant.
| Parts | Size | Shape | Color |
|---|---|---|---|
| Rhizome | 2 cm in diameter | Elongated and branched | – |
| Stem | 30 cm–120 cm | Obtuse quadrangular | Green |
| Blade | 1.5–4.5 cm long, 0.5–1.2 cm wide | Lanceolate to linear-lanceolate | Black-green |
| Raceme | 7–15 cm long | Conical | – |
| Bract | 4–11 mm | Lanceolate to linear-lanceolate (basal bract); Oval-lanceolate to lanceolate (upper bract) | – |
| Calyx | 4 mm (flowering stage); 5 mm (fruiting stage) | – | – |
| Scutellum | 1.5 mm (flowering stage); 4 mm (fruiting stage) | – | – |
| Corolla | 2.3–3 cm long | – | Purple, fuchsia, or blue |
| Nutlet | 1.5 × 1 mm | Ovoid | Black-brown |
4. Ethnobotanical uses
4.1. Traditional historical applications
SR is a common Chinese herbal medicine, with the traditional efficacy of clearing heat and drying dampness, eliminating fire and removing toxicity, stopping bleeding and tranquilizing fetus to prevent miscarriage [14]. It was widely used in many ancient Chinese prescriptions, formulated for its diverse effects.
As early as the Eastern Han Dynasty, there were many prescriptions involving SR recorded in Shanghan Zabing Lun, such as Gegenqinlian decoction, Huangqin decoction, Huanglian Ejiao decoction, and Banxia Xiexin decoction [15]. Among these, Gegen Qinlian decoction was a formula for relieving both superficial and internal disorders [16]. Huangqin decoction had the efficacy in clearing heat and relieving dysentery, harmonizing the middle Jiao and alleviating pain [17]. In the formulation compositions of Gegen Qinlian decoction and Huangqin decoction, SR developed an effective use in clearing heat and drying dampness. This efficacy of SR had persisted through other dynasties, such as Longdan Xiegan decoction and Baixianpi powder in the Song Dynasty and Huangqin Huashi decoction in the Qing Dynasty. In Huanglian Ejiao decoction and Banxia Xiexin decoction, SR showed its ability to clear heat and lower fire. Huanglian Ejiao decoction, consisting of SR, Coptidis rhizoma, and Asini Corii Colla, had the effects of nourishing Yin and clearing heat. Banxia Xiexin decoction was used to mildly regulate cold and heat, relieve oppression and resolve hard mass. During the Tang Dynasty, Huanglian Jiedu decoction, which contained SR for its eliminating fire and detoxifying toxins, had been recorded in the Waitai Miyao. In the prescription, SR played a crucial role in clearing the fire of the Upper Jiao. In the Yuan and Ming Dynasties, SR was added in several gynecological prescriptions for nourishing Yin, tonifying blood, and tranquilizing fetus to prevent miscarriage. For instance, the Antai pill recorded in Danxi Xinfa was formulated with the SR, which had the efficacy of tonifying the kidneys and calming the fetus. Yixue Rumen recorded a formula called Gujing pill, which was used to treat Yin deficiency and blood heat, pre-menstruation and abnormal color of menstruation in women. The main uses of SR in the traditional prescriptions of various dynasties are detailed in Table 2.
Table 2.
Traditional uses of Scutellariae radix.
| Dynasty | Work | Prescriptions | Form | Effect |
|---|---|---|---|---|
| Eastern Han Dynasty (202–220 BCE) | Shanghan Zabing Lun | Gegen Huangqin Huanglian Decoction | Puerariae Lobatae Radix, Scutellariae Radix, Coptidis Rhizoma, Glycyrrhizae Radix Et Rhizoma, Praeparata Cum Melle | Relieving superficies and clearing interior |
| Huangqin Decoction | Scutellariae Radix, Paeoniae Radix Alba, Glycyrrhizae Radix Et Rhizoma, Jujubae Fructus | Clearing heat and stopping dysentery, harmonizing the spleen and stomach, and relieving pain | ||
| Ganjiang Huangqin Huanglian Renshen Decoction | Zingiberis Rhizoma, Scutellariae Radix, Coptidis Rhizoma, Ginseng Radix Et Rhizoma | Warming spleen and stomach for dispelling cold, reveling heat, bloated stomach, and astriction | ||
| Huanglian Ejiao Decoction | Scutellariae Radix, Coptidis Rhizoma, Asini Corii Colla, Paeoniae Radix Alba | Nourishing yin for lowering fire and clearing heat | ||
| Banxia Xiexin Decoction | Pinelliae Rhizoma, Scutellariae Radix, Zingiberis Rhizoma, Ginseng Radix Et Rhizoma, etc | Mildly regulating cold and heat, relieving oppression and resolving hard mass | ||
| Huangqin Jia Banxia Shengjiang Decoction | Scutellariae Radix, Paeoniae Radix Alba, Jujubae Fructus, Zingiberis Rhizoma | Clearing heat and stopping vomiting, nourishing lung qi | ||
| Tang Dynasty (618 CE–907) | Waitai Miyao | Huanglian Jiedu Decoction | Scutellariae Radix, Coptidis Rhizoma, Phellodendri Chinensis Cortex, Gardeniae Fructus | Purging fire and removing toxicity |
| Song Dnasty (960 CE–1279) | Xiaoer Yaozheng Zhijue | Longdan Xiegan Decoction | Conyzae Herba, Scutellariae Radix, Alismatis Rhizoma, Gardeniae Fructus, etc. | Clearing excessive fire of liver and gallbladder and eliminating dampness-heat in xiajiao |
| Song Dnasty (960 CE–1279) | Taiping Shenghui Fang | Baixianpi Powder | Dictamni Cortex, Scutellariae Radix, Gentianae Macrophyllae Radix, Glycyrrhizae Radix Et Rhizoma, etc | Clearing heat and removing dampness |
| Yuan Dynasty (1271 CE–1368) | Danxi Xinfa | Antai Pill | Scutellariae Radix, Atractylodis Macrocephalae Rhizoma | Nourishing blood and tranquilizing fetus to prevent miscarriage |
| Ming Dynasty (1368 CE–1644) | Yuji Weiyi | Huangqin Shaoyao Decoction | Scutellariae Radix, Glycyrrhizae Radix Et Rhizoma, Glycyrrhizae Radix Et Rhizoma | Clearing heat and stopping bleeding |
| Ming Dynasty (1368 CE–1644) | Waike Zhengzong | Huangqin Qingfei Drink | Scutellariae Radix, Chuanxiong Rhizoma, Angelicae Sinensis Radix, Paeoniae Radix Rubra, etc | Cooling blood and harmonizing nutrient |
| Ming Dynasty (1368 CE–1644) | Yixue Rumen | Gujing Pill | Scutellariae Radix, Paeoniae Radix Alba, Testudinis Carapax Et Plastrum, Phellodendri Chinensis Cortex, etc | Nourishing yin and clearing heat, consolidating channel for hemostasis |
| Qing dynasty (1616 CE–1912) | Wenbing Tiaobian | Huanglian Huangqin Decoction | Scutellariae Radix, Coptidis Rhizoma | Clearing heat and removing turbidity |
| Huangqin Huashi Decoction | Scutellariae Radix, Amomi Fructus Rotundus, Tetrapanacis Medulla, Poriae Cutis, etc | clearing heat and promoting diuresis |
4.2. Modern uses
SR has a variety of effects for treating numerous clinical diseases. For instance, SR could be used to treat colds during pregnancy without adverse effects on the fetus after birth [6]. The pain of migraine could be alleviated by treatment with SR [10]. A retrospective clinical analysis indicated that SR reduced the symptoms of fever, blisters, rash, and oral lesions in hand-foot-and-mouth diseases because of its inhibitory effect on enterovirus 71, the causative agent of HFMD [8]. A double-blinded randomized clinical test suggested that SR enhanced the efficacy of metformin in type 2 diabetes patients. SR in combination with metformin increased the glucose tolerance and inhibited the expression of inflammatory markers [18]. The extract of SR performed well in inhibiting the plaque development and alleviating gingivitis [7]. SR could modulate cell proliferation and apoptosis, as well as influence the infiltration of T cells and macrophages in the tumor microenvironment [17]. A clinical study showed that baicalin capsules could improve the immune ability and the liver function of cancer patients, reducing the side effects caused by chemotherapy [9].
Furthermore, with the help of modern pharmaceutical processes, SR is combined with several Chinese herbs to create more widely accepted Chinese patent medicines. For instance, Niuhuang Qinggan capsule, Fufang Qinlan oral liquid, and Pudilan Xiaoyan capsule have the main effects of clearing heat and eliminating toxins. Gong Liu Qing capsule and Gongning grain are effective in nourishing blood and removing blood stasis, thus treating gynecological diseases. Some commonly used Chinese patent medicines that contain SR are shown in Table 3.
Table 3.
Chinese patent medicines containing Scutellariae radix.
| Type | Preparation Name | Main Compositions | Dosage of SR | Function | References |
|---|---|---|---|---|---|
| Tablet | Shiduqing Pian | Rehmanniae Radix, Angelicae Sinensis Radix, Salviae Miltiorrhizae Radix Et Rhizoma, Scutellariae Radix, etc | 125 g | Nourishing blood and moisturizing skin, dispelling pathogenic wind for relieving itching | [1] |
| Tablet | Chaihuang Pian | Bupleuri Radix, Scutellariae Radix | 1000 g | Clearing heat and resolving superficies syndrome | [1] |
| Tablet | Tongxuan Lifei Pian | Perillae Folium, Peucedani Radix, Platycodonis Radix, Scutellariae Radix, etc | 120 g | Resolving superficies syndrome for dispelling cold and unblocking stuffy orifice | [1] |
| Tablet | Hexue Mingmu Pian | Typhae Pollen, Rehmanniae Radix, Ecliptae Herba, Scutellariae Radix, etc | 45 g | Cooling blood for hemostasis, nourishing yin and improving eyesight | [1] |
| Tablet | Biyankang Pian | Pogostemon cablin Benth, Centipedae Herba, Dendranthema indicum, Scutellariae Radix,etc | 109 g | Clearing heat and removing toxicity | [1] |
| Tablet | Niuhuang Jiangya Pian | Saigae Tataricae Cornu, Margarita, Bovis Calculus Artifactus, Scutellariae Radix, etc | – | Clearing heart and dissipating phlegm, suppressing hyperactive liver for tranquilizing the mind | [1] |
| Pill | Qingfei Yihuo Wan | Gardeniae Fructus, Anemarrhe Naerhizoma, Fritillariae Thunbergii Bulbus, Scutellariae Radix, etc | 140 g | Clearing lung to stopping cough, expectorant and facitating feces excretion | [1] |
| Pill | Antai Wan | Angelicae Sinensis Radix, Chuanxiong Rhizoma, Atractylodis Macrocephalae Rhizoma, Scutellariae Radix, etc | 200 g | Nourishing blood and tranquilizing fetus to prevent miscarriage | [1] |
| Pill | Qingre Liangxue Wan | Scutellariae Radix, Rehmanniae Radix | 500 g | Cooling blood, clearing heat, and nourshing yin | [1] |
| Pill | Gegen Qinlian Wan | Puerariae Lobatae Radix, Coptidis Rhizoma, Glycyrrhizae Radix Et Rhizoma Praeparata Cum Melle, Scutellariae Radix | 375 g | Clearing heat and removing toxicity, promoting diuresis and relieving diarrhea | [1] |
| Pill | Yinhuang Wan | Lonicerae Japonicae Flos Extract, Scutellariae Radix Extract | 160 g | Clearing heat and dispelling wind pathogen, relieving sore throat and removing toxicity | [1] |
| Pill | Tongqiao Erlong Wan | Bupleuri Radix, Gentianae Radix Et Rhizoma, Aloe, Scutellariae Radix, etc | 120 g | Clearing liver-fire, unblocking stuffy orifice, and moistening dryness for relaxing bowels | [1] |
| Capsule | Niuhuang Qinggan Jiaonang | Lonicerae Japonicae Flos, Forsythiae Fructus, Margaritifera Concha, Scutellariae Radix, etc | 166.7 g | Clearing heat and removing toxicity, resolving superficies syndrome | [1] |
| Capsule | Pudilan Xiaoyan Jiaonang | Taraxaci Herba, Corydalis Bungeanae Herba, Isatidis Radix, Scutellariae Radix, etc | 271 g | Clearing heat and removing toxicity, reduce swelling and relieving sore throat | [1] |
| Capsule | Jiuwei Gantai Jiaonang | Notoginseng Radix Et Rhizoma, Curcumae Radix, Tribuli Fructus, Scutellariae Radix, etc | 160 g | Invigorating the spleen and dispersing the stagnated liver-energy, resolving blockages, and unblocking veins | [1] |
| Capsule | Gongliuqing Jiaonang | Murrayae Folium Et Cacumen, Zanthoxyli Radix, Aucklandiae Radix, Scutellariae Radix, etc | 769 g | Promoting blood circulation for removing blood stasis | [1] |
| Capsule | Fufang Niuhuang Xiaoyan Jiaonang | Bovis Calculus Artifactus, Gardeniae Fructus, Cinnabaris, Scutellariae Radix, etc | 190.6 g | Clearing heat and removing toxicity, sedative and tranquilizer | [1] |
| Grain | Sanjiu Weitai Keli | Murrayae Folium Et Cacumen, Zanthoxyli Radix, Poria, Scutellariae Radix, etc | 153.85 g | Clearing heat and drying dampness, activating blood and relieving pain | [1] |
| Grain | Qinghou Liyan Keli | Chebulae Fructus Immaturus, Bambusae Caulis In Taenias, Platycodonis Radix, Scutellariae Radix, etc | 36 g | Clearing heat, relieving sore throat, and moistening throat | [1] |
| Grain | Huanglian Shangqing Keli | Coptidis Rhizoma, Gardeniae Fructus, Forsythiae Fructus, Scutellariae Radix, etc | 192 g | Clearing heat and dispelling wind pathogen, clearing heat and reveling pain | [1] |
| Grain | Gongning Keli |
Rubiae Radix Et Rhizoma, Typhae Pollen, Sanguisorbae Radix, Scutellariae Radix, etc |
117 g | Removing blood stasis and clearing heat, consolidating channel for hemostasis | [1] |
| Grain | Xiao'er tuire keli | Isatidis Folium, Isatidis Radix, Moutan Cortex, Scutellariae Radix, etc | 180 g | Dispelling wind pathogen and resolving superficies syndrome, removing toxicity and relieving sore throat | [1] |
| Mixture | Fufang Yuxingcao Heji | Houttuyniae Herba, Isatidis Radix, Forsythiae Fructus, Scutellariae Radix, etc | 25 g | Clearing heat and removing toxicity | [1] |
| Mixture | Fufang Qinlan Koufuye | Lonicerae Japonicae Flos, Forsythiae Fructus, Isatidis Radix, Scutellariae Radix, etc | 500 g | Dispelling the evil in the superficies with drugs of pungent taste and cool nature, Clearing heat and removing toxicity | [1] |
| Mixture | Bidouyan Koufuye | Magnoliae Flos, Schizonepetae Herba, Menthae Haplocalycis Herba, Scutellariae Radix, etc | 112 g | Clearing heat and promoting diuresis, unblocking stuffy orifice | [1] |
5. Processing
5.1. Evolution of processing methods of SR
Processing is a conventional pharmaceutical procedure that transforms crude drugs into decoction pieces. Some auxiliary materials, such as wine, vinegar, and honey, are often added during processing process to enhance the efficacy and reduce the toxicity of Chinese herbs [19]. Although SR was applied early, its processing was not officially recorded until the Tang Dynasty. The fine cutting of SR was first recorded in the Qianjin Yaofang [20]. During the Song Dynasty, stir-frying processing, wine soaking, and vinegar processing emerged. Stir-frying involved gently heating SR to enhance its medicinal properties and directing its effects upward in the body. Vinegar processing promoted the efficacy of SR in the blood, commonly used in treating menstrual disorders [21,22]. In the Yuan and Ming dynasties, wine-processed SR and charred SR were widely used [20]. Charred SR had the effects of stopping bleeding and clearing heat, which was mostly used in the treatment of vomiting and epistaxis. Wine processing included wine stir-frying, wine washing, wine steaming, wine boiling, and other forms. With the ascending power of wine led to the medical effects of clearing the heat of the upper jiao [23]. In brief, the various processing methods of SR in the past dynasties were diversified. However, there were only six processing methods still in use today, including SR piece, wine-processed SR, charred SR, stir-fried SR, honey-processed SR, and ginger-processed SR [24]. SR piece and wine-processed SR are listed in the Chinese Pharmacopoeia (version 2020), but the difference in their quality is not distinguished. The evolution history of SR processing is shown in Table 4.
Table 4.
Evolution of Scutellariae radix processing methods.
| Dynasty | Processing methods | Classic sources | References |
|---|---|---|---|
| Tang dynasty (618 CE–907) | Fine cutting | Qianjin Yaofang | [20] |
| Song dynasty (960 CE–1279) | Stir-frying processing, Wine soaking, Vinegar processing | Taiping Huimin Heji Ju Fang, Renzhai Zhi Zhi Fang, Weisheng Jianyifang | [21,22] |
| Yuan dynasty (1271 CE–1368) | Wine washing, ginger processing | Danxi xinfa | [20] |
| Ming dynasty (1368 CE–1644) | Wine stir-frying, Wine steaming, Making charcoal, Vinegar processing, Salt processing, | Yinhai Jingwei, Yizong Bidu, Paozhi Dafa, Shoushi Baoyuan, Renshu bianlan | [20,22] |
| Modern | Stir-frying, wine processing, making charcoal, cutting, honey processing, ginger processing | – | [24] |
5.2. Modern research on processing SR
Modern analytical techniques showed the chemical components and their in vivo absorption were altered after different processing. Using ultrahigh performance liquid chromatography (UHPLC), the flavonoid content in charred SR, wine-processed SR, and crude SR could be determined [25]. The results revealed that the processing led to the decomposition of flavonoid glycosides and a decrease in their content. The charring of SR reduced the content of volatile components of SR [26]. An ultrahigh performance liquid chromatography coupled with electrospray ion source-mass spectrometry (UHPLC-ESI-MS/MS) method was employed to determine the absorption of ten flavonoids of crude and wine-processed SR in mice, such as baicalin, baicalein, scutellarin, scutellarein, and others. It indicated that the pharmacokinetic parameters of most flavonoids in wine-processed SR were significantly different from those of the crude SR, which portrayed that wine processing could improve the bioavailability of main flavonoids [27]. In addition, compared to crude SR, wine-processed SR was more effective in reducing the inflammatory factors in a lipopolysaccharide-induced murine model of acute lung injury. Non-targeted metabolomics implied that crude SR and wine-processing SR act on different metabolic pathways [28].
In recent years, the differentiation and identification of SR and its processed products had also begun to attract the attention of researchers. Utilizing fingerprinting combined with back propagation-artificial neural network modeling, baicalein, baicalin, wogonin, and wogonoside were selected as quality control markers for crude SR, wine-pressed SR, and charred SR, and their content was determined. Based on quantitative data, principal component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA) were employed to discriminate between them [29]. Following sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) experiment, SR was found to possess β-glucuronidase, which catalyzed the conversion from flavonoid glycosides to aglycones. However, this enzyme was inactivated in wine-processed SR and steamed SR. Thus, the conversion rate between flavonoid glycosides and aglycones could be determined to distinguish crude SR from processed samples [30]. Interestingly, electronic tongue technology detected that the bitterness of SR decreased while the saltiness increased after wine-processing. Subsequently, PCA analysis of various taste monitoring values showed that the differences between these two tastes could distinguish different SR before and after wine-processing. Based on the different taste response values of the electronic tongue, a fisher discriminant model was established to realize the rapid classification of SR and its processed products [31].
In summary, there are obvious differences between crude SR and processed SR in terms of component contents, metabolic absorption, and pharmacological activity. Furthermore, the similarity in appearance between crude SR and its processed products (mainly wine-processed SR and steamed SR) can easily lead to clinical misuse and affect therapeutic efficacy. Therefore, the comprehensive analytical methods should be developed and the corresponding quality control standards for processed SR should be established to ensure their rational application.
6. Phytochemistry
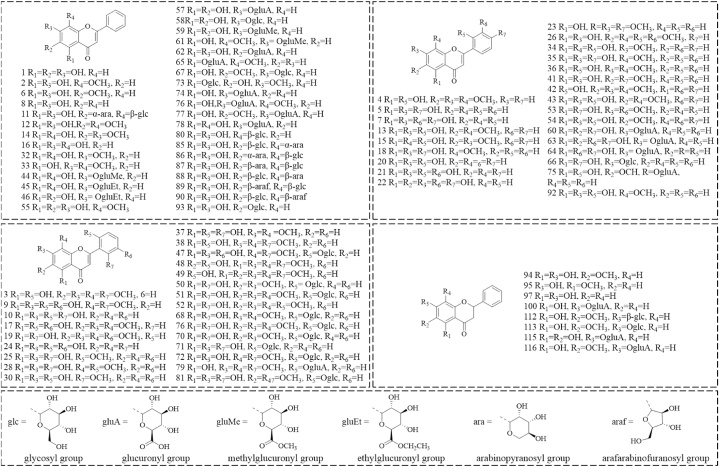
Hitherto, 210 components have been isolated from SR, including flavonoids and their glycosides, phenylpropanoids, phenylethanoid glycosides, phenolic acids, polysaccharides, volatile components, and others. The main characteristic components are flavonoids and their glycosides, which are also the most abundant secondary metabolites in SR. The various types of chemical components and their representative compounds in SR are illustrated in Fig. 2. The detailed information about 210 components from SR is seen in Table 5.
Fig. 2.
Types of chemical components and their representative components in Scutellariae radix.
Table 5.
Phytochemical components of Scutellariae radix.
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| Flavonoids and flavonoid glycosides | ||||
| 1 | baicalein | C15H10O5 | 1H NMR,13C NMR | [32] |
| 2 | wogonin | C16H12O5 | 1H NMR,13C NMR | [32] |
| 3 | skullcapflavone II | C19H18O8 | TLC, 1H NMR,13C NMR | [33] |
| 4 | tenaxin I | C18H16O7 | TLC, 1H NMR,13C NMR | [33] |
| 5 | apigenin | C15H10O5 | 1H NMR,13C NMR | [34] |
| 6 | oroxylinA | C16H12O5 | 1H NMR,13C NMR | [32] |
| 7 | luteolin | C15H10O6 | 1H NMR,13C NMR | [35] |
| 8 | chrysin | C15H10O4 | 1H NMR,13C NMR | [36] |
| 9 | 5,7,2′,5′-tetrahydroxy-8,6′-dimethoxyflavone | C17H14O8 | 1H NMR,13C NMR | [37] |
| 10 | 5,7,2′,6′-tetrahydroxyflavone | C15H10O6 | 1H NMR,13C NMR | [32] |
| 11 | chrysin-6-C-α-L-arabinopyranosyl-8-C-β-D-glueopyranosid | C26H28O13 | 1H NMR,13C NMR | [32] |
| 12 | 5,7-dihydroxy-6,8-dimethoxyflavone | C17H14O6 | 1H NMR,13C NMR, APCI-MS | [11] |
| 13 | 5,7,2′-trihydroxy-6,8-dimethoxyflavone | C17H14O7 | 1H NMR,13C NMR, EI-MS | [38] |
| 14 | 5,8-dihydroxy-6,7-dimethoxyflavone | C17H14O7 | 1H NMR,13C NMR, MS | [39] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 15 | 5,8,2′-trihydroxy-6,7-dimethoxyflavone | C17H14O7 | UV, IR, TLC, 1H NMR,13C NMR, | [40] |
| 16 | norwogonin | C15H10O5 | 1H NMR,13C NMR, APCI-MS | [11] |
| 17 | 5,2′,5′-trihydroxy-6,7,8-trimethoxyflavone | C18H16O8 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| 18 | 4′-hydroxywogonin | C16H12O6 | 1H NMR,13C NMR, EI-MS | [38] |
| 19 | 5,2′-dihydroxy-6,7,8,3′-tetramethoxyflavone | C19H18O8 | 1H NMR,13C NMR, FAB-MS | [42] |
| 20 | 2′-hydroxychrysin | C15H10O5 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| 21 | 5,7,2′,3′-tetrahydroxyflavone | C15H10O6 | IR, 1H NMR,13C NMR | [43] |
| 22 | 6-hydroxyluteolin | C15H10O7 | HPLC-MS/MS | [44] |
| 23 | salvigenin | C18H16O6 | 1H NMR,13C NMR, APCI-MS | [11] |
| 24 | 5,7,2′,5′-tetrahydroxyflavone | C15H10O6 | 1H NMR,13C NMR,FAB-MS | [45] |
| 25 | 5,7,6′-trihydroxy-2′-methoxyflavone | C16H12O6 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| 26 | 5,7-dihydroxy-6,8,2′,3′-tertramethoxyflavone | C19H18O7 | 1H NMR,13C NMR, APCI-MS | [11] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 27 | 3,5,4′-trihydroxy-6,7,8-trimethoxyflavone | C18H16O8 | HPLC-MS/MS | [44] |
| 28 | 5,7,6′-trihydroxy-8,2′-dimethoxyflavone | C17H14O7 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 29 | viscidulin Ⅲ | C17H14O8 | 1H NMR,13C NMR | [32] |
| 30 | 5,7,2′-trihydroxy-6′-methoxyflavone | C16H12O6 | 1H NMR,13C NMR, APCI-MS | [11] |
| 31 | 5,7-dihydroxy-8,2′,3′,6′-tetramethoxyflavone | C19H18O8 | 1H NMR,13C NMR, ESI-MS | [47] |
| 32 | 5,8-dihydroxy-7-methoxyflavone | C16H12O5 | TLC, 1H NMR,13C NMR | [48] |
| 33 | 7-O-methylwogonin | C17H14O5 | IR, 1H NMR, 13C NMR | [49] |
| 34 | 5,8,2′-trihydroxy-7-methoxyflavone | C16H12O6 | UV, IR, TLC, 1H NMR,13C NMR, | [40] |
| 35 | 5,7,4′-trihydroxy-8-methoxyflavone | C16H12O6 | 1H NMR,13C NMR, EI-MS | [38] |
| 36 | skullcapflavone I | C17H14O6 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| 37 | viscidulin II | C17H14O7 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| 38 | rivularin | C18H16O7 | 1H NMR,13C NMR,FAB-MS | [45] |
| 39 | 6′-hydroxy-5,6,7,8,2′-pentamethoxyflavone | C20H20O8 | 1H NMR,13C NMR, EI-MS | [38] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 40 | 6,6′-dihydroxy-5,7,8,2′-tetramethoxyflavone | C19H18O8 | 1H NMR,13C NMR, EI-MS | [38] |
| 41 | 5,7-dihydroxy-6,4′-dimethoxyflavone | C17H14O6 | TLC, 1H NMR,13C NMR, | [50] |
| 42 | 2′-hydroxy-6,7,8-trimethoxyflavone | C18H16O6 | 1H NMR,13C NMR | [51] |
| 43 | 5,7,2′-trihydroxy-6,8-dimethoxyflavone | C17H14O7 | 1H NMR,13C NMR | [51] |
| 44 | norwogonin-7-O-β-D-methylglucuronide | C22H20O11 | 1H NMR,13C NMR | [51] |
| 45 | wogonin-7-O-β-D-ethylglucuronide | C24H24O11 | 1H NMR,13C NMR, ESI-MS | [52] |
| 46 | baicalein-7-O-β-D-ethylglucuronide | C23H22O11 | 1H NMR,13C NMR, ESI-MS | [52] |
| 47 | viscidulin Ⅲ-2′-O-β-D-glucopyranoid | C23H24O13 | 1H NMR,13C NMR, EI-MS | [38] |
| 48 | 6,2′-dihydroxy-5,7,8,6′-tetramethoxyflavone | C19H18O8 | 1H NMR,13C NMR, EI-MS | [38] |
| 49 | 2′-hydroxy-5,6,7,8,6′-pentamethoxyflavone | C20H20O8 | 1H NMR,13C NMR, EI-MS | [38] |
| 50 | 5,2′,6′-trihydroxy-6,7-dimethoxyflavone 2′-O-β-D-glucoside | C23H24O12 | 1H NMR,13C NMR, SI-MS | [53] |
| 51 | 5,2′,6′-trihydroxy-6,7,8-trimethoxyflavone 2′-O-β-D-glucoside | C24H26O13 | 1H NMR,13C NMR, SI-MS | [53] |
| 52 | 5,6′-dihydroxy-6,7,8,2′tetramethoxyflavone | C19H18O8 | UPLC-Q-orbitrap MS | [54] |
| 53 | velutin | C17H14O6 | 1H NMR,13C NMR | [55] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 54 | tenaxin II | C16H12O6 | 1H NMR,13C NMR | [55] |
| 55 | 5,6,7-trihydroxy-8-methoxyflavone | C16H12O6 | 1H NMR,13C NMR | [55] |
| 56 | 8,8″-bibaicalein | C30H18O10 | 1H NMR,13C NMR, EI-MS | [38] |
| 57 | baicalin | C21H18O11 | 1H NMR,13C NMR | [55] |
| 58 | baicalein-7-O-β-D-glucoside | C21H20O10 | 1H NMR,13C NMR | [55] |
| 59 | baicalein-7-O-β-D- methylglucuronide | C22H20O11 | 1H NMR,13C NMR | [55] |
| 60 | scutellarin | C21H18O12 | TLC, HPLC, 1H NMR,13C NMR | [56] |
| 61 | wogonin-7-O-β-D-methylglucuronide | C23H22O11 | TLC, 1H NMR, 13C NMR | [57] |
| 62 | baicalein-6-O-β-D-glucuronide | C21H18O11 | HPLC-MS/MS | [44] |
| 63 | 6-hydroxyluteolin-7-O-β-D-glucoronide | C21H18O13 | HPLC-MS/MS | [44] |
| 64 | luteolin-7-O-β-D-glucuronide | C21H18O12 | HPLC-MS/MS | [44] |
| 65 | 8-methoxyflavone-5-O-β-D-glucoside | C22H20O10 | HPLC-MS/MS | [44] |
| 66 | apigenin-7-O-β-D-glucoside | C21H20O10 | 1H NMR,13C NMR, APCI-MS | [11] |
| 67 | oroxylin A-7-O-β-D-glucoside | C22H22O10 | HPLC-MS/MS | [44] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 68 | 5,6′-dihydroxy-7,8-dimethoxyflavone2′-O-β-D-glucoside | C23H24O12 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| 69 | 5,6′-dihydroxy-6,7,8-trimethoxyflavone 2′-O-β-D-glucoside | C24H26O13 | 1H NMR,13C NMR, SI-MS | [53] |
| 70 | 5,6′-dihydroxy-6,7-dimethoxyflavone 2′-O-β-D-glucoside | C23H24O12 | 1H NMR,13C NMR, APCI-MS | [11] |
| 71 | 5,7,6′-trihydroxyflavone-2′-O-β-D-glucoside | C21H20O11 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 72 | viscidulin III-6′-O-β-D-glucoside | C23H24O13 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 73 | wogonin-5-O-β-D-glucoside | C22H22O10 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 74 | chrysin-7-O-β-D-glucuronide | C21H18O10 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 75 | 5,2′-dihydroxy-6-methoxyflavone-7-O-β-D-glucuronide | C22H20O12 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 76 | wogonoside | C22H20O11 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 77 | oroxyloside | C22H20O11 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 78 | norwogonin-7-O-β-D-glucuronide | C21H18O11 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 79 | 5-hydroxy-7,8,6′-trimethoxyflavone2′-O-β-D-glucuronide | C24H24O13 | UV, IR, 1H NMR, 13C NMR | [58] |
| 80 | chrysin-8-C-β-D-glucoside | C21H20O9 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 81 | viscidulin III-2′-O-β-D-glucopyranoside | C23H22O14 | 1H NMR,13C NMR, APCI-MS | [11] |
| 82 | quercetin-3-O-β-D-glucuronide | C21H18O13 | HPLC-MS/MS | [44] |
| 83 | 5,6,8-trimethoxy-3′,4′-methylenedioxyflavone-7-O-β-D-glucoside | C26H28O12 | 1H NMR,13C NMR, HPLC-ESI-MS | [59] |
| 84 | 3,5,8-trimethoxy-3′,4′-methylenedioxyflavone7-O-β-D-glucoside | C26H28O12 | 1H NMR,13C NMR, HPLC-ESI-MS | [59] |
| 85 | chrysin-6-C-β-D-glucoside-8-C-α-L-arabinopyranoside | C26H28O13 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 86 | chrysin-6-C-α-L-arabinopyranoside-8-C-β-D-glucoside | C26H28O13 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 87 | chrysin-6-C-β-L-arabinopyranoside-8-C-β-D-glucoside | C26H28O13 | HPLC-MS/MS | [44] |
| 88 | chrysin-6-C-β-D-glucoside-8-C-β-L-arabinopyranoside | C26H28O13 | HPLC-MS/MS | [44] |
| 89 | chrysin-6-C-β-arabinofuranoside-8-C-β-D-glucoside | C26H28O13 | HPLC-MS/MS | [44] |
| 90 | chrysin-6-C-β-D-glucoside-8-C-β-arabinofuranoside | C26H28O13 | HPLC-MS/MS | [44] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 91 | chrysin-3-C-α-arabinopyranoside-8-C-β-D-glucoside | C26H28O13 | 1H NMR,13C NMR, HPLC-ESI-MS | [59] |
| 92 | scutevulin | C16H12O6 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| 93 | chrysin-6-C-β-D-glucoside | C21H20O9 | 1H NMR,13C NMR,FAB-MS | [45] |
| 94 | dihydrooroxylin A | C16H14O5 | TLC, 1H NMR,13C NMR | [33] |
| 95 | alpinetin | C16H14O4 | TLC, 1H NMR,13C NMR | [33] |
| 96 | naringenin | C15H12O5 | HPLC-MS/MS | [44] |
| 97 | pinocembrin | C15H12O4 | HPLC-MS/MS | [44] |
| 98 | isocarthamidin | C15H12O6 | HPLC-MS/MS | [44] |
| 99 | carthamidin | C15H12O6 | HPLC-MS/MS | [44] |
| 100 | (2S)-5,7,4′-trihydroxy-6-methoxyflavanone | C16H14O6 | 1H NMR,13C NMR, APCI-MS | [11] |
| 101 | (+)-eriodictyol(2S)-5,7,3′,4′-tetrahydroxyflavanone | C15H12O6 | 1H NMR,13C NMR, APCI-MS | [11] |
| 102 | (2S)-5,7,2′,5′-tetrahydroxyflavanone | C15H12O6 | 1H NMR,13C NMR, APCI-MS | [11] |
| 103 | (2S)-5,7,2′,6′-tetrahydroxyflavanone | C15H12O6 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 104 | (2S)-7,2′,6′-trihydroxy-5-methoxyflavanone | C16H14O6 | 1H NMR,13C NMR, APCI-MS | [11] |
| 105 | 5,6,7-trihydroxy-4′-methoxyflavonone | C16H14O6 | TLC, 1H NMR,13C NMR, | [50] |
| 106 | 5,7,2′-trihydroxy-6-methoxyflavonone | C16H14O6 | TLC, 1H NMR,13C NMR, | [50] |
| 107 | 5,7,2′-trihydroxyflavone | C15H12O5 | 1H NMR,13C NMR | [51] |
| 108 | (2S)-5,7,6′-trihydroxyflavanone 2′-O-β-D-glucopyranoside | C21H22O11 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 109 | naringenin-7-O-glucoronide | C21H20O11 | HPLC-MS/MS | [44] |
| 110 | pinocembrin-7-O-glucoronide | C21H20O10 | HPLC-MS/MS | [44] |
| 111 | (2S)-5,7,2′,5′-tetrahydroxyflavanone-7-O-β-D-glucoside | C21H22O10 | 1H NMR,13C NMR, APCI-MS | [11] |
| 112 | (2S)-5,7-dihydroxy-6-methoxyflavanone-7-O-β-D-glucoside | C22H24O9 | 1H NMR,13C NMR, APCI-MS | [11] |
| 113 | (2S)-5-hydroxy-6-methoxyflavanone-7-O-β-D-glucoside | C22H24O10 | 1H NMR,13C NMR, APCI-MS | [11] |
| 114 | (2S)-5,7,6′-trihydroxyflavanone-2′-O-β-D-glucoside | C20H20O11 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 115 | dihydrobaicalin | C21H20O11 | 1H NMR,13C NMR, APCI-MS | [11] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 116 | (2S)-5-hydroxy-6-methoxyflavanone-7-O-β-D-glucuronide | C22H22O11 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 117 | (2S)-5,6,3′,4′-tetrahydroxyflavanone-7-O-β-D-glucuronide | C21H20O13 | 1H NMR,13C NMR, APCI-MS | [11] |
| 118 | isocarthamidin-7-O-β-D-glucuronide | C21H20O12 | HPLC-MS/MS | [44] |
| 119 | 3,5,7,6′-tetrahydroxyflavone-2′-O-β-D-glucoside | C21H20O12 | 1H NMR,13C NMR, APCI-MS | [11] |
| 120 | patuletin-7-O-β-D-glucuronide | C22H20O14 | 1H NMR,13C NMR, HPLC-ESI-MS | [59] |
| 121 | 5,7,6′-trihydroxy-2′-methoxyflavonol | C16H12O7 | 1H NMR,13C NMR, ESI-MS | [50] |
| 122 | 5,7,2′,6′-tetrahydroxyflavonol | C15H10O7 | 1H NMR,13C NMR, EI-MS | [38] |
| 123 | (2R,3R)-3,5,7,2′,6′-pentahydroxyflavanone | C15H12O7 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| 124 | 4′,5,7-trihydroxy-6-methoxyflavanone | C16H16O5 | UV, IR, TLC, 1H NMR,13C NMR, | [40] |
| 125 | 2′,6′,5,7-tetrahydroxyflavanone | C15H14O5 | UV, 1H NMR,13C NMR | [60] |
| 126 | delphinidin-3-O-(6-O-malonyl)-β-D-glucoside-5-O-β-D-glucoside | C30H33O20 | 1H NMR,13C NMR, ESI-MS | [61] |
| 127 | 2,6,2′,4′-tetrahydroxy-6′-methoxychalcone | C16H14O6 | UV, IR, TLC, 1H NMR,13C NMR | [41] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 128 | 2,4′-dihydroxydihydrochalcone-3′-C-β-glucoside-6′-O-β-D-glucoside | C27H34O15 | 1H NMR,13C NMR, ESI-MS | [62] |
| Phenylpropanoids | ||||
| 129 | 4'-(β-D-glucopyranosyloxy)-3,3′,5,5′-tetramethoxy-9,9′-epoxylignane-4,7′-diol | C28H38O13 | 1H NMR,13C NMR, ESI-MS | [63] |
| 130 | 4'-(β-D-glucopyranosyloxy)-3,3′,5′-trimethoxy-9,9′-epoxylignane-4,7′-diol | C27H36O12 | 1H NMR,13C NMR, ESI-MS | [63] |
| 131 | 4'-(β-D-glucopyranosyloxy)-3,3-dimethoxy-9,9′-epoxylignane-4,7′-diol | C26H34O11 | 1H NMR,13C NMR, ESI-MS | [63] |
| 132 | (+)-syringaresinol-4-O-β-D-glucoside | C28H36O13 | 1H NMR,13C NMR | [64] |
| 133 | veraguensin | C22H28O5 | 1H NMR,13C NMR | [55] |
| 134 | galgravin | C22H28O5 | 1H NMR,13C NMR | [55] |
| 135 | denudanolide B | C20H22O6 | 1H NMR,13C NMR | [55] |
| 136 | denudatin B | C21H24O5 | 1H NMR,13C NMR | [55] |
| 137 | (2R,3R,3aS)-5-allyl-2-(3,4-dimethoxy-phenyl)-3a-methoxy-3-methyl-3,3a-dihydrobenzofuran-6(2H)-one | C21H24O5 | 1H NMR,13C NMR | [55] |
| 138 | eupomatenoid-7 | C20H20O4 | 1H NMR,13C NMR | [55] |
| 139 | trans-caffeic acid methyl ester | C10H10O4 | 1H NMR,13C NMR | [51] |
| 140 | trans-caffeic acid | C9H8O4 | 1H NMR,13C NMR | [51] |
| 141 | 4-O-β-D-glucosyl-trans-p-coumaricacid | C15H18O8 | 1H NMR,13C NMR | [65] |
| 142 | 4-O-β-D-glucosyl-cis-p-coumaricacid | C15H18O8 | 1H NMR,13C NMR | [65] |
| 143 | ferulic acid methyl ester | C11H12O4 | 1H NMR,13C NMR, ESI-MS | [66] |
| Phenylethanol glycosides | ||||
| 144 | salidroside | C14H20O7 | UV, IR, 1H NMR, 13C NMR | [58] |
| 145 | darendoside A | C19H28O11 | 1H NMR,13C NMR | [64] |
| 146 | darendoside B | C21H32O12 | 1H NMR,13C NMR | [64] |
| 147 | martynoside | C31H40O15 | UV, IR, 1H NMR, 13C NMR | [58] |
| 148 | acteoside | C29H36O15 | UV, IR, 1H NMR, 13C NMR | [58] |
| 149 | isomartynoside | C31H40O15 | 1H NMR,13C NMR | [64] |
| 150 | leucosceptoside A | C30H38O15 | UV, IR, 1H NMR, 13C NMR | [58] |
| 151 | cistanoside D | C31H40O15 | IR, UV, 1H NMR,13C NMR, ESI-MS | [46] |
| Phenolic acids | ||||
| 152 | benzoic acid | C7H6O2 | TLC, 1H NMR,13C NMR | [33] |
| 153 | phenylacetic acid | C8H8O2 | 1H NMR,13C NMR | [65] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 154 | syringaldehyde | C9H10O4 | 1H NMR,13C NMR, ESI-MS | [66] |
| 155 | vanillin | C8H8O3 | 1H NMR,13C NMR, ESI-MS | [66] |
| 156 | p-hydroxybenzoic acid | C7H6O3 | 1H NMR,13C NMR | [51] |
| 157 | protocatechuic acid | C7H6O4 | 1H NMR,13C NMR | [51] |
| Others | ||||
| 158 | N1,N5,N10-Tri-p-(E,E,E)-coumaroylspermidine | C33H33O6N3 | HPLC-MS/MS | [44] |
| 159 | pellitorine | C14H25NO | 1H NMR,13C NMR, ESI-MS | [66] |
| 160 | (E)-4-[(2-methylpropyl)amino]-4-oxo-2-butenoicacid | C8H13NO3 | 1H NMR,13C NMR, ESI-MS | [66] |
| 161 | 4,5-dihydropiperlonguminine | C16H21NO3 | 1H NMR,13C NMR, ESI-MS | [66] |
| 162 | futoamide | C18H23NO3 | 1H NMR,13C NMR, ESI-MS | [66] |
| 163 | piperlonguminine | C16H19NO3 | 1H NMR,13C NMR, ESI-MS | [66] |
| 164 | sinapoylhexoside | C16H20O10 | HPLC-MS/MS | [44] |
| 165 | 7-O-Acetylloganic acid | C18H26O11 | HPLC-MS/MS | [44] |
| 166 | lutein | C40H56O2 | 1H NMR, 13C NMR | [67] |
| 167 | β-carotene | C40H56 | 1H NMR, 13C NMR | [67] |
| 168 | stigmasterol | C29H48O | 1H NMR,13C NMR, EI-MS | [38] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 169 | β-sitosterol | C29H50O | 1H NMR,13C NMR, EI-MS | [38] |
| 170 | daucosterin | C35H60O6 | 1H NMR,13C NMR, EI-MS | [38] |
| 171 | (+)-crotepoxide | C18H18O8 | 1H NMR,13C NMR, ESI-MS | [66] |
| Volatile components | ||||
| 172 | benzylalcohol | C7H8O | GC-MS | [67] |
| 173 | isopropylcyclohexane | C9H18 | GC-MS | [68] |
| 174 | octane | C8H18 | GC-MS | [68] |
| 175 | hexanoicacid | C6H12O2 | GC-MS | [68] |
| 176 | 3,5-Difluoro-N,N-dimethylaniline | C8H9F2N | GC-MS | [68] |
| 177 | benzaldehyde | C7H6O | GC-MS | [68] |
| 178 | 3, 7-dimethyl nonane | C11H24 | GC-MS | [68] |
| 179 | benzeneacetaldehyde | C8H8O | GC-MS | [68] |
| 180 | acetyl valeryl | C7H12O2 | GC-MS | [68] |
| 181 | 3, 7-dimethyl decane | C12H26 | GC-MS | [68] |
| 182 | undecane | C11H24 | GC-MS | [68] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 183 | camphor | C10H16O | GC-MS | [68] |
| 184 | 2, 5, 9-trimethyl decane | C13H28 | GC-MS | [68] |
| 185 | octanoic acid | C8H16O2 | GC-MS | [68] |
| 186 | 3, 8-dimethyl undecane | C13H28 | GC-MS | [68] |
| 187 | dodecane | C12H26 | GC-MS | [68] |
| 188 | benzylideneacetone | C10H10O | GC-MS | [68] |
| 189 | 3-ethyl-3-methyl decane | C13H28 | GC-MS | [68] |
| 190 | nonanoic acid | C9H18O2 | GC-MS | [68] |
| 191 | 4, 6-dimethyl dodecane | C14H30 | GC-MS | [68] |
| 192 | tridecane | C13H28 | GC-MS | [68] |
| 193 | 1, 2-dihydro-1, 1, 6-trimethyl-naphthalene | C13H16 | GC-MS | [68] |
| 194 | succinicacid, diisobutylester | C12H22O4 | GC-MS | [68] |
| 195 | β-caryophyllene | C15H24 | GC-MS | [68] |
| 196 | butanedioicacid,methyl-,bis(1-methylpropyl)ester | C13H24O4 | GC-MS | [68] |
| 197 | pentadecane | C15H32 | GC-MS | [68] |
| Number | Name | Chemical formula | Identification methods | References |
|---|---|---|---|---|
| 198 | GermacreneD | C15H24 | GC-MS | [68] |
| 199 | 9-Cedranone | C15H24O4 | GC-MS | [68] |
| 200 | diphenyl amine | C12H11N | GC-MS | [68] |
| 201 | benzophenone | C13H10O | GC-MS | [68] |
| 202 | hexanedioic acid,bis(2-methylpropyl)ester | C14H26O4 | GC-MS | [68] |
| 203 | 13-tetradecenylacetate | C16H30O2 | GC-MS | [68] |
| 204 | diisobutyl phthalate | C16H22O4 | GC-MS | [68] |
| 205 | eicosane | C20H42 | GC-MS | [68] |
| 206 | heneicosane | C21H44 | GC-MS | [68] |
| 207 | 1,2-benzenedicarboxylic acid, butyl8-methylnonylester | C22H34O4 | GC-MS | [68] |
| 208 | 2, 2′-methylenebis[6- (1, 1-dimethylethyl)-4-methyl- phenol | C23H32O2 | GC-MS | [68] |
| 209 | hexatriacontane | C36H74 | GC-MS | [68] |
| 210 | tetratetracontane | C44H90 | GC-MS | [68] |
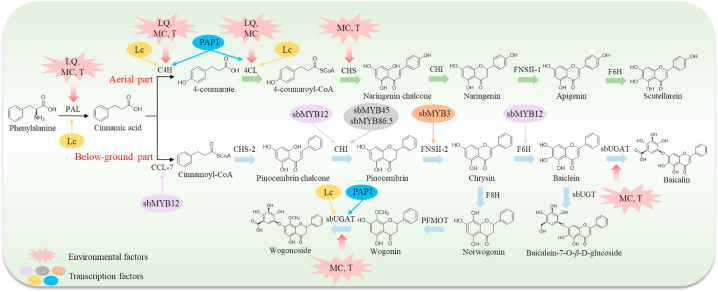
6.1. Flavonoids and their glycosides
At present, there were 93 flavones and their glycosides (1-93) [11,[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]], 29 flavanones and their glycosides (94–118, 124–126) [11,33,36,44,46,50,51], [40,60,61], 5 flavonols (119–123) [11,38,46,50,59], and 2 chalcones (127, 128) [41,62] were isolated from SR (Fig. 3). Flavonoids are rich in pharmacological activities. Baicalin has wide clinical applications with multifarious pharmacological effects, such as antitumor, antimicrobial, antiviral, and antioxidant effects [69]. Baicalein is the aglycone of baicalin. It has promising antineoplastic activity and potent neuroprotective activity [70,71]. Wogonin had the anti-inflammation and anti-oxidation effects [72]. Scutellarein has been verified with anti-cancer properties [73]. Oroxylin A is regarded as a potential antitumor agent, which possesses a great potential in the treatment of multiple cancers such as brain, liver, lung, breast, cervical, gall bladder, and others [74].
Fig. 3.
The structures of flavonoids and their glycosides (1–128).
6.2. Phenylpropanoids
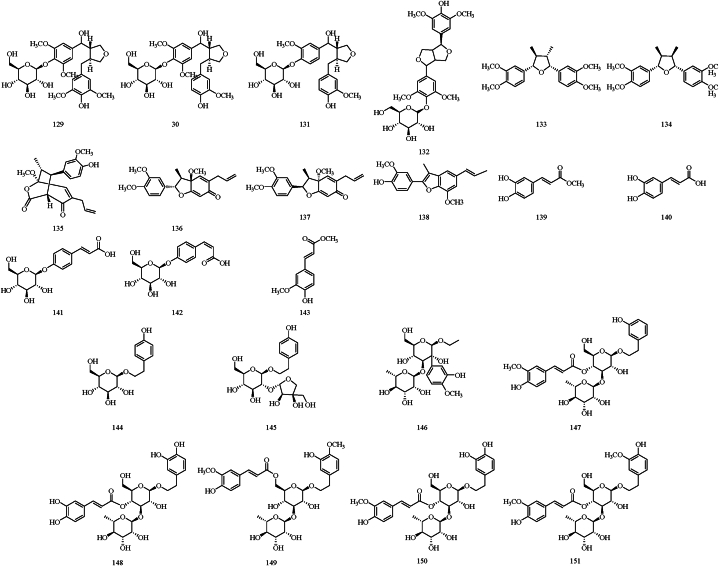
Currently, the phenylpropanoids isolated from SR include four lignan glycosides, six lignans, and five simple phenylpropanoids [51,55,[63], [64], [65], [66]]. Among these, veraguensin (133), galgravin (134), denudanolide B (135), denudatin B (136), (2R,3R,3S)-5-allyl-2-(3,4-dimethoxy-phenyl)-3a-methoxy-3-methyl-3,3a-dihydrobenzofuran-6(2H)-one (137), and eupomatenoid-7 (138) were obtained by SR aqueous extracts. Notably, 4’-(β-D-glucopyranosyloxy)-3,3′,5,5′-tetramethoxy-9,9′-epoxylignane-4,7′-diol (129), 4’-(β-D-glucopyranosyloxy)-3,3′,5′-trimethoxy-9,9′-epoxylignane-4,7′-diol (130), and 4’-(β-D-glucopyranosyloxy)-3,3-dimethoxy-9,9′-epoxylignane-4,7′-diol (131), three lignan glycosides isolated from SR for the first time, were shown to have good anti-osteoporotic properties in an in vitro experiment [63]. The structures of phenylpropanoids are displayed in Fig. 4.
Fig. 4.
The structures of phenylpropanoids (129–143) and phenylethanoid glycosides (144–151).
6.3. Phenylethanoid glycosides
Phenylethanoid glycosides possess multiple pharmacological effects such as anti-inflammatory, antioxidant, antibacterial, antitumor, and antiviral effects, as evidenced by both in vivo and in vitro studies [75]. In the literature reported, a total number of 8 phenylethanoid glycosides of SR are documented including salidroside (144), darendoside A (145) darendoside B (146), martynoside (147), acteoside (148), isomartynoside (149), leucosceptoside A (150), and cistanoside D (151) [46,58,64]. Their structures are shown in Fig. 4.
6.4. Phenolic acids
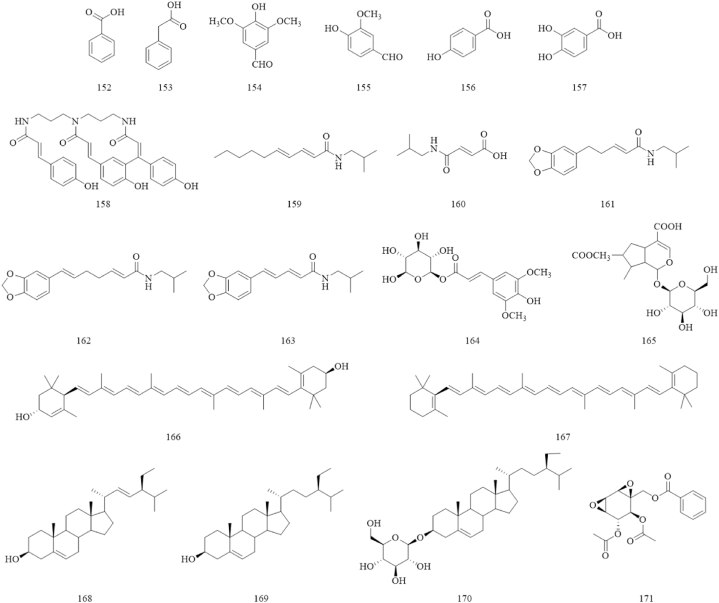
Phenolic acids are commonly found in fruits and vegetables, and are known for their biological activities such as antioxidant and antitumor [76,77]. A total of 6 common phenolic acids were isolated and identified from SR, such as benzoic acid (152) [33], phenylacetic acid (153) [65], syringaldehyde (154), vanillin (155) [66], p-hydroxybenzoic acid (156), and protocatechuic acid (157) [51]. The structures of phenolic acids are shown in Fig. 5.
Fig. 5.
The structures of phenolic acids (152–157) and others (158–171).
6.5. Volatile components
Volatile oils in plants generally have an aromatic odor and are not miscible with water. Using GC-MS, various parts from SR plants were found to contain different kinds of volatile components with different contents. Specifically, 39 volatile components (172–210) were detected from the roots of SR [68].
6.6. Polysaccharides
Polysaccharides play an essential part in medicine because of their extremely rich pharmacological activities, such as antioxidant, antiviral, antitumor, and immunomodulation. They are also used in the development of functional food [78]. Presently, two pure polysaccharides, SP1-1 and SP2-1, were isolated from SR. These polysaccharides exhibited anti-inflammatory activity, reduced the levels of pro-inflammatory cytokines and enhanced the intestinal barrier, thereby improving the symptoms of colitis in mice [,[79], [80]].
6.7. Others components
Other components such as alkaloids, terpenoids, and sterols are also found in SR. Five alkaloids: pellitorine (159), (E)-4-[(2-methylpropyl)amino]-4-oxo-2-butenoicacid (160), 4,5-dihydropiperlonguminine (161), futoamide (162), and piperlonguminine (163), and an epoxycyclohexene (171) were isolated from SR [66]. Terpene components such as sinapoylhexoside (164), 7-O-acetylloganic acid (165), lutein (166), and β-carotene (167) were detected by HPLC and HPLC-MS [44,67]. Detailed structures of these compounds are displayed in Fig. 5.
7. Pharmacological effects
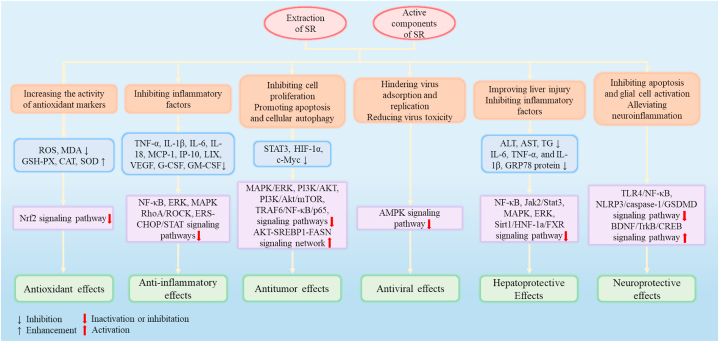
Numerous studies showed that SR possesses lots of pharmacological activities such as antioxidant, anti-inflammatory, antitumor, antiviral, hepatoprotective, and neuroprotective effects. Some of the pharmacological activities of SR and its representative components are found in Table 6. The related action mechanisms are shown in Fig. 6.
Table 6.
Pharmacological exprimental studies of Scutellariae radix.
| Extract/Compound | In vivo/in vitro expriment | Dosage | Administration approach | Result | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Antioxidant effects | ||||||
| ethanol extract of SR (60 %) | In vivo, TNBS-induced UC murine model | 100 mg/kg and 200 mg/kg | Oral administration | Increasing the activity of GSH-PX, CAT, and SOD | Upregulation of TGF-β expression | [81] |
| baicalin | In vitro, LPS-induced bEnd.3 cells model | 8 μg/mL | – | Scavenging ROS and MDA, increasing SOD | Activating of the Nrf-2 signaling pathway | [82] |
| wogonin | In vivo, TAC- induced cardiac hypertrophy murine model; In vitro, angiotensin II-induced H9C2 cells and neonatal rat cardiomyocytes model | 10 mg/kg | Intragastric administration | Improving cardiac hypertrophy and inhibiting oxidative stress | Regulating of Nrf-2 signaling pathway | [83] |
| wogonin | In vivo, murine testicular dysfunction caused by cadmium model | 10 mg/kg | Oral administration | Inhibiting the production of MDA and elevating the levels of SOD, CAT and GPx | Upregulating mRNA levels of Nrf-2 | [84] |
| norwogonin | In vitro, hypoxia-induced PC12 cells model | 100 μmol/L | – | Enhancing the activity of SOD, CAT, and GSH-Px and inhibiting the production of intracellular ROS and MDA and the expression of HIF-1α and VEGF proteins | Regulating of mitochondrial-dependent apoptosis pathway | [85] |
| Anti-inflammatory | ||||||
| water extract of SR | In vitro, LPS-induced macrophages model | 0.1 μg/mL, 1 μg/mL and 10 μg/mL | – | Inhibiting the pro-inflammatory cytokines | Downregulating of the NF-κB signaling pathway | [86] |
| baicalin | In vitro, Mϕs and DCs cell inflammation model caused by Pam3CSK4 and PGN and the Mϕs cell inflammation model caused by HK-MSRA | 50–200 μM | – | Dose-dependently inhibited the inflammatory reactions and the production of IL-6, TNF-α, and IL-1β | Inhibiting the activation of ERK, JNK MAPK, and NF-κB pathways | [87] |
| baicalein | In vitro, poly I:C-induced RAW 264.7 cells | 100 μM | – | Inhibiting calcium release, excessive production of NO, and inflammatory factors | Inhibiting the endoplasmic reticulum stress-CHOP/STAT pathways | [88] |
| skullcapflavone II | In vitro, TNF-α/IFN-γ-induced atopic dermatitis cells model | 25 μg/mL | – | Down-regulate the expression levels of TARC, CTSS, and MDC | Restraining the NF-κB, STAT1, and p38 MAPK signaling pathways | [89] |
| scutellarin | In vitro, IL-1β induced ATDC5 cells model | 50 μM | – | Down-regulating the expression levels of MMPs family | Blocking the activiation of NF-κB/MAPK signalings induced by IL-1β | [90] |
| Antitumor | ||||||
| 80 % ethanolic extract of SR | In vitro, EGFR TKI-Resistant PC9 Cell Lines | 25 μg/mL, 50 μg/mL, 100 μg/mL | – | Inhibiting cell viability and colony formation in the four cell lines | Inhibiting STAT3 activity triggering apoptosis | [91] |
| The extract of SR | In vitro, Ovarian cancer cell lines | 100 mg/mL | – | Down-regulating HIF-1α expression | Inhibiting MAPK/ERK and PI3K/AKT signaling pathways | [92] |
| baicalin | In vitro, colon cancer cells | IC50 = 165.5 μM | – | Inhibiting the growth of HT-29 colon cancer cells and preventing its apoptosis in a dose-dependent manner | Down-regulating the expression of oncomiSR | [93] |
| wogonoside | In vitro, PANC-1 andSW1990 cells; In vivo, inoculated PANC-1 cells murine model | 100 μM; 80 mg/kg | Gastric lavage | Inhibiting the stem cell-like transition and mesenchymal transition and suppressing tumour enlargement | Inhibiting the activation of the TRAF6/NF-κB/p65 signal pathways | [94] |
| wogonin | In vitro, DU145 and 22Rv1 prostate cancer cell lines; In vivo, subcutaneous DU145 and 22Rv1 xenograft murine model | 100 μM;100 mg/kg | Intravenous injection | Inhibiting the tumor growth, modulating the metabolism of fatty acid, and inducing apoptosis in human prostate cancer cells. | Activating the AKT-SREBP1-FASN signaling network | [95] |
| Antiviral | ||||||
| SR extract | In vivo, CVB3-induced myocarditis murine model; In vitro, CVB3-infected Hela cells and primary myocardial cells | 400 mg/kg; 400 μg/mL | Intra-gastric administration | Reducing the high mortality rate caused by CVB3 and preventing the replication of CVB3 | Inhibiting the expression of p38 and AKT | [96] |
| baicalin | In vitro, Marek's disease virus-infected chicken embryonic fibroblasts | 20 μg/mL | – | Reducing viral infectivity, inhibiting the viral expression, and down-regulating IRF7 expression | – | [97] |
| baicalin | In vivo, RSV-infected murine model; In vitro, RSV-infected lung adenocarcinoma HEp-2 cell line | 200 mg/kg; IC50 = 0.0894 mg/mL | Oral administration | Respiratory syncytial virus were inhibited | Activating IFN, inhibiting the transcription of NS1 and NS2 mRNAs, down-regulating the expression of viral protein M, and promoting the release of ribosomal protein L13a | [98] |
| baicalein | In vitro, DENV-2-infected VERO cells | IC50 = 5.39 μg/mL | – | Dengue virus type 2 were supressed | Presenting a good virucidal activity, inhibiingt virus replication, and resisting virus adsorption | [99] |
| wogonin | In vitro, human lung epithelial cells (A549) and MadinDarby canine kidney cells | 10 μg/mL | – | Preventing the replication of the influenza A virus and the formation of influenza B virus plaques | Inhibiting the AMPK signaling pathway | [100] |
| Hepatoprotective Effects | ||||||
| methanolic extract of SR | In vivo, acute alcohol-induced liver injury murine model and TM-induced liver injury murine model | 160 mg/kg | Intragastric administration | Increasing concentration of GSH and decreasing the concentration of MDA and hepatocyte injury markers (ALT, AST, and TG) in a dose-dependent manner | Inhibiting ERS and down-regulating GRP78 protein expression | [101] |
| baicalin | In vivo, ethanolic -induced liver injury murine model | 50 mg/kg | Intragastric administration | Up-regulating microRNA-205 expression and promoting its binding to importinα5 | Suppress NF-κB signaling transduction | [102] |
| baicalein | In vivo, CCL4-induced liver injury murine model | 33 mg/mL | Intragastric administration | Ameliorating liver injury induced by CCl4 in rats | Activating cellular autophagy and inhibiting endoplasmic ERS | [103] |
| baicalin | In vivo, 17α-ethinylestradiol-induced cholestatic liver injury murine model | 200 mg/kg | Oral administration | Decreasing the expression levels of inflammatory factors and hepatic uptake transporters, but increasing the hepatic efflux transporters | Activating the Sirt1/HNF-1a/FXR signal pathway | [104] |
| scutellarin | In vivo, CCL4-induced liver injury murine model | 0.12 mmol/kg | Oral administration | Reducing biochemical markers of liver injury and inflammatory factors, improving centrilobular necrosis and hepatocyte apoptosis in liver tissue | Inhibiting CYP2E1 and IκBα/NF-κB signaling pathways, and modulating the endogenous metabolites involved in lipid metabolism and bile acid homeostasis | [105] |
| Neuroprotective effects | ||||||
| baicalin | In vivo, APP/PS1 mice | 103 mg/kg | Intragastric administration | Improving the dyskinesia, promoting the inactivation of microglia, reducing the number of pro-inflammatory cytokines, and inhibiting neuronal apoptosis induced by neuroinflammation | Inactivating the NLRP3 inflammasome and blocking the TLR4/NF-κB signal pathway | [106] |
| baicalin | In vivo, chronic unpredictable mild stress-induced depression murine model | 50 mg/kg | Intragastric administration | Improve cognitive dysfunction, elevating the protein ratios of p-ERK/ERK and p-CREB/CREB and up-regulating the expression levels of ERK mRNA and CREB mRNA | Modulating the BDNF/ERK/CREB signalling pathway | [107] |
| baicalein | In vivo, MPTP-induced PD-like murine model | 560 mg/kg | Intragastric administration | Reducing the loss of dopamine neurons and inhibitting glial cell activation and proliferation | Preventing NLRP3/caspase-1/GSDMD signal pathway | [108] |
| baicalein | In vivo, rotenone-induced depression-like murine model | 300 mg/kg | Oral administration | Improving depression-like behaviour, reducing levels of pro-inflammatory cytokines, decreasing α-synuclein accumulation, and maintaining neurotransmitter homeostasis | Activating BDNF/TrkB/CREB signaling pathways | [109] |
| wogonin | In vivo, the middle cerebral artery occlusion murine model | 10 μmol/L | Oral administration | Reducing nerve injury and improving nerve function | Regulating TGF-β1 signal pathway | [110] |
Fig. 6.
Changes in biomarkers and signaling pathways of Scutellariae radix on pharmacological effects.
7.1. Antioxidant effects
When excessive free radicals accumulate in the body, oxidative stress may lead to many serious diseases [111]. It was reported that SR had prominent antioxidant activity and its flavonoids were also recognized as natural antioxidants. These components inhibit the overproduction of free radicals, reduce the accumulation of reactive oxygen species (ROS) and malondialdehyde (MDA), thereby alleviating oxidative stress and increase the activity of antioxidant markers in vivo. The activation of the Nrf2 signaling pathway is the key mechanism by which they exert an antioxidant effect.
The ethanolic extract of SR (60 %) effectively inhibited the excessive production of ROS and enhanced the activities of glutathione peroxidase (GSH-PX), catalase (CAT), and superoxide dismutase (SOD) in LPS-induced RAW264.7 cells and 2,4,6-trinitro-benzene sulfonic acid-induced murine model of ulcerative colitis (UC). Moreover, the 60 % ethanolic extract of SR at a concentration of 200 mg/kg significantly suppressed myeloperoxidase (MPO) activity and transforming growth factor β1 protein expression compared with mesalazine (active control, 100 mg/kg) [81].
Baicalin, wogonin, norwogonin, and scutellarin, compounds extracted from SR, also had antioxidant activities. In a methotrexate-induced mitochondrial damage model, the activities of antioxidant enzymes (CAT, SOD, and GSH-PX) were restored by baicalin [112]. In addition, baicalin (8 μg/mL) could decrease ROS and MDA and augment the activity level of SOD in bEnd.3 cells, through the activation of the Nrf-2 signaling pathway [82]. Wogonin (10 mg/kg) similarly reduced the production of ROS and MDA and activated SOD, CAT, and GSH-PX, which were achieved by the same pathway [83,84]. Norwogonin (100 μmol/L) inhibited the release of lactate dehydrogenase, improved cell morphology, and alleviated hypoxia-induced cell damage. It was good at enhancing the activity of SOD, CAT, and GSH-PX, inhibiting the production of intracellular ROS and MDA, and repressing the expression of HIF-1α and VEGF proteins in hypoxia-induced PC12 cells [85]. Scutellarin (100 mg/kg), in the rats of transient middle cerebral artery occlusion injury, decreased the oxidative-related damage factors, including 3-nitrotyrosine, 4-hydroxynonenal, 8-hydroxydeoxy-guanosine, neurotrophin-3, PARP1 and ROS [113]. In addition, a method of HPLC-PDA-ESI-MS combined with free radical reaction was developed to detect the antioxidant activities of baicalin, baicalein, scutellarin, scutellarein, wogonoside, and chrysin-7-glucuronide. Among these, baicalein showed the highest scavenging capacity against superoxide radicals and lipid radicals; wogonoside showed the highest scavenging ability against hydroxyl radicals; scutellarein displayed the highest reactivity during lipid peroxidation [114].
Antioxidants play an important role in human health. There is no doubt that the excellent antioxidant properties of SR can reduce the effects of ROS on cellular functions, heralding its potential for the treatment of cardiovascular diseases, Alzheimer's disease, cancer, and other disorders related to oxidative stress. Consequently, researchers should focus on this phenomenon and conduct appropriate clinical studies to explore its therapeutic applications in the future.
7.2. Anti-inflammatory effects
The aerial parts extract and water extract of SR have been shown to inhibit the expression of VEGFR1, TNF-α, IL-1β, and IL-6, and block the NF-κB signaling transduction in LPS-induced cell inflammatory models [115,86]. In the Mϕs and DCs cell inflammation models induced by Pam3CSK4, PGN, and HK-MSRA, the administration of varying concentrations of baicalin (50–200 μM) dose-dependently inhibited the inflammatory reactions and the production of IL-6, TNF-α, and IL-1β. Further study suggested that it was related to the inactivation of ERK, JNK, MAPK, and NF-κB signaling pathways [87]. In complete Freund's adjuvant-induced inflammatory pain rats, 60 % ethanolic extract of SR (3–6 g/kg) relieved inflammatory pain, suppressed the expression of NF-κB, COX-2 and P2X3, and inhibited the release of inflammatory factors [116].
Other studies also showed that baicalin inhibited the production of inflammatory factors by negatively modulating the RhoA/ROCK signaling pathways [[117], [118], [119]]. At a concentration of 100 μM, baicalein inhibited calcium release, excessive production of NO, and inflammatory factors, including IL-1α, IL-6, MCP-1, IP-10, LIX, VEGF, G-CSF, GM-CSF, in cells induced by polyinosinic-polycytidylic acid. It also suppressed the mRNA expression of STAT1, STAT3, and Fas, which were mediated through the ERS-CHOP/STAT pathway [88]. Wogonin was able to suppress the production of proinflammatory cytokines, reduce the phosphorylation of p65, and increase the expression of Iκ-Bα protein in inflammatory tissues. It was concerned with the inhibition of NF-κB-P65 signaling pathway [120,121]. Skullcapflavone II (25 μg/mL) was found to downregulate the expression levels of atopic dermatitis-associated cytokines, including thymus- and activation-regulated chemokine, cathepsin S, and macrophage-derived chemokine, by restraining the NF–κB, STAT1, and p38 MAPK signaling pathways [89]. At a concentration of 50 μM, scutellarin downregulated the expression levels of MMPs family and blocked the activation of NF-κB/MAPK signaling induced by IL-1β in an osteoarthritis model [90]. Besides, the dextran sulfate sodium salt-induced mice were administered 200 mg/kg SP1-1 by gavage for 10 days, a polysaccharide isolated from SR, which markedly inhibited the production of IL-1β, IL-18, and TNF-α. The reduction of infiltration in colon macrophages and the inactivation of caspase-1 in peritoneal macrophages could be detected in mice treated with SP1-1. The anti-inflammatory effects of SP1-1 were attributed to the activation of the NF-κB signal pathway and the inhibition of NLRP3 inflammasome [79].
The levels of several proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-18, MCP-1, and IP-10, were inhibited by the extract of SR, baicalin, baicalein, wogonin, skullcapflavone II, and the polysaccharide of SR. Furthermore, the NF-κB signaling pathway is critical for them in down-regulating the expression of inflammatory factors, up-regulating the expression of anti-inflammatory cytokines, and improving the inflammatory response. These provide strong evidence that SR treats inflammatory diseases.
7.3. Antitumor effects
The ethanolic extract of SR (80 %), at the concentration of 25–100 μg/mL, had excellent antitumor activity against the EGFR TKI-resistant human lung cancer cells: H1299, H1975, PC9/ER, and PC9/GR, by inhibiting STAT3 activity triggering apoptosis [91]. Hypoxia inducible factor-1α (HIF-1α) was often considered to be one of the key factors in controlling tumor growth and metastasis [122]. The extract of SR (100 mg/mL) was able to down-regulate HIF-1α expression, block the synthesis of HIF-1α, and accelerate the catabolism in ovarian cancer cell lines after three days of treatment. Its action mechanism was attributed to inhibiting MAPK/ERK and PI3K/AKT signaling pathways [92]. The extract of SR (250 μg/mL) could target and inhibit the viability of human hepatocellular carcinoma cells SK-Hep-1, promote its apoptosis, and prevent its migration, thus preventing the development of hepatocellular carcinoma [123]. The total flavonoid aglycones extract of SR showed eminent inhibitory effects on pancreatic cancer cell lines, especially BxPC3 tumor (IC50 = 6.5 g/mL). It could regulate apoptosis and autophagy to prevent tumor cell survival by activating the caspase signaling pathway and inhibiting the PI3K/Akt/mTOR signaling pathway respectively [124].
Growing studies indicated that compounds from SR exhibited inhibitory activities against the development of a variety of cancer cells, including colon, pancreatic, bladder, gastric, and prostate cancer. Baicalin was found to inhibit the growth of HT-29 colon cancer cells (IC50 = 165.5 μM), prevent dose-dependently its apoptosis, and suppress the expression of oncogenic transcription factor c-Myc. It down-regulated the expression of oncogenic miRNAs [93]. In addition, baicalin induced ferroptotic cell death and showed inhibitory activities against 5637 (at the concentration of 20 μg/ml) and KU-19-19 (at the concentration of 40 μg/ml), two types of bladder cancer tumor cells [125]. Wogonoside (100 μM) inhibited the stem cell-like transition and mesenchymal transition in PANC-1 and SW1990 cells. Moreover, tumor mass enlargement was suppressed by wogonoside (80 mg/kg) in the inoculated PANC-1 cells murine model. Inactivation of the TRAF6/NF-κB/p65 signaling pathways was the main action mechanism [94]. Wogonin equally inhibited tumor growth, which modulated the metabolism of fatty acid, and induced apoptosis in human prostate cancer cells. It was achieved by activating the AKT-SREBP1-FASN signaling network [95]. 7-O-methylwogonin (IC50 = 30 μM) selectively inhibited Polo-like kinase 1 (Plk1) activity, induced mitotic delay, and increased mitotic aggregation in Hep3B cells, similar to the mechanism of BI 2536, a specific Plk1 inhibitor [126]. Oroxylin A modulated the polarization of M1-like macrophages, reduced the number of M2-like macrophages, and promoted the infiltration of T cells, thereby inhibiting the development of hepatocellular carcinoma [127]. A recent study showed that oroxylin A, as a natural inhibitor of Recepteur d'origine Nantais, inhibited osteoclasts and reduced the number of osteoclasts induced by breast cancer [128].
SR and its isolated compounds had broad-spectrum antitumor activity. They do well in inhibiting cell proliferation, promoting apoptosis, and inducing cellular autophagy, thus inhibiting the further development of tumors. However, most of the studies on the antitumor activities related to SR were carried out in vitro intracellularly and lacked corresponding in vivo experimental validation. Various animal cancer models should be utilized to advance in vivo studies of the effects of these compounds, and subsequent clinical validation is necessary. This will greatly facilitate the development of lead compounds for antitumor drugs and the screening of potential antitumor drugs.
7.4. Antiviral effects
SR, along with its compounds baicalin, baicalein, and wogonin, have outstanding preventive and curative effects on a variety of viruses. In a vitro experiment, 400 μg/mL SR extract prevented the replication of Coxsackievirus B3 (CVB3) in primary cardiomyocytes and attenuated the toxicity of CVB3 in HeLa cells. In a vivo experiment, SR extract (400 mg/kg) reduced the high mortality rate caused by CVB3, and inhibited the expression of p38 and protein kinase B to inhibit the replication of CVB3 [96]. The flavonoid extract of SR effectively hindered the process of adsorption and replication of the tick-borne encephalitis virus at a concentration of 9 μg/mL. The virus was directly inactivating at concentrations of the extract greater than or equal to 5 μg/mL [129]. Additionally, the flavonoid extract of SR showed potential against the influenza A virus [130].
Baicalin (20 μg/mL) significantly reduced viral infectivity, inhibited the viral expression, and down-regulated IRF7 expression in chicken embryonic fibroblasts infected with Marek's disease virus [97]. When respiratory syncytial virus (RSV)-infected rats were orally administered 200 mg/kg baicalin for five days, type I interferon was activated, the expression of viral protein M was suppressed, and the release of ribosomal protein L13a was increased. These mechanisms determined the superior inhibitory effect of baicalin against RSV [98]. Notably, the combination of baicalin and resveratrol could enhance its anti-RSV effect [131]. Before and after dengue virus type 2 (DENV-2) infection, VERO cells were treated with baicalein. The results showed that baicalein had a good activity of anti-DENV-2 (IC50 = 1.55 μg/mL) and inhibited virus replication (IC50 = 6.46 μg/mL). Added to that, pretreatment of cells with baicalein prevented virus adsorption (IC50 = 7.14 μg/mL) [99]. In human lung epithelial cells and Madin-Darby canine kidney cells infected by influenza virus, wogonin prevented the replication of the influenza A virus and the formation of influenza B virus plaques, by inhibiting the AMPK signaling pathway [100].
7.5. Hepatoprotective effects
SR and its root-specific flavonoids had multiple protective effects against alcoholic liver diseases and liver injury. These hepatoprotective effects involve the inhibition of oxidative markers and inflammatory factors, which are closely related to the antioxidant and anti-inflammatory properties.
After treatment with the methanolic extract of SR (160 mg/kg), the concentration of GSH in mice with alcoholic liver injury increased while the concentration of MDA and hepatocyte injury markers (alanine transaminase, aspartate transaminase, and triglyceride) decreased in a dose-dependent manner. Immunohistochemical examination and ELISA assay showed the expression level of the glucose regulated protein 78 kDa (a typical marker of ERS) decreased [101]. Additionally, the aqueous extract of SR (100 mg/mL) showed hepatoprotective effect in mice with a high-fat diet and long-term alcohol consumption. It was able to reduce the activities of liver enzymes such as alanine transaminase, aspartate transaminase and lactate dehydrogenase, inhibit endogenous cholesterol synthesis and act as a 3-hydroxy-3-methylglutaryl-coenzymeA reductase inhibitor in mice [132].
Baicalin (50 mg/kg) was also effective in the treatment of alcoholic liver disease. However, its mechanisms were up-regulating microRNA-205 expression and promoting its binding to importinα5 to suppress NF-κB signaling transduction [102]. Baicalein, at a concentration of 33 mg/mL, ameliorated liver injury induced by CCl4 in rats, through activating cellular autophagy and inhibiting endoplasmic ERS [103]. Interestingly, baicalein and baicalin inhibited elevated IL-6, TNF-α, and IL-1β, reduced serum alanine transferase levels, and suppressed hepatic myeloperoxidase activity in the acetaminophen-induced liver injury. Therefore, these compounds could ameliorate autophagy, which involved JAK2/STAT3, MAPK, and ERK signaling pathways respectively [133,134]. In 17α-ethinylestradiol-induced liver injury of rats, baicalin (200 mg/kg) decreased the expression levels of inflammatory factors and hepatic uptake transporters but increased the hepatic efflux transporters. Its potential mechanism was associated with modulating the sirtuin 1/hepatic nuclear receptor-1a/farnesoid X receptor signal pathway [104]. Besides, baicalin attenuated cirrhosis induced by TAA via NOX4/NF-κB/NLRP3 inflammatory vesicles [135]. Scutellarin also had a hepatoprotective effect, which reduced biochemical markers of liver injury and inflammatory factors, and improved centrilobular necrosis and hepatocyte apoptosis in liver tissue [105].
7.6. Neuroprotective effects
An ethanolic extract of SR was administered orally to rats with spinal cord injury at a dose of 100 mg/kg. It inhibited the expression of pro-inflammatory factors as well as the carbonylation and nitration of proteins, significantly restrained the apoptosis of neurons and oligodendrocytes, and improved the functional recovery after spinal cord injury [136]. The ethanolic extract of SR (50–100 μg/mL) was able to exhibit neuroprotective effects against excitotoxic neuronal cell death by blocking N-methyl-D-aspartate receptors [137]. The water extract of SR had potential antidepressant effects, and it improved depressive-like behavior in chronic unpredictable mild stress mice. Daily administration of 0.75 g/kg or 1.5 g/kg of SR water extract up-regulated the levels of transforming growth factor-β3, p-SMAD2/3, and NEDD9 proteins and increased the number of doublecortin-, microtubule-associated protein 2-, and neuronal nucleus-positive cells in the hippocampus of the model mice [138].
Baicalin, baicalein, and scutellarin can alleviate neuroinflammation as well as inhibit neuroapoptosis and glial cell activation, thus exerting neuroprotective effects. It implies their potential to treat neurological diseases, such as Alzheimer's disease, Parkinson's disease, and depression. Baicalin has shown promise in improving symptoms in mouse models of Alzheimer's disease and depression. In the development of Alzheimer's disease, neuroinflammation is closely related to neuronal apoptosis [139]. In APP/PS1 mice, baicalin (103 mg/kg) effectively improved dyskinesia, promoted the inactivation of microglia, reduced the number of proinflammatory cytokines, and inhibited neuronal apoptosis induced by neuroinflammation. It acted as a neuroprotective agent by inactivating the NLRP3 inflammasome and blocking the TLR4/NF-κB signaling pathway [106]. Besides, compared with the positive drug fluoxetine treatment, baicalin (50 mg/kg) also improved cognitive dysfunction in CUMS-induced depression mice. Moreover, baicalin elevated the protein ratios of p-ERK/ERK and p-CREB/CREB and up-regulated the expression levels of ERK mRNA and CREB mRNA, by modulating the BDNF/ERK/CREB signaling pathways [107].
Baicalein was able to improve MPTP-induced motor dysfunction and depression caused by Parkinson's. Intragastric administration of 560 mg/kg baicalein for 9 days reduced the loss of dopamine neurons and inhibited glial cell activation and proliferation in MPTP-induced mice. Baicalein developed a neuroprotective effect by preventing the NLRP3/caspase-1/Gasdermin D signal pathway [108]. Correspondingly, it had been demonstrated that treatment of SH-SY5Y cells with 50 μM baicalin could alleviate the neurotoxicity caused by the neurotoxic agent MPP+ (the active metabolite of MPTP) [140]. In a rotenone-induced depression murine model, the treatment of 300 mg/kg baicalin improved depression-like behavior, reduced the levels of proinflammatory cytokines, decreased the accumulation of α-synuclein, maintained neurotransmitter homeostasis and activated BDNF/TrkB/CREB signaling pathways [109].
Scutellarin (100 mg/kg) promoted the conversion of microglia phenotype from M1 to M2, thereby exerting anti-inflammatory and neuroprotective effects to moderate neuroinflammation. It acted by blocking the p38 and JNK signaling pathways and activating the ERK1/2 signaling pathway [141]. Studies also manifested that wogonin attenuated cortical damage in irradiation-induced rats and neurological damage in cerebral ischemia rats to improve neurological function [110,142]. However, the precise mechanisms underlying these effects remain unclear and warrant further investigation.
It can be seen that the extract and active components of SR exhibit excellent neuroprotective effects. Moreover, it is necessary to use a variety of classical animal models of neurological diseases to comprehensively evaluate their mechanism of action, which can provide new insights for the development of drugs and clinical treatments for neurological disorders.
8. Quality control
Quality control is the key basis for guaranteeing the quality and clinical efficacy of traditional Chinese medicines (TCMs) [143]. It is of great significance to employ analytical techniques to optimize extraction methods, establish the fingerprints, determine content, characterize components, and screen the quality markers for the effectiveness and safety of SR.
8.1. Green extraction methods
At present, several green extraction technologies have been developed for SR, including ultrasound-assisted deep eutectic solvent extraction, deep eutectic solvent combined with ultrahigh pressure extraction, enzyme-assisted extraction, ultrasound-assisted enzyme extraction, microwave-assisted ionic liquid extraction, and ultrasound-assisted ionic liquid extraction. The optimal extraction conditions and extracted components are summarized in Table 7.
Table 7.
Green extraction methods of Scutellariae radix.
| Methods | Optimal extraction conditions | Extracted components | Reference |
|---|---|---|---|
| Ultrasound-assisted deep eutectic solvents | Betaine: acetic molar ratio 1:4, water content 40 %, extraction temperature 52 °C, extraction time 23 min, and liquid-solid ratio 1:100 g/mL | Total flavonoids from SR | [144] |
| Microwave-assisted deep eutectic solvents | L-proline: urea molar ratio 2:1, water content 40 %, extraction temperature 110 °C, extraction time 20 min, and liquid-solid ratio 40 mL/g | Baicalin, baicalein and wogonin | [145] |
| Deep eutectic solvent-based ultrahigh pressure extraction | Choline chloride: lactic acid molar ratio 1:1, water content 40 %, pressure 400 MPa, extraction time 4 min, and liquid-solid ratio 110 mL/g | Baicalin | [146] |
| Ultrasound-assisted enzymatic extractin | Cellulase concentration 1.1 %, pH 5.5, extraction temperature 56.5 °C, extraction time 39.4 min, and ultrasonic power 200 W | Baicalein and wogonin | [147] |
| Ultrasound-assisted enzymatic extraction | Cellulase concentration 165.6 U/mL, extraction temperature 57.3 °C, extraction time 50 min, ultrasonic power 225 W, and liquid-solid ratio 44.8 mL/g | The polysaccharides of SR | [148] |
| Enzyme-assisted extraction | Cellulase concentration 20 U/mL, cellulase digestion time 24 h, pH 7.0, and extraction time 3 h | Baicalin | [149] |
| Ionic liquid-based microwave-assisted extraction | [C8mim]Br concentration 1.0 M, irradiation time 90 s, irradiation power 400 W, and liquid-solid ratio 1:6 (g/mL) | Flavonoids | [150] |
| Ultrasound-assisted ionic liquid-based liquid-liquid extraction | Volume ratio 3:3, extraction time 60 min, ultrasound-assisted extraction time 5 min, temperature 70 °C, and liquid-solid ratio 1:40 | Baicalin and baicalein | [151] |
Deep eutectic solvents (DES) are widely used because of their low toxicity, chemical stability, and high biodegradability properties [144]. Using ultrasound-assisted extraction with betaine: acetic (molar ratio 1:4), under optimal extraction conditions, the extraction of total flavonoids of SR was achieved with high efficiency and the activity of the extract was not affected [145]. Similarly, efficient extraction of baicalin, baicalein and wogonin could be achieved using a natural DES (L-proline and urea, molar ratio 2:1) combined with microwave-assisted extraction. The results of in vitro bioactivity assay showed that the extracts possessed desirable antioxidant activity [146]. The use of choline chloride: lactic acid (molar ratio 1:1) assisted by ultrahigh pressure extraction could exceptionally disrupt the root tissue, facilitating the efficient extraction of baicalin [152]. It was remarkable that a green and cyclically switchable supramolecular DES was developed and prepared for the extraction of flavonoids from SR. It combined excellent extraction efficiency with reduced processing time and could be recycled up to 5 times while maintaining an extraction efficiency of more than 90 % [153].
An ultrasound-assisted enzyme pretreatment extraction method was developed. It could effectively break the glycosidic bonds of baicalin and wogonoside in SR, thus enhancing their extractive rate [147]. In addition, the extraction of polysaccharides in SR could also be achieved by using ultrasound-assisted cellulase extraction [148]. It was worth noting that the cellulase from the endophytic bacteria of SR roots could be enriched by the enzymatic production process, and was subsequently used to assist in the extraction of baicalin, which created a new reference pathway for the extraction of active components in Chinese medicine [149].
The use of ionic liquids is an excellent method for extracting the active components of SR, with the advantages of low volatility, easy miscibility with water, and easy recycling [154]. An ionic liquid microwave-assisted extraction method was employed to disrupt the internal structure of SR, which achieved a high yield of total flavonoid extracts within 90s [150]. The extraction and separation of baicalin and baicalein could be achieved by an ionic liquid ultrasound-assisted extraction method based on a liquid-liquid dispersion system. In this system, the strongly polar baicalin was dispersed in the aqueous phase, while the weakly polar baicalein was dispersed in the ionic liquid phase [151].
The continuous development and innovation of extraction methods can help us to extract more components of SR faster, and even achieve a rapid targeted extraction of active components only by optimizing the extraction conditions. It also provides a powerful reference for research on extracting components from natural products containing flavonoids.
8.2. Fingerprints
Chinese herb fingerprint technology is a comprehensive and quantifiable quality control tool for herbs, characterized by wholeness, ambiguity, and specificity [155,156]. Fingerprints established by capillary electrophoresis, high performance liquid chromatography (HPLC), ultra-fast liquid chromatography with a diode array detector (UFLC-DAD), and ultra-high performance liquid chromatography coupled with mass spectrometry (UHPLC-MS) are used to rapidly evaluate the quality of SR from different batches, regions, and growth patterns. Moreover, the integration of fingerprints with chemometrics as well as activity-integrated fingerprints can elucidate the relationship between the components and their efficacy, aiding in the screening of effective substances.
Capillary electrophoresis was used to establish fingerprints of SR to distinguished samples from different origins by combining quantitative analysis of identified components such as baicalin, baicalein, and wogonin [157]. HPLC-guided fingerprints of 58 batches of wild and cultivated SR were established, and the quantification of baicalin, baicalein, and wogonin were completed, revealing that the flavonoid glycoside content of cultivated SR was higher than that of wild SR [158]. A UFLC-DAD method was developed to establish fingerprints of SR from different regions to achieve a rapid quality evaluation. Compared with the conventional HPLC method, it had a shorter run time, better compound separation, and a higher resolution [159]. Additionally, UHPLC-MS was developed to establish SR fingerprints. 23 compounds were identified which enhanced the characterization of common peaks in the fingerprints and improved the analytical efficiency [160]. The correlation analysis between HPLC fingerprints and the results of screening antibacterial components of SR were performed by multivariate statistical analysis in chemometrics. Baicalin, baicalein, wogonin, wogonoside, and oroxylin A-7-O-β-D-glucuronide were screened as key antibacterial components [161]. A multi-dimensional-multi-information integrated Xanthine oxidase and superoxide anion fingerprint has been established. It offered an activity-integrated fingerprint that evaluated the quality of SR from different batches and origins in terms of both chemical components and efficacy [162].
8.3. Analytical methods
Content determination and characterization of the components are also part of quality evaluation of SR. Several methods are employed to analyze the chemical components of SR, such as isocratic high performance liquid chromatography with diode array detection (isocratic HPLC-DAD), ultra-high performance liquid chromatography with photo diode array (UHPLC-PDA), ultra high performance liquid chromatography coupled with a Q-Exactive hybrid quadrupole-orbitrap mass spectrometry, ultra- high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC-Q-TOF-MS), and high performance liquid chromatography-diode array detection coupled with quadrupole time-of-flight mass spectrometry (HPLC-DAD-Q-TOF-MS).
For the quantification of the main chemical components of SR, an isocratic HPLC-DAD method was developed to determine the content of baicalin, baicalein, wogonoside, and wogonin in 70 % ethanolic extracts of SR [163]. Subsequently, using a UHPLC-PDA method, the quantification of ten flavonoids of SR, including baicalin, baicalein, wogonoside, wogonin, chrysin, apigenin, and others, were accurately and rapidly completed [164].
In the qualitative analysis of SR, the method of UHPLC coupled with a Q-Exactive hybrid quadrupole-orbitrap mass spectrometry with a combination of a four-step analytical strategy successfully identified 132 chemical components [165]. Similarly, five major groups of 118 chemical components in SR were identified by UHPLC-Q-TOF-MS with a combination of diagnostic ions and neutral loss [166]. These methods facilitate the rapid identification of chemical components by avoiding the analysis of extensive non-targeted mass spectrometry data. In addition, the HPLC-DAD-Q-TOF-MS method allowed qualitative and quantitative determination of active components in SR roots and leaves after ultraviolet-B radiation [167].
8.4. Quality markers
Quality markers are chemical substances that reflect the intrinsic quality of TCMs and are closely related to their functional properties [168]. The key to the quality control of TCMs is their material basis [169]. Therefore, screening of quality markers is an important element to improve quality of SR.
An epidermal growth factor receptor (EGFR)/carboxymethylcellulose (CMC) column-online-HPLC-MS method was successfully established to achieve the screening of the tumor-targeting active components in SR. The results showed that baicalein was the potential quality marker acting on the epidermal growth factor [170]. An ultrafiltration HPLC-MS method was developed to screen of α-glucosidase, lipoxidase, and superoxide dismutase inhibitors of SR [171,172]. Based on the technical approach, 13 potential markers inhibiting α-glucosidase such as wogonin, chrysin, oroxylin A, skullcapflavone II, viscidulin III, tenaxin I and others, as well as 5 potential markers inhibiting lipoxidase and superoxide dismutase such as baicalin, wogonoside, chrysin-6-C-arabinosyl-8-C-glucoside, and oroxylin A-7-O-glucuronide, were screened. HPLC-PAD-QTOF-MS combined with a multivariate statistical strategy achieved the characterization of raw and processing SR and screening for chemical markers. The quantification of the selected markers, mainly including baicalin, baicalein, wogonin, wogonoside, and skullcapflavone II were also accomplished. It could be used for the quality evaluation of SR and its processing products [29,173]. Additionally, the establishment of LC-MS integrated with a bioassay for cellular NO generation inhibitory activity and an assay for DPPH/ABTS free radical scavenging activity allowed the online screening of quality markers related to anti-inflammatory and antioxidant in SR [174].
It is clear that baicalin, baicalein, wogonin, wogonoside, skullcapflavone II, oroxylin A, and chrysin are considered the potential quality markers of SR. However, there are many gaps in the study of the efficacy-related quality markers. Therefore, it is necessary to conduct biological-level validation to establish the evaluation methods that truly reflect the intrinsic quality of SR.
9. Influence factors of biosynthesis
Flavonoids, as the dominant secondary metabolites and medicinal active components in SR, have pharmacological effects such as anti-inflammatory [175], antioxidant [176], anti-cancer [177], antiviral, immunomodulation effects [178], and neuroprotective effects [179]. It manifests that they can serve as a source for the development of new drugs. However, these secondary metabolites are accumulated in specific parts of SR plants, and their total content is low, resulting in a few medicinal parts and a high cost of medicinal use. Notably, the application of synthetic biology and metabolic engineering based on biosynthesis can solve this problem [180]. Therefore, the analysis of the influence factors and regulatory mechanism of flavonoids biosynthesis in SR have attracted wide attention.
9.1. Biosynthetic pathway of flavonoids in SR
Biosynthesis is a chemical synthesis process that takes place in living organisms, which is controlled by enzymes to produce various secondary metabolites [181]. The biosynthetic pathway of SR flavonoids, mainly divided into above-ground and below-ground parts, has been well studied (Fig. 7). For the above-ground part of SR, naringenin, a product of phenylalanine via phenylpropanoid metabolism, is generally considered a precursor for the synthesis of scutellarein and scutellarin. Its biosynthetic pathway is as follows. Firstly, phenylalanine is catalyzed by phenylalanine ammonia-lyase (PAL) to form cinnamic acid, which is used as a substrate for cinnamoyl 4 hydroxylase (C4H) to produce 4-coumarate. Then, it sequentially undergoes 4 Coumarate:coenzyme A ligase (4CL) activation, chalcone synthase (CHS) catalyzed condensation, and chalcone isomerase (CHI) catalyzed isomerization to form naringenin. Next, the C-ring of naringenin is oxidized to produce apigenin under the action of flavonoid synthase II-1 (FNSII-1). Finally, apigenin undergoes cyclic hydroxylation under the modification of flavonoid 6-hydroxylase (F6H) to produce scutellarein, which is glycosylated to form scutellarin [182].
Fig. 7.
The biosynthetic pathway of flavonoids in Scutellariae radix and the influence of environmental factors and transcription factors on related enzymes. LR: light quality; MC: Moisture content; T: temperature.
Nevertheless, biosynthesis of root-specific flavonoids in SR are more complex than the aerial parts. First of all, cinnamic acid is sequentially decorated by cinnamate-CoA ligase-like 7 (CCL-7), chalcone synthase (CHS), and chalcone isomerase (CHI) to produce pinocembrin [183]. Subsequently, under the conversion of flavonoid synthase II-2 (FNSII-2), pinocembrin is used as a substrate to generate chrysin. It is catalyzed by flavonoid 8-hydroxylase (F8H) to produce norwogonin or by flavonoid 6-hydroxylase (F6H) to produce baicalein [184]. In the end, norwogonin is converted to wogonin in the effect of phenylpropanoid and flavonoid O-methyltransferases (PFMOT). Baicalein is converted to baicalin with the participation of glucuronosyltransferases (sbUGATs) or oroxin A (baicalein-7-O-β-D-glucoside) with the participation of glucuronosyltransferases (sbUGTs) [[185], [186], [187]].
9.2. Influence of environmental factors on flavonoids biosynthesis
9.2.1. Light quality
Ultraviolet-A (UV-A) accounts for 98 % of UV radiation and affect the secondary metabolites of plants [188,189]. Biosynthesis in the both above-ground parts and roots of SR can be affected by UV-A. It increased the content of total flavonoids and signaling molecules (NO and H2O2) of SR and augmented the key enzymes activity (PAL, C4H, and 4CL) in the biosynthesis of aerial parts [190]. The content of baicalin, baicalein, wogonin, and wogonoside increased and new compounds (including pectolinarigenin, puerarin, isokaempferide, and jaceosidin) appeared in the roots of SR after 5 days of post-harvest UV-A irradiation [191].
Ultraviolet-B (UV–B) is one of the known abiotic stresses and is capable of causing the accumulation of secondary metabolites in plants [192,193]. UV-B effectively led to intracellular NO production, which was seen as a signaling molecule regulating the synthetic pathway of baicalin. Therefore, in SR cell cultures stimulated by UV-B, the content of baicalin was 3.1 times higher than that in unstimulated SR cell cultures [194]. UV-B also contributed to the increased content of glycoside ligands (baicalein, wogonin, and scutellarein), which led to the decrease in the content of glucuronides (baicalin and wogonoside) [195]. In addition, white light emitting diodes treatment for two weeks resulted in the accumulation of flavonoid secondary metabolites in the SR roots, mainly baicalin, baicalein, and wogonin [196].
The findings provide a reference for how to select appropriate light quality conditions to increase the content of active components during the SR cultivation process.
9.2.2. Moisture content
Water deficit is a stress factor for the product quality and affect the secondary metabolites of medicinal plants [197]. The total flavonoid content of 3-month-old SR increased markedly after 50 and 70 days of cultivation in 12 % soil water content. Further research found that the expression of PAL, CHS, and sbUGAT were elevated in the absence of water. Meanwhile, lack of water reduced the content of endogenous gibberellin protein but increased the content of indoleacetic acid-related protein in the roots and leaves of SR. The application of these inhibitors could return the flavonoid content to a normal level. Therefore, the lack of water affected flavonoid synthesis by modulating hormone metabolism in SR [198]. The effects of different degrees of drought on baicalin biosynthesis in SR had been studied. Under mild drought, PAL, 4CL, C4H, and CHS activities were elevated and their corresponding gene expression levels increased, promoting baicalin biosynthesis. However, in the case of severe drought, the opposite is true [199].
Collectively, harvesting under a prolonged water deficit should be avoided in the cultivation of SR. Appropriate reduction of water in the soil can increase the content of flavonoids in SR, especially baicalin.
9.2.3. Temperature
The low temperature (10 °C) led to the decrease in the content of baicalin and total flavonoids of SR, while the high temperature (40 °C) promoted the conversion of baicalein to baicalin. It was caused by the decrease of β-glucuronidase expression and the increase of sbUGAT expression at high temperatures [200]. At 25 °C, SR plants showed good growth of callus and increased content as well as accumulation of baicalin. The correlation analysis implied that baicalin content was positively associated with the expression of PAL, C4H, and CHS [201]. Interestingly, the content of baicalin, baicalein, and wogonin in SR seedlings, cultivated in a 4 °C growth chamber, was higher than those cultivated at room temperature. It indicated that appropriate lower temperature also promoted the accumulation of major flavonoids [202].
The regulation of secondary metabolite synthesis in SR is a balance in response to changes in abiotic stress factors. Environmental factors act on enzymes in the flavonoid biosynthetic pathway. Therefore, using the effects of environmental factors on the biosynthesis rationally, it is possible to cultivate high-quality SR. However, how to make a good balance between yield and the effects of abiotic stress factors needs to be further explored.
9.3. Influence of transcription factors on flavonoids biosynthesis
Transcription factors are important regulators at the level of gene transcription that activate or repress the expression of specific genes to affect biological processes including metabolism and biosynthesis [203].
SbMYB3, SbMYB12, SbMYB45, and SbMYB86.1 are key transcription factors in the biosynthesis of flavonoids in SR and are all R2R3-MYBs, which are considered to be the largest MYB subfamily in plants. Among them, SbMYB3 and SbMYB12 promoted the accumulation of root-specific flavonoids. SbMYB3 bound to SbFNSII-2 promoters and strengthened its transcription. Thus, the overexpression of SbMYB3 increased the content of baicalin, baicalein, wogonoside, and wogonin in the subsequent biosynthesis of hairy roots [204]. SbMYB12, a novel S20 R2R3-MYB transcription factor, was found to bind to SbCHI-2, SbCCL7-4, and SbF6H-1 promoters. It could promote the production of baicalin and wogonoside in the root of SR [205]. Nevertheless, SbMYB45 and SbMYB86.1 identified from SR played a key role in the flavonoid biosynthesis in SR leaves. The expression of SbCHI promoter was activated and the accumulation of flavonoids, especially baicalin, was augmented by them [206]. In addition, SbMYB18/32/46/60/70/74 genes were identified from the nucleus of SR. Among them, SbMYB18/32/60/70 had transcriptional activation properties, which might play a role in the regulatory mechanisms of biological processes [207]. Whether they affect the biosynthesis of flavonoids in SR and the related regulatory mechanisms deserves further study. Another study portrayed that the transcription factor Lc activated the expression of SbPAL1, SbC4H, Sb4CL and SbUGAT as well as the transcription factor PAP1 activated the expression of SbPAL1, SbPAL2, SbPAL3, SbC4H, Sb4CL, SbCHI, and SbUGAT to increase the content of flavonoids in the root of SR [208].
In summary, the relationship between transcription factors and genes involved in biosynthesis of SR was revealed (Fig. 7). The understanding of the mechanisms of formation and regulation of active components in SR was strengthened. Overexpression of transcription factors increases the flavonoid content and accumulation in aerial parts and root of SR, which sheds new light on the metabolic engineering.
10. Conclusion and future perspectives
SR is an intriguing traditional herbal medicine that has been widely valued since ancient times. This paper provided an updated review of its ethnobotanical uses, processing, phytochemistry, pharmacological effects, quality control, as well as regulatory factors of biosynthesis. In the field of traditional historical applications, different efficacies of SR had been developed, such as harmonizing the liver and spleen, clearing heat and removing toxicity, tonifying blood and calming the fetus, combining it with other traditional Chinese medicines to provide good therapeutic effects in different types of diseases. Phytochemical studies had demonstrated a total of 210 components were isolated and identified from SR, including flavonoids, phenylpropanoids, phenylethanoid glycosides, phenolic acids, volatile components, polysaccharides and others. On this basis, the pharmacological activities of SR extract and its bioactive components were further investigated. The results showed the extract of SR, baicalin, baicalein, wogonin, wogonoside, and scutellarin had abundant pharmacological effects. In addition, skullcapflavone II showed an anti-inflammatory effect, and total flavonoids extract of SR, 7-O-methylwogonin, and oroxylin A showed antitumor effects.
Nevertheless, while reviewing the detailed contents of SR, some key problems were exposed and deserved further exploration. Firstly, the processed SR are increasingly used in clinical practice, but its quality control indicators are not perfect. Processing plays an important role in altering the efficacy of SR, and further studies are needed to understand its influence mechanism. Secondly, numerous components have been isolated from SR and the proportion of flavonoids is the highest, so most of the studies on the pharmacological activity have been focused on these components, ignoring the biological activity of other components. Thirdly, SR had conspicuous medicinal value, but the in vivo mechanisms of action related to antitumor had not been fully elucidated and the relationship between hepatoprotective effects and anti-inflammatory effects is unclear. Fourthly, though “nature and flavor”, “channel tropism”, “ascending and descending, floating and sinking” and “traditional efficacy” of SR are clear, there are few modern pharmacological studies that are based on the traditional efficacy. Fifthly, in the quality control of SR, analytical methods and fingerprints had been widely studied. However, most of the results in quality markers stay at the level of chemical analysis and lack corresponding biological experiments. Sixthly, there is insufficient research that exists on the regulatory mechanisms of environmental factors, especially on the development of new transcription factors in flavonoid biosynthesis.
In response to the above problems, the following viewpoints are proposed: (1) Analytical tools such as near-infrared spectroscopy, liquid chromatography, mass spectrometry combined with auxiliary tools including chemometrics and electronic tongue technology can be used to establish quality control indicators for processed SR. In addition, experiments on differences in animal tissue distribution of the chemical components and comparative pharmacokinetic experiments should be applied to visualize the reasons why the processing alters the effects of SR. (2) In future pharmacological studies, advanced techniques and methods should be utilized to complement the pharmacological effects of other components in SR, so as to expand the therapeutic material basis more comprehensively. (3) With the aid of network pharmacology, in vivo pharmacological experiments, metabolomics, proteomics, and transcriptomics, the action mechanisms of SR in terms of antitumor and hepatoprotective effects can be unraveled to support its clinical applications. (4) Under the guidance of the TCM theory of treatment based on syndrome differentiation, animal models of TCM syndrome related to the efficacy of SR are supposed to establish. Combined with modern pharmacological methods to clarify the action mechanisms and targets of SR's efficacy. It is of great significance for the clinical use and secondary development of SR. (5) To obtain more bioactive markers related to the efficacy, corresponding activity screening, pharmacokinetic and pharmacological assays of potential quality markers should be conducted. It is helpful to exhaustively elucidate the pharmacodynamic material basis of SR. (6) Studies oriented towards action mechanisms of regulatory factors linked to flavonoid biosynthesis should be carried out. It would provide theoretical references for the cultivation of SR with excellent quality and large-scale production of root-specific flavonoids.
Conclusively, SR has numerous bioactive components and is rich in the pharmacological effects, which has outstanding medicinal values. It is reviewed in terms of the ethnobotanical uses, processing, phytochemistry, pharmacological effects, quality control and influence factors of biosynthesis. The purpose is to provide a foundation for secondary exploitation and subsequent studies on clinical applications of SR.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Wentao Ma: Writing – original draft, Methodology, Data curation. Tianyu Liu: Writing – original draft, Data curation. Omachi Daniel Ogaji: Writing – review & editing. Jin Li: Supervision. Kunze Du: Writing – review & editing, Supervision. Yanxu Chang: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Tianjin Committee of Science and Technology in China (23ZYJDSS00010).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36146.
Contributor Information
Kunze Du, Email: dkztcm@tjutcm.edu.cn.
Yanxu Chang, Email: tcmcyx@tjutcm.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Commission C.P. China Medical Science Press; Peking: 2020. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 2.Qian X.J., Meng W.W., Zhao J.C., Wang Y.H., Jin Y., Zhan Z.L. Herbal textual research on scutellariae radix in famous classical formulas. Chin. J. Exp. Tradit. Med. Formulae. 2023;29(5):84–93. doi: 10.13422/j.cnki.syfjx.20220458. [DOI] [Google Scholar]
- 3.Zhang L., Cao B., Bai C., Li G., Mao M. Predicting suitable cultivation regions of medicinal plants with Maxent modeling and fuzzy logics: a case study of Scutellaria baicalensis in China. Environ. Earth Sci. 2016;75(5):361. doi: 10.1007/s12665-015-5133-9. [DOI] [Google Scholar]
- 4.Liu R.X., Song G.H., Wu P.G., Zhang X.W., Hu H.J., Liu J., Miao X.S., Hou Z.Y., Wang W.Q., Wei S.L. Distribution patterns of the contents of five biologically activate ingredients in the root of Scutellaria baicalensis. Chin. J. Nat. Med. 2017;15(2):152–160. doi: 10.1016/S1875-5364(17)30030-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N., Su F.S., Zhou Y.T., Gao Q.G., Chen J.L., Xu X.L., Han L.Y. High quality standard of Scutellariae Radix based on plant metabolomics and index components, China J. Chin. Mater. Med. 2022;47(10):2681–2688. doi: 10.19540/j.cnki.cjcmm.20211123.203. [DOI] [PubMed] [Google Scholar]
- 6.Chu W.M. Experiences of single Scutellaria baicalensis in ttreating colds during pregnancy, inner Mongolia. J. Tradit. Chin. Med. 2010;29(16):15. doi: 10.16040/j.cnki.cn15-1101.2010.16.038. [DOI] [Google Scholar]
- 7.Arweiler N.B., Pergola G., Kuenz J., Hellwig E., Sculean A., Auschill T.M. Clinical and antibacterial effect of an anti-inflammatory toothpaste formulation with Scutellaria baicalensis extract on experimental gingivitis. Clin. Oral Invest. 2011;15(6):909–913. doi: 10.1007/s00784-010-0471-1. [DOI] [PubMed] [Google Scholar]
- 8.Lin H., Zhou J., Lin K., Wang H., Liang Z., Ren X., Huang L., Xia C. Efficacy of Scutellaria baicalensis for the treatment of hand, foot, and mouth disease associated with encephalitis in patients infected with EV71: a multicenter, retrospective analysis. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/5697571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G.T., Yang X.W., Wang Q., Wang X. The efficacy of baicalin capsule combined with hepatic artery chemoembolization in the treatment of primary hepatocellular carcinoma. Chin. J. Integr. Tradit. West. Med. Liver Dis. 2016;26(6):369–370. [Google Scholar]
- 10.Liao C.C., Liao K.R., Lin C.L., Li J.M. The effectiveness of Scutellaria baicalensis on migraine: implications from clinical use and experimental proof, evid. Based complement alternat. Méd. 2021;2021 doi: 10.1155/2021/8707280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018;56(1):465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao T., Tang H., Xie L., Zheng Y., Ma Z., Sun Q., Li X. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019;71(9):1353–1369. doi: 10.1111/jphp.13129. [DOI] [PubMed] [Google Scholar]
- 13.Song J.W., Long J.Y., Xie L., Zhang L.L., Xie Q.X., Chen H.J., Deng M., Li X.F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. and its probably potential therapeutic effects on COVID-19: a review. Chin. Med. 2020;15:102. doi: 10.1186/s13020-020-00384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma D., Li X.Q., Xue X.P., Tian Y., Jin L., Cui Z.J., Ma Y. A study on the "quality evaluation through morphological identification" of Scutellaria baicalensis by decipherment of ancient literature. J. Chin. Med. Mater. 2022;45(10):2516–2521. doi: 10.13863/j.issn1001-4454.2022.10.043. [DOI] [Google Scholar]
- 15.Xue Y., Zhu W.K., Li Y., Zhu H.Q., Luo Y.B., Wang Y.L., Cui C. Herbal textual research on scutellariae radix in treatise on febrile diseases. Shanghai. J. Trad. Chin. 2021;55(5):33–37. doi: 10.16305/j.1007-1334.2021.2008048. [DOI] [Google Scholar]
- 16.Zeng S.Y. Beijing Univ Of Chin Med; Beijing: 2017. RevMan Meta-Analysis and Evaluation of Gegen Huangqin Huanglian Decoction Treatment of Pediatric Rotavirus Enteritis Randomized Controlled Trials. ([In Chinese]) [Google Scholar]
- 17.Liu D., Wang Z., Zhong L., Xie C., Huang X., Zhi Y., Zhang Y., Liang J., Shi Z., Huang J., Zhang S., Zhang J., Ding F. Targets and potential mechanism of Scutellaria baicalensis in treatment of primary hepatocellular carcinoma based on bioinformatics analysis. JAMA Oncol. 2022;2022 doi: 10.1155/2022/8762717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin N.R., Gu N., Choi H.S., Kim H. Combined effects of Scutellaria baicalensis with metformin on glucose tolerance of patients with type 2 diabetes via gut microbiota modulation. Am. J. Physiol. Endocrinol. Metab. 2020;318(1):E52–E61. doi: 10.1152/ajpendo.00221.2019. [DOI] [PubMed] [Google Scholar]
- 19.Chen L.L., Verpoorte R., Yen H.R., Peng W.H., Cheng Y.C., Chao J., Pao L.H. Effects of processing adjuvants on traditional Chinese herbs. J. Food Drug Anal. 2018;26(2S):S96–S114. doi: 10.1016/j.jfda.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X.S., Cheng J.H. Progress of research on the processing of Scutellaria baicalensis, inner Mongolia. J. Tradit. Chin. Med. 2012;31(23):124–126. doi: 10.16040/j.cnki.cn15-1101.2012.23.051. [DOI] [Google Scholar]
- 21.Zhang C.F., Zhang Y.Q. Research on Scutellaria baicalensis processing, res. Pract. Chin. Med. 2003;(5):26–28. [Google Scholar]
- 22.Fan M.H., Yu X.H., Zhu L., Zhang Q., Ju C.G. Study on the historical evolution of Scutellaria baicalensis processing. Chin. J. Ethnomed. Ethnopharmacy. 2021;30(20):53–57. [Google Scholar]
- 23.Lin H.H., Shao C.X., Peng T., Yao J. Historical evolution and modern research progress of processing of scutellariae radix. Chin. J. Exp. Tradit. Med. Formulae. 2023;30(3):279–289. doi: 10.13422/j.cnki.syfjx.20230861. [DOI] [Google Scholar]
- 24.Gu Z.W. History of Scutellaria baicalensis processing and progress of modern research on processed products. Shandong J. Tradit. Chin. Med. 2013;32(3):211–212. doi: 10.16295/j.cnki.0257-358x.2013.03.036. [DOI] [Google Scholar]
- 25.Luo L., Wei S.H., Zhang Y., Wu M.H., Cao H., Ma Z.G. UPLC-Based comparison of five flavonoids in different processed products of Scutellaria baicalensis. Chin. Tradit. Pat. Med. 2021;43(4):966–970. [Google Scholar]
- 26.Huang Q., Wu D.L., Wang Y., Ma Y.L., Yu D.R., Zhang C. GC-MS analysis of volatile components in scutellariae radix before and after being charred. Chin. J. Exp. Tradit. Med. Formulae. 2016;22(24):9–12. doi: 10.13422/j.cnki.syfjx.2016240009. [DOI] [Google Scholar]
- 27.Cui X.B., Qian X.C., Huang P., Zhang Y.X., Li J.S., Yang G.M., Cai B.C. Simultaneous determination of ten flavonoids of crude and wine-processed Radix Scutellariae aqueous extracts in rat plasma by UPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study. Biomed. Chromatogr. 2015;29(7):1112–1123. doi: 10.1002/bmc.3398. [DOI] [PubMed] [Google Scholar]
- 28.Hu L., Wang Y., Sun H., Xiong Y., Zhong L., Wu Z., Yang M. An untargeted metabolomics approach to investigate the wine-processed mechanism of Scutellariae radix in acute lung injury. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112665. [DOI] [PubMed] [Google Scholar]
- 29.Wang F., Wang B., Wang L., Xiong Z.Y., Gao W., Li P., Li H.J. Discovery of discriminatory quality control markers for Chinese herbal medicines and related processed products by combination of chromatographic analysis and chemometrics methods: radix Scutellariae as a case study. J. Pharm. Biomed. Anal. 2017;138:70–79. doi: 10.1016/j.jpba.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Huang F., Zhang X., Li W., Zhao Y., Mu Q., Wang X., Wang Y. Discovery of conversion driven by β-glucuronidase from flavone glycoside to aglycone and application in identifying the raw Scutellariae Radix. Arab. J. Chem. 2022;15(11) doi: 10.1016/j.arabjc.2022.104216. [DOI] [Google Scholar]
- 31.Chai C.C., Cao Y., Mao M., Wang J.Y., Liu N., Li X.X., Zhang K., Chen D.L., Wei L.Y., Yi Y.H., Li F. Evaluation of taste changes of Scutellariae Radix before and after wine-frying based on electronic ongue technology and its application in identification of Scutellariae Radix pieces. China J. Chin. Mater. Med. 2020;45(11):2552–2559. doi: 10.19540/j.cnki.cjcmm.20200328.303. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X.Q., Liang H., Lu X.H., Cai S.Q., Wang B., Zhao Y.Y. Flavonoids from Scutellaria baicalensis and their bioactivities. J. Peking Univ., Health Sci. 2009;41(5):578–584. [PubMed] [Google Scholar]
- 33.Xu D.Y., Cheng P.D., Zhang L., Cao Y.D., Ding A.W. Study on chemical constituents of Scutellaria baicalensis. Chin. J. Exp. Tradit. Med. Formulae. 2011;17(1):78–80. doi: 10.13422/j.cnki.syfjx.2011.01.036. [DOI] [Google Scholar]
- 34.Ma J.L. Study on chemical constituents from stems and leaves of Scutellaria baicalensis. Chin. J. Exp. Tradit. Med. Formulae. 2013;19(7):147–149. [Google Scholar]
- 35.Guo X.Y. Chengde Medical Univ, Hebei; 2015. Study on Flavonoids from the Stems and Leaves of Scutellaria Baicalensis Georgi. [Google Scholar]
- 36.Wang H.W., Yin Z.F., Li H.B., Yuan X.X., Yang M.M., Zhao G.Q. Chemical constituents from stems and leaves of Scutellaria baicalensis. Chin. J. Exp. Tradit. Med. Formulae. 2016;22(22):41–44. doi: 10.13422/j.cnki.syfjx.2016220041. [DOI] [Google Scholar]
- 37.Song C.Y., Liu Y., Piao J.H., Piao C.G., Lv H.Z., Li G. Chemical constituents of Scutellaria radix and their antioxidant activity. Lishizhen Med. Mater. Med. Res. 2007;(4):856–857. [Google Scholar]
- 38.Wang H.Y. Shenyang Pharmaceutical Univ; Liaoning: 2002. Studies on Anxiolytic Constituents of Scutellaria Baicalensis Georgi. [Google Scholar]
- 39.Tomimori T., Miyaichi Y., Kizu H. On the flavonoid constituents from the roots of Scutellaria baicalensis Georgi. I. Yakugaku Zasshi. 1982;102(4):388–391. doi: 10.1248/yakushi1947.102.4_388. [DOI] [PubMed] [Google Scholar]
- 40.Takagi S., Yamaki M., Inoue K. Studies on the water-soluble constituents of the roots of Scutellaria baicalensis Georgi (Wogon) Yakugaku Zasshi. 1980;100(12):1220–1224. doi: 10.1248/yakushi1947.100.12_1220. [DOI] [PubMed] [Google Scholar]
- 41.Tomimori T., Miyaichi Y., Imoto Y., Kizu H., Tanabe Y. Studies on the constituents of Scutellaria species. III. On the flavonoid constituents of the root of Scutellaria baicalensis Georgi (3) Yakugaku Zasshi. 1984;104(5):524–528. doi: 10.1248/yakushi1947.104.5_524. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa K., Furukawa H., Fujioka T., Fujii H., Mihashi K., Shimomura K., Ishimaru K. Flavone production in transformed root cultures of Scutellaria baicalensis Georgi. Phytochemistry. 1999;52(5):885–890. doi: 10.1016/S0031-9422(99)00306-4. [DOI] [Google Scholar]
- 43.Tomimori T., Miyaichi Y., Imoto Y., Kizu H., Suzuki C. Studies on the constituents of Scutellaria species. IV. On the flavonoid constituents of the root of Scutellaria baicalensis Georgi (4) Yakugaku Zasshi. 1984;104(5):529–534. doi: 10.1248/yakushi1947.104.5_529. [DOI] [PubMed] [Google Scholar]
- 44.Seo O.N., Kim G.-S., Kim Y.-H., Park S., Jeong S.W., Lee S.J., Jin J.S., Shin S.C. Determination of polyphenol components of Korean Scutellaria baicalensis Georgi using liquid chromatography–tandem mass spectrometry: contribution to overall antioxidant activity. J. Funct.Foods. 2013;5(4):1741–1750. doi: 10.1016/j.jff.2013.07.020. [DOI] [Google Scholar]
- 45.Zhang Y.-Y., Guo Y.-Z., Onda M., Hashimoto K., Ikeya Y., Okada M., Maruno M. Four flavonoids from Scutellaria baicalensis. Phytochemistry. 1994;35(2):511–514. doi: 10.1016/S0031-9422(00)94792-7. [DOI] [Google Scholar]
- 46.Ji S., Li R., Wang Q., Miao W.J., Li Z.W., Si L.L., Qiao X., Yu S.W., Zhou D.M., Ye M. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J. Ethnopharmacol. 2015;176:475–484. doi: 10.1016/j.jep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Long H.L., Xu G.Y., Deng A.J., Li Z.H., Ma L., Lu Y., Zhang Z.H., Wu F., Qin H.L. Two new flavonoids from the roots of Scutellaria baicalensis. J. Asian Nat. Prod. Res. 2015;17(7):756–760. doi: 10.1080/10286020.2014.999048. [DOI] [PubMed] [Google Scholar]
- 48.Popova T.P., Litvinenko V.I., Kovalev I.P. Flavones of the roots of Scutellaria baicalensis. Chem. Nat. Compd. 1973;9(6):699–702. doi: 10.1007/BF00565789. [DOI] [Google Scholar]
- 49.Tomimori T., Miyaichi Y., Imoto Y., Kizu H., Tanabe Y. Studies on the constituents of Scutellaria species. II. On the flavonoid constituents of the root of Scutellaria baicalensis GEORGI (2) Yakugaku Zasshi. 1983;103(6):607–611. doi: 10.1248/yakushi1947.103.6_607. [DOI] [Google Scholar]
- 50.Long H.L., Deng A.J., Li Z.H., Qin H.L. Session 21 of the 15th Annual Meeting of the Chinese Association for Science and Technology: Symposium on Modern Research on Traditional Chinese Medicine and Natural Medicines, Guiyang. 2013. Chemical constituents from Scutellaria baicalensis Georgi; pp. 123–125. Guizhou, China. [Google Scholar]
- 51.Du Z.Q. Shenyang Pharmaceutical Univ; Liaoning: 2012. Studies on Chemical Constituents of Scutellaria Baicalensis and Metabolism of its Extract in Rat. [Google Scholar]
- 52.Wang M.H., Li L.Z., Sun J.B., Wu F.H., Liang J.Y. A new antioxidant flavone glycoside from Scutellaria baicalensis Georgi. Nat. Prod. Res. 2014;28(20):1772–1776. doi: 10.1080/14786419.2014.931391. [DOI] [PubMed] [Google Scholar]
- 53.Ishimaru K., Nishikawa K., Omoto T., Asai I., Yoshihira K., Shimomura K. Two flavone 2'-glucosides from Scutellaria baicalensis. Phytochemistry. 1995;40(1):279–281. doi: 10.1016/0031-9422(95)00200-q. [DOI] [PubMed] [Google Scholar]
- 54.Qiao X., Li R., Song W., Miao W.J., Liu J., Chen H.B., Guo D.A., Ye M. A targeted strategy to analyze untargeted mass spectral data: rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J. Chromatogr. A. 2016;1441:83–95. doi: 10.1016/j.chroma.2016.02.079. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z.B., Sun C.P., Xu J.X., Morisseau C., Hammock B.D., Qiu F. Phytochemical constituents from Scutellaria baicalensis in soluble epoxide hydrolase inhibition: kinetics and interaction mechanism merged with simulations. Int. J. Biol. Macromol. 2019;133:1187–1193. doi: 10.1016/j.ijbiomac.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 56.Ma S.L. Hebei Normal Univ, Hebei; 2012. Study on Chemical Compositions of Stems an Leaves of Scutellaria Baicalensis Georgi. [Google Scholar]
- 57.Li R. Sauropus Spatulifolius and Scutellaria Baicalensis. Suzhou Univ; Jiangsu: 2019. Study on the chemical composition Anti-acute Lung Injury activities of Gnaphalium hypoleucum. [Google Scholar]
- 58.Zhou Y., Hirotani M., Yoshikawa T., Furuya T. Flavonoids and phenylethanoids from hairy root cultures of Scutellaria baicalensis. Phytochemistry. 1997;44(1):83–87. doi: 10.1016/S0031-9422(96)00443-8. [DOI] [Google Scholar]
- 59.Lin W.H., Liu S., Wu B. Structural identification of chemical constituents from Scutellaria baicalensis by HPLC-ESI-MS/MS and NMR spectroscopy. Asian J. Chem. 2013;25(7):3799–3805. doi: 10.14233/ajchem.2013.13788. [DOI] [Google Scholar]
- 60.Kubo M., Kimura Y., Odani T., Tani T., Namba K. Studies on Scutellariae radix. Part II: the antibacterial substance. Planta Med. 1981;43(2):194–201. doi: 10.1055/s-2007-971499. [DOI] [PubMed] [Google Scholar]
- 61.Oszmiański J., Bakowska A., Piacente S. Thermodynamic characteristics of copigmentation reaction of acylated anthocyanin isolated from blue flowers of Scutellaria baicalensis Georgi with copigments. J. Sci. Food Agric. 2004;84(12):1500–1506. doi: 10.1002/jsfa.1815. [DOI] [Google Scholar]
- 62.Yao X., Cheng Y.X., Cheng L., Liu W., Wang X., Li F.S., Li B., Dong H.J. Chemical constituents from Scutellaria baicalensis. Chin. Tradit. Pat. Med. 2020;42(11):2935–2940. [Google Scholar]
- 63.Long H., Zhang H., Deng A., Ma L., Wu L., Li Z., Zhang Z., Wang W., Jiang J., Qin H. Three new lignan glucosides from the roots of Scutellaria baicalensis. Acta Pharm. Sin. B. 2016;6(3):229–233. doi: 10.1016/j.apsb.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.M. Y., T. T. Studies on the constituents of Scutellaria species XVI phenol glycosides of the root of Scutellaria baicaleinsis Georgi. J. Nat. Med. 1994;48:215–218. [Google Scholar]
- 65.Liu Y.X., Liu Z.G., Su L., Yang R.P., Hao D.F., Pei Y.H. Chemical constituents from Scutellaria baicalensis Georgi, chin. J. Med. Chem. 2009;19(1):59–62. [Google Scholar]
- 66.Xu J.X., Ding L.Q., Jiang M.M., Qiu F. Studies on the non-flavonoid components of Scutellaria baicalensis. Chin. J. Med. Chem. 2016;26(6):480–483+489. doi: 10.14142/j.cnki.cn21-1313/r.2016.06.007. [DOI] [Google Scholar]
- 67.Tuan P.A., Kim Y.B., Kim J.K., Arasu M.V., Al-Dhabi N.A., Park S.U. Molecular characterization of carotenoid biosynthetic genes and carotenoid accumulation in Scutellaria baicalensis Georgi. EXCLI J. 2015;14:146–157. doi: 10.17179/excli2014-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song S.H., Wang Z.Z. Analysis of essential oils from different organs of Scutellaria baicalensis. J. Chin. Med. Mater. 2010;31(8):1265–1270. doi: 10.13863/j.issn1001-4454.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 69.Huang T., Liu Y., Zhang C. Pharmacokinetics and bioavailability enhancement of baicalin: a review. Eur. J. Drug Metab. Pharmacokinet. 2019;44(2):159–168. doi: 10.1007/s13318-018-0509-3. [DOI] [PubMed] [Google Scholar]
- 70.Sowndhararajan K., Deepa P., Kim M., Park S.J., Kim S. Baicalein as a potent neuroprotective agent: a review. Biomed. Pharmacother. 2017;95:1021–1032. doi: 10.1016/j.biopha.2017.08.135. [DOI] [PubMed] [Google Scholar]
- 71.Tuli H.S., Aggarwal V., Kaur J., Aggarwal D., Parashar G., Parashar N.C., Tuorkey M., Kaur G., Savla R., Sak K., Kumar M. Baicalein: a metabolite with promising antineoplastic activity. Life Sci. 2020;259 doi: 10.1016/j.lfs.2020.118183. [DOI] [PubMed] [Google Scholar]
- 72.Zhuang J., Peng Y., Gu C., Chen H., Lin Z., Zhou H., Wu X., Li J., Yu X., Cao Y., Zeng H., Fu X., Xu C., Huang P., Cao S., Wang C., Yan F., Chen G. Wogonin accelerates hematoma clearance and improves neurological outcome via the PPAR-gamma pathway after intracerebral hemorrhage, transl. Stroke. Res. 2021;12(4):660–675. doi: 10.1007/s12975-020-00842-9. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Wang J., Zhong S., Li J., Du W. Scutellarein inhibits the development of colon cancer via CDC4-mediated RAGE ubiquitination. Int. J. Mol. Med. 2020;45(4):1059–1072. doi: 10.3892/ijmm.2020.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sajeev A., Hegde M., Daimary U.D., Kumar A., Girisa S., Sethi G., Kunnumakkara A.B. Modulation of diverse oncogenic signaling pathways by oroxylin A: an important strategy for both cancer prevention and treatment. Phytomedicine. 2022;105 doi: 10.1016/j.phymed.2022.154369. [DOI] [PubMed] [Google Scholar]
- 75.Tian X.Y., Li M.X., Lin T., Qiu Y., Zhu Y.T., Li X.L., Tao W.D., Wang P., Ren X.X., Chen L.P. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur. J. Med. Chem. 2021;209 doi: 10.1016/j.ejmech.2020.112563. [DOI] [PubMed] [Google Scholar]
- 76.Weng C.J., Yen G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38(1):76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Mu K., Yao Y., Wang D., Kitts D.D. Prooxidant capacity of phenolic acids defines antioxidant potential. Biochim. Biophys. Acta Gen. Subj. 2023;1867(7) doi: 10.1016/j.bbagen.2023.130371. [DOI] [PubMed] [Google Scholar]
- 78.Zeng P., Li J., Chen Y., Zhang L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog Mol Biol Transl Sci. 2019;163:423–444. doi: 10.1016/bs.pmbts.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui L., Wang W., Luo Y., Ning Q., Xia Z., Chen J., Feng L., Wang H., Song J., Tan X., Tan W., Wang C., Jia X. Polysaccharide from Scutellaria baicalensis Georgi ameliorates colitis via suppressing NF-κB signaling and NLRP3 inflammasome activation. Int. J. Biol. Macromol. 2019;132:393–405. doi: 10.1016/j.ijbiomac.2019.03.230. [DOI] [PubMed] [Google Scholar]
- 80.Cui L., Guan X., Ding W., Luo Y., Wang W., Bu W., Song J., Tan X., Sun E., Ning Q., Liu G., Jia X., Feng L. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021;166:1035–1045. doi: 10.1016/j.ijbiomac.2020.10.259. [DOI] [PubMed] [Google Scholar]
- 81.Jin Y., Yang J., Lin L., Lin Y., Zheng C. The attenuation of scutellariae radix extract on oxidative stress for colon injury in lipopolysaccharide-induced RAW264.7 cell and 2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis rats. Phcog. Mag. 2016;12(46):153–159. doi: 10.4103/0973-1296.177913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X., Yu J.Y., Sun Y., Wang H., Shan H., Wang S. Baicalin protects LPS-induced blood-brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int. Immunopharm. 2021;96 doi: 10.1016/j.intimp.2021.107725. [DOI] [PubMed] [Google Scholar]
- 83.Yu W., Xu Z., Gao Q., Xu Y., Wang B., Dai Y. Protective role of wogonin against cadmium induced testicular toxicity: involvement of antioxidant, anti-inflammatory and anti-apoptotic pathways. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118192. [DOI] [PubMed] [Google Scholar]
- 84.Shi X., Zhang B., Chu Z., Han B., Zhang X., Huang P., Han J. Wogonin inhibits cardiac hypertrophy by activating nrf-2-mediated antioxidant responses. Cardiovasc Ther. 2021;2021 doi: 10.1155/2021/9995342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jing L., Gao R., Zhang J., Zhang D., Shao J., Jia Z., Ma H. Norwogonin attenuates hypoxia-induced oxidative stress and apoptosis in PC12 cells. BMC Complement Med Ther. 2021;21(1):18. doi: 10.1186/s12906-020-03189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu X., An W., Li X., Zhou B., Li H. Anti-inflammatory effects of Scutellaria baicalensis water extract in LPS-induced THP-1 Macrophages through metabolomics study. Arab. J. Chem. 2023;16(3) doi: 10.1016/j.arabjc.2022.104507. [DOI] [Google Scholar]
- 87.Shi T., Li T., Jiang X., Jiang X., Zhang Q., Wang Y., Zhang Y., Wang L., Qin X., Zhang W., Zheng Y. Baicalin protects mice from infection with methicillin-resistant Staphylococcus aureus via alleviating inflammatory response. J. Leukoc. Biol. 2020;108(6):1829–1839. doi: 10.1002/JLB.3AB0820-576RRR. [DOI] [PubMed] [Google Scholar]
- 88.Kim Y.J., Kim H.J., Lee J.Y., Kim D.H., Kang M.S., Park W. Anti-inflammatory effect of baicalein on polyinosinic-polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses. 2018;10(5):224. doi: 10.3390/v10050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee H., Lee D.H., Oh J.H., Chung J.H. Skullcapflavone II suppresses TNF-alpha/IFN-gamma-Induced TARC, MDC, and CTSS production in HaCaT cells. Int. J. Mol. Sci. 2021;22(12):6428. doi: 10.3390/ijms22126428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang H., Wang Z., Wang L., Li Y., Guo J., Yang X., Zhao J., Rong K., Zhang P., Ye B., Zhang K., Ma H. Scutellarin ameliorates osteoarthritis by protecting chondrocytes and subchondral bone microstructure by inactivating NF-kappaB/MAPK signal transduction. Biomed. Pharmacother. 2022;155 doi: 10.1016/j.biopha.2022.113781. [DOI] [PubMed] [Google Scholar]
- 91.Park H.J., Park S.H., Choi Y.H., Chi G.Y. The root extract of Scutellaria baicalensis induces apoptosis in EGFR TKI-resistant human lung cancer cells by inactivation of STAT3. Int. J. Mol. Sci. 2021;22(10):5181. doi: 10.3390/ijms22105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hussain I., Waheed S., Ahmad K.A., Pirog J.E., Syed V. Scutellaria baicalensis targets the hypoxia-inducible factor-1alpha and enhances cisplatin efficacy in ovarian cancer. J. Cell. Biochem. 2018;119(9):7515–7524. doi: 10.1002/jcb.27063. [DOI] [PubMed] [Google Scholar]
- 93.Tao Y., Zhan S., Wang Y., Zhou G., Liang H., Chen X., Shen H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang H., Li X., Yu L., Liu L., Zhu H., Cao W., Sun Z., Yu X. Wogonoside inhibits TNF receptor-associated factor 6 (TRAF6) mediated-tumor microenvironment and prognosis of pancreatic cancer. Ann. Transl. Med. 2021;9(18):1460. doi: 10.21037/atm-21-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun Y., Guo W., Guo Y., Lin Z., Wang D., Guo Q., Zhou Y. Apoptosis induction in human prostate cancer cells related to the fatty acid metabolism by wogonin-mediated regulation of the AKT-SREBP1-FASN signaling network. Food Chem. Toxicol. 2022;169 doi: 10.1016/j.fct.2022.113450. [DOI] [PubMed] [Google Scholar]
- 96.Fu Q., Gao L., Fu X., Meng Q., Lu Z. Scutellaria baicalensis inhibits Coxsackievirus B3-induced Myocarditis via AKT and p38 pathways. J. Microbiol. Biotechnol. 2019;29(8):1230–1239. doi: 10.4014/jmb.1904.04050. [DOI] [PubMed] [Google Scholar]
- 97.Yang F., Feng C., Yao Y., Qin A., Shao H., Qian K. Antiviral effect of baicalin on Marek's disease virus in CEF cells. BMC Vet. Res. 2020;16(1):371. doi: 10.1186/s12917-020-02595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qin S., Huang X., Qu S. Baicalin induces a potent innate immune response to inhibit respiratory syncytial virus replication via regulating viral non-structural 1 and matrix RNA. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.907047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M.R., Abubakar S. Novel antiviral activity of baicalein against dengue virus. BMC Compl. Alternative Med. 2012;12:214. doi: 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seong R.K., Kim J.A., Shin O.S. Wogonin, a flavonoid isolated from Scutellaria baicalensis, has anti-viral activities against influenza infection via modulation of AMPK pathways. Acta Virol. 2018;62(1):78–85. doi: 10.4149/av_2018_109. [DOI] [PubMed] [Google Scholar]
- 101.Dong Q., Chu F., Wu C., Huo Q., Gan H., Li X., Liu H. Scutellaria baicalensis Georgi extract protects against alcoholinduced acute liver injury in mice and affects the mechanism of ER stress. Mol. Med. Rep. 2016;13(4):3052–3062. doi: 10.3892/mmr.2016.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang L., Wang H.F., Chen Y.M., Bai R.X., Du S.Y. Baicalin confers hepatoprotective effect against alcohol-associated liver disease by upregulating microRNA-205. Int. Immunopharm. 2022;107 doi: 10.1016/j.intimp.2022.108553. [DOI] [PubMed] [Google Scholar]
- 103.Ustuner M.C., Tanrikut C., Ustuner D., Kolac U.K., Koroglu Z.O., Burukoglu D., Entok E. The effect of baicalein on endoplasmic reticulum stress and autophagy on liver damage. Hum. Exp. Toxicol. 2021;40(10):1624–1633. doi: 10.1177/09603271211003634. [DOI] [PubMed] [Google Scholar]
- 104.Yang J.Y., Xiang D.C., Xiang D., He W.X., Liu Y.A., Lan L.L., Li G.D., Jiang C., Ren X.H., Liu D., Zhang C.L. Baicalin protects against 17alpha-ethinylestradiol-induced cholestasis via the sirtuin 1/hepatic nuclear receptor-1alpha/farnesoid X receptor pathway. Front. Pharmacol. 2019;10:1685. doi: 10.3389/fphar.2019.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miao Z., Lai Y., Zhao Y., Chen L., Zhou J., Li C., Wang Y. Protective property of scutellarin against liver injury induced by carbon tetrachloride in mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.710692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin X., Liu M.Y., Zhang D.F., Zhong X., Du K., Qian P., Yao W.F., Gao H., Wei M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia‐mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF‐κB signaling pathway. CNS Neurosci. Ther. 2019;25(5):575–590. doi: 10.1111/cns.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jia Z., Yang J., Cao Z., Zhao J., Zhang J., Lu Y., Chu L., Zhang S., Chen Y., Pei L. Baicalin ameliorates chronic unpredictable mild stress-induced depression through the BDNF/ERK/CREB signaling pathway. Behav. Brain Res. 2021;414 doi: 10.1016/j.bbr.2021.113463. [DOI] [PubMed] [Google Scholar]
- 108.Rui W., Li S., Xiao H., Xiao M., Shi J. Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP induced mice model of Parkinson's disease. Int. J. Neuropsychopharmacol. 2020;23(11):762–773. doi: 10.1093/ijnp/pyaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao X., Kong D., Zhou Q., Wei G., Song J., Liang Y., Du G. Baicalein alleviates depression-like behavior in rotenone- induced Parkinson's disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed. Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111556. [DOI] [PubMed] [Google Scholar]
- 110.Kong Z., Shen Q., Jiang J., Deng M., Zhang Z., Wang G. Wogonin improves functional neuroprotection for acute cerebral ischemia in rats by promoting angiogenesis via TGF-beta1. Ann. Transl. Med. 2019;7(22):639. doi: 10.21037/atm.2019.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 112.Ijaz M.U., Shahzadi S., Ashraf A., Mahboob S., Al-Ghanim K.A., Riaz M.N., Al-Misned F., Sultana S. Protective effect of baicalin on methotrexate-induced mitochondrial damage in testicular tissues of rats. J. King Saud Univ. Sci. 2022;34(8) doi: 10.1016/j.jksus.2022.102343. [DOI] [Google Scholar]
- 113.Deng M., Sun J., Peng L., Huang Y., Jiang W., Wu S., Zhou L., Chung S.K., Cheng X. Scutellarin acts on the AR-NOX axis to remediate oxidative stress injury in a mouse model of cerebral ischemia/reperfusion injury. Phytomedicine. 2022;103 doi: 10.1016/j.phymed.2022.154214. [DOI] [PubMed] [Google Scholar]
- 114.Shi G.F., Yao R.X., Wang G.Y., Wang Z.J., Chen F.W. Liquid chromatography-tandem mass spectrometry screening method for the detection of radical-scavenging natural antioxidants from the whole scutellariae (radix, stem and leaf) J. Chromatogr. Sci. 2015;53(7):1140–1146. doi: 10.1093/chromsci/bmu176. [DOI] [PubMed] [Google Scholar]
- 115.Xia Y.T., Hu W.H., Wu Q.Y., Dong T.T., Duan R., Xiao J., Li S.P., Qin Q.W., Wang W.X., Tsim K.W. The herbal extract deriving from aerial parts of Scutellaria baicalensis shows anti-inflammation and anti-hypoxia responses in cultured fin cells from rabbit fish. Fish Shellfish Immunol. 2020;106:71–78. doi: 10.1016/j.fsi.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao L., Zhao J.X., Qin X.M., Zhao J. The ethanol extract of Scutellaria baicalensis Georgi attenuates complete Freund's adjuvant (CFA)-induced inflammatory pain by suppression of P2X3 receptor. J. Ethnopharmacol. 2023;317 doi: 10.1016/j.jep.2023.116762. [DOI] [PubMed] [Google Scholar]
- 117.Rizzo V., Ferlazzo N., Curro M., Isola G., Matarese M., Bertuccio M.P., Caccamo D., Matarese G., Ientile R. Baicalin-induced autophagy preserved LPS-stimulated intestinal cells from inflammation and alterations of paracellular permeability. Int. J. Mol. Sci. 2021;22(5):2315. doi: 10.3390/ijms22052315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang H., Liu B., Jiang S., Wu J.F., Qi C.H., Mohammadtursun N., Li Q., Li L., Zhang H., Sun J., Dong J.C. Baicalin ameliorates cigarette smoke-induced airway inflammation in rats by modulating HDAC2/NF-kappaB/PAI-1 signalling. Pulm. Pharmacol. Ther. 2021;70 doi: 10.1016/j.pupt.2021.102061. [DOI] [PubMed] [Google Scholar]
- 119.Cai X., Shi Y., Dai Y., Wang F., Chen X., Li X. Baicalin clears inflammation by enhancing macrophage efferocytosis via inhibition of RhoA/ROCK signaling pathway and regulating macrophage polarization. Int. Immunopharm. 2022;105 doi: 10.1016/j.intimp.2022.108532. [DOI] [PubMed] [Google Scholar]
- 120.Khan S., Zhang D., Zhang Y., Li M., Wang C. Wogonin attenuates diabetic cardiomyopathy through its anti-inflammatory and anti-oxidative properties. Mol. Cell. Endocrinol. 2016;428:101–108. doi: 10.1016/j.mce.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 121.Li H.D., Chen X., Yang Y., Huang H.M., Zhang L., Zhang X., Zhang L., Huang C., Meng X.M., Li J. Wogonin attenuates inflammation by activating PPAR-gamma in alcoholic liver disease. Int. Immunopharm. 2017;50:95–106. doi: 10.1016/j.intimp.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 122.Masoud G.N., Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015;5(5):378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu T.H., Lin T.Y., Yang P.M., Li W.T., Yeh C.T., Pan T.L. Scutellaria baicalensis induces cell apoptosis and elicits mesenchymal-epithelial transition to alleviate metastatic hepatocellular carcinoma via modulating HSP90beta. Int. J. Mol. Sci. 2024;25(5):3073. doi: 10.3390/ijms25053073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu J., Wang H., Wang J., Chang Q., Hu Z., Shen X., Feng J., Zhang Z., Wu X. Total flavonoid aglycones extract in Radix Scutellariae induces cross-regulation between autophagy and apoptosis in pancreatic cancer cells. J. Ethnopharmacol. 2019;235:133–140. doi: 10.1016/j.jep.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 125.Kong N., Chen X., Feng J., Duan T., Liu S., Sun X., Chen P., Pan T., Yan L., Jin T., Xiang Y., Gao Q., Wen C., Ma W., Liu W., Zhang M., Yang Z., Wang W., Zhang R., Chen B., Xie T., Sui X., Tao W. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin. B. 2021;11(12):4045–4054. doi: 10.1016/j.apsb.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woo S.U., Jang H.R., Chin Y.W., Yim H. 7-O-Methylwogonin from Scutellaria baicalensis disturbs mitotic progression by inhibiting Plk1 activity in Hep3B cells. Planta Med. 2019;85(3):217–224. doi: 10.1055/a-0731-0394. [DOI] [PubMed] [Google Scholar]
- 127.Wang P., Cao J., Feng Z., Tang Y., Han X., Mao T., Li S., Guo Q., Ke X., Zhang X. Oroxylin a promoted apoptotic extracellular vesicles transfer of glycolytic kinases to remodel immune microenvironment in hepatocellular carcinoma model. Eur. J. Pharmacol. 2023;957 doi: 10.1016/j.ejphar.2023.176037. [DOI] [PubMed] [Google Scholar]
- 128.Chen Y., Zheng J., Mo L., Chen F., Li R., Wang Y., Liang Q., Chen Z., Dai W., Chen L., Yan P., Zhou H., Li X. Oroxylin A suppresses breast cancer-induced osteoclastogenesis and osteolysis as a natural RON inhibitor. Phytomedicine. 2024;129 doi: 10.1016/j.phymed.2024.155688. [DOI] [PubMed] [Google Scholar]
- 129.Li C., Lin G., Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011;32(8):427–445. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- 130.Zhi H., Jin X., Zhu H., Li H., Zhang Y., Lu Y., Chen D. Exploring the effective materials of flavonoids-enriched extract from Scutellaria baicalensis roots based on the metabolic activation in influenza A virus induced acute lung injury. J. Pharm. Biomed. Anal. 2020;177 doi: 10.1016/j.jpba.2019.112876. [DOI] [PubMed] [Google Scholar]
- 131.Cheng K., Wu Z., Gao B., Xu J. Analysis of influence of baicalin joint resveratrol retention enema on the TNF-alpha, SIgA, IL-2, IFN-gamma of rats with respiratory syncytial virus infection. Cell Biochem. Biophys. 2014;70(2):1305–1309. doi: 10.1007/s12013-014-0055-9. [DOI] [PubMed] [Google Scholar]
- 132.Lee I.S., Park S., Park K., Choue R. Hepatoprotective activity of scutellariae radix extract in mice fed a high fat diet with chronic alcohol exposure. Phytother Res. 2011;25(9):1348–1353. doi: 10.1002/ptr.3370. [DOI] [PubMed] [Google Scholar]
- 133.Liao C.C., Day Y.J., Lee H.C., Liou J.T., Chou A.H., Liu F.C. ERK signaling pathway plays a key role in baicalin protection against acetaminophen-induced liver injury. Am. J. Chin. Med. 2017;45(1):105–121. doi: 10.1142/S0192415X17500082. [DOI] [PubMed] [Google Scholar]
- 134.Zhou H.C., Wang H., Shi K., Li J.M., Zong Y., Du R. Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules. 2018;24(1):131. doi: 10.3390/molecules24010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zaghloul R.A., Zaghloul A.M., El-Kashef D.H. Hepatoprotective effect of Baicalin against thioacetamide-induced cirrhosis in rats: targeting NOX4/NF-kappaB/NLRP3 inflammasome signaling pathways. Life Sci. 2022;295 doi: 10.1016/j.lfs.2022.120410. [DOI] [PubMed] [Google Scholar]
- 136.Yune T.Y., Lee J.Y., Cui C.M., Kim H.C., Oh T.H. Neuroprotective effect of Scutellaria baicalensis on spinal cord injury in rats. J. Neurochem. 2009;110(4):1276–1287. doi: 10.1111/j.1471-4159.2009.06214.x. [DOI] [PubMed] [Google Scholar]
- 137.Yang J., Wu X., Yu H., Liao X., Teng L. NMDA receptor-mediated neuroprotective effect of the Scutellaria baicalensis Georgi extract on the excitotoxic neuronal cell death in primary rat cortical cell cultures. 2014. ScientificWorldJournal 2014. [DOI] [PMC free article] [PubMed]
- 138.Zhao F., Zhang C., Xiao D., Zhang W., Zhou L., Gu S., Qu R. Radix scutellariae ameliorates stress-induced depressive-like behaviors via protecting neurons through the TGFbeta3-smad2/3-Nedd9 signaling pathway. Neural Plast. 2020;2020 doi: 10.1155/2020/8886715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galimberti D., Scarpini E. Inflammation and oxidative damage in Alzheimer's disease: friend or foe? Front. Biosci. 2011;3(1):252–266. doi: 10.2741/s149. [DOI] [PubMed] [Google Scholar]
- 140.Song Q., Peng S., Zhu X. Baicalein protects against MPP(+)/MPTP-induced neurotoxicity by ameliorating oxidative stress in SH-SY5Y cells and mouse model of Parkinson's disease. Neurotoxicology. 2021;87:188–194. doi: 10.1016/j.neuro.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 141.Chen H.L., Yang L., Zhang X.L., Jia Q.Y., Duan Z.D., Li J.J., Zheng L.Y., Liu T.T., Qi Z., Yuan Y., Wu C.Y. Scutellarin acts via MAPKs pathway to promote M2 polarization of microglial cells. Mol. Neurobiol. 2023;60(8):4304–4323. doi: 10.1007/s12035-023-03338-3. [DOI] [PubMed] [Google Scholar]
- 142.Wang L., Li C., Sreeharsha N., Mishra A., Shrotriya V., Sharma A. Neuroprotective effect of Wogonin on Rat's brain exposed to gamma irradiation. J. Photochem. Photobiol., B. 2020;204 doi: 10.1016/j.jphotobiol.2020.111775. [DOI] [PubMed] [Google Scholar]
- 143.Ren J.L., Zhang A.H., Kong L., Han Y., Yan G.L., Sun H., Wang X.J. Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153165. [DOI] [PubMed] [Google Scholar]
- 144.Li X., Wang M., Zhao J., Guo H., Gao X., Xiong Z., Zhao L. Ultrasound-assisted emulsification liquid phase microextraction method based on deep eutectic solvent as extraction solvent for determination of five pesticides in traditional Chinese medicine. J. Pharm. Biomed. Anal. 2019;166:213–221. doi: 10.1016/j.jpba.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 145.Hao C., Chen L., Dong H., Xing W., Xue F., Cheng Y. Extraction of flavonoids from scutellariae radix using ultrasound-assisted deep eutectic solvents and evaluation of their anti-inflammatory activities. ACS Omega. 2020;5(36):23140–23147. doi: 10.1021/acsomega.0c02898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo X., Ren T., Li H., Lu Q., Di X. Antioxidant activity and mechanism exploration for microwave-assisted extraction of flavonoids from Scutellariae Radix using natural deep eutectic solvent. Microchem. J. 2024;200 doi: 10.1016/j.microc.2024.110300. [DOI] [Google Scholar]
- 147.Yun C., Wang S., Gao Y., Zhao Z., Miao N., Shi Y., Ri I., Wang W., Wang H. Optimization of ultrasound-assisted enzymatic pretreatment for enhanced extraction of baicalein and wogonin from Scutellaria baicalensis roots. J. Chromatogr. B. 2022;1188 doi: 10.1016/j.jchromb.2021.123077. [DOI] [PubMed] [Google Scholar]
- 148.Yun C., Ji X., Chen Y., Zhao Z., Gao Y., Gu L., She D., Ri I., Wang W., Wang H. Ultrasound-assisted enzymatic extraction of Scutellaria baicalensis root polysaccharide and its hypoglycemic and immunomodulatory activities. Int. J. Biol. Macromol. 2023;227:134–145. doi: 10.1016/j.ijbiomac.2022.12.115. [DOI] [PubMed] [Google Scholar]
- 149.Ma X.D., Zhang X.G., Guo S.J., Ma G.Y., Liu W.J., Wang N., Feng M., Su Y. Application of enzyme-assisted extraction of baicalin from Scutellaria baicalensis Georgi. Prep. Biochem. Biotechnol. 2021;51(3):241–251. doi: 10.1080/10826068.2020.1808791. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Q., Zhao S.H., Chen J., Zhang L.W. Application of ionic liquid-based microwave-assisted extraction of flavonoids from Scutellaria baicalensis Georgi. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2015;1002:411–417. doi: 10.1016/j.jchromb.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 151.Wang L., Cai C., Liu J., Tan Z. Selective separation of the homologues of baicalin and baicalein from Scutellaria baicalensis Georgi using a recyclable ionic liquid-based liquid-liquid extraction system. Process Biochem. 2021;103:1–8. doi: 10.1016/j.procbio.2021.02.001. [DOI] [Google Scholar]
- 152.Wang H., Ma X., Cheng Q., Wang L., Zhang L. Deep eutectic solvent-based ultrahigh pressure extraction of baicalin from Scutellaria baicalensis Georgi. Molecules. 2018;23(12):3233. doi: 10.3390/molecules23123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang Y., Li H., Hai X., Guo X., Di X. Designing green and recyclable switchable supramolecular deep eutectic solvents for efficient extraction of flavonoids from Scutellariae Radix and mechanism exploration. J. Chromatogr. A. 2024;1730 doi: 10.1016/j.chroma.2024.465084. [DOI] [PubMed] [Google Scholar]
- 154.Xiao J., Chen G., Li N. Ionic liquid solutions as a green tool for the extraction and isolation of natural products. Molecules. 2018;23(7):1765. doi: 10.3390/molecules23071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li X., Wang R.Y., Ma L., Cui J.X., Hong W.X. Application of the quality evaluation of traditional Chinese herbal medicines using chromatography of fingerprints. J. Biomed. Eng. 2012;29(1):192–196. [PubMed] [Google Scholar]
- 156.Han Q.W., Zhou B., Li Y.P., Gu Z.H. Quality evaluation of Shenlian capsules by HPLC fingerprint and pattern recognition. Chin. J. Pharm. Anal. 2020;40(7):1300–1308. doi: 10.16155/j.0254-1793.2020.07.19. [DOI] [Google Scholar]
- 157.Yu K., Gong Y., Lin Z., Cheng Y. Quantitative analysis and chromatographic fingerprinting for the quality evaluation of Scutellaria baicalensis Georgi using capillary electrophoresis. J. Pharm. Biomed. Anal. 2007;43(2):540–548. doi: 10.1016/j.jpba.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen C.Y., Chen J.X., Li W., Li H., Yang B. Comparative chemical and statistical analysis of cultivated and wild Radix Scutellariae. Am. J. Chin. Med. 2011;39(5):1029–1041. doi: 10.1142/S0192415X1100938X. [DOI] [PubMed] [Google Scholar]
- 159.Long T., Yu J., Huang Y., Shi Z., Xu L. Rapid fingerprint analysis of radix scutellariae by UFLC-DAD. J. Chromatogr. Sci. 2013;51(10):939–942. doi: 10.1093/chromsci/bms193. [DOI] [PubMed] [Google Scholar]
- 160.Yan G.L., Fang Y., Liu S.M., Fan Z.Q., Zu J.X. Studies on UPLC-MS characteristic fingerprint of Scutellaria baicalensis. Chin. J. Exp. Tradit. Med. Formulae. 2012;18(24):89–93. doi: 10.13422/j.cnki.syfjx.2012.24.026. [DOI] [Google Scholar]
- 161.Huang Z., Yu Y., Yang H.L., Wang Y.F., Huang J.L., Xiao L., Liang M., Qi J. Screening antibacterial constituents of Scutellaria radix based on spectrum-effect relationships between HPLC fingerprints and the inhibition of oral bacteria. J. Chromatogr. Sci. 2023;62(1):74–84. doi: 10.1093/chromsci/bmad013. [DOI] [PubMed] [Google Scholar]
- 162.Zhu Y., Sun L.Q., Yang Y.D., Qi J., Yu B.Y. Application of multi-dimensional and multi-informational (MD-MI) integrated xanthine oxidase and superoxide anion fingerprint in quality evaluation of Scutellariae Radix. J. Pharm. Biomed. Anal. 2020;191 doi: 10.1016/j.jpba.2020.113595. [DOI] [PubMed] [Google Scholar]
- 163.Islam M.N., Chung H.J., Kim D.H., Yoo H.H. A simple isocratic HPLC method for the simultaneous determination of bioactive components of Scutellariae radix extract. Nat. Prod. Res. 2012;26(21):1957–1962. doi: 10.1080/14786419.2011.631134. [DOI] [PubMed] [Google Scholar]
- 164.Cui X., Cai H., Li H., Tao Y., Huang P., Qian X., Li J., Cai B. Simultaneous determination of 10 flavonoids in crude and wine-processed radix scutellariae by UHPLC. J. Chromatogr. Sci. 2016;54(3):312–317. doi: 10.1093/chromsci/bmv143. [DOI] [PubMed] [Google Scholar]
- 165.Qiao X., Li R., Song W., Miao W.J., Liu J., Chen H.B., Guo D.A., Ye M. A targeted strategy to analyze untargeted mass spectral data: rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J. Chromatogr., A. 2016;1441:83–95. doi: 10.1016/j.chroma.2016.02.079. [DOI] [PubMed] [Google Scholar]
- 166.Zhang F., Li Z., Li M., Yuan Y., Cui S., Chen J., Li R. An integrated strategy for profiling the chemical components of Scutellariae Radix and their exogenous substances in rats by ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2020;34(18) doi: 10.1002/rcm.8823. [DOI] [PubMed] [Google Scholar]
- 167.Tang W.T., Fang M.F., Liu X., Yue M. Simultaneous quantitative and qualitative analysis of flavonoids from ultraviolet-B radiation in leaves and roots of Scutellaria baicalensis Georgi using LC-UV-ESI-Q/TOF/MS. J Anal Methods Chem. 2014;2014 doi: 10.1155/2014/643879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu C.X., Chen S.L., Xiao X.H., Zhang T.J., Hou W.B., Liao M.L. A new concept on quality marker of Chinese materia medica: quality control for Chinese medicinal products. Chin. Tradit. Herb. Drugs. 2016;47(9):1443–1457. [Google Scholar]
- 169.Chen L.H., Xiao F.L., Huang S.Y., Zhu W.F., GuanN Y.M., Ying P.Y., lV Z.H. Research ideas and innovation development trends of quality evaluation of Chinese materia medica. Chin. Tradit. Herb. Drugs. 2021;52(9):2541–2547. [Google Scholar]
- 170.He H., Han S., Zhang T., Zhang J., Wang S., Hou J. Screening active compounds acting on the epidermal growth factor receptor from Radix scutellariae via cell membrane chromatography online coupled with HPLC/MS. J. Pharm. Biomed. Anal. 2012;62:196–202. doi: 10.1016/j.jpba.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 171.Wang L., Tan N., Wang H., Hu J., Diwu W., Wang X. A systematic analysis of natural alpha-glucosidase inhibitors from flavonoids of Radix scutellariae using ultrafiltration UPLC-TripleTOF-MS/MS and network pharmacology. BMC Complement Med Ther. 2020;20(1):72. doi: 10.1186/s12906-020-2871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xia J., Liu C., Niu H., Hou W., Li S. Screening and isolation of potential lipoxidase and superoxide dismutase inhibitors from Scutellaria baicalensis Georgi using high-speed countercurrent chromatography target-guided by ultrafiltration-liquid chromatography-mass spectrometry. J. Separ. Sci. 2021;44(7):1371–1382. doi: 10.1002/jssc.202001072. [DOI] [PubMed] [Google Scholar]
- 173.Hu L., Xiong Y., Zou Z., Yang Y., He J., Zhong L., Wang Y., Yang M. Identifying the chemical markers in raw and wine-processed Scutellaria baicalensis by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry coupled with multiple statistical strategies. Biomed. Chromatogr. 2020;34(8):e4849. doi: 10.1002/bmc.4849. [DOI] [PubMed] [Google Scholar]
- 174.Han Y.K., Kim H., Shin H., Song J., Lee M.K., Park B., Lee K.Y. Characterization of anti-inflammatory and antioxidant constituents from Scutellaria baicalensis using LC-MS coupled with a bioassay method. Molecules. 2020;25(16):3617. doi: 10.3390/molecules25163617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dinda B., Dinda S., DasSharma S., Banik R., Chakraborty A., Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 176.Wozniak D., Drys A., Matkowski A. Antiradical and antioxidant activity of flavones from Scutellariae baicalensis radix. Nat. Prod. Res. 2015;29(16):1567–1570. doi: 10.1080/14786419.2014.983920. [DOI] [PubMed] [Google Scholar]
- 177.Wang L., Feng T., Su Z., Pi C., Wei Y., Zhao L. Latest research progress on anticancer effect of baicalin and its aglycone baicalein. Arch Pharm. Res. (Seoul) 2022;45(8):535–557. doi: 10.1007/s12272-022-01397-z. [DOI] [PubMed] [Google Scholar]
- 178.Syafni N., Devi S., Zimmermann-Klemd A.M., Reinhardt J.K., Danton O., Grundemann C., Hamburger M. Immunosuppressant flavonoids from Scutellaria baicalensis. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112326. [DOI] [PubMed] [Google Scholar]
- 179.Pan L., Cho K.S., Yi I., To C.H., Chen D.F., Do C.W. Baicalein, baicalin, and wogonin: protective effects against ischemia-induced neurodegeneration in the brain and retina. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/8377362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li Q.Q., Liu Z.Z., Zhang J., Xu D.L. Advances in the biosynthesis of medicinally active ingredients in Chinese medicine. Chin. Tradit. Pat. Med. 2022;44(11):3603–3608. [Google Scholar]
- 181.Nabavi S.M., Samec D., Tomczyk M., Milella L., Russo D., Habtemariam S., Suntar I., Rastrelli L., Daglia M., Xiao J., Giampieri F., Battino M., Sobarzo-Sanchez E., Nabavi S.F., Yousefi B., Jeandet P., Xu S., Shirooie S. Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol. Adv. 2020;38 doi: 10.1016/j.biotechadv.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 182.Liu W., Feng Y., Yu S., Fan Z., Li X., Li J., Yin H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222312824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao Q., Zhang Y., Wang G., Hill L., Weng J.K., Chen X.Y., Xue H., Martin C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016;2(4) doi: 10.1126/sciadv.1501780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao Q., Yang J., Cui M.Y., Liu J., Fang Y., Yan M., Qiu W., Shang H., Xu Z., Yidiresi R., Weng J.K., Pluskal T., Vigouroux M., Steuernagel B., Wei Y., Yang L., Hu Y., Chen X.Y., Martin C. The reference genome sequence of Scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol. Plant. 2019;12(7):935–950. doi: 10.1016/j.molp.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 185.Nagashima S., Hirotani M., Yoshikawa T. Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. cell suspension cultures. Phytochemistry. 2000;53(5):533–538. doi: 10.1016/s0031-9422(99)00593-2. [DOI] [PubMed] [Google Scholar]
- 186.Zhao Q., Cui M.Y., Levsh O., Yang D., Liu J., Li J., Hill L., Yang L., Hu Y., Weng J.K., Chen X.Y., Martin C. Two CYP82D enzymes function as flavone hydroxylases in the biosynthesis of root-specific 4'-deoxyflavones in Scutellaria baicalensis. Mol. Plant. 2018;11(1):135–148. doi: 10.1016/j.molp.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pei T., Yan M., Li T., Li X., Yin Y., Cui M., Fang Y., Liu J., Kong Y., Xu P., Zhao Q. Characterization of UDP-glycosyltransferase family members reveals how major flavonoid glycoside accumulates in the roots of Scutellaria baicalensis. BMC Genom. 2022;23(1):169. doi: 10.1186/s12864-022-08391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jeon Y.-M., Son K.-H., Kim S.-M., Oh M.-M. Growth of dropwort plants and their accumulation of bioactive compounds after exposure to UV lamp or LED irradiation. Hortic. Environ. Biotechnol. 2018;59(5):659–670. doi: 10.1007/s13580-018-0076-1. [DOI] [Google Scholar]
- 189.Lim Y.J., Lyu J.I., Kwon S.J., Eom S.H. Effects of UV-A radiation on organ-specific accumulation and gene expression of isoflavones and flavonols in soybean sprout. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.128080. [DOI] [PubMed] [Google Scholar]
- 190.Miao N., Yun C., Shi Y., Gao Y., Wu S., Zhang Z., Han S., Wang H., Wang W. Enhancement of flavonoid synthesis and antioxidant activity in Scutellaria baicalensis aerial parts by UV-A radiation. Ind. Crop. Prod. 2022;187 doi: 10.1016/j.indcrop.2022.115532. [DOI] [Google Scholar]
- 191.Miao N., Yun C., Han S., Shi Y., Gao Y., Wu S., Zhao Z., Wang H., Wang W. Postharvest UV-A radiation affects flavonoid content, composition, and bioactivity of Scutellaria baicalensis root. Postharvest Biol. Technol. 2022;189 doi: 10.1016/j.postharvbio.2022.111933. [DOI] [Google Scholar]
- 192.Holl J., Lindner S., Walter H., Joshi D., Poschet G., Pfleger S., Ziegler T., Hell R., Bogs J., Rausch T. Impact of pulsed UV-B stress exposure on plant performance: how recovery periods stimulate secondary metabolism while reducing adaptive growth attenuation. Plant Cell Environ. 2019;42(3):801–814. doi: 10.1111/pce.13409. [DOI] [PubMed] [Google Scholar]
- 193.Takshak S., Agrawal S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol., B. 2019;193:51–88. doi: 10.1016/j.jphotobiol.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 194.Zhang J.J., Li X.Q., Sun J.W., Jin S.H. Nitric oxide functions as a signal in ultraviolet-B-induced baicalin accumulation in Scutellaria baicalensis suspension cultures. Int. J. Mol. Sci. 2014;15(3):4733–4746. doi: 10.3390/ijms15034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yun C., Zhao Z., Ri I., Gao Y., Shi Y., Miao N., Gu L., Wang W., Wang H. How does UV-B stress affect secondary metabolites of Scutellaria baicalensis in vitro shoots grown at different 6-benzyl aminopurine concentrations? Physiol. Plantarum. 2022;174(5) doi: 10.1111/ppl.13778. [DOI] [PubMed] [Google Scholar]
- 196.Yeo H.-J., Park C.-H., Park S.-Y., Chung S.-O., Kim J.-K., Park S.-U. Metabolic analysis of root, stem, and leaf of Scutellaria baicalensis plantlets treated with different LED lights. Plants. 2021;10(5):940. doi: 10.3390/plants10050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Albergaria E.T., Oliveira A.F.M., Albuquerque U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. South Afr. J. Bot. 2020;131:12–17. doi: 10.1016/j.sajb.2020.02.002. [DOI] [Google Scholar]
- 198.Wu K., Yuan Y., Liu Y., Wu C., Chen S., Wang Z., Yang Z., Qin S., Huang L. Water deficit affected flavonoid accumulation by regulating hormone metabolism in Scutellaria baicalensis Georgi roots. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0042946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cheng L., Han M., Yang L.-m., Li Y., Sun Z., Zhang T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crop. Prod. 2018;122:473–482. doi: 10.1016/j.indcrop.2018.06.030. [DOI] [Google Scholar]
- 200.Yuan Y., Shuai L., Chen S., Huang L., Qin S., Yang Z. Flavonoids and antioxidative enzymes in temperature-challenged roots of Scutellaria baicalensis Georgi. Z. Naturforsch., C: J. Biosci. 2012;67(1–2):77–85. doi: 10.1515/znc-2012-1-210. [DOI] [PubMed] [Google Scholar]
- 201.Hou Y.L., Han M., Yang L.M., Cheng L., Luo Y. Effect of temperature on content of baicalin and expression of key enzyme genes in callus of Scutellaria baicalensis, China J. Chin. Mater. Med. 2018;43(13):2670–2675. doi: 10.19540/j.cnki.cjcmm.20180510.010. [DOI] [PubMed] [Google Scholar]
- 202.Yeo H.J., Park C.H., Kim J.K., Sathasivam R., Jeong J.C., Kim C.Y., Park S.U. Effects of chilling treatment on baicalin, baicalein, and wogonin biosynthesis in Scutellaria baicalensis plantlets. Plants. 2022;11(21):2958. doi: 10.3390/plants11212958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weidemuller P., Kholmatov M., Petsalaki E., Zaugg J.B. Transcription factors: bridge between cell signaling and gene regulation. Proteomics. 2021;21(23–24) doi: 10.1002/pmic.202000034. [DOI] [PubMed] [Google Scholar]
- 204.Fang Y., Liu J., Zheng M., Zhu S., Pei T., Cui M., Chang L., Xiao H., Yang J., Martin C., Zhao Q. SbMYB3 transcription factor promotes root-specific flavone biosynthesis in Scutellaria baicalensis. Hortic. Res. 2023;10(2) doi: 10.1093/hr/uhac266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang W., Hu S., Yang J., Zhang C., Zhang T., Wang D., Cao X., Wang Z. A novel r2r3-MYB transcription factor SbMYB12 positively regulates baicalin biosynthesis in Scutellaria baicalensis Georgi. Int. J. Mol. Sci. 2022;23(24) doi: 10.3390/ijms232415452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fang S., Qiu S., Chen K., Lv Z., Chen W. The transcription factors SbMYB45 and SbMYB86.1 regulate flavone biosynthesis in Scutellaria baicalensis. Plant Physiol. Biochem. 2023;200 doi: 10.1016/j.plaphy.2023.107794. [DOI] [PubMed] [Google Scholar]
- 207.Wang W., Hu S., Zhang C., Yang J., Zhang T., Wang D., Cao X., Wang Z. Systematic analysis and functional characterization of r2r3-MYB genes in Scutellaria baicalensis Georgi. Int. J. Mol. Sci. 2022;23(16):9342. doi: 10.3390/ijms23169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Park C.H., Xu H., Yeo H.J., Park Y.E., Hwang G.-S., Park N.I., Park S.U. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metab. Eng. 2021;64:64–73. doi: 10.1016/j.ymben.2021.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.